Safety and Effectiveness of Intraoperative Neuromonitoring in Peripheral Nerve Stimulation: A Single-Center Real World Data Analysis, and Review
by Ioannis M Skaribas1*, Peter D Vu2, Gregory Blazek2
1Expert Pain Care, Houston, TX, USA
2McGovern Medical School at The University of Texas Health Science Center at Houston, Houston, TX, USA
*Corresponding author: Ioannis M. Skaribas, Expert Pain, 11451 Katy Fwy, Suite 340, Houston, TX 77079, USA
Received Date: 05 November 2025
Accepted Date: 12 November 2025
Published Date: 17 November 2025
Citation: Skaribas IM, Vu PD, Blazek G (2025) Safety and Effectiveness of Intraoperative Neuromonitoring in Peripheral Nerve Stimulation: A Single-Center Real World Data Analysis, and Review. Chron Pain Manag 9: 176. https://doi.org/10.29011/2576-957X.100076
Abstract
Background: Peripheral Nerve Stimulation (PNS) is an effective treatment for chronic peripheral neuropathic pain. Accurate lead placement traditionally relies on patient feedback, which may be unreliable under sedation. Intraoperative Neuromonitoring (IONM), long used in spinal cord stimulation, offers an alternative for guiding PNS implantation. The objective of this study was to demonstrate that PNS can be implanted accurately and safely under general anesthesia with IONM. Methods: This single-center, retrospective case series evaluated consecutive patients with chronic lower back pain who underwent permanent PNS implantation with the micro-implantable pulse generator (Nalu™ Neurostimulation System [Carlsbad, CA]) between April 2024 and February 2025. All procedures were performed under general anesthesia with fluoroscopic guidance and IONM. Pain outcomes were assessed using a numeric rating scale (NRS) at baseline and 3 months post-implant. Safety was evaluated by monitoring for intraoperative and postoperative adverse events through 3 months of follow-up. Results: A total of 30 patients (mean age 76.7 years, 66.7% female) were included and underwent successful implantation targeting the cluneal (90%) or lumbar medial branch nerves (10%). Mean NRS pain scores decreased from 8.9 at baseline to 2.6 at 3 months, representing a 70.5% mean reduction. All patients met responder criteria (≥50% pain reduction), and 16.7% were high responders (≥80% reduction). No adverse events were reported. Conclusions: PNS implantation guided by IONM under general anesthesia was safe, accurate, and effective in this case series. These findings support IONM as a valuable adjunct in neuromodulation and warrant further prospective study.
Keywords: Chronic pain; Electromyography; General anesthesia; Monitored anesthesia care; Neuromodulation; Micro-IPG
Introduction
Chronic nerve pain, including pain of peripheral nerve origin, is difficult to treat [1], and is a substantial burden for patients, who commonly experience reduced physical activity, compromised daily functioning, decreased productivity, sleep disturbances, depression, anxiety, and diminished health-related quality of life [2-11]. Only 30% to 40% of patients with chronic nerve pain achieve adequate response to conventional stepwise treatment, primarily pharmacotherapy [1]. For patients who fail to receive sufficient relief with conventional medical management, clinical guidelines now recommend interventional strategies, including neuromodulation via Peripheral Nerve Stimulation (PNS) [12,13]. A substantial clinical and real-world evidence base supports the ability of PNS to treat chronic pain of peripheral nerve origin [1418]. Similar to spinal cord stimulation (SCS) [19], PNS delivers targeted electrical impulses to modulate neural activity and reduce pain, using implanted leads and a pulse generator [20]. Recent technologic advances have enabled PNS to provide the broad, complex programming capabilities and sophisticated stimulation protocols once only available with SCS systems [17,21,22].
