Improvement of Bowel Movement in Healthy Adults Through Intake of Natto Powder For 4 Weeks - A Randomized, Placebo-Controlled, Double-Blind, Clinical Study
by Kenji Kikushima1, Keisuke Suzuki2, Kengo Saitou2, Tomoki Matsuura2, Kouji Sakurai2, Kana Matsuzaki3, Keiko Ohnuki3, Koichiro Ohnuki3,4*
1Department of Nutrition, Faculty of Health Science, Kochi Gakuen University, 780-0955 Kochi, Japan
2Yamada Foods Co., Ltd., 019-1301 Akita, Japan
3User Life Science Co., Ltd., 820-0115 Fukuoka, Japan
4Department of Biological and Environmental Chemistry, Kindai University, 820-8555 Fukuoka, Japan
*Corresponding author: Koichiro Ohnuki, Department of Biological and Environmental Chemistry, Kindai University11-6, Kayanomori, Iizuka, Fukuoka 820-8555 Japan
Received Date: 20 August 2025
Accepted Date: 29 August 2025
Published Date: 03 September 2025
Citation: Kikushima K, Suzuki K, Saitou K, Matsuura T, Sakurai K, et al. (2025) Improvement of Bowel Movement in Healthy Adults Through Intake of Natto Powder For 4 Weeks - A Randomized, Placebo-Controlled, Double-Blind, Clinical Study. Food Nutr J 10: 329. https://doi.org/10.29011/2575-7091.100329
Abstract
Natto is a traditional Japanese food, made from whole soybeans fermented with Bacillus subtilis var. natto. Although natto bacteria regulate intestinal flora, little research has been conducted on its effects on bowel movements. This study examined the effects of natto powder on human health, including bowel movements, mood, fatigue, and sleep quality. This randomized, double-blind, placebo-controlled, parallel-group comparative study was conducted on 30 healthy adults. For 4 weeks, the subjects consumed either 3 g of natto powder or an equivalent amount of placebo powder daily. In Visual Analogue Scale, almost all items showed improvement in bowel movements in both groups. However, the change in pain during defecation was greater in the test group than in the placebo group, resulting in a significant difference between the two groups. Changes in the stool color and odor were also to be greater in the test group than in the placebo group. Additionally, we found that the test powder reduced fatigue and improved sleep quality more effectively than the placebo powder. For those not accustomed to natto, powdered natto is a convenient way to enjoy its health benefits.
Keywords: Natto; Fermented Foods; Bowel Movement; Clinical Trial; Traditional Food
Introduction
Fermented foods offer a wide range of health benefits. As well as being a great source of probiotics, they contain bacteria that can produce bioactive metabolites which promote health. Natto is a traditional Japanese food, made from whole soybeans fermented with Bacillus subtilis var. natto. Natto has a long history and is possibly derived from Chinese douchi, a food made from fermented black soybeans, which is mentioned in Engishiki, a comprehensive Japanese legal compendium completed in 927 [1]. The Japanese people have long been familiar with natto, and the book Honchoushokukagami (published in 1697) states that natto "regulates the stomach, promotes appetite, and detoxifies," indicating that its health benefits were recognized early on [2].
Natto has a sticky texture and distinctive flavor. In addition to the nutrients contained in soybeans, natto is renowned for its nutritional profile and potential health benefits. It is rich in protein, fiber, vitamins, and minerals. The sticky texture is the result of poly-γ-glutamic acid (γ-PGA) forming during fermentation [3]. γ-PGA has been reported to offer several health benefits, including improved calcium [4] and vitamin absorption [5], enhanced sleep quality [5], and potential effects on blood glucose and lipid levels [6]. Natto is the richest dietary source of vitamin K2. Consuming natto increases the amount of vitamin K2 in the blood [7,8], which is important for bone health and blood clotting. Moreover, nattokinase, a byproduct of the fermentation, is renowned for its ability to break down blood clots and reduce blood pressure [9]. B. natto is non-pathogenic to humans and one of the 40 probiotics approved by the US FDA [10,11]. B. natto can easily reach the intestines alive [10,12]. The presence of natto bacteria and the enzymes and dietary fiber they produce are expected to improve intestinal flora boost immunity [13]. Natto bacteria also produce a powerful antibacterial substance called dipicolinic acid [14]. Dipicolinic acid has a broad antibacterial spectrum [8] and plays a role in maintaining a healthy intestinal flora. However, research on the effects of natto on the intestines is still insufficient.
