Exploring the Microbial Diversity and Bioactivity of Lebanese Artisanal Dairy Products Running title: Microbial Diversity in Lebanese Dairy Products
by Rayane Khaled1,2#, Mélissa Tourret1#, Sandy Theysgeur1, Elodie Dussert1, Bernard Taminiau3, Georges Daube3, Khaled El Omari2, Djamel Drider1, Imad Al Kassaa2,4, Anca Lucau-Danila1*
1 UMRT 1158 BioEcoAgro, University of Lille, F-59000 Lille, France
2 Department of Nutrition, Lebanese University, CR8J+792, Tripoli, Lebanon
3 Department of Food Sciences-Microbiology, FARAH, University of Liege, 4000 Liege, Belgium
4 Fonterra Research and Development Center, Dairy Farm Road, Palmerston North 4472, New Zealand
#The authors contributed equaly to this work
*Corresponding author: Lucau-Danila A, UMRT 1158 BioEcoAgro, University of Lille, F-59000 Lille, France
Received Date: 15 January 2025
Accepted Date: 21 January 2025
Published Date: 27 January 2025
Citation: Khaled R, Tourret M, Theysgeur S, Dussert E, Taminiau B, et al. (2025) Exploring the Microbial Diversity and Bioactivity of Lebanese Artisanal Dairy products Running title: Microbial Diversity in Lebanese Dairy Products. Food Nutr J 10: 321. https://doi.org/10.29011/2575-7091.100221
Abstract
The aim of this study was to assess the potential impact of two traditional Lebanese fermented dairy products, Darfiyeh and Labneh, on human health. Using targeted metagenomic analysis, we identified the bacterial and fungal taxa present in these products. Food safety was evaluated by screening for microbial antibiotic resistance genes and assessing the pathogenic, antagonistic, and probiotic potential of the microorganisms. In vitro tests were conducted to investigate antioxidant, antihypertensive, and anti-inflammatory activities, linking microbial and metabolic compositions to potential health effects. The tested dairy products exhibited a diverse range of bacteria (such as Lactobacillus, Lactococcus, Leuconostoc, and Streptococcus) and fungi (including Pichia, Debaryomyces, Kluyveromyces, and Saccharomyces). Few microorganisms were common to both types of dairy products with the majority forming distinct microbial profiles that varied significantly based on the artisanal production methods and the geographical origins of the producers. This variability was discussed in the light of food safety concerns. A significant antioxidant effect was detected and attributed to the synthesis of antioxidant molecules and the microbial composition of the products. The antihypertensive effects were mainly associated with the ripening duration, which enhanced the probiotic, postbiotic and metabiotic components, whereas the anti-inflammatory activity correlated with the microbial composition of each product. These findings highlight the health-promoting potential properties of traditional Lebanese dairy products, revealing their complex microbial ecosystems. However, the study also underscores the importance of monitoring for potential contaminants and health risks to ensure their safety and maximize their benefits.
Keywords: Dairy Products; Metagenomics; Antioxidant Effect; Antihypertensive Effect; Anti-Inflammatory Effect
Introduction
Fermentation has long been used as a process to transform and preserve food. In fact, before the 19th century, fermentation processes were carried out without understanding of the underlying microbial mechanisms [1]. The health benefits of consuming fermented dairy products have been empirically observed for a very long time, such as Metchnikoff’s observations [2]. However, fermented foods were not considered functional foods until the early 1980s [3]. Dairy products are currently known to contain probiotics and postbiotics including metabiotics, all of which have been shown to have health benefits [4]. Fermented foods, in particular, contain an increased amount of probiotics - live microorganisms that, when consumed in sufficient quantities, provide health benefits to the host [5]. Dairy products, such as yogurt, kefir, cheese, and fermented milk drinks, are among the most common vehicles for delivering probiotics to the human diet [6]. Postbiotics are substances derived from microorganisms that are no longer alive, i.e. inanimate, dead, or inactivated. Postbiotics are substances derived from microorganisms after their life cycle, including intentionally inanimate intact cells, cell fragments, and metabolites, with or without additional components [7]. Dairy products most commonly containing postbiotics include yogurt, cheese, and artisanal fermented milk [8]. Metabiotics, on the other hand, are metabolites or end-products of probiotic activity that provide beneficial effects to the host. Unlike postbiotics, metabiotics are mixtures of bioactive compounds produced by microorganisms during fermentation, such as short-chain fatty acids (SCFAs), peptides, polysaccharides, enzymes, vitamins, and more [9]. Dairy products that most commonly contain metabiotics include yogurt [10], cheese [11], artisanal fermented milk, and kefir [12].
Many studies have been conducted on lactic acid bacteria (LAB), an important group of fermenters known as starter cultures in many dairy and non-dairy foods [13]. In addition to their efficiency in food conversion, LAB are known to produce many bioactives such as primary and secondary metabolites that are beneficial for human health [14]. Foods containing LAB offer numerous health benefits, making them a valuable addition to a balanced diet [1516]. Many LAB strains support gut homeostasis by increasing microbiome diversity, which significantly improves digestion and nutrient absorption [17]. Additionally, LAB strains, through their structural components or bioactive metabolites, enhance immune function, regulate immune responses, and exhibit antihypertensive activity, thereby reducing the risk of infectious and chronic diseases [18-20]. Far fewer studies have examined other bacteria and yeasts involved in milk fermentation, particularly in artisanal dairy products.
Our interest in this study was to analyze and evaluate the composition and functional properties of several artisanal dairy products of Lebanese origin. Lebanon, a Mediterranean country, is known for its cultural diversity and rich variety of traditional foods. Among the extensive list of artisanal food products, fermented dairy products are an important part of the daily dietary habits of the Lebanese population. Our study aimed to evaluate the health benefits of two artisanal Lebanese dairy products, Darfiyeh (D) and Labneh (L), collected from three different traditional producers in different areas of Lebanon. The microbiota composition was analyzed using targeted metagenomics. Food safety was evaluated considering the presence of microbial Antibiotic Resistance Genes (ARGs), as well as the pathogenic, antagonistic and probiotic potential of these microorganisms. To assess the pro-, post-, and metabiotic effects, three solutions were prepared for each dairy product: a crude solution containing live microorganisms, a heated solution containing inanimate microorganisms and a filtered solution containing mainly metabolites. Each solution underwent antioxidant testing by measuring the Hydroxyl Radical (HO•) via a Fenton reaction. The antihypertensive activity was tested using the Angiotensin-Converting Enzyme (ACE) inhibition method. Finally, the anti-inflammatory activity was tested on U937 cells in vitro.
Materials and Methods
Traditional dairy products, producers selection and sample collection
Two types of traditional Lebanese dairy products were analyzed: Darfiyeh and Labneh, both of which are considered soft cheeses. Darfiyeh is a type of goat’s milk cheese made by hand by draining raw milk that has spontaneously fermented due to the natural microorganisms present in goat’s milk. This product continued to ferment for 3 to 6 months inside a goat skin. On the other hand, Labneh, a regional specialty of Lebanon with a history spanning over two thousand years, was made from cow’s milk. Raw cow’s milk was fermented with a homemade inoculum and then strained through a fine cloth for filtering.
Three different preparations of each type of dairy product were collected from three artisanal producers located in northern Lebanon (Figure 1). Producers were selected based on two main criteria : maintaining an artisanal production level and having over 15 years of experience in traditional craft production. A survey of six breeders/producers was conducted, covering socio-demographic information, breeding activities, and production practices, including commercialization. The survey gathered information on producer demographics, livestock size, breeding methods, milk production volumes, production seasons, raw materials, and marketing strategies. Additionally, a detailed observation of the processing was performed to create a production flow chart for each product type, highlighting differences among producers.
Figure 1: Geographical origin of artisanal dairy products. Three producers were selected for the Darfiyeh samples (D1, D2, and D3) and three others for the Labneh samples (L1, L2, and L3) in North Lebanon.
Samples of Darfiyeh cheese from the three producers (D1, D2, and D3) and Labneh samples from three other producers (L1, L2, and L3) were collected at the end of the manufacturing process in 2023. The samples were placed in 100 mL sterile plastic containers, sealed, refrigerated, and transported to the microbiology laboratory at the Chamber of Commerce, Industry, and Agriculture of Tripoli & North Lebanon. Upon arrival, samples were divided: 15 g were stored at -80 °C for long-term DNA extraction, while 30.5 g were used to prepare working solutions for functional analyzes. DNA extraction and amplicon sequencing
DNA extraction from both product types and the three different producers was performed using the NucleoSpin® DNA Soil kit (Macherey-Nagel, Düren, Germany) according to manufacturer’s protocol. DNA extraction was performed in triplicate on samples under identical conditions, and the DNA from the replicates was subsequently pooled. DNA quantities were measured with a BioSpectrometer (Eppendorf, Hamburg, Germany), and DNA quality was assessed using the 2100 Bioanalyzer (Agilent, Santa Clara, USA). Sequencing was conducted by the GIGA genoproteomic platform at Liège University (Belgium). For bacterial amplicon sequencing, the V1-V3 region of the 16S rDNA was amplified and libraries were prepared using the following primers: forward (5’-GAGAGTTTGATYMTGGCTCAG-3’) and reverse (5’-GAGAGTTTGGCTCAG-3’). For fungal amplicon sequencing, the Internal Transcribed Spacer (ITS) region 5.8S-ITS2 was amplified and libraries were prepared using universal primers with Illumina overhand adapters targeting the ITS2 region. The forward primer ITS3KYO2 (5’-GATGAAGAACGYAGYRAA-3’) and the reverse primer ITS4 (5’-TCCTCCGCTTATTGATATGC-3’) were chosen for their broad coverage of fungal taxa. Each PCR product was purified with the Agencourt AMPure XP Ball Kit (Beckman Coulter, Pasadena, USA), then subjected to a second PCR round for indexing with Nextera XT index 1 and 2 primers. Following purification, PCR products were quantified using Quant-IT PicoGreen (Thermo-
Fisher Scientific, Waltham, USA) and diluted to 10 ng·µL-1. Final quantification of each library sample was performed with the KAPA SYBR FAST qPCR Kit (KapaBiosystems, Wilmington, USA) before standardization, pooling, and sequencing on a MiSeq sequencer with v3 reagents (ILLUMINA, San Diego, USA). Data processing involved the MOTHUR v1.44 package and the VSearch algorithm [21] for alignment, clustering, and chimera detection as described by Gérard et al. [22]. After cleaning, sequences were clustered into operational taxonomic units (OTUs) at 97% identity. Alignment and taxonomic identification were carried out with MOTHUR using the SILVA v1.32 database for full-length 16S rDNA and the UNITE database v9.0 for 5.8S rDNA gene sequences. A rarefied table of 10,000 reads per sample was used for further analysis. Reads were aggregated into phylotypes at the phylum and genus taxonomic levels. All analyses compared experimental groups to their respective controls. Normality was tested using the Shapiro–Wilk test, and homogeneity of variances was checked with Bartlett’s test. Statistical differences were assessed using ANOVA and Tukey’s test. Data analysis was performed with PRISM 7 (GraphPad Prism 6.0, Windows Inc., San Diego, CA, USA), and differences were considered significant at p < 0.05. All raw reads from biosamples were deposited at the National Center for Biotechnology Information (NCBI) under BioProject accession number PRJNA1209664. Data from NGS analysis were examined for alpha diversity using the Shannon index, and graphical representations were created with GraphPad Prism 7 software for Windows (San Diego, CA, USA). Beta diversity was analyzed using principal component analysis (PCoA) with the FactoMineR package in R version 3.5.2 (r-project.org).