PNS systems are inserted using a minimally invasive procedure, with successful implantation requiring precise lead placement proximal to the target peripheral nerve, facilitated by image guidance using ultrasound and/or fluoroscopy [12]. PNS implantation under local anesthesia alone is uncommon [23], and is generally limited to the temporary placement of small devices [24]. Currently, most PNS procedures are performed with the patient awake (conscious) but sedated under Monitored Anesthesia Care (MAC), or with the patient asleep (unconscious) under general endotracheal anesthesia [23,25,26]. Confirmation of PNS-induced paraesthesia over the area of pain is typically elicited by intraoperative verbal feedback from the sedated patient during awake procedures, or by arousing patients during asleep procedures [12,27]. However, reliance on verbal patient feedback during lead implantation can be unreliable due to factors including patient stress, sedative-related confusion, impaired ability to communicate, and hearing or language difficulties [25,27-29]. Patients woken from general anesthesia may become acutely aware of pain, increasing their risk for agitation, medication-related disorientation, and impaired ability to communicate [27]. There are also safety concerns with MAC, as accidental oversedation can cause central respiratory depression, airway obstruction (due to an unprotected airway), brain damage, and death [28,30,31]. In cases of respiratory distress, the patient’s prone position during the PNS procedure can also delay resuscitation [31]. Additional risks include patient movement during the procedure [29,32], and MAC may be contraindicated in individuals with sensitivity to sedatives or local anaesthetics [27].
The use of Intraoperative Neuromonitoring (IONM) with PNS can provide continuous, objective feedback on neural function. IONM involves Electromyography (EMG), Somatosensory Evoked Potentials (SSEP), and motor-evoked potentials (MEP) to ensure accurate lead placement [25,29] while facilitating procedural safety through continuous surveillance [25,29,33]. This added layer of monitoring may support safer and more efficient procedures and improved patient comfort and satisfaction. These advantages are clinically relevant because inaccurate or incomplete patient feedback can contribute to device-related problems; for example, in a 2023 analysis of PNS from the Manufacturer and User Facility Device Experience (MAUDE) database, 8.6% of 1012 devicerelated Adverse Events (AEs) were related to reports of “unwanted stimulation” [34].
IONM has been used with SCS for over two decades, with increasing frequency [26,35,36] and with studies showing its safety and efficacy to be comparable, if not superior, to awake implantation [26,27,35,37-39]. A 2023 systematic review identified a substantial body of Level II evidence indicating superior pain relief, less extraneous paraesthesia, fewer postoperative neurologic deficits, and a 27% shorter operating time with IONM versus asleep placement for SCS [26]. To mitigate the complications of neurostimulation, the most recent evidence-based guidance from the International Neuromodulation Society (INS) recommends IONM for procedures performed under general anesthesia [25].
However, the use of IONM during PNS procedures remains underevaluated, with no published reports of IONM in PNS surgery.
In this manuscript, we report the first known case series of standardized use of IONM for permanent PNS therapy using the micro-implantable pulse generator (micro-IPG; Nalu™ Neurostimulation System [Carlsbad, CA]). The micro-IPG has shown high treatment efficacy, effectiveness, and safety across two randomized controlled clinical trials [17,40] and one large-scale real-world registry study in patients with chronic neuropathic pain [18]. The objective of this study was to demonstrate that PNS can be implanted accurately and safely under general anesthesia with IONM.
Materials and Methods
This observational, retrospective real-world study was conducted at a single, outpatient private practice pain clinic (Expert Pain, Houston, TX). All patients who initially presented with chronic peripheral neuropathic pain and numbness over the lower back and superior buttock area, had successful PNS trial procedures (i.e., achieved ≥50% pain reduction during the 7-day trial period), and were permanently implanted with PNS targeting the cluneal or lumbar medial branch nerves between April 2024 and February 2025 were included in this case series. The same surgeon (study author IS) performed all trial and permanent implantation procedures; IS is a double board-certified anaesthesiologist and interventional pain management specialist who has completed more than 2,000 implant procedures over the last 25 years.
To ensure ethical compliance and patient safety, Institutional Review Board (IRB) approval was obtained from WCG IRB, Puyallup, WA (IRB reference: 1331269), and all participants provided informed consent following standards of Good Clinical Practice [41] and Committee of Publication Ethics (COPE) guidelines [42]. The study followed IRB guidelines for data confidentiality and regulatory adherence. Data used herein were collected per usual clinical practice and stored in the patient records at the clinic. The PROCESS guidelines for case series reporting were used to draft this manuscript [43].