In Japan, natto is commonly eaten for breakfast alongside rice. While over 70% of Japanese people enjoy natto and consider worth eating for its numerous health benefits [15], some people may need time to get used to its taste [16]. For those unfamiliar with natto, powdered natto is one of the best ways to enjoy this intriguing superfood. In this study, we examined the effect of natto powder on the human health including bowel movement, mood, fatigue, and sleep quality. A randomized, double-blind, placebo-controlled, parallel-group comparative study was conducted on 32 healthy adults between the ages of 30 and 70 to examine the effects of natto powder intake on intestinal regulation. For 4 weeks, the subjects consumed either 3 g of natto powder or an equivalent amount of placebo powder daily. Their bowel movements and health status were evaluated using a questionnaire before and after the intervention.
Materials and Methods
The study was conducted by User Life Science Co., Ltd. (Fukuoka, Japan) commissioned by Yamada Foods Co., Ltd. (Akita, Japan). All examinations were conducted by mail correspondence. All studies strictly adhered to the Declaration of Helsinki (revised in Fortaleza in 2013) and the Ethical Guidelines for Medical and Health Research Involving Human Subjects, which were published by the Japanese government in 2021. Written informed consent was obtained before the study, by carefully explaining the purpose and methods of the study and confirming that all subjects recognized them and were participating in the study of their own volition. The study protocol is registered at the UMIN Clinical Trials Registry (UMIN-CTR; ID = UMIN000056978).
In this study, a randomized, double-blind, placebo-controlled, parallel-group comparative trial was conducted to verify the intestinal effects of natto powder consumption in healthy adults. Subjects were carefully chosen following the selection and the exclusion criteria. Selection criteria: (1) those with age between 30-70 years old, (2) those regarded as generally healthy, (3) those submitting the agreement for participating in the trial by their own will, and (4) those who are aware of constipation. Exclusion Criteria: (1) those who has regularly took any other functional foods, drugs or supplements for the similar effects to our tests, (2) those who changed or began to take any health foods in the past 4 weeks, (3) those working at the nighttime or by the day and night shift, (4) those who needed to have any medical treatments such as hormone replacement therapy, drug treatment, exercise therapy or food therapy and so on, (5) those who had previously had severe disorders of sugar metabolism, lipid metabolism, liver function, renal function, heart, circulatory organ, respiratory organs, endocrine system, immune system, or neurological diseases, (6) those who had a history of alcoholic or drug addiction, (7) those who had risk of allergic reaction against the food, (8) those who were pregnant or lactating at the informed consent, or wanted to become pregnant during the trial period, (9) those who participated in another human clinical trial within 1 month prior to the time of consent, and (10) those who are judged inappropriate by the primary investigator of the study.
A randomization coordinator unrelated to the trial randomly divided the 32 subjects into test and control groups of 16 subjects each, using stratified randomization by age and BMI (body mass index) to ensure that there was no significant bias between the two groups. Each subject took either 3 g of natto powder or 3 g of placebo powder made from the same soybeans per day for 4 weeks (28 days). The test powder and the placebo powder were provided by Yamada Foods Co., Ltd. The appearance and smell of the test powder and the placebo powder are indistinguishable. The test powder was made from Ryuhou soybean grown in Akita, Japan, fermented by bacterium Bacillus subtilis natto isolated from the Shirakami Mountains, a World Natural Heritage site. The nutritional information for 1 g of the test powder is as follows: energy 4 kcal, protein 0.4 g, fat 0.2 g, carbohydrates 0.3 g (of which sugars 0.1 g and dietary fiber 0.2 g), and salt equivalent 0.2 mg. The placebo powder was not fermented by Bacillus natto and was dried in the same manner as the test powder.
The initial measurement was performed in mid-February 2025, and the final measurement was conducted in mid-March. Changes in the subjects' condition were evaluated using a questionnaire consisting of the following 5 items: a visual analogue scale to evaluate stool consistency (VAS-S), a mood scale (POMS2) for adults, the Chalder Fatigue Scale (CFS), the OSA Sleep Inventory-MA version (OSA-MA) and a VAS to evaluate fatigue severity (VAS-F). All these tests were performed at the initial and final measurements, which were taken with intervals of 28 days, before and after the intervention.
Any problems that occurred during the trial were considered adverse events. However, events that were recognized before informed consent was obtained, or that were predictable based on the subjects past daily experiences and conditions before the trial began, were not considered adverse events. Subjects were instructed to report any adverse events that occurred following the intervention.
The mean values and standard errors for each measurement for the test and placebo groups were calculated. To compare values before and after the trial, a paired t-test was performed. Additionally, an independent t-test was performed on the scores of the test and the placebo groups at the pre- and the post-intervention, respectively.
The significance level was set at 5% for two-sided tests in both analyses, and values below 5% were considered significant (*p < 0.05; **p < 0.01).