Evaluation of antibiotic resistance, pathogenicity, antagonistic, and probiotic activity
To assess potential health risks posed by microorganisms in artisanal fermented dairy products, Antibiotic Resistance Genes (ARGs) were identified in the genomes of the detected bacterial species using the RAST server [23,24]. A score was then calculated for each gene based on the relative abundance of the bacterial species harboring it. A comprehensive literature review was also conducted to evaluate the pathogenicity, antagonistic properties, and probiotic activity of all identified bacterial and fungal species.
Preparation of working solutions for functional assays
Crude dairy product solutions were prepared by mixing 10 g of fermented dairy products with 90 mL of pure water (110 mg/mL) in a sterile stomacher bag. The bag was then placed in a Stomacher® 400 Circulator (Radnor, USA) and homogenized for 5 minutes at 230 rpm. Heated dairy product solutions were prepared by heating the crude solutions at 60 °C for 20 minutes. Filtered solutions were prepared from crude solutions by centrifugation at 1200 rpm for 3 minutes, followed by filtration through a 0.45 µm filter.
Cytotoxicity assays
The toxicity of various solutions prepared from Darfiyeh and Labneh dairy products was evaluated using the U937 monocytic cell line (access number 85011440, lot N°11D008, American Type Culture Collection (ATTC), Manassas, USA). U937 cells were cultured in vitro in T75 flasks using RPMI-1640 medium supplemented with 10% Fetal Bovine Serum (FBS), 1% l-glutamine, 1% penicillin-streptomycin (P/S), 1% amphotericin B, and 0.2% antimycoplasma antibiotic. The cultures were maintained at 37 °C with 5% CO₂. Differentiation of U937 cells into macrophages was induced by treatment with phorbol 12-myristate 13-acetate (PMA, 81 mM) for 48 hours. After differentiation, the medium was replaced with antibiotic-free medium, and the cells were incubated for an additional three days under the same conditions. For the cytotoxicity assay, the cells were scraped and seeded at a density of 2 × 10⁵ cells/well in 96-well plates. Serial dilutions of the dairy product solutions (final concentrations of 0.25, 0.50, 0.75, 1.00, and 1.50 mg/mL) were prepared and tested for cytotoxicity using the Cell Counting Kit-8 (CCK-8). After a 24-hour incubation with the cells, CCK-8 was added to each well, and absorbance at 450 nm was measured following a 1.5-hour incubation.
Antioxidant assays
Hydroxyl radical inhibition was evaluated using a cell-free model adapted from Halliwell et al. [25]. The hydroxyl radical (HO·) was generated from hydrogen peroxide (H₂O₂) via the Fenton reaction, and the antioxidant activity of the samples was measured based on their inhibition rate of HO· production. Sample concentrations were standardized to 15 mg/mL, with successive dilutions tested at final concentrations of 0.25, 0.50, 0.75, 1.00, and 1.50 mg/mL. The Fenton reaction was initiated in tubes containing 20 mM KH₂PO₄ buffer (pH 7.4), an EDTA-Fe²+-AA mix (15 µM FeCl₃, 15 µM EDTA, and 840 µM ascorbic acid), approximately 12 nmol of H₂O₂ per mL (9.6 µM), sample solutions at varying concentrations, and deoxyribose (30 mM). The tubes were incubated in a water bath at 37 °C for 30 minutes. During the reaction, the produced HO· was partially inhibited by the samples, while the remaining radicals degraded deoxyribose to form Malondialdehyde (MDA). To measure MDA, the reaction mixture was heated at 95 °C for 13 minutes in the presence of thiobarbituric acid (69 mM) and trichloroacetic acid (367 mM). The reaction was then stopped by placing the tubes in an ice-water bath. The resulting pink chromogen (MDA) was measured at 532 nm using a spectrophotometer (SpectraMax® iD3, Molecular Devices, USA). The absorbance used to calculate the HO· concentration was determined by subtracting the absorbance of the blank samples, which contained all reagents except H₂O₂, from the absorbance of the experimental samples. In parallel, a positive inhibition control was used, L-cysteine (3.98 mM). All reagents were purchased from Sigma (Saint-Quentin Fallavier, France). The level of hydroxyl radical was quantified in mg/mL using a standard curve generated with increasing H₂O₂ concentrations and expressed as a ratio relative to the average values of the negative control (water). Data were presented in bar graphs ± SD based on fold of control.
Antihypertensive assays
The ACE inhibitory activity of different dairy product samples was tested following the protocol described by Sentandreu and Toldrá, with some modifications [26]. Briefly, 25 µL of samples, diluted to final concentrations of 4.2, 8.31, 16.7, and 25.0 mg/mL in Tris buffer (150 mM, pH 8.3) for Darfiyeh, and 33.3, 50.0, 66.7, and 83.3 mg/mL in Tris buffer (150 mM, pH 8.3) for Labneh, were pre-incubated with 25 µL of ACE (A6778, Sigma-Aldrich, SaintQuentin Fallavier, France) working solution (0.2 mU/mL enzyme activity) in a 96-well plate. The enzymatic reaction was initiated by adding 100 µL of a fluorescent substrate working solution containing o-aminobenzoylglycyl-p-nitro-L-phenylalanylL-proline (Abz-Gly-p-nitro-Phe-Pro-OH; M-1100, Bachem, Bubendorf, Switzerland) at a concentration of 0.45 mM. A negative control, used to assess ACE activity inhibition, consisted of replacing the sample with 25 µL of Tris buffer (150 mM, pH 8.3). A positive control for ACE activity inhibition was prepared by substituting the sample with 10 µL of captopril (0.6 µM) and 15 µL of Tris buffer (150 mM, pH 8.3). Fluorescence readings were taken every 2 minutes for 1 hour at 37 °C, with excitation and emission wavelengths set to 365 nm and 415 nm, respectively, using a fluorescent spectrophotometer (SpectraMax® iD3, Molecular Devices, USA). The sample concentration required to achieve 50% inhibition of ACE activity (IC50) was determined by plotting % ACE inhibition against the natural logarithm of the final sample concentration. The percentage of ACE activities inhibition was calculated using the obtained slope (linear portion) with the following formula:
In this formula, “a” represents the obtained slope from the fluorescence values over time in the sample. The IC50 was then determined using the following linear relation:
In this equation, “a” represents the slope, and “b” is the intercept, both calculated by averaging the percentage of inhibition for each concentration and expressed as natural logarithm. The IC50 was expressed in mg/mL with nine replicates used for each sample, and captopril serving as the positive control.
In vitro cytokines secretion assay
The human promonocytic cell line U937 (access number 85011440, lot N°11D008, American Type Culture Collection (ATCC), Manassas, USA) was cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), 1% l-glutamine, 1% penicillin-streptomycin (P/S), 1% amphotericin B, and 0.2% anti-mycoplasma antibiotic in a humidified 5% CO₂ atmosphere at 37 °C. For macrophage differentiation, U937 cells were seeded at a density of 12 × 10⁶ cells per 75 cm² flask and treated with 81 mM phorbol-12-myristate-13-acetate (PMA) for 48 hours. After this incubation, PMA was removed and replaced with antibiotic-free RPMI medium, and the cells were incubated for an additional 3 days at 37 °C in a 5% CO₂ atmosphere. Adherent cells were gently washed with PBS, scraped, and reseeded in antibiotic-free RPMI medium at a density of 2 × 10⁶ cells per well in 12-well plates. Cells were exposed to lipopolysaccharides (LPS from E. coli O26:B6, Millipore Corporation) at a final concentration of 200 µg/mL and to test samples at a final concentration of 1.5 mg/mL for 4 hours at 37 °C. Non-inflamed cells (without LPS) served as Negative Controls (NI), while two additional controls were included: an inflammation control (cells treated with LPS alone, denoted as I) and a positive control for inflammation inhibition (cells treated with LPS and dexamethasone (D4902, Sigma), denoted as CtI). After 4 hours of incubation, cell supernatants were collected and centrifuged at 1,200 rpm for 5 minutes at 4 °C. The supernatants were aliquoted and stored at -20 °C until further analysis. Secreted human TNF-α and IL-10 levels were quantified using the Biotechne® Quantikine™ ELISA kit (Biotechne, Minneapolis, USA) according to the manufacturer’s instructions. Quantification was performed using a SpectraMax® spectrophotometer. Samples and controls were diluted 10-fold for IL-10 and 50-fold for TNF-α quantification, except for non-inflamed cells.
Statistics
Statistical analyses were conducted for antoxidant, antihypertensive and anti-inflammatory assays using GraphPad Prism 7 software for Windows (San Diego, CA, USA). If the data met normality (Shapiro-Wilk test, α = 0.05) and homogeneity of variances (nonsignificant Bartlett’s test, α = 0.05), ANOVA was performed (overall Fisher’s test, p < 0.05, followed by Tukey’s post-hoc test, p < 0.05). Non-parametric analysis (Kruskal-Wallis test, p < 0.05, followed by Dunn’s test, p < 0.05) was used when ANOVA assumptions were not met.
Results
Darfiyeh and Labneh producer’s survey
According to the questionnaire results, 83% of respondents were both producers and farmers, with only one farm exclusively engaged in production. Production relied entirely on female housekeepers (100%) aged 45 to 55, with only 33% having access to the local marketplace. Producers who owned livestock processed up to 1,900 liters of goat milk or 5,500 liters of cow’s milk annually from their animals. Overall, goat milk processing per farm averaged 5,500 liters per season (March to October), slightly less than cow milk, which averaged 6,500 liters per season (year-round). Details of farming and production for each product are summarized in Table 1.
|
Artisanal dairy products |
D1 |
D2 |
D3 |
L1 |
L2 |
L3 |
|
Producer only or both farmer and producer |
Farmer and producer |
Farmer and producer |
Farmer and producer |
Farmer and producer |
Producer |
Farmer and producer |
|
Rural, urbanized or urban area |
Rural |
Rural |
Urbanized |
Rural |
Urban |
Rural |
|
Person who carried out the production |
Female housekeeper |
Female housekeeper |
Female housekeeper |
Female housekeeper |
Female housekeeper |
Female housekeeper |
|
Age of producer (years) |
45 |
55 |
48 |
50 |
49 |
53 |
|
Manual, industrial or combined production |
Manual |
Manual |
Manual |
Manual |
Manual |
Manual |
|
Artisanal, industrial or mixed materials for production |
Mixed material |
Mixed material |
Mixed material |
Artisanal |
Artisanal |
Artisanal |
|
Access to the local marketplace |
No |
No |
Yes |
No |
Yes |
No |
|
Market or private price (€/kg) |
5.5 |
6 |
6 |
3 |
3.5 |
3 |
|
Animal nutrition |
Natural herb and cereals |
Natural herb |
Natural herb and cereals |
Natural herb |
Natural herb and cereals |
Natural herb and cereals |
|
Milk processed from own production (L/season) |
120-1,900 |
100-980 |
110-1,700 |
200-2,500 |
140-1,800 |
|
|
Total milk processed (L/season) |
200-4,500 |
240-5,500 |
170-2,300 |
230-6,500 |
210-6,000 |
190-5,600 |
|
Range of production (kg/season) |
40-600 |
20-450 |
35-500 |
50-1,200 |
40-700 |
30-650 |
|
Race of goat/cow |
Baladi goat |
Baladi goat |
Baladi goat |
Baladi cow |
Baladi cow |
Baladi cow |
|
Grazing place |
Mountains of Hilane |
Mountains of Ehden |
Mountains of Hasroun |
Montains of Mrahat |
Coastal plains of Abou Samra |
Montains ofAkkar |
|
Manufacturing time |
6 months |
6 months |
4 months |
30 days |
15 days |
7 days |
Table 1 : Production parameters and rearing practices related to Darfiyeh (D1-D3) and Labneh (L1-L3) dairy products.