Permanent PNS implantation procedures were performed in accordance with the clinic’s standard of care, with patients under general endotracheal anesthesia in a prone position, utilizing fluoroscopy to guide anatomic lead placement and IONM (Cadwell Cascade Pro, Cadwell Industries, Kennewick, WA) to ensure accurate electrode placement. Anesthesia consisted of midazolam, fentanyl, and propofol for induction and a volatile agent for maintenance, typically sevoflurane. Trained and experienced technicians operated the IONM device. IONM feedback alone was used to determine if PNS coverage was appropriate; patients were not woken during the surgery. All patients were administered intraoperative antibiotics, and all incisions were closed using Vicryl and Monocryl sutures. American Society of Regional Anesthesia and Pain Medicine (ASRA) guidelines for anticoagulation were followed for any patients on antithrombotic therapy [44].
Procedures lasted approximately 1.5 hours. Afterward, patients were given detailed postoperative instructions, prescribed a 7-day course of antibiotics, and were instructed to return for evaluation 72 hours after the procedure. After sufficient site healing, all PNS devices were programmed to alternate every 3 minutes between tonic and sub-paraesthesia stimulation.
To evaluate treatment effectiveness, pain scores were obtained from patients using a standard numeric rating scale (NRS, 0 to 10) prior to the PNS trial (i.e., at baseline) and at 3 months after permanent PNS implantation. Response and high response were defined as ≥50% and ≥80% reductions in pain scores from baseline, respectively. To evaluate procedure safety, patients were evaluated immediately post-operation, before discharge, and via phone call within 24 hours. Descriptive statistics were used to calculate and summarize outcomes, including change from baseline in pain scores and response rate.
Results
A total of 30 patients received permanent PNS implantation targeting the cluneal nerve (27/30; 90.0%) or lumbar medial branch nerves (3/30; 10.0%) for chronic intractable lower back pain. Patients were a mean 76.7 years of age (range, 66-89) and 66.7% were female (Table 1). Table 2 provides detailed clinical profiles for each patient, including treatment muscle group targets. The majority (66.7%) had previous back surgery and 83.3% had previously implanted, operating SCS devices. Mean patient baseline pain score was 8.9 (range, 8-10).
|
Characteristic |
N=30 |
|
Age, mean (range) |
76.7 (66-89) years |
|
Median |
77 years |
|
Sex, female |
66.7% (20/30) |
|
Mean baseline NRS pain score (range), pre-PNS |
8.9 (8-10) |
|
Mean NRS pain score (range), 3 months post-PNS |
2.6 (1-4) |
|
Mean pain percent reduction (range), 3 months post-PNS |
70.5% (55.6%-88.9%) |
|
Responder rate* 3 months post-PNS |
100.0% (30/30) |
|
High responder rate** 3 months post-PNS |
16.7% (5/30) |
|
Abbreviation: NRS: numeric rating scale; PNS: peripheral nerve stimula *Defined as ≥50% pain reduction. ** Defined as ≥80% pain reduction. |
tion. |
Table 1: Patient Characteristics (N=30).
|
Age |
Sex |
SCS Implant |
Previous Spinal Surgery |
Patient Diagnosis Summary |
IONM Muscle Group Target |
NRS |
||
|
Baseline |
3 Mo |
% Change |
||||||
|
74 |
F |
Yes |
Lumbar fusion, lumbar decompression, cervical fusion |
Bilateral cluneal, low back pain, leg pain, left side worse |
Left: Iliopsoas, vastus lateralis, tibialis anterior, gastrocnemius, abductor hallucis Right: Iliopsoas, vastus lateralis |
8 |
2 |
75.0% |
|
78 |
M |
Yes |
Cervical fusion, cervical laminectomy, thoracic laminectomy, lumbar laminectomy, L1 and L2 fusion |
Bilateral cluneal, low back and buttocks |