Results
Completion status
No adverse events were reported during the trial period. Two subjects withdrew from the study for reasons unrelated to the trial: one male from the test group and one male from the placebo group. This left 15 subjects in each group who completed the study. The remaining 30 subjects were analyzed for VAS-S, POMS2 and CFS. The analyzed subjects' data is shown in Table 1. There were no significant differences between the two groups in any of the profiles.
|
Group |
Male |
Female |
Age (years) |
Height (cm) |
Weight (Kg) |
BMI |
|
Test group (n = 15) |
8 |
7 |
53.8 (1.5) |
164.5 (2.0) |
60.0 (2.5) |
22.0 (0.6) |
|
Placebo group (n = 15) |
7 |
8 |
53.2 (2.3) |
164.9 (2.5) |
63.8 (3.5) |
23.5 (0.8) |
|
P-value between two groups |
- |
- |
0.8 |
0.9 |
0.4 |
0.2 |
|
() denotes standard error |
||||||
Table 1: Profiles of the subjects who completed the trial.
Additionally, 2 subjects from the placebo group were excluded from the OSA-MA analysis due to incomplete documentation, resulting in 15 subjects from the test group and 13 subjects from the placebo group being included. One subjects from the test group was excluded from the VAS-F analysis, resulting in 14 test group subjects and 15 placebo group subjects being included.
VAS-S
The VAS-S is a questionnaire-style survey that evaluates the consistency and shape of stools using the Visual Analogue Scale (VAS) method. The questionnaire consists of six items: pain during defecation, sensation of incomplete evacuation, straining during defecation, stool color, stool volume, and stool odor. For each test item, a 100 mm long horizontal straight line is drawn, with the left endpoint (0 mm) representing poor condition and the right endpoint (100 mm) representing excellent condition. Subjects self-assess their current stool condition by drawing a short vertical line on the long horizontal line. The VAS score was determined by measuring the distance from the left end point to the vertical short line made by the subjects (1 mm = 1). Higher scores indicate better condition.
The results showed an increase in scores of all items in both the test group and the placebo group (Table 2). When comparing before and after scores in each group, significant differences (p < 0.05) were observed in all items except for stool odor in the placebo group. Additionally, significant differences were observed in post- intervention scores for pain during defecation (p < 0.05) when comparing the two groups.
|
Group |
Mean score at pre-intervention |
P-value between two groups |
Mean score at post-intervention |
P-value between two groups |
P-value between pre- and post-intervention |
|
|
Pain during defecation |
Test group (n = 15) |
64.9 (7.6) |
0.7 |
93.7 (2.3) |
0.03* |
0.002** |
|
Placebo group (n = 15) |
60.4 (7.2) |
79.9 (5.4) |
0.02* |
|||
|
Sensation of incomplete evacuation |
Test group (n = 15) |
39.3 (6.6) |
0.2 |
69.3 (6.5) |
0.4 |
0.001** |
|
Placebo group (n = 15) |
29.6 (3.9) |
60.9 (5.7) |
<0.001** |
|||
|
Straining during defecation |
Test group (n = 15) |
41.5 (6.4) |
0.2 |
69.1(6.6) |
0.3 |
0.002** |
|
Placebo group (n = 15) |
31.4 (4.2) |
59.9 (6.4) |
<0.001** |
|||
|
Stool color |
Test group (n = 15) |
49.9 (5.1) |
0.3 |
77.0 (5.6) |
0.07 |
0.004 ** |
|
Placebo group (n = 15) |
46.1 (4.6) |
61.0 (5.8) |
0.008** |
|||
|
Stool volume |
Test group (n = 15) |
28.7 (4.1) |
0.08 |
61.3 (7.3) |
1 |
<0.001** |
|
Placebo group (n = 15) |
36.2 (5.2) |
60.7 (4.3) |
0.002** |
|||
|
Stool odor |
Test group (n = 15) |
34.2 (4.0) |
0.8 |
59.8 (5.4) |
0.4 |
0.005** |
|
Placebo group (n = 15) |
45.1 (4.3) |
53.1 (4.3) |
0.06 |
|||
|
() denotes standard error < * p < 0.05 ** p < 0.01 |
||||||
Table 2: Change in the visual analogue scale to evaluate stool consistency (VAS-S) before and after the intake of test or placebo powder for 4 weeks.
POMS2
The Mood Scale (POMS2) is a questionnaire designed to evaluate mood in adults. For each question, subjects rate themselves on a five-point scale from 0 to 4, with 0 indicating 'not at all' and 4 indicating 'very much'. The questions are classified into seven scales and a Total Mood Disturbance (TMD) score, which comprehensively represents negative mood. For the five scales (AH: Anger-Hostility; CB: Confusion-Bewilderment; DD: Depression-Dejection; FI: Fatigue-Inertia; and TA: Tension-Anxiety), lower scores indicate a better state. For the other two scales, VA (Vigor-Activity) and F (Friendliness), higher scores are better. A higher TMD score indicates higher levels of negative emotion.