Darfiyeh and Labneh artisanal dairy production
Darfiyeh products were made from raw milk of Baladi goats grazing in the mountainous areas of Hilane (D1), Ehden (D2) and Hasroun (D3). All production phases were carried out manually using a mix of traditional and modern materials. Production occured during the goat milking season. No starters were added; the microbiota of this cheese originates exclusively from goat’s milk and contamination by adventitious microorganisms. Rennet was added to coagulate the casein in the milk. The amount of the microbial rennet powder from Mucor miehei (Strength 1: 150,000 Soxhlet units) used was variable, but ensured a firm coagulation within 60–90 min. Following coagulation, the curd was compressed for the first drainage of the whey. Ripening was conducted by wrapping the cheese in cleaned goat skin for up to six months, resulting in a semi-hard goat’s cheese. Few differences were observed among the producers located in Zgharta, Ehden and Bsharri and consisted mainly in the ripening time (the longest for D1 and D2 and the shortest for D3).
The Labneh product for our analyses was obtained from cow’s milk from the black-and-white Baladi cow race that grazed in the mountainous area of Mrahat (L1), coastal plains of Abou Samra (L2) and Akkar Mountains (L3). All production phases were completed manually, and both the tools and materials used were artisanal. Production occurred year-round. The first step in the production process was the preparation of yogurt from whole milk. The milk was first heated to 85 °C and then cooled to a lukewarm temperature of about 40–45 °C before adding a small amount of yogurt from the previous day to initiate fermentation. This homemade starter (inoculum) consisted of the prior yogurt sample primarily containing lactobacilli and streptococci. This inoculum has been previously studied, with 96 strains of Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus identified [27]. The product was then left to ferment for several hours, usually overnight, until it reached the desired consistency and acidity. Once ready, the Labneh product was poured into a fine cloth or cheesecloth, traditionally made of cotton or linen, for filtering. It was wrapped in cloth and hung to allow the whey to drain. This process can take from 12 to 24 hours, or even several days, depending on the desired consistency and acidity level. In the three localities where this dairy product was obtained (Akkar Al Atika, Tripoli, and Akkar), random differences were observed among producers regarding the manufacturing time: seven days for L2, two weeks for L3, and one month for L1 (Table 1).
Microbial diversity in dairy products
To estimate changes in the number of identified OTUs in the different dairy products, the Shannon index, representing alpha diversity, was calculated for all samples (Figure 2). We observed that the bacterial alpha diversity is quite similar in the two products but is more variable in Darfiyeh compared to Labneh (Figure 2A). In contrast, the Shannon index for fungal OTUs was higher and more variable in Labneh than in Darfiyeh (Figure 2B).
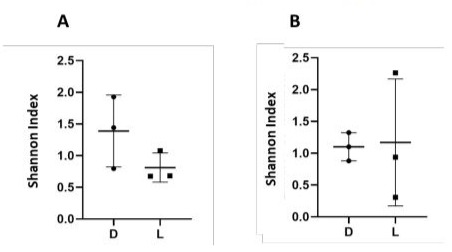
Figure 2: Microbial diversity of Darfiyeh and Labneh samples. Alpha-diversity of bacteria (A) and fungi (B) is illustrated by the Shannon index for each sample. D1-D3 – Darfiyeh samples from three producers, L1-L3 – Labneh samples from three producers.
To observe the specific distribution of microbial OTUs, betadiversity was calculated for bacteria and fungi identified in the different dairy products (Figure 3). Each type of dairy product exhibited a distinct microbial composition, with notable differences in taxa distribution between the two product types. The distribution of bacterial OTUs (Figure 3A) and fungal OTUs (Figure 3B) was broader for Labneh products (red circles) compared to Darfiyeh products (blue circles).
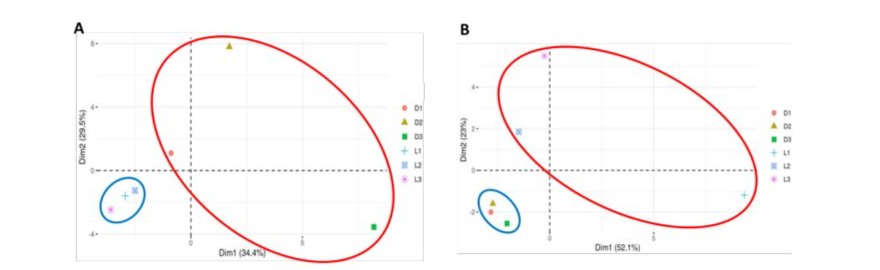
Figure 3: Principal component analysis representing beta-diversity for bacteria (A) and fungi (B). D1-D3 – Darfiyeh samples from three producers, L1-L3 – Labneh samples from three producers.
Microbial abundance in dairy products
Metagenomic analyses revealed the relative abundances of bacterial and fungal taxa in both Darfiyeh and Labneh products.
For bacterial phyla (Figure 4), Bacillota (formerly Firmicutes) were dominant across all tested dairy products. Pseudomonadota (formerly Proteobacteria) appeared to be more prevalent in Darfiyeh cheese, with high variability observed among producers.
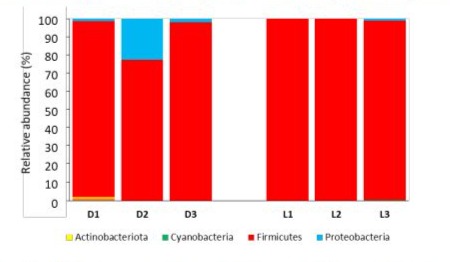
Figure 4 : Relative abundance of bacterial phyla identified in each dairy products (D - Darfiyeh, L - Labneh). Numbers 1, 2, and 3 correspond to three distinct producers.
With regard to the analysis of bacterial genera (Figure 5), the results showed clear differences in the dominant taxa. Two bacterial taxa were found to be dominant in Darfiyeh products: Lactococcus, predominantly in D1 and D3, and Leuconostoc, predominantly in D2. Labneh consisted mainly of Lactobacillus (dominant in L1 and L2) and Streptococcus (dominant in L3).
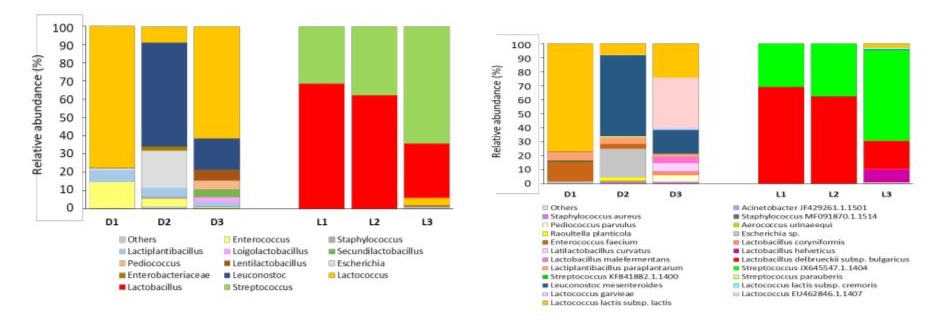 Figure 5 : Relative abundance of bacterial genera identified in different types of dairy products (D - Darfiyeh, L - Labneh). Numbers 1, 2, and 3 correspond to three distinct producers. «Other» refers to genera with less than 0.1% relative abundance.
Figure 5 : Relative abundance of bacterial genera identified in different types of dairy products (D - Darfiyeh, L - Labneh). Numbers 1, 2, and 3 correspond to three distinct producers. «Other» refers to genera with less than 0.1% relative abundance.
At species level, we observed that for each type of dairy product there are 2 or 3 species which are dominant in turn and which constitute the majority of the bacterial community (Figure 6).
Darfiyeh cheese contained Lactococcus lactis subsp. lactis that was consistently present in all productions (77% in D1, 7% in D2 and 24% in D3), Lactococcus EU462846.1.1407(0.03% in D1, 0.35% in D2 and 35% in D3)and Leuconostoc mesenteroides that was identified in high abundance in D2 (57%) and D3 (17%) preparations. Less abundant species mainly present in D1 and D3 included Lactobacillus coryniformis (0.01 - 2.3%), Latilactobacillus curvatus (5.5%), Lactobacillus malefermentans
(0.03 - 4.4%), Lactobacillus delbrueckii subsp. bulgaricus (0.01-0.08%), Lactiplantibacillus paraplantarum (2.1 - 6.2%), and Lactococcus garvieae (1.4%). Enterococcus faecium and Escherichia sp. were also identified in highly variable proportions in all Darfiyeh preparations (from 0.02 to 20%). Other species were sporadically observed in certain preparations at very less abundance (below 5%).
In contrast, Labneh consistently contained Lactobacillus delbrueckii subsp. bulgaricus (68% in L1, 62% in L2 and 20% in L3), and Streptococcus JX645547.1.1404 (30% in L1, 37% in L2 and 64% in L3). Other LAB were detected at low concentrations (betwen 0.01 and 9.3%) and mainly in L3 preparation as Lactobacillus coryniformis, Latilactobacillus curvatus, Lactobacillus helveticus, Lactobacillus malefermentans, Lactiplantibacillus paraplantarum, Leuconostoc mesenteroides,
Lactococcus lactis subsp. cremoris, Lactococcus garvieae, Lactococcus EU462846.1.1407, Lactococcus lactis subsp. lactis, Streptococcus KF841882.1.1400.
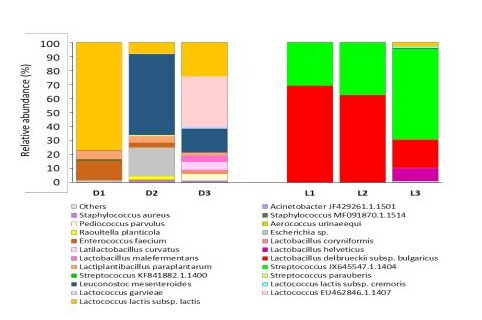
Figure 6 : Relative abundance of bacterial species identified in different types of dairy products (D - Darfiyeh, L - Labneh). Numbers 1, 2, and 3 correspond to three distinct producers. «Other» refers to genera with less than 0.1% relative abundance.