Left and right: Iliopsoas, vastus lateralis, tibialis anterior, gastrocnemius, abductor hallucis |
8 |
1 |
87.5% |
|
75 |
F |
Yes |
Back surgery, back fusion |
Bilateral cluneal, low back (entire back), right side worse |
Left and right: Iliopsoas, vastus lateralis |
8 |
2 |
75.0% |
|
74 |
F |
Yes |
Right hip replacement |
Bilateral cluneal, low back, starts on right side, spreads to left |
Left and right: Iliopsoas, vastus lateralis, tibialis anterior, gastrocnemius, abductor hallucis |
9 |
2 |
77.8% |
|
89 |
M |
Yes |
None |
Bilateral cluneal, low back, starts on right side, spreads to left |
Left and right: Tibialis anterior |
9 |
2 |
77.8% |
|
78 |
M |
Yes |
Lumbar laminectomy and fusion |
Bilateral cluneal, low back, equal pain on both sides |
Left: Rectus abdominus, tibialis anterior, gastrocnemius Right: Tibialis anterior, gastrocnemius |
9 |
1 |
88.9% |
|
79 |
F |
Yes |
Back surgery, right SI fusion, left SI fusion, total hip replacement |
Bilateral cluneal, low back, equal pain on both sides |
Left: Iliopsoas vastus lateralis, tibialis anterior, gastrocnemius, abductor hallucis Right: Iliopsoas vastus lateralis, tibialis anterior, gastrocnemius |
9 |
1 |
88.9% |
|
88 |
M |
Yes |
None |
Bilateral cluneal, low back, equal pain on both sides, leg pain |
Left: Rectus abdominis, vastus lateralis/medialis, tibialis anterior, gastrocnemius, abductor hallucis Right: Rectus abdominis, tibialis anterior, gastrocnemius, abductor hallucis |
9 |
4 |
55.6% |
|
78 |
F |
Yes |
Unspecified lumbar surgery |
Bi-lateral medial branch, low back, equal pain on both sides, both leg pain |
Left and right: Iliopsoas vastus lateralis |
9 |
1 |
88.9% |
|
76 |
F |
Yes |
Lumbar laminectomy, L4-5 SI fusion, right SI fusion |
Bilateral cluneal, low back, across back |
Left and right: Rectus abdominis, iliopsoas vastus lateralis/medialis |
9 |
3 |
66.7% |
|
80 |
F |
Yes |
None |
Bilateral cluneal, low back, radiates to both sides |
Left and right: Iliopsoas, vastus lateralis, tibialis anterior, gastrocnemius, abductor hallucis |
9 |
3 |
66.7% |
|
81 |
M |
No |
C3 laminectomy, back surgery |
Bilateral cluneal, low back |
Left: Iliopsoas, vastus lateralis/medialis, tibialis anterior, gastrocnemius, abductor hallucis Right: Iliopsoas, vastus lateralis/medialis, abductor hallucis |
9 |
2 |
77.8% |
|
76 |
F |
Yes |
None |
Bi-lateral medial branch, mid/low back pain |
Left: Gluteus maximus, iliopsoas, vastus lateralis Right: Iliopsoas, vastus lateralis, tibialis anterior, gastrocnemius |
8 |
1 |
87.5% |
|
73 |
F |
Yes |
Cervical laminectomy, lumbar laminectomy |
Bilateral cluneal, low back, both sides, down buttocks and legs |
Left and right: Iliopsoas, vastus lateralis, tibialis anterior, gastrocnemius, abductor hallucis |
9 |
2 |
77.8% |
|
84 |
F |
Yes |
Lumbar fusion |
Bilateral cluneal, low back |
Left and right: Iliopsoas, vastus lateralis, tibialis anterior, gastrocnemius, abductor hallucis |
9 |
4 |
55.6% |
|
69 |
M |
Yes |
C5-6 discectomy, lumbar laminectomy |
Bilateral cluneal, low back |
Left and right: Iliopsoas, vastus lateralis, tibialis anterior, gastrocnemius, abductor hallucis |
8 |
3 |
62.5% |
|
81 |
F |
Yes |
None |
Bilateral cluneal, low back |
Left and right: Iliopsoas, vastus lateralis, tibialis anterior, gastrocnemius, abductor hallucis |
10 |
3 |
70.0% |
|
68 |
F |
Yes |
None |
Bilateral cluneal, low back |
Left and right: Iliopsoas, vastus lateralis, tibialis anterior, gastrocnemius, abductor hallucis |
9 |
3 |
66.7% |
|
77 |
F |
Yes |
None |
Bilateral cluneal, low back |
Left: Rectus abdominis, vastus lateralis/medialis, tibialis anterior, gastrocnemius Right: Rectus abdominis, tibialis anterior, gastrocnemius |
9 |
3 |