Comparison before and after the intervention showed a significant difference (p < 0.05) in VA (vitality) in the test group (Table 3). In the placebo group, a significant difference was observed in FI (fatigue-lethargy). No significant differences were observed between the two groups in all items.
|
Group |
Mean score at pre-intervention |
P-value between two groups |
Mean score at post-intervention |
P-value between two groups |
P-value between pre- and post-intervention |
|
|
Anger- Hostility |
Test group (n = 15) |
3.9 (1.1) |
0.7 |
4.5 (1.2) |
0.3 |
0.4 |
|
Placebo group (n = 15) |
3.3 (0.8) |
2.9 (0.6) |
0.5 |
|||
|
Confusion- Bewilderment |
Test group (n = 15) |
4.1 (1.0) |
0.9 |
3.5 (1.0) |
0.9 |
0.2 |
|
Placebo group (n = 15) |
4.0 (0.9) |
3.7 (1.0) |
0.6 |
|||
|
Depression- Dejection |
Test group (n = 15) |
4.2 (1.2) |
0.7 |
3.5 (1.0) |
0.7 |
0.4 |
|
Placebo group (n = 15) |
3.5 (0.9) |
2.9 (0.9) |
0.4 |
|||
|
Fatigue-Inertia |
Test group (n = 15) |
5.1 (1.2) |
0.7 |
4.2 (1.0) |
1 |
0.07 |
|
Placebo group (n = 15) |
5.9 (1.3) |
4.3 (1.0) |
0.03 * |
|||
|
Tension-Anxiety |
Test group (n = 15) |
5.9 (1.4) |
1 |
6.1 (1.5) |
0.8 |
0.8 |
|
Placebo group (n = 15) |
5.9 (0.9) |
5.7 (0.9) |
0.7 |
|||
|
Vigor-Activity |
Test group (n = 15) |
7.9 (1.3) |
0.7 |
9.8 (1.5) |
0.8 |
0.04 * |
|
Placebo group (n = 15) |
8.7 (1.2) |
9.2 (1.4) |
0.5 |
|||
|
Friendliness |
Test group (n = 15) |
11.1 (1.2) |
0.6 |
12.9 (1.1) |
0.1 |
0.07 |
|
Placebo group (n = 15) |
10.2 (1.1) |
10.4 (1.0) |
0.8 |
|||
|
TMD score |
Test group (n = 15) |
15.3 (6.0) |
0.9 |
12.1 (5.9) |
0.8 |
0.2 |
|
Placebo group (n = 15) |
14.9 (4.8) |
10.3 (4.5) |
0.09 |
|||
|
() denotes standard error 0.01 < * p < 0.05 |
||||||
Table 3: Change in the Mood Scale (POMS2) before and after the intake of test or placebo powder for 4 weeks.
CFS
The Chalder Fatigue Scale (CFS) is a questionnaire that evaluates subjective fatigue. Consisting of 14 items, it asks subjects to self-evaluate on a 4-point scale ranging from 0 (none) for the best condition to 3 (very high) for the worst. A higher total score indicates a higher degree of fatigue. Both the test group and the placebo group showed a decrease in scores after intake, and a significant difference was obtained in the pre- and post-comparison (p < 0.05) (Table 4). However, no significant difference was observed between the two groups.
|
Group |
Mean score at pre-intervention |
P-value between two groups |
Mean score at post-intervention |
P-value between two groups |
P-value between pre- and post-intervention |
|
|
Fatigue |
Test group (n = 15) |
18.5 (2.2) |
0.6 |
12.3 (2.1) |
0.9 |
0.03 * |
|
Placebo group (n=15) |
16.6 (2.8) |
12.7 (2.1) |
0.04 * |
|||
|
() denotes standard error 0.01 < * p < 0.05 |
||||||
Table 4: Change in the Chalder Fatigue Scale (CFS) before and after the intake of test or placebo powder for 4 weeks.
OSA-MA
The OSA Sleep Inventory MA Version is a psychological scale designed to evaluate sleep introspection upon waking in middle-aged and elderly individuals. This questionnaire consists of 16 items organized into five factors: Factor 1: Sleepiness upon waking, Factor 2: Initiation and maintenance of sleep, Factor 3: Frequent dreaming, Factor 4: Refreshing, and Factor 5: Sleep length. Higher scores indicate better sleep quality.