Metagenomic analysis of fungal relative abundance revealed that Ascomycota phylum was highly abundant in both Darfiyeh and Labneh products. Additionally, Basidiomycota phylum was detected in Labneh, along with other, less-characterized fungal phyla (Figure 7).
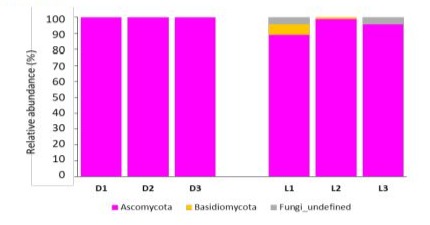
Figure 7: Relative abundance of fungal phyla identified in different types of dairy products (D - Darfiyeh, L - Labneh). Numbers 1, 2, and 3 correspond to three distinct producers.
For fungal taxa identified at the genus level (Figure 8), we detected several genera overal present in all Darfiyeh and Labneh preparations: Debaryomyces, Pichia, Saccharomyces, Kluyveromyces, and Candida. In Darfiyeh products, two genera were highly abundant, Pichia (dominant in D1) and Debaryomyces (dominant in D2 and D3). In Labneh samples, Saccharomyces was dominant in L2 and L3, while Dipodascus was dominant in L1.
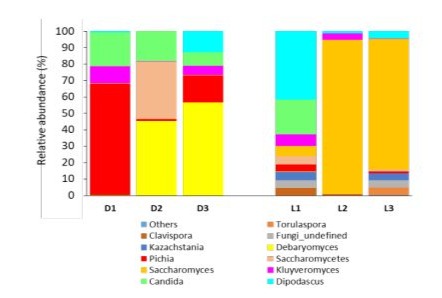
Figure 8 : Relative abundance of fungal genera identified in different types of dairy products (D - Darfiyeh, L - Labneh). Numbers 1, 2, and 3 correspond to three distinct producers. «Other» refers to genera with less than 0.1% relative abundance.
At the species level (Figure 9), Pichia fermentans (67%, 1.2%, 16%) and Debaryomyces hansenii (0.3%, 45%, 56%) were dominant in Darfiyeh preparations, while Saccharomyces eubayanus (6%, 93%, 79%) and Dipodascus geotrichum (41%, 1.2%, 4.5%) were dominant in Labneh samples. As with bacteria, it appears that each type of dairy product there are 2 species which are dominant in turn and which constitute the majority of the fungal community. Other species aincluding Clavispora lusitaniae, Kazachstania servazzii, Kluyveromyces lactis, Kluyveromyces marxianus, Candida sake, Candida argentea and Candida zeylanoides were observed at variable relative abundances in all preparations (less than 20%). Wickerhamiella pararugosa and Torulaspora delbrueckii were exclusively identified in Labneh samples (less than 5%).
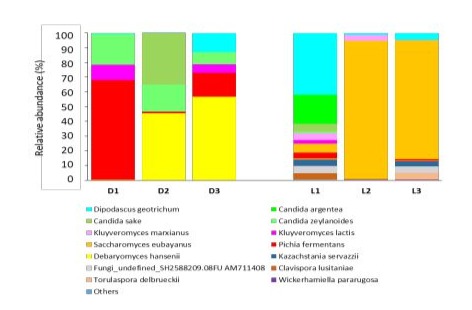
Figure 9 : Relative abundance of fungal species identified in different types of dairy products (D - Darfiyeh, L - Labneh).
Numbers 1, 2, and 3 correspond to three distinct producers.
«Other» refers to genera with less than 0.1% relative abundance.
Antibiotic resistance potential
The search for ARGs was performed on the bacterial species identified in the Darfiyeh and Labneh samples using the RAST server. A total of 11 genes associated with antibiotic resistance were identified. For all samples analyzed through targeted metagenomics, a score was calculated for each gene, based on the relative abundance of the bacterial species harboring this gene in their genome (Figure 10). ARGs were detected in all samples, with significant variability between producers. Darfiyeh samples appeared to contain a higher number of these genes. In contrast, Labneh samples showed considerable variability: while samples L1 and L2 exhibited a reduced number of ARGs, sample L3 contained all 11 identified antibiotic resistance genes. The detected ARGs confer resistance to various classes of antibiotics, including fluoroquinolones, tetracycline, penicillin, acriflavine, fosfomycin, and streptothricin.
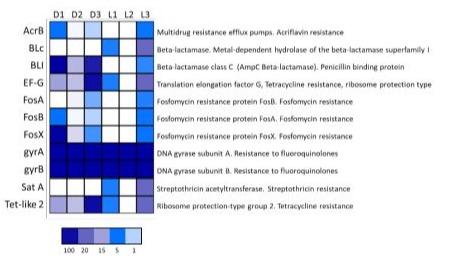
Figure 10 : Distribution of antibiotic resistance genes in Darfiyeh (D) and Labneh (L) products. For each sample, a score was assigned to each gene based on the relative abundance of the bacterial species containing that gene in its genome.
Estimation of pathogenic, antagonistic, and probiotic potential
To assess the pathogenic, antagonistic, and probiotic potential of the microorganisms identified in Darfiyeh and Labneh dairy products, a detailed literature review was conducted on all identified bacterial species (Table 2) and fungal species (Table 3). Figure 11 selectively displays the relative abundance of these species across different samples.
Regarding the pathogenic potential of bacteria, eight bacterial taxa were identified as potential human pathogens at very low levels, most of which are opportunistic pathogens that affect immunocompromised individuals. Staphylococcus aureus appeared to be the most potentially aggressive pathogen but showed a low relative abundance (between 0.01% and 0.95%) in the Darfiyeh samples. In the Labneh products, only sample L3 indicated the presence of S. aureus, and again with a very low abundance of 0.02%. Some strains of Escherichia coli are known to be aggressive pathogens in humans. The genus Escherichia was present in the samples with low abundance (≤ 1%), except in sample D2, where it reached 20.40%. Since this taxon is not a lactic acid bacterium involved in milk fermentation, it is likely a contaminant introduced during the milking or manufacturing process. All other bacterial species with pathogenic potential (Enterococcus faecium, Lactococcus garvieae, Raoultella planticola, Aerococcus urinaeequi, Acinetobacter sp., Streptococcus parauberis) were present in the samples with very low abundances (≤ 2%). This low proportion suggested the presence of antagonistic mechanisms that inhibit the activity of these potential pathogens, thereby mitigating health risks for the consumers. Overall, the Labneh samples contained far fewer bacteria with pathogenic potential than the Darfiyeh samples (Figure 11).
The antagonistic activity of bacterial species was indeed more significant in the Darfiyeh cheese samples, with numerous bacterial species known for their ability to synthesize bacteriocins and other antimicrobial molecules (Lactiplantibacillus paraplantarum, Lactococcus lactis subsp. lactis, Leuconostoc mesenteroides, Pediococcus parvulus, Latilactobacillus curvatus, Lactobacillus delbrueckii subsp. bulgaricus, Lactobacillus helveticus). These species are also recognized for their probiotic activity, exhibitingimmunomodulatory properties and numerous beneficial effects on gut health (Table 2). They constituted a significant proportion of the microbiota. Specifically, Lactococcus lactis subsp. lactis was the predominant beneficial species in the Darfiyeh samples (7.79%– 77.56%), while Lactobacillus delbrueckii subsp. bulgaricus was the dominant species in the Labneh samples (20.19%–68.96%) (Figure 6).
Among the fungal species, Dipodascus geotrichum, Clavispora lusitaniae, Candida zeylanoides, and Candida sake are known to cause opportunistic infections, and their abundance varied widely across different samples (Table 3). Similar to pathogenic bacteria, the Labneh samples contained significantly fewer fungal species with pathogenic potential compared to the Darfiyeh samples (Figure 11). Counterbalancing this potential health risk, species with antagonistic activity, such as Pichia fermentans, Debaryomyces hansenii, Kluyveromyces lactis, and Candida sake, were identified. These species were present in large proportions (up to 67.73%) across different samples and were generally more abundant in the Darfiyeh samples. It is worth noting that Candida sake, although reported as a potential pathogen in rare infection cases, is also known as a biocontrol agent in food products. Two fungal species, Kluyveromyces marxianus and Kluyveromyces lactis, have been described as probiotics. These species are present in all Darfiyeh and Labneh samples in highly variable proportions, reaching up to 10.43% in D1.
Species | Effects | Reference | |
Pathogenicity | Enterococcus faecium | Opportunistic pathogen involved in nosocomial infections resistant to antibiotics (vancomycin) | [28] |
Lactococcus garvieae | Opportunistic infections in immunocompromised individuals | [29] | |
Escherichia sp. | Some strains are major pathogens | [30] | |
Staphylococcus aureus | Major human pathogen, resistant to multiple antibiotics | [31] | |
Raoultella planticola | Opportunistic infections in immunocompromised individuals | [32] | |
Aerococcus urinaeequi | Associated with urinary tract infections, sepsis, and endocarditis | [33] | |
Acinetobacter sp. | Opportunistic pathogen | [34] | |
Streptococcus parauberis | Rare infections in humans, particularly in immunocompromised individuals | [35] | |
Antagonistic activity | Lactiplantibacillus paraplantarum | Produces lactocin F and lactocin 705 that can inhibit the growth of pathogens | [36] |
Lactococcus lactis subsp. lactis | Produces nisin, which can inhibit the growth of foodborne pathogens | [37] | |
Leuconostoc mesenteroides | Produces leuconocins and organic acids that inhibit pathogens | [38] | |
Pediococcus parvulus | Produces pediocins that inhibit pathogens in fermented foods | [39] | |
Latilactobacillus curvatus | Produces sakacin, which can inhibit the growth of pathogens | [40] | |
Lactobacillus delbrueckii subsp. bulgaricus | Produces bulgarican and lactocin that help suppress pathogens in fermented products | [41] | |
Lactobacillus helveticus | Produces helveticin J and M that help suppress pathogens in fermented products | [42] | |
Probiotics | Lactiplantibacillus paraplantarum | Beneficial effects on gut health and modulation of the immune response | [43] |
Lactococcus lactis subsp. lactis | Beneficial effects on gut health and immunomodulatory properties | [44] | |
Leuconostoc mesenteroides | Contributes to gut health and the balance of intestinal microbiota | [45] | |
Pediococcus parvulus | Beneficial effects on gut health | [46] | |
Latilactobacillus curvatus | Potential probiotic properties, contributing to microbial stability and digestive health | [47] | |
Lactobacillus delbrueckii subsp. bulgaricus | Recognized for its benefits on gut health and probiotic properties | [48] | |
Lactobacillus helveticus | Improves digestion and reduces symptoms of lactose intolerance | [49] |
Table 2: Estimation of pathogenicity, antagonistic activity, and probiotic potential of bacterial species identified in Darfiyeh and Labneh dairy products.
|
Species |
Effects |
Reference |
|
|
Dipodascus geotrichum |
Geotrichosis in immunocompromised patients |
[50] |
|
|
Clavispora lusitaniae |
Opportunistic pathogen in immunocompromised patients |
[51] |
|
|
Candida zeylanoides |
Rare infections in immunocompromised patients |
[52] |
|
|
Candida sake |
Infections in immunocompromised patients |
[53] |
|
|
Pichia fermentans |
Biocontrol agent. Inhibits the growth of certain pathogenic fungi |
[54] |
|
|
Debaryomyces hansenii |
Biocontrol agent. Produces antifungal substances to inhibit the growth of pathogenic fungi |
[55-56] |
|
|
Kluyveromyces lactis |
Produces enzymes, antimicrobial substances, and killer toxins to inhibit other yeasts |
[57-58] |
|
|
Candida sake |
Produces enzymes, antimicrobial substances, and killer toxins to inhibit other yeasts |
[59] |
|
|
Kluyveromyces marxianus |
Biocontrol agent |
[60] |
|
|
Kluyveromyces lactis |
Enhances digestive health and modulates the immune system |
[61] |
Table 3: Estimation of pathogenicity, antagonistic activity, and probiotic potential of fungal species identified in Darfiyeh and Labneh dairy products.