66.7% |
|
77 |
M |
Yes |
Right L4-5 discectomy |
Bilateral cluneal, low back, mostly left side |
Left: Vastus lateralis/ medialis, tibialis anterior Right: Vastus lateralis/ medialis, tibialis anterior, abductor hallucis |
9 |
4 |
55.6% |
|
82 |
F |
Yes |
Laminectomy |
Bilateral cluneal, low back and legs |
Left and right: Iliopsoas, vastus lateralis, tibialis anterior, gastrocnemius, abductor hallucis |
9 |
3 |
66.7% |
|
79 |
M |
Yes |
L5-S1 laminectomy, posterior fusion |
Bilateral cluneal, low back, right side only |
Left and right: Iliopsoas, vastus lateralis, tibialis anterior, gastrocnemius, abductor hallucis |
10 |
3 |
70.0% |
|
76 |
F |
No |
None |
Medial branch, bra line/mid back |
Left and right: Iliopsoas, vastus lateralis, tibialis anterior, gastrocnemius, abductor hallucis |
9 |
3 |
66.7% |
|
78 |
F |
Yes |
Back surgery x3 |
Bilateral cluneal, low back |
Left: Rectus abdominis, vastus lateralis/medialis, tibialis anterior, gastrocnemius, abductor hallucis Right: Rectus abdominis, tibialis anterior, gastrocnemius, abductor hallucis |
9 |
2 |
77.8% |
|
73 |
M |
No |
None |
Bilateral cluneal, low back |
Left and right: Iliopsoas, vastus lateralis, tibialis anterior, gastrocnemius, abductor hallucis |
9 |
3 |
66.7% |
|
71 |
F |
No |
Neck fusion |
Left cluneal/SI, low back/hip |
Left and right: Iliopsoas, vastus lateralis, tibialis anterior, gastrocnemius, abductor hallucis |
9 |
4 |
55.6% |
|
84 |
M |
No |
L4-5 disc surgery, facet rhizotomy |
Bilateral cluneal, low back, across back |
Left and right: Iliopsoas, vastus lateralis |
9 |
4 |
55.6% |
|
70 |
F |
Yes |
Unspecified back surgery |
Bilateral cluneal, low back |
Left and right: Iliopsoas, vastus lateralis, tibialis anterior, gastrocnemius, abductor hallucis |
9 |
3 |
66.7% |
|
68 |
F |
Yes |
Unspecified lumbar surgery |
Bilateral cluneal, low back and legs, left side worse |
Left and right: Iliopsoas, vastus lateralis, tibialis anterior, gastrocnemius, abductor hallucis |
9 |
4 |
55.6% |
|
66 |
F |
Yes |
None |
Bilateral cluneal, low back |
Left and right: Iliopsoas, vastus lateralis, tibialis anterior, gastrocnemius, abductor hallucis |
9 |
3 |
66.7% |
|
F: Female; M: Male; NRS: Numeric Rating Scale; PNS: Peripheral Nerve Stimulation; SCS: Spinal Cord Stimulation; SI, Sacroiliac |
||||||||
Table 2: Detailed Patient Profiles.
All PNS surgeries were performed and completed without incident. All devices were activated within 10 days following surgery, with programming optimized and tailored to patient preferences. All patients’ postoperative courses were unremarkable, with no reports of infection, site pain, electrode repositioning, lead migration, loss of stimulation or unpleasant/unwanted stimulation, or serious AEs through 3 months of follow-up.
At 3 months, mean patient pain score was 2.6 (range, 1-4), indicating an average pain reduction of 70.5% (range, 55.6% to 88.9%) (Table 1). All (100%) patients met response criteria (≥50% pain reduction), and 16.7% were high responders (≥80% pain reduction).
Discussion
This case series confirms the effectiveness and safety of using IONM during PNS implantation. All patients achieved meaningful (i.e., ≥50%) pain relief. Notably, 16.7% were high responders with ≥80% pain relief, while the overall reduction in pain intensity was 70.5%. These results align with published data from two large randomized controlled trials (COMFORT and COMFORT 2) evaluating the micro-IPG system for the treatment of peripheral neuropathic pain, without any specified IONM use [40,45]. In these studies, pooled 3-month data (N=103) indicated an 81% responder rate with a 30% high responder rate and an average pain reduction of 66% [40].