A comparison of the results before and after intake showed a significant change in factor IV (fatigue recovery) in the placebo group (p < 0.05) (Table 5). However, no significant differences were observed in any of the items, including factor IV, when comparing the test and placebo groups.
|
Group |
Mean score at pre- intervention |
P-value between two groups |
Mean score at post- intervention |
P-value between two groups |
P-value between pre- and post-intervention |
|
|
Factor 1 |
Test group (n=15) |
18.5 (1.6) |
0.4 |
22.0 (1.7) |
0.1 |
0.2 |
|
Sleepiness upon waking |
Placebo group (n=13) |
16.2 (2.2) |
18.3 (1.5) |
0.3 |
||
|
Factor 2 |
Test group (n=15) |
14.3 (1.4) |
0.09 |
19.5 (1.8) |
0.4 |
0.05 |
|
Initiation and maintenance of sleep |
Placebo group (n=13) |
18.0 (1.5) |
17.3 (1.9) |
0.8 |
||
|
Factor 3 |
Test group (n=15) |
21.2 (2.3) |
0.7 |
22.4 (1.7) |
0.7 |
0.6 |
|
Frequent dreaming |
Placebo group (n=13) |
19.9 (1.8) |
21.4 (2.4) |
0.8 |
||
|
Factor 4 |
Test group (n=15) |
17.4 (2.0) |
0.9 |
22.4 (1.6) |
0.3 |
0.06 |
|
Refreshing |
Placebo group (n=13) |
17.1 (2.2) |
19.7 (1.7) |
0.04* |
||
|
Factor 5 |
Test group (n=15) |
21.2 (1.9) |
0.4 |
20.1 (1.7) |
0.8 |
0.7 |
|
Sleep length |
Placebo group (n=13) |
19.0 (1.5) |
20.7 (1.9) |
0.1 |
||
|
() denotes standard error 0.01 < * p < 0.05 |
||||||
Table 5: Change in the OSA Sleep Inventory (OSA-MA) before and after the intake of test or placebo powder for 4 weeks.
VAS-F
VAS-F is a questionnaire-style survey that evaluates mental and physical fatigue using the Visual Analogue Scale (VAS) method. The questionnaire consists of 12 questions. For each item, the subject self-evaluates their current mental and physical fatigue on a scale of 0 (does not apply at all) to 100 (applies most), by drawing a short vertical line on a long horizontal line.
In the test group, significant differences were observed in the scores before and after the intervention in the items of overall fatigue, drowsiness, and refreshment, while in the placebo group, significant differences were observed in the items of tension and irritability (Table 6). When comparing the changes between the two groups, no statistically significant differences were observed between the groups in all items.
|
Group |
Mean score at pre- intervention |
P-value between two groups |
Mean score at post- intervention |
P-value between two groups |
P-value between pre- and post-intervention |
|
|
Overall fatigue |
Test group (n = 14) |
50.9 (7.1) |
0.8 |
35.1 (6.8) |
0.4 |
0.05 * |
|
Placebo group (n = 15) |
47.5 (7.4) |
43.0 (6.1) |
0.5 |
|||
|
Mental fatigue |
Test group (n = 14) |
46.6 (7.8) |
0.6 |
36.1 (7.5) |
0.5 |
0.2 |
|
Placebo group (n = 15) |
51.5 (6.4) |
43.7 (6.4) |
0.4 |
|||
|
Physical fatigue |
Test group (n = 14) |
51.5 (7.5) |
0.9 |
36.3 (7.2) |
0.7 |
0.06 |
|
Placebo group (n = 15) |
50.1 (7.9) |
40.9 (6.7) |
0.2 |
|||
|
Perceived stress |
Test group (n = 14) |
44.2 (8.6) |
0.8 |
37.6 (7.7) |
0.6 |
0.4 |
|
Placebo group (n = 15) |
47.3 (6.8) |
42.6 (5.9) |
0.6 |
|||
|
Tension |
Test group (n = 14) |
40.1 (7.6) |
0.6 |
32.2 (7.6) |
0.9 |
0.3 |
|
Placebo group (n = 15) |
44.8 (6.2) |
33.1 (5.8) |
0.04* |
|||
|
Drowsiness |
Test group (n = 14) |
57.1 (5.7) |
0.6 |
32.8 (5.9) |
0.3 |
0.002** |
|
Placebo group (n = 15) |
51.8 (6.9) |
41.9 (5.6) |
0.1 |
|||
|
Boredom |
Test group (n = 14) |
35.5 (6.5) |
0.9 |
30.6 (5.9) |
1 |
0.6 |
|
Placebo group (n = 15) |
36.1 (5.4) |
30.2 (5.4) |
0.3 |
|||
|
Willingness |
Test group (n = 14) |
53.5 (5.7) |
0.4 |
63.8 (5.9) |
0.2 |
0.2 |
|
Placebo group (n = 15) |
45.7 (5.8) |
54.3 (5.1) |
0.3 |
|||
|
Hunger |
Test group (n = 14) |
55.8 (5.2) |
0.9 |
45.2 (7.6) |
0.8 |
0.1 |
|
Placebo group (n = 15) |
55.2 (4.3) |
47.9 (5.2) |
0.3 |
|||
|
Thirst |
Test group (n = 14) |
45.6 (6.4) |
0.3 |
32.2 (6.5) |
0.3 |
0.1 |
|
Placebo group (n = 15) |
54.7 (4.2) |
41.8 (5.1) |
0.1 |
|||
|
Irritability |
Test group (n = 14) |
42.6 (7.8) |
0.7 |
29.6 (6.5) |
0.4 |
0.1 |
|
Placebo group (n = 15) |
46.1 (5.9) |
36.8 (5.9) |
0.02* |
|||
|
Refreshment |
Test group (n = 14) |
40.4 (6.7) |
0.7 |
60.0 (5.1) |
0.1 |
0.03* |
|
Placebo group (n = 15) |
44.5 (5.6) |
48.0 (5.7) |
0.7 |
|||
|
() denotes standard error 0.01 < * p < 0.05 ** p < 0.01 |
||||||
Table 6: Change in the visual analogue scale to evaluate fatigue severity (VAS-F) before and after the intake of test or placebo powder for 4 weeks.