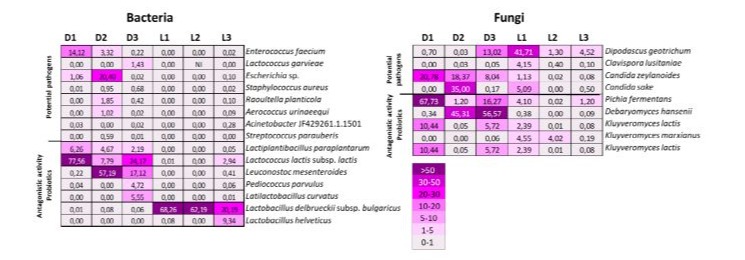
Figure 11: Relative abundance of bacterial and fungal species identified in Darfiyeh (D) and Labneh (L) samples from different producers (1-3), selected for their pathogenic, antagonistic, or probiotic potential.
Antioxidant effect of dairy products
The antioxidant effect of dairy products was assessed in a cell-free model for hydroxyl radical inhibition. Crude, heated, and filtered solutions from Darfiyeh and Labneh dairy products were tested at increasing final concentrations between 0.25 and 1.5 mg/mL (Figure 12). Both types of dairy products demonstrated antioxidant activity, with Darfiyeh products showing a more pronounced effect, beginning at a concentration of 0.75 mg/mL, lower than that of Labneh (1.5 mg/mL). A significant antioxidant activity was noted in crude and heated solutions (for D1, D2, D3, L1 and L2 products) as well as in filtered solutions (for D2 and D3), indicating a stronger probiotic, postbiotic and metabiotic effect. Slight variability was also observed according to the origin of the producers, likely due to the differing composition of microorganisms.
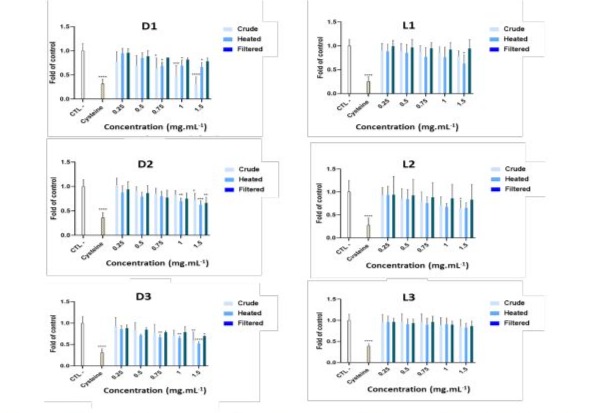
Figure 12: Inhibition of hydroxyl radical production by Lebanese fermented dairy products. The effects of each dairy product (D - Darfiyeh, L - Labneh) from different producers (1-3) and prepared as solutions (C - crude, H - heated, and F - filtered) were tested at increasing concentrations between 0.25 and 1.5 mg/mL. Distilled water served as the negative control (CTL), and cysteine was used as the positive control. HO. levels were expressed as a ratio of CTL. Statistical analysis for n = 6 independent assays was performed using ANOVA: Kruskal-Wallis test, with * p < 0.05, ** p < 0.005, *** p < 0.0005, and **** p < 0.00005.
Antihypertensive effect of dairy products
As dairy products are reputed to have antihypertensive effects, this activity was tested using the ACE inhibitory activity assay. Darfiyeh demonstrated a stronger antihypertensive effect than Labneh products, with lower IC50 values. Significant variability was observed across the different origins of these products as D1 and D2 exhibited significantly stronger antihypertensive effects than D3 (Figure 13A). L1 also appeared to have a stronger effect than L2 and L3 (Figure 13B).
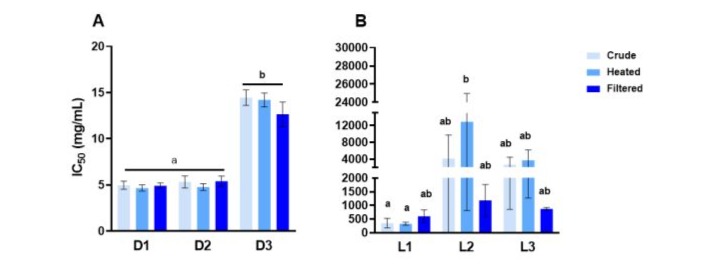
Figure 13 : Angiotensin converting enzyme (ACE) inhibition by Darfiyeh (A) and Labneh (B) dairy products. Crude, heated, and filtered solutions from different producers (1-3) were tested at increasing final concentrations ranging from 4.2 to 25 mg/mL for Darfiyeh (D) and from 33.3 to 83.3 mg/mL for Labneh (L). The inhibition was expressed as the IC50 value, presented with standard deviations. Statistical analysis was performed for n=9 independent assays using one-way ANOVA or Kruskal Wallis test followed by Tuckey or Dunn’s multiple comparisons, respeectively. In the same histogram, bars not sharing a common letter indicate significant differences (p < 0.05).
Anti-inflammatory effect of dairy products
The different solutions (crude, heated and filtered) of Darfiyeh and Labneh products at a final concentration of 1.5 mg/mL non-cytotoxic concentration (Supplementary file), were tested for their anti-inflammatory activity in vitro using U937 cells differentiated in macrophages. Their capacity to secrete the anti-inflammatory cytokine IL-10 and the pro-inflammatory cytokine TNF-α was quantified in cell cultures maintained in antibiotic-free media. The positive control of inflammation (I) was used to assess cytokine expression levels (Figure 14). The addition of dairy products revealed that crude solutions significantly increased the secretion of the anti-inflammatory cytokine IL10, with significant effects observed in the D3, L1, and L2 samples. Significant postbiotic and metabiotic effects were also recorded for the D3 samples (Figure 14A). In contrast, a slight but statistically non-significant decrease in TNF-α secretion was observed. However, variations were noted between different samples and the positive control of inflammation (Figure 14B).
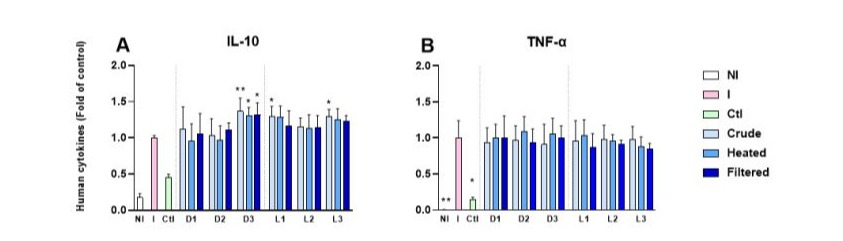
Figure 14 : Effects of Darfiyeh and Labneh crude, heated and filtered solutions on IL-10 (A) and TNF-α (B) production by human U937 macrophages. U937 were differentiated with PMA before being inflamed with LPS and put in contact with samples for 4 h. NI correspond to no-inflammed cells. Efficiency of LPS inflammation was controlled in each test (I). Dexamethasone was used as a positive control of inflammation inhibition (CtI). Cytokine levels in samples were expressed as a ratio of the positive control of inflammation (I) level. Statistical analysis was conducted on n=8 independent assays and performed against the I control using one-way ANOVA and the Kruskal-Wallis multiple comparisons test (* p<0.05 ; ** p<0.01).
Discussion
Microbial originality of traditional lebanese fermented milk products
We analyzed two traditional dairy products from Lebanon: Darfiyeh and Labneh. Handmade from goat and cow milk, respectively, these products were the focus of metagenomic analyses that provided an overview of their developing microbiota. As these were produced using traditional methods, three different producers were selected for each type of dairy product (Figure 1) to observe the variability in microbiota composition. Alpha and beta diversity (Figures 2 and 3) showed significant variability in the number and distribution of taxa among the different producers. Darfiyeh products varied mainly in the number of bacterial OTUs, and Labneh appeared to be more variable in the number of fungal OTUs and overall taxa distribution among producers. Microbial abundance analysis showed that each product contained two or three dominant bacterial and fungal species, whose abundance varied depending on the producer and together constituted the majority of the microbial community. These species clearly distinguished Darfiyeh from Labneh samples.
In Darfiyeh cheese (Figure 6), dominant bacterial species included Lactococcus lactis subsp. lactis, Lactococcus EU462846.1.1407, and Leuconostoc mesenteroides, with varying dominance in samples from different producers. In addition, other LAB species were observed in varying proportions, including Latilactobacillus curvatus and Enterococcus faecium. Serhan et al. [62] had previously identified L. lactis subsp. lactis, L. curvatus, and E. faecium in different Darfiyeh samples using PCR-TTGE and qPCR. Therefore, despite the variability between producers, this presence may represent a consistent microbiological signature of this type of dairy product. These strains are all part of routine microbial culture collections and are used in various research and industrial applications [63-67].
In terms of fungi, Pichia fermentas and Debaryomyces hansenii were dominant in Darfiyeh products (Figure 9). Other species including Kluyveromyces lactis, Kluyveromyces marxianus and Candida zeylanoides were observed at variable relative abundances across all preparations (less than 20%). P. fermentans, D. hansenii, Kluyveromyces ssp. and C. zelanoydes have been previously identified in various fermented foods and dairy products due to their roles in the fermentation process, biocontrol properties, and probiotic potential [68-69]. Overall, substantial variability was observed in the abundance of different taxa among Darfiyeh producers. This variability could be attributed to the specific characteristics of local goat milk and differences in handling, production, and storage practices. Made from raw milk and relying on natural microorganisms, the traditional packaging of Darfiyeh in goatskin containers, as well as differences in maturation times, influenced the microbial composition through the release of metabolites affecting microbial growth and community interactions.
Labneh samples were found to consistently contain Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus JX645547.1.1404 (Figure 6). This composition resulted from the production method using a yogurt-like homemade inoculum containing L. delbrueckii subsp. bulgaricus and streptococci strains such as Streptococcus thermophilus [70]. As a homemade inoculum, the composition of the strains may vary; however, this initial inoculation may explain the more consistent alpha diversity of bacterial OTUs observed in Labneh samples. The roles of L. delbrueckii subsp. bulgaricus and Streptococcus ssp. in yogurt production have been extensively studied. These two bacterial taxa work synergistically, with streptococci releasing amino acids and other nutrients that promote the growth of lactobacilli. This cooperation enhances the efficiency of fermentation and contributes to the texture and flavor of yogurt [71-73]. Other LAB species were recorded in low concentrations (less than 10%) in Labneh samples (Figure 6). All these strains are cultivable and used in various research and industrial applications [63]. The L3 product appeared to have the highest bacterial variability, probably due to specific handling and production practices, including a shorter draining duration (7 days) compared to L1 and L2, which were drained for 1 month and 2 weeks, respectively.