IONM used during PNS plays a distinct technical and physiologic role from its use in SCS, but provides similar benefits in terms of optimizing lead placement accuracy and reducing the risk of nontarget stimulation. In SCS, IONM is used to confirm appropriate activation of the dorsal column pathways and ensure safe epidural lead placement by avoiding the stimulation of ventral motor roots or other non-target tracts. This process relies on monitoring central conduction pathways, sometimes with the aid of fluoroscopic guidance, to confirm dorsal column engagement [46]. In contrast, PNS targets specific peripheral nerves corresponding to the patient’s area of pain, typically involving local peripheral nerve mapping under visualization and low-threshold stimulation to ensure accurate lead placement within the intended nerve distribution [47].
By confirming nerve proximity and adequacy in real time, IONM during PNS enhances the precision of lead positioning and minimizes the likelihood of off-target activation. Evidence from the current study and prior research [25,28,29] shows that IONM offers valuable real-time assessment to guide safe and accurate lead placement without the need for intraoperative patient feedback. With IONM, SSEPs and MEPs monitor the functional integrity of sensory and motor pathways, respectively, during neurointerventional procedures, ensuring that these critical structures are not compromised [29]. The accuracy of electrode placement in anesthetized patients is achieved by eliciting and capturing EMG responses from specific muscle groups innervated by the targeted peripheral nerves [25,28,29]. In addition, intraoperative SSEP collision testing may be used as a physiologic marker of paraesthesia [25,28]. Figures 1 shows examples of the IONM display in the operating room, demonstrating that individual channels can be stimulated independently, and highlighting the extent of therapeutic coverage. Figure 2 shows lead placement along a patient’s iliac crest, along with the IONM display during active stimulation.

Figure 1: Examples of IONM display, demonstrating that individual channels can be stimulated independently (left and right targets shown), highlighting the extent of therapeutic coverage for the neuromodulation therapy.
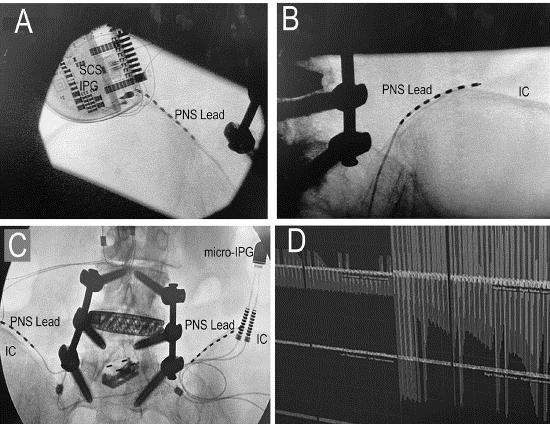
Figure 2: The upper left image shows the placement of the PNS lead along the superior margin of the patient’s left Iliac crest (IC) and the upper right image shows placement along the right IC. The lower left image shows the bilateral configuration of the leads along with the pre-existing spinal stabilization hardware. The lower right image shows intraoperative neuromonitoring during stimulation, in which the patient’s left side was being actively stimulated showing adequate coverage (left side of image).
As IONM capabilities are increasingly being integrated directly into device platforms [48,49], this is a timely moment for physicians to consider the roles and application of this technology in clinical practice. The potential exists for IONM to decrease healthcare resource utilization related to PNS implantation by increasing lead placement accuracy, leading to fewer revision or repeat procedures [27], and decreasing intraoperative time [26,27]. IONM also eliminates the need for intraoperative wake-up testing, which can otherwise add an average of 35 minutes to procedures [25,27]. Despite these potential advantages, IONM has not been widely adopted for use with PNS. Several factors contribute to this situation, including resource availability, healthcare provider experience, and reimbursement [29,50,51]. While support for IONM is typically available in urban teaching hospitals, this is not always the case in nonteaching hospitals, rural centers, or smaller surgical centers [51]. Likewise, in some settings, IONM has been shown to reduce overall operating room and anesthesia time [27], while in other cases, IONM setup and monitoring requirements can lead to longer procedure durations [52]. Additionally, engaging third-party neuromonitoring companies can present challenges [53].