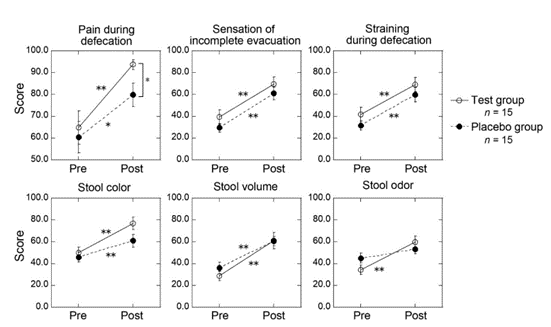
Figure 1: Change in the visual analogue scale to evaluate stool consistency (VAS-S) before and after the intake of test or placebo powder for 4 weeks. Higher scores indicate better condition. Most items in both the test and the placebo group showed significant increase (0.01 < * p < 0.05, **p < 0.01) after the trial, except for the stool odor in the placebo group. When comparing the test and the placebo groups, significant difference was detected in post intervention scores for pain during defecation (* p < 0.05). Error bar: standard error.
Discussion
Natto has long been considered a health food in Japan, with various health benefits attributed to it [13]. However, despite the fact that natto bacteria live in the intestines, little research has been conducted on how natto affects intestinal regulation. In this trial, we examined the effects of taking natto powder on the bowel movements and health status of subjects with constipation. The subjects took 3g of either the test powder or the placebo powder for 4 weeks. No adverse events were reported during the intervention, suggesting that the test powder can be regarded as generally safe. The effects of the test powder were evaluated using a questionnaire consisting of 5 items. At the initial measurement, no significant differences were observed between the test group and the placebo group for any of the test items, indicating that there was no bias between the groups.
In the VAS-S, which evaluates stool consistency and shape, scores for nearly all items increased within 4 weeks. Significant differences were observed between the pre- and post-measurement scores of these items in both the test and placebo groups. We speculate that the increase in the placebo group is not simply due to the placebo effect. In previous studies in which we examined bowel movements using the same method [17,18], we did not observe a statistically significant difference between the pre- and post-measurement scores in the placebo group, as was observed in this study. Several studies have reported that soybeans or their derivatives including lecithin, which is a mixture of phospholipids, have effects on the intestinal regulation [19-21]. In our study, pain during defecation improved after the 4-week intervention in both groups, but the change in the test group was greater than in the placebo group, resulting in a significant difference between the two groups that was not evident prior to the intervention. Additionally, the changes in the stool color and odor were greater in the test group than in the placebo group, suggesting the possibility that Bacillus subtilis natto and their metabolites reach the intestines and change the stool condition. Natto consumption is known to improve the intestinal environment [22]. A clinical study conducted on healthy adults who consumed natto showed that it maintained healthy intestinal flora, increased organic acids, and reduced putrefactive products, such as skatole and ammonia [23]. The probiotic effects of natto help relieve constipation. Based on these results, we hypothesize that the bacteria and metabolites in the test powder may regulate bowel movements and reduce pain during defecation.