Fungi found in Labneh products were mostly Saccharomyces eubayanus and Dipodascus geotrichum. As with bacteria, it seems that for each Labneh product there were 2 species which were dominant in turn and which constituted the majority of the fungal community. S. eubayanus plays a significant role in brewing [74] and has been studied in various natural habitats in association with certain plants and soil [75] but it is not commonly associated with dairy products. We can assume that due to its fermentation capabilities, it could increase the transformation of milk and participate in the organoleptic and functional properties of the final product. D. geotrichum is known to be present in milk and cheese, contributing to the ripening and flavor of cheese and other fermented dairy products [76]. With low abundance, we also identified Wickerhamiella pararugosa and Torulaspora delbrueckii (less than 5%). W. pararugosa is recognized for its role in various fermentation processes, particularly in non-dairy contexts [77]. Similar to S. eubayanus, it may represent a newly observed species with the potential to enhance milk transformation. T. delbrueckii has been more extensively studied and is recognized for its biotechnological importance in various food fermentations, including dairy products [78-79]. Apart from the bacterial species introduced by the initial inoculum, the diversity of other bacterial and fungal taxa in Labneh products may be influenced by different sources of milk, processing methods, and environmental factors [80-81].
Food safety of artisanal dairy products
Different dairy products in the Mediterranean region have already been the subject of numerous studies [82] due to their historical and cultural uniqueness. The farming systems, characterized by extensive grazing, traditional technologies, and specific milking and transformation processes, enhance the organoleptic and functional properties of these dairy products. Despite their popularity, there are concerns regarding the fragility of sanitary conditions and the non-standardized transformation processes, which could introduce significant variability in quality [83-85]. To assess the risk posed by microorganisms present in artisanal and traditionally produced Darfiyeh and Labneh dairy products from Lebanon, we searched for ARGs in the genomes of the identified bacteria (Figure 10). We found that nearly all identified bacterial species could harbor one or more ARGs, including those for fluoroquinolones, tetracycline, penicillin, acriflavine, fosfomycin, and streptothricin. The levels of species harboring these genes varied among samples from different producers. The concern about antibiotic resistance genes in dairy products is linked to public health and food safety issues, as these genes can be transferred to pathogenic bacteria present in the products or within the human microbiome, thereby increasing the risk of spreading antibiotic resistance. LAB used traditionally as well as industrially, such as Lactobacillus and Streptococcus, also carry antibiotic resistance genes [86]. Although these bacteria are not pathogenic, their resistance genes can be transferred to pathogenic bacteria under certain conditions. In any case, a thorough understanding of the presence of these genes in dairy products can inform food safety monitoring measures to mitigate the risks associated with antibiotic resistance [87].
The pathogenic potential of all identified bacterial and fungal species was also assessed through the literature (Tables 2 and 3, Figure 11). While no strains closely related to clinical pathogens were identified, the presence of Escherichia species, sometimes at very high levels (e.g. in sample D2), and Staphylococcus aureus, present at low levels but in all Darfiyeh cheese samples, may indicate a potential risk. In contrast, the antagonistic activity of the identified species was notably represented. The antagonistic activity of microorganisms in artisanal dairy products helps reduce health risks by inhibiting pathogen growth. In the tested products, LAB dominated, outcompeting pathogens for nutrients and limiting their proliferation. Many of the strains of identified LAB are known to produce various antimicrobial compounds, including organic acids (such as lactic acid and acetic acid) and bacteriocins (e.g., lactocin F and lactocin 705 produced by Lactiplantibacillus paraplantarum, nisin produced by Lactococcus lactis subsp. lactis, leuconocins produced by Leuconostoc mesenteroides, pediocins produced by Pediococcus parvulus, sakacin produced by Latilactobacillus curvatus, bulgarican and lactocin produced by Lactobacillus delbrueckii subsp. bulgaricus, helveticin J and M produced by Lactobacillus helveticus etc.), which are known for their effectiveness in inhibiting the growth of foodborne pathogens. Additionally, these substances can create an inhospitable environment for many pathogens by lowering the product’s pH, which prevents their survival and proliferation [42].
Probiotics, which include various bacterial and fungal species, play a vital role in promoting human and animal health. In Darfiyeh and Labneh products, we identified a significant number of bacterial and fungal species with previously described probiotic activity, such as Lactiplantibacillus paraplantarum, Lactococcus lactis subsp. lactis, Leuconostoc mesenteroides, Pediococcus parvulus, Latilactobacillus curvatus, Lactobacillus delbrueckii subsp. bulgaricus, Lactobacillus helveticus, Kluyveromyces marxianus and Kluyveromyces lactis (Tables 2 and 3). Their numerous benefits highlight the functional aspects of the two dairy products studied and emphasize the importance of exploiting these microorganisms for therapeutic and preventive purposes.
Our results suggest that, despite variations in microbial composition between producers, the microbial ecosystem in each tested dairy product is diverse and with many health benefits. This diversity may contribute to a protective effect. A rich and well-balanced microbial community can resist invasion by pathogens, thereby reducing the risk of contamination [88-89]. However, hygiene conditions during the production and storage of these products must be consistently maintained to prevent contamination with pathogenic microorganisms.
Health effects of artisanal dairy products
Regarding the antioxidant effect, observational studies have suggested a potential link between dairy consumption and a reduced risk of chronic diseases associated with oxidative stress, such as cardiovascular diseases and certain cancers [90]. Additionally, fermented dairy has been described as being able to enhance antioxidant activity due to the presence of probiotics and their metabolic by-products [91]. Our findings indicated that Darfiyeh products exhibited a stronger antioxidant effect than Labneh, with significant probiotic, postbiotic, and metabiotic impacts observed across different samples (Figure 12). These results may be attributed to various antioxidant molecules, some of which are generated by microorganisms. Given the remarkable differences between Darfiyeh and Labneh products, we hypothesize that one or more dominant microorganisms present in Darfiyeh may contribute to the enhanced antioxidant effect. Lactococcus lactis ssp. lactis and Leuconostoc mesenteroides that are dominant in Darfiyeh products (Figure 6), are well-known probiotics and have been extensively studied and used in various probiotic formulations and research [92-93]. In Labneh products, the antioxidant activity was also observed in L1 and L2 samples, and may be attributed to Lactobacillus delbrueckii subsp. bulgaricus that are dominant in these samples (Figure 6) and that was largely studied for its probiotic potential [42,94].
Numerous studies have demonstrated that a significant number of peptides are generated from milk proteins during cheese ripening. Some of these peptides exhibit antihypertensive effects by inhibiting ACE [95-97]. Additionally, the accumulation of SCFAs increases during ripening. SCFAs have been shown to influence blood pressure regulation through various mechanisms, including the modulation of the renin-angiotensin system [98-99]. In our analyses, Darfiyeh samples demonstrated stronger antihypertensive activity than Labneh (Figure 13). A key distinction between the two types of dairy products lies in their ripening periods: Darfiyeh ripens over 4 to 6 months, while Labneh ripens within 1 to 4 weeks. Among Darfiyeh samples from different producers, D1 and D2 showed significantly greater effects than D3, regardless of the solution preparation method (Figure 13A). Similarly, L1 samples exhibited stronger antihypertensive effects compared to L2 and L3 (Figure 13B). This difference may be attributed to the ripening duration, as D1 and D2 had longer ripening periods than D3, and L1 had a longer ripening period than L2 and L3 (Table 1). We hypothesize that extended ripening periods likely facilitate the accumulation of probiotic strains, postbiotic residues, and metabolites, including bioactive compounds, as previously demonstrated in other dairy products.
The health benefits of microorganisms in dairy products have also been shown to include roles in disease prevention and immune system modulation [100]. Both dairy products tested showed a significant increase in the anti-inflammatory cytokine IL-10 (Figure 14A) and a subtle decrease in pro-inflammatory TNF-α levels (Figure 14B). These effects appear to be influenced by the presence of microorganisms, microbial particles, and metabolite content. Labneh solutions may contain enhanced microbial cells, particles and metabolites from Lactobacillus delbrueckii subsp. bulgaricus (Figure 6) which have been described as possessing anti-inflammatory properties [101]. Darfiyeh is characterized by a significantly higher level of Lactococcus spp. (Figure 6), which has been shown to reduce IL-1β-induced IL-8 secretion in Caco-2 cells [102]. It also has an increased proportion of Debaryomyces hansenii (Figure 9), which has been found to trigger an antiinflammatory effect in human monocyte-derived dendritic cells in vitro [103,104].
Conclusion
This study highlighted the constitutive and functional originality of two traditional dairy products made manually in Lebanon: Darfiyeh and Labneh. The Darfiyeh product was prepared with raw goat milk that retained all of its natural microbiota (notably Lactococcus lactis subsp. lactis, Leuconostoc mesenteroides, Enterococcus faecium) with particularities depending on the origin of the milk and the specific preparation methods of each producer. This study also highlighted the presence of fungi among which Pichia fermentas and Debaryomyces hansenii were dominant.
Labneh product was prepared with heated cow’s milk and inoculated with a homemade inoculum containing lactobacilli and streptococci. Following fermentation, the initial strains of the inoculum (Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus JX645547.1.1404) were retained, while other LABs were present in variable proportions depending on the milk’s origin and production specifics. The dominant fungi associated with Labneh products were Saccharomyces eubayanus and Dipodascus geotrichum.
Potential ARGs were identified in all samples, with Labneh products exhibiting a lower pathogenic potential and Darfiyeh demonstrating a higher antagonistic potential. Despite variations in microbial composition among producers and the risks of contamination, our results suggest that the antagonistic and probiotic potential of the microbial ecosystem remains consistently high across all tested dairy products.
Antioxidant, antihypertensive, and anti-inflammatory activities were tested in vitro on various samples of Darfiyeh and Labneh, specifically highlighting their probiotic, postbiotic, and metabiotic effects. Overall, Darfiyeh products exhibited stronger antioxidant and antihypertensive effects, while both dairy products demonstrated significant anti-inflammatory activity.
While these findings provide valuable insights, several limitations should be addressed in future research to build on the current results. Firstly, the study relied on a limited number of samples, which may not fully represent the variability inherent to artisanal production methods. Secondly, the metagenomic analyses identified a wide array of bacterial and fungal species, but further research is needed to isolate and characterize their individual contributions to the observed functional properties. Thirdly, while the study identified ARGs and assessed pathogenic potential, it did not confirm the presence of these genes in the working strains, nor did it quantify their expression levels or evaluate the transferability of ARGs within microbial communities. Finally, for a better interpretation of health effects, controlled experimental setups are necessary to establish causal relationships and determine the precise mechanisms underlying these effects. Addressing these limitations will enhance our understanding of the microbiota and functional properties of traditional dairy products, paving the way for improved health-oriented applications and preservation of artisanal production methods.