It is also essential for healthcare providers to be aware that there are applicable reimbursement codes (Current Procedural Terminology [CPT] 95940, 95941) when utilizing IONM services. Many facilities will either directly contract with IONM companies or reimburse them, subsequently billing insurance carriers for these expenses. For facility administrators, effective communication and a thorough understanding of the clinical benefits of IONM during PNS are essential.
Limitations
This study lacked a comparison or control group, limiting the ability to directly assess outcomes against alternative approaches. However, selection bias was minimized by including all patients who received a permanent PNS implant at the clinic during the study period, with none excluded from analysis. This study focused exclusively on patients who received the micro-IPG PNS system; therefore, its findings may not be generalizable to larger PNS devices. Additionally, this research was conducted at a single center with a physician highly experienced in both IONM and PNS permanent implants, which precludes drawing conclusions regarding the learning curve for these procedures. While these early (3-month) outcomes are expected to best reflect the procedural benefits of IONM, all patients will be followed for up to 12 months to assess durability of response.
Finally, only two nerve sites were tested in this study. Additional research is warranted to investigate outcomes when IONM is used during PNS procedures for chronic peripheral nerve pain affecting the upper and more distal lower limbs.
Conclusion
The use of IONM in PNS procedures for chronic lower back pain is safe and effective and can result in outcomes comparable or superior to published PNS research without IONM. These positive results warrant additional research and consideration.
Acknowledgements
The authors would like to thank Caitlin Rothermel, MPH and Naseem Bazargan, MPH of MedLitera for their writing and editorial assistance.
Ethics Approval
This study involves human participants and was approved by WCG IRB Tracking Number: 20221779. Participants gave informed consent to participate in the study before taking part.
Conflicts of Interest
IS reports consultancy for Abbott Medical and Nalu Medical. DV and GB report no conflicts.
Funding: Support was provided by Nalu Medical, Inc.
Data Availability Statement
All data relevant to the study are included in the article.
Contributors
All authors either made a substantial contribution to the study concept, design, and/or analysis. All authors approved the final version of the manuscript. IS is responsible for the overall content as the guarantor and accepts full responsibility for the work and/ or the conduct of the study, had access to the data, and controlled decision to publish.
References
- Attal N, Bouhassira D, Colvin L (2023) Advances and challenges in neuropathic pain: a narrative review and future directions. Br J Anaesth 131: 79-92.
- Kawai K, Kawai AT, Wollan P, Yawn BP (2017) Adverse impacts of chronic pain on health-related quality of life, work productivity, depression and anxiety in a community-based study. Fam Pract 34: 656-661.
- Saykal TU, Uysal N (2024) The effect of peripheral neuropathy on disability and anxiety. Florence Nightingale J Nurs 32: 30-35.
- Meyer-Rosberg K, Kvarnstrom A, Kinnman E, Gordh T, Nordfors LO, et al. (2001) Peripheral neuropathic pain–a multidimensional burden for patients. Eur J Pain 5: 379-389.
- Novak CB, Anastakis DJ, Beaton DE, Katz J (2009) Patient-reported outcome after peripheral nerve injury. J Hand Surg Am 34: 281-287.
- Doth AH, Hansson PT, Jensen MP, Taylor RS (2010) The burden of neuropathic pain: a systematic review and meta-analysis of health utilities. Pain 149: 338-344.
- Smith BH, Torrance N, Bennett MI, Lee AJ (2007) Health and quality of life associated with chronic pain of predominantly neuropathic origin in the community. Clin J Pain 23: 143-149.
- Crane AM, Levitt RC, Felix ER, Sarantopoulos KD, McClellan AL, et al. (2017) Patients with more severe symptoms of neuropathic ocular pain report more frequent and severe chronic overlapping pain conditions and psychiatric disease. Br J Ophthalmol 101: 227-231.
- Nicholson B, Verma S (2004) Comorbidities in chronic neuropathic pain. Pain Med 5: S9-S27.
- Vieira WF, Coelho DRA, Litwiler ST, McEachern KM, Clancy JA, et al. (2024) Neuropathic pain, mood, and stress-related disorders: a literature review of comorbidity and co-pathogenesis. Neurosci Biobehav Rev 161: 105673.
- Radat F