Not only in the stool conditions, but significant changes in fatigue were observed in both groups using CFS. Soybeans are a significant source of lecithin. A previous clinical study suggested that consuming soy lecithin increases vigor in middle-aged women experiencing fatigue [24]. Therefore, the lecithin contained in both test and placebo powders may be responsible for these changes. Although no significant difference was observed between the two groups, the test group showed a greater change in score. This suggests that the test product may be more effective at alleviating fatigue. This finding is supported by the results of the VAS-F study, in which only the test group experienced a significant decrease in overall fatigue and refreshment scores. A clinical trial of subjects with peripheral neuropathy found that vitamin K2 alleviated associated symptoms, including fatigue [25]. Although no direct mechanisms have been found to show that vitamin K2 directly alleviates fatigue, we hypothesize that vitamin K2, which is plentiful in natto, plays a role in calcium absorption and utilization [26], contributing to bone health, energy levels, and reduced fatigue.
In the OSA-MA study, sleep quality improved after the intervention, as indicated by increased scores on all items in the test group except sleep length. The VAS-F study also showed that drowsiness improved statistically significantly only in the test group. Previous studies have shown that taking natto capsules daily for eight weeks improves sleep quality and reduces feelings of stress [27]. The authors of the paper hypothesized that natto bacteria activate serotonin production by stimulating enterochromaffin (EC) cells in the intestinal tract, thereby promoting melatonin synthesis in the pineal gland. Additionally, we speculate that the sticky component of natto, called γ-PGA, improves sleep quality. Studies have shown that γ-PGA supplementation significantly increases serum concentrations of glutamate and GABA [28]. GABA acts as an inhibitory neurotransmitter and sleep inducer [29]. The intestinal environment and sleep influence each other reciprocally [30]. Good sleep improves the intestinal environment and, conversely, a well-organized intestinal environment improves sleep quality. The results of our study demonstrated that natto has a positive effect on brain-gut interaction.
Conclusion
In this study, we demonstrated that taking natto powder regulates bowel movements and reduces defecation pain. Additionally, we found that the test powder can reduce fatigue and improve sleep quality possibly by maintaining healthy intestinal flora. However, in the VAS-S test in particular, statistically significant changes in scores were observed in many items in the placebo group, as well as in the test group. This is thought to be due to the effects of the indigents of soybeans, the raw material of natto. Nevertheless, one conclusion we can certainly draw from the study is that natto is more effective for these items. To clarify this further, using a placebo made from a different raw material is needed to determine whether the observed changes are unique to natto or common to soybeans. Further research is needed to confirm these promising results and elucidate the underlying mechanisms.
Conflict of Interest
This study was conducted by User Life Science Co., Ltd. with funding and samples provided by Yamada Foods Co., Ltd.
References
- Shurtleff W, Aoyagi A (2012) History of natto and its relatives (1405-2012). Soyinfo Center.
- Sumi H (2015) Natto, the pride of Japan. Chemical Education 63: 358-359.
- Tamura M, Watanabe J, Noguchi T, Nishikawa T (2023) High poly-γ-glutamic acid-containing natto improves lipid metabolism and alters intestinal microbiota in mice fed a high-fat diet. Journal of Clinical Biochemistry and Nutrition 74: 47-56.
- Lei P, Ma Y, Xiao W, Wang L, Fu H, et al. (2024) A calcium delivery system fabricated by poly-γ-glutamic acid: Preparation, characterization, and delivery mechanism studies. LWT 210: 116846.
- García-García C, Baik I (2021) Effects of poly-gamma-glutamic acid and vitamin B6 supplements on sleep status: a randomized intervention study. Nutrition research and practice 15: 309-318.
- Li Y, Zhang W, Tang C, Wang C, Liu C, et al. (2024) Antidiabetic effects and mechanism of γ- polyglutamic acid on type II diabetes mice. International journal of biological macromolecules 261: 129809.
- Fujita Y, Iki M, Tamaki J, Kouda K, Yura A, et al. (2012) Association between vitamin K intake from fermented soybeans, natto, and bone mineral density in elderly Japanese men: the Fujiwara-kyo Osteoporosis Risk in Men (FORMEN) study. Osteoporosis International 23: 705-714.
- Afzaal M, Saeed F, Islam F, Ateeq H, Asghar A, et al. (2022) Nutritional health perspective of natto: A critical review. Biochemistry Research International 2022: 5863887.
- Li X, Long J, Gao Q, Pan M, Wang J, et al. (2023) Nattokinase supplementation and cardiovascular risk factors: a systematic review and meta-analysis of randomized controlled trials. Reviews in Cardiovascular Medicine 24: 234.
- Wang P, Gao X, Li Y, Wang S, Yu J, Wei Y (2020) Bacillus natto regulates gut microbiota and adipose tissue accumulation in a high-fat diet mouse model of obesity. Journal of Functional Foods 68: 103923.