Acknowledgments
The authors would like to thank the Lebanese Association for Scientific Research (LASeR) and Futur Digital Consultancy for their generous financial support of the RK PhD project. The authors also acknowledge the CPER BiHauts Eco de France (2021–2027) program for its financial support of this work.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Supplementary Materials
Supplementary file. Cytotoxicity assays
References
- Paul Ross R, Morgan S, Hill C (2002) Preservation and fermentation: Past, present and future. International Journal of Food Microbiology 79: 3‑16.
- Anukam KC, Reid G (2007) Probiotics: 100 Years (1907‑2007) after Elie Metchnikoff’s Observation. Communicating Current Research and Educational Topics and Trends in Applied Microbiology 2: 466‑474.
- Arai S (1996) Studies on Functional Foods in Japan—State of the Art. Bioscience, Biotechnology, and Biochemistry 60: 9‑15.
- Gill P, Staudacherb HM (2023) Are postbiotics key to the potential benefits of fermented foods?.The Lancet Gastroenterology & Hepatology 8: 509.
- Gupta V, Garg R (2009) Probiotics. Indian Journal of Medical Microbiology 27: 202‑209.
- Gao J, Li X, Zhang G, Sadiq FA, Simal-Gandara J, et al. (2021) Probiotics in the dairy industry ‑ Advances and opportunities. Comprehensive reviews in food science and food safety 20: 39373982.
- Vinderola G, Sanders ME, Salminen S (2022) The Concept of Postbiotics. Foods 11: 1077.
- Tomasik P, Tomasik P (2020) Probiotics, Non‑Dairy Prebiotics and Postbiotics in Nutrition. Appl Sci 10: 1470.
- Polyanskaya I, Stoyanova L, Popova V (2021) Concept of metabiotics in fermented dairy products. Journal of Hygienic Engineering and Design 37: 209‑213.
- Pihurov M, Păcularu-Burada B, Cotarlet M, Vasile M, Bahrim GE (2021) Novel Insights for Metabiotics Production by Using Artisanal Probiotic Cultures. Microorganisms 9: 2184.
- Shigina ES, Polyanskaya IS, Popova VL (2022) Metabiotics in fermented milk product. Journal of Hygienic Engineering & Design 40: 266.
- Fan D, Stoyanova LG and Netrusov AI (2022) Microbiome and Metabiotic Properties of Kefir Grains and Kefirs Based on Them. Microbiology 91: 339‑355.
- Ağagündüz D, Yılmaz B, Şahin TÖ, Güneşliol BE, Ayten Ş, et al. (2021) Dairy Lactic Acid Bacteria and Their Potential Function in Dietetics: The Food–Gut‑Health Axis. Foods 10: 3099.
- Wessels S, Axelsson L, Bech Hansen E, De Vuyst L, Laulund S, et al. (2004) The lactic acid bacteria, the food chain, and their regulation. Trends in Food Science & Technology 15: 498-505.
- Widyastuti Y, Febrisiantosa R and Febrisiantosa A (2014) The Role of Lactic Acid Bacteria in Milk Fermentation. Food and Nutrition Sciences 5: 435‑442.
- Leeuwendaal NK, Stanton C, O’Toole PW and Beresford TP (2022) Fermented Foods, Health and the Gut Microbiome. Nutrients 14: 1527.
- Kaur H, Kaur G and Ali SA (2022) Dairy‑Based Probiotic‑Fermented Functional Foods: An Update on Their Health‑Promoting Properties. Fermentation 8: 425.
- Mathur H, Beresford TP and Cotter PD (2020) Health Benefits of Lactic Acid Bacteria (LAB) Fermentates. Nutrients 12: 1679.
- Zhang X, Luo Q, Guan X, Tang Y, Chen X, et al. (2023) Effects of fermented dairy products on inflammatory biomarkers: A metaanalysis. Nutrition, Metabolism and Cardiovascular Diseases 33: 471‑482.
- Chourasia R, Chiring Phukon L, Abedin MM, Padhi S, Singh SP et al. (2023) Bioactive peptides in fermented foods and their application: A critical review. Systems Microbiology and Biomanufacturing 3: 88‑109.
- Rognes T, Flouri T, Nichols B, Quince C, Mahé F (2016) VSEARCH: A versatile open source tool for metagenomics. Peer J 4: e2584.
- Gérard A, El-Hajjaji S, Burteau S, Fall PA, Pirard B, et al. (2021) Study of the microbial diversity of a panel of Belgian artisanal cheeses associated with challenge studies for Listeria ocytogenes. Food Microbiology 100: 103861.
- Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, et al. (2008) The RAST Server: rapid annotations using subsystems technology. BMC Genomics 9: 75.
- Zhao G, Nyman M, Jönsson J (2006) Rapid determination of short chain fatty acids in colonic contents and faeces of humans and rats by acidified water-extraction and direct-injection gas chromatography. Biomedical Chromatography 20: 674‑682.
- Halliwell B, Gutteridge JM, Aruoma OI (1987) The deoxyribose method: A simple “test‑tube” assay for determination of rate constants for reactions of hydroxyl radicals. Analytical biochemistry 165: 215– 219.
- Sentandreu MÁ and Toldrá F (2006) A rapid, simple and sensitive fluorescence method for the assay of angiotensin-I converting enzyme. Food Chemistry 97: 546‑554.
- Chammas GI, Saliba R, Corrieu G and Béal C (2006) Characterisation of lactic acid bacteria isolated from fermented milk “laban”. International Journal of Food Microbiology 110: 52‑61.
- Arias CA and Murray BE (2012) The rise of the Enterococcus: Beyond vancomycin resistance. Nature Reviews Microbiology 10: 266‑278.
- Vendrell D, Balcázar JL, Ruiz-Zarzuela I, de Blas I, Gironés O, et al. (2006) Lactococcus garvieae in fish: A review. Comparative Immunology, Microbiology and Infectious Diseases 29: 177‑198.
- Kaper JB, Nataro JP and Mobley HL (2004) Pathogenic Escherichia coli. Nature Reviews Microbiology 2: 123‑140.
- Lowy FD (1998) Staphylococcus aureus infections. The New England Journal of Medicine 339: 520‑532.
- Sękowska A (2017) Raoultella spp. – clinical significance, infections and susceptibility to antibiotics. Folia Microbiologica 62: 221‑227.
- Cattoir V, Kobal A, Legrand P (2010) Aerococcus urinae and Aerococcus sanguinicola, two frequently misidentified uropathogens. Scandinavian Journal of Infectious Diseases 42: 775‑780.
- Peleg AY, Seifert H and Paterson DL (2008) Acinetobacter baumannii: Emergence of a successful pathogen. Clinical Microbiology Reviews 21: 538‑582.
- Agnew W, Barnes AC (2007) Streptococcus iniae: an aquatic pathogen of global veterinary significance and a challenging candidate for reliable vaccination. Veterinary microbiology 122: 1‑15.
- Rouse S, Harnett D, Vaughan A and van Sinderen D (2008) Lactic acid bacteria with potential to eliminate fungal spoilage in foods. Journal of Applied Microbiology 104: 915‑923.
- Delves-Broughton J, Blackburn P, Evans RJ and Hugenholtz J (1996) Applications of the bacteriocin, nisin. Antonie van Leeuwenhoek 69: 193‑202.
- Stiles ME (1994) Bacteriocins produced by Leuconostoc species. Journal of Dairy Science 77: 2718‑24.
- Talarico TL and Dobrogosz WJ (1989) Chemical characterization of an antimicrobial substance produced by Lactobacillus reuteri. Antimicrobial Agents and Chemotherapy 33: 674‑679.
- Leroy F and De Vuyst L (1999) Temperature and pH conditions that prevail during fermentation of sausages are optimal for production of the antilisterial bacteriocin sakacin K. Applied and Environmental Microbiology 65: 974‑981.
- Servin AL (2004) Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiology Reviews 28: 405440.
- Gänzel MG (2015) Lactic metabolism revisited: Metabolism of lactic acid bacteria in food fermentations and food spoilage. Current Opinion in Food Science 2: 106‑117.
- Tamminen MT, Ouwehand AC, Mäki M, Joutsjoki T, Sjöblom M, et al. (2012) Viability of Lactobacillus paraplantarum DSM 14485 in human gastrointestinal tract and its molecular and biochemical identification after fermented vegetable consumption. Agricultural and Food Science 21: 182–196.
- Khemariya P, Singh S, Nath G, Gulati AK (2017) Probiotic Lactococcus lactis: A Review. Turkish Journal of Agriculture ‑ Food Science and Technology 5: 556–562.
- Shin, SY, Han NS (2015) Leuconostoc spp. as Starters and Their Beneficial Roles in Fermented Foods. In: Liong MT (eds) Beneficial Microorganisms in Food and Nutraceuticals. Microbiology Monographs, Springer Pg No: 111–132.
- Todorov SD, Dioso CM, Liong MT, Nero LA, Khosravi-Darani K, Ivanova IV (2022) Beneficial features of pediococcus: from starter cultures and inhibitory activities to probiotic benefits. World Journal of Microbiology & Biotechnology 39: 4.
- Bhattacharya D, Nanda PK, Pateiro M, Lorenzo JM, Dhar P, Das AK (2022) Lactic Acid Bacteria and Bacteriocins: Novel Biotechnological Approach for Biopreservation of Meat and Meat Products. Microorganisms 10: 2058.
- Kailasapathy K, Chin J (2000) Survival and therapeutic potential of probiotic organisms with reference to Lactobacillus acidophilus and Bifidobacterium spp. Immunology and Cell Biology 78: 80-88.
- Griffiths MW, Tellez AM (2013) Lactobacillus helveticus: the proteolytic system. Frontiers in Microbiology 4: 30.
- Walsh TJ, Groll A, Hiemenz J, Fleming R, Roilides E, et al. (2004) Infections due to emerging and uncommon medically important fungal pathogens. Clinical microbiology and infection 10: 48‑66.
- Beck-Sagué C, Jarvis WR (1993) Secular trends in the epidemiology of nosocomial fungal infections in the United States, 1980‑1990. National Nosocomial Infections Surveillance System. The Journal of Infectious Diseases 167: 1247‑1251.
- Sandven P, Lassen J (1999) Importance of Candida species other than C. albicans as opportunistic pathogens. Mycoses, 42: 85‑88.
- Trofa D, Gácser A, Nosanchuk JD (2008) Candida parapsilosis, an emerging fungal pathogen. Clinical Microbiology Reviews 21: 606625.
- Zhang X, Li B, Zhang Z, Chen Y, Tian S (2020) Antagonistic Yeasts: A Promising Alternative to Chemical Fungicides for Controlling Postharvest Decay of Fruit. Journal of fungi (Basel) 6: 158.
- Medina-Córdova N, Rosales-Mendoza S, Hernández-Montiel LG, Angulo C (2018) The potential use of Debaryomyces hansenii for the biological control of pathogenic fungi in food. Biological Control 121: 216‑222.
- Alía A, Córdoba JJ, Rodríguez A, García C, Andrade MJ (2020) Evaluation of the efficacy of Debaryomyces hansenii as protective culture for controlling Listeria monocytogenes in sliced dry-cured ham. Food Science and Technology - LWT 119: 108886.
- van den Berg JA, van der Laken KJ, van Ooyen AJ, Renniers TC, Rietveld K, Schaap A, Brake AJ, Bishop RJ, Schultz K, Moyer D, et al. (1990) Kluyveromyces as a host for heterologous gene expression: expression and secretion of prochymosin. Biotechnology 8: 135‑139.
- Zolfaghari A, Beheshti-Maal K, Ahadi AM, Monajemi R (2024) A novel inhibitory strategy of Leishmania major using Kluyveromyces lactis and Saccharomyces cerevisiae killer toxins. Future Microbiology 1‑11.
- Teixidó N, Usall J, Viñas I (1999) Efficacy of preharvest and postharvest Candida sake biocontrol treatments to prevent blue mould on apples during cold storage. International Journal of Food Microbiology 50: 203‑210.
- Baptista M and Domingues L (2022) Kluyveromyces marxianus as a microbial cell factory for lignocellulosic biomass valorisation. Biotechnology Advances 60: 108027.
- Parvez S, Malik KA, Ah Kang S, Kim HY (2006) Probiotics and their fermented food products are beneficial for health. Journal of Applied Microbiology 100: 1171‑1185.
- Serhan M, Cailliez-Grimal C, Borges F, Revol-Junelles AM, Hosri C, et al. (2009) Bacterial diversity of Darfiyeh, a Lebanese artisanal raw goat’s milk cheese. Food Microbiology 26: 645-52.
- Hamese S, Mugwanda K, Takundwa M, et al. (2023) Recent advances in genome annotation and synthetic biology for the development of microbial chassis. Journal of Genetic Engineering and Biotechnology 21: 156.
- Colombo E, Franzetti L, Frusca M, Scarpellini M (2010) Phenotypic and Genotypic Characterization of Lactic Acid Bacteria Isolated from Artisanal Italian Goat Cheese. Journal of Food Protection 73: 657‑662.
- Delavenne E, Mounier J, Asmani K, Jany JL, Barbier G et al. (2011) Fungal diversity in cow, goat and ewe milk. International Journal of Food Microbiology 151: 247‑251.
- Martín-Platero AM, Valdivia E, Maqueda M, Martínez-Bueno M (2009) Characterization and safety evaluation of enterococci isolated from Spanish goats’ milk cheeses. International journal of food microbiology 132: 24‑32.
- Terzic-Vidojevic A, Vukasinovic M, Veljovic K, Ostojic M, Topisirovic L (2007) Characterization of microflora in homemade semi-hard white Zlatar cheese. International Journal of Food Microbiology 114: 36-42.
- Gürkan Özlü B, Terzi Y, Uyar E, Shatila F, Yalçın HT (2022) Characterization and determination of the potential probiotic yeasts isolated from dairy products. Biologia 77: 1471–1480.
- Hatoum R, Labrie S, Fliss I (2013) Identification and Partial Characterization of Antilisterial Compounds Produced by Dairy Yeasts. Probiotics and Antimicrobial Proteins 5: 8–17.
- Sørensen KI, Curic-Bawden M, Junge MP, Janzen T, Johansen E (2016) Enhancing the Sweetness of Yoghurt through Metabolic Remodeling of Carbohydrate Metabolism in Streptococcusthermophilus and Lactobacillus delbrueckii subsp. bulgaricus. Applied and Environmental Microbiology Journal 82: 3683‑3692.
- Tamime AY, Robinson RK (2007) 6‑Microbiology of yoghurt and related starter cultures. In: In Woodhead Publishing Series in Food Science, Technology and Nutrition. Tamime and Robinson (eds), Tamime and Robinson’s Yoghurt (Third Edition), Woodhead Publishing, pp 468534.
- Ozer BH, Kirmaci HA (2010) Functional milks and dairy beverages. International Journal of Dairy Technology 6: 1‑15.
- Mayo B, Aleksandrzak-Piekarczyk T, Fernández M, Kowalczy, M, Álvarez-Martín P, et al. (2010) Updates in the Metabolism of Lactic Acid Bacteria. In: Biotechnology of Lactic Acid Bacteria: Novel Applications pp. 3-48. Mozzi, Raya and Vignolo (eds), Blackwell Publishing.
- Hittinger CT, Steele JL, Ryder DS (2018) Diverse yeasts for diverse fermented beverages and foods. Current Opinion in Biotechnology 49: 199‑206.
- Libkind D, Hittinger CT, Valério E, Gonçalves C, Dover J, et al. (2011) Microbe domestication and the identification of the wild genetic stock of lager‑brewing yeast. Proceedings of the National Academy of Sciences, 108: 14539‑14544.
- Gente S, Sohier D, Coton E, Duhamel C, Gueguen M (2006) Identification of Geotrichum candidum at the species and strain level: proposal for a standardized protocol. Journal of Industrial Microbiology and Biotechnology 33: 1019–1031.
- Jensen SL, Umiker NL, Arneborg N, Edwards CG (2009) Identification and characterization of Dekkera bruxellensis, Candida pararugosa, and Pichia guilliermondii isolated from commercial red wines. Food Microbiology 26: 915‑921.
- Fernandes T, Silva‑Sousa F, Pereira F, Rito T, et al. (2021) Biotechnological Importance of Torulaspora delbrueckii: From the Obscurity to the Spotlight. Journal of Fungi 7: 712.
- Albertin W, Chasseriaud L, Comte G, Panfili A, Delcamp A, et al. (2014) Winemaking and Bioprocesses Strongly Shaped the Genetic Diversity of the Ubiquitous Yeast Torulaspora delbrueckii. PLoS ONE 9: e94246.
- Banjara N, Suhr MJ, Hallen-Adams HE (2015) Diversity of Yeast and Mold Species from a Variety of Cheese Types. Current Microbiology 70: 792‑800.
- Behera SS, El Sheikha AF, Hammami R, Kumar A (2020) Traditionally fermented pickles : How the microbial diversity associated with their nutritional and health benefits? Journal of Functional Foods 70: 103971.
- Boyazoglu J, Morand-Fehr P (2001) Mediterranean dairy sheep and goat products and their quality. A critical review. Small Ruminant Research 40: 1‑11.
- Centers for Disease Control and Prevention (CDC) (2012) The Dangers of Raw Milk: Unpasteurized Milk Can Pose a Serious Health Risk.
- EFSA BIOHAZ Panel (2015) Scientific opinion on the public health risks related to the consumption of raw drinking milk. EFSA Journal 13: 3940.
- Food Standards Agency (FSA) (2018) Raw Drinking Milk and Cream Guidance.
- Mathur S, Singh R (2005) Antibiotic resistance in food lactic acid bacteria—a review. International Journal of Food Microbiology 105: 281‑295.
- Van Boeckel TP, Pires J, Silvester R, Zhao C, Song J, et al. (2019) Global trends in antimicrobial resistance in animals in low‑ And middleincome countries. Science 365: eaaw1944.
- Yang S, Bai M, Kwok LY, Zhong Z, Sun, Z (2023) The intricate symbiotic relationship between lactic acid bacterial starters in the milk fermentation ecosystem. Critical Reviews in Food Science and Nutrition 1–18.
- Ravyts F, Vuyst LD, Leroy F (2012) Bacterial diversity and functionalities in food fermentations. Engineering in Life Sciences 12: 356-367.
- Soedamah-Muthu SS, Verberne LD, Ding EL, Engberink MF, Geleijnse JM, et al. (2012) Dairy consumption and incidence of hypertension: a dose‑response meta‑analysis of prospective cohort studies. Hypertension 60: 1131‑1137.
- Virtanen SM, Jousilahti P (2006) Fermented dairy products, probiotics, and their effect on oxidative stress and antioxidant status. Nutrition Research Reviews 19: 1‑9.
- Paritova A, Nurgaliyev A, Nurgaliyeva G, Abekeshev N, Abuova A, Zakirova F, et al. (2024) The dietary effects of two strain probiotics (Leuconostoc mesenteroides, Lactococcus lactis) on growthperformance, immune response and gut microbiota in Nile tilapia (Oreochromis niloticus). PLoS ONE 19: e0312580.
- Swain MR, Anandharaj M, Ray RC, Parveen Rani R (2014) Fermented fruits and vegetables of Asia: a potential source of probiotics. Biotechnology Research International 2014: 250424.
- Salminen S, van Loveren H (2012) Probiotics and prebiotics: health claim substantiation. Microbial Ecology in Health and Disease 23.
- Sieber R, Bütikofer U, Egger C, Portmann R, Walther B, et al. (2010) ACE-inhibitory activity and ACE-inhibiting peptides in different cheese varieties. Dairy Science & Technology 90: 47-73.
- Hao X, Xia Y, Wang Y, Zhang X, Liu L (2023) The addition of probiotic promotes the release of ACE‑I peptide of Cheddar cheese : Peptide profile and molecular docking. International Dairy Journal 137: 105507.
- Helal A, Cattivelli A, Conte A, Tagliazucchi D (2023) Effect of Ripening and In Vitro Digestion on Bioactive Peptides Profile in Ras Cheese and Their Biological Activities. Biology 12: 948.
- Pluznick JL (2014) Gut microbiota in renal physiology: focus on shortchain fatty acids and their receptors. Kidney international 90: 11911198.
- Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, et al. (2015) Gut dysbiosis is linked to hypertension. Hypertension 65: 1331‑1340.
- Bordoni A, Danesi F, Dardevet D, Dupont D, Fernandez AS, et al. (2017) Dairy products and inflammation: A review of the clinical evidence. Critical Reviews in Food Science and Nutrition 57: 2497– 2525.
- Moro-García MA, Alonso-Arias R, Baltadjieva M, Fernández Benítez C, Fernández Barrial MA, et al. (2013) Oral supplementation with Lactobacillus delbrueckii subsp. bulgaricus 8481 enhances systemic immunity in elderly subjects. Age 35: 1311-1326.
- Luerce TD, Gomes-Santos AC, Rocha CS, Moreira TG, Cruz DN, et al. (2014) Anti-inflammatory effects of Lactococcus lactis NCDO 2118 during the remission period of chemically induced colitis. Gut Pathogens 6: 33.
- Ochangco HS, Gamero A, Smith IM, Christensen JE, Jespersen L, et al. (2016) In vitro investigation of Debaryomyces hansenii strains for potential probiotic properties. World Journal of Microbiology and Biotechnology 32: 141.
- Du Y, He C, An Y, Huang Y, Zhang H, et al. (2024) The Role of Short Chain Fatty Acids in Inflammation and Body Health. International Journal of Molecular Sciences 25: 7379.
Supplementary File
Cytotoxicity Assays
Figure S1: Cytotoxicity test of darfiyeh and labneh crude, heated and filtered solutions. U937 cells were differentiated into macrophages using PMA and then exposed to dairy product solutions at concentrations of 0.25, 0.50, 0.75, 1.00, and 1.50 mg/mL for 24 hours. Subsequently, the CCK-8 reagent was added, and the cells were incubated for 1.5 hours to evaluate cytotoxicity.
The results of the cytotoxicity test, shown in Figure S1, indicated a slight decrease in cell viability at the highest concentration tested (1.5 mg/mL). However, with viability remaining around 80%, none of the dairy product solutions can be considered cytotoxic. Therefore, a final concentration of 1.5 mg/mL was deemed suitable for use in the anti-inflammatory test.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.