- Jiang X, Ding H, Liu Q, Wei Y, Zhang Y, et al. (2020) Effects of peanut meal extracts fermented by Bacillus natto on the growth performance, learning and memory skills and gut microbiota modulation in mice. British Journal of Nutrition 123: 383-393.
- Kato A, Yoshifuji A, Komori K, Aoki K, Taniyama D et al. (2022) A case of Bacillus subtilis var. natto bacteremia caused by ingestion of natto during COVID-19 treatment in a maintenance hemodialysis patient with multiple myeloma. Journal of Infection and Chemotherapy 28: 1212-1215.
- Wang C, Chen J, Tian W, Han Y, Xu X, et al. (2023) Natto: A medicinal and edible food with health function. Chinese herbal medicines 15: 349-359.
- Mawatari K, Atsumi M, Nakamura F, Yasuda M, Fukuuchi T, et al. (2019) Determination of dipicolinic acid in “Natto” by high-performance liquid chromatography coupled with postcolumn photoirradiation with zinc acetate. International Journal of Tryptophan Research 12: 1178646919852120.
- Tanno Y (2019) The Consumer Aspect of the Expansion of Natto Consumption in Japan: Focusing on Acceptance Among Young People. Annals of the Research Center for Regional Community Innovation, Ehime University 14: 76-93.
- Matsushita E, Yamanaka K (2015) A preference survey for Japanese food among international students with a comparison to Japanese students. Nagoya Journal of Nutritional Sciences 1: 127-133.
- Kikushima K, Nagae M, Miyazaki H, Setoyama S, Ohnuki K, et al. (2022) Improvement in Immune Functions by Daily Taking Sea Buckthorn Fruit Beverage for 4 Weeks. Japanese Pharmacology & Therapeutics 50: 1079-1094.
- Takahashi M, Hara K, Shimizu K, Ohnuki K (2022) Improvement Effect of Intestinal Environment, Defecation Symptoms and Skin Quality by Inulin‒containing Supplements. Japanese Pharmacology & Therapeutics 50: 927-935.
- Basson AR, Ahmed S, Almutairi R, Seo B, Cominelli F (2021) Regulation of Intestinal Inflammation by Soybean and Soy-Derived Compounds. Foods 10: 774.
- Lee GS, Kim SK, Ban JY, Oh CH (2025) Ameliorative Effects of Soybean Powder Fermented by Bacillus subtilis on Constipation Induced by Loperamide in Rats. International Journal of Molecular Sciences 26: 7615.
- Akashi T, Muto A, Takahashi Y, Nishiyama H (2017) Enteral Formula Containing Egg Yolk Lecithin Improves Diarrhea. Journal of Oleo Acience 66: 1017-1027.
- Ngampuak V, Thongmee A, Pongpoungphet N, Wongwailikhit K, Kanchanaphum P (2023) Probiotic Properties of Exopolysaccharide-Producing Bacteria from Natto. International Journal of Food Science 2023: 3298723.
- Terada A, Yamamoto M, Yoshimura E (1999) Effect of the Fermented Soybean Product "Natto" on the Composition and Metabolic Activity of the Human Fecal Flora. Japanese Journal of Food Microbiology 16: 221-230.
- Hirose A, Terauchi M, Osaka Y, Akiyoshi M, Kato K, Miyasaka N (2018) Effect of soy lecithin on fatigue and menopausal symptoms in middle-aged women. Nutrition Journal 17: 4.
- Mehta DS, Dound YA, Jadhav SS, Bhave AA, Devale M, Vaidya AD (2018) A novel potential role of Vitamin K2-7 in relieving peripheral neuropathy. Journal of Pharmacology and Pharmacotherapeutics 9: 180-185.
- Maresz K (2015) Proper Calcium Use: Vitamin K2 as a Promoter of Bone and Cardiovascular Health. Integrative medicine (Encinitas, Calif.) 14: 34-39.
- Sano Y, Ichikawa Y, Mizuyama K, Nakamura N (2022) Effects of Consumption of Bacillus subtilis subsp. natto QOL (QOL Bacillus natto) Containing Food on the Quality of Sleep. Japanese Pharmacology & Therapeutics 50: 1201-1212.
- Lee H, Chang MJ, Kim SH (2010) Effects of poly-γ-glutamic acid on serum and brain concentrations of glutamate and GABA in diet-induced obese rats. Nutrition Research and Practice 4: 23-29.
- Varinthra P, Anwar SNMN, Shih SC, Liu IY (2024) The role of the GABAergic system on insomnia. Tzu chi medical journal 36: 103-109.
- Sejbuk M, Siebieszuk A, Witkowska AM (2024) The Role of Gut Microbiome in Sleep Quality and Health: Dietary Strategies for Microbiota Support. Nutrients 16: 2259.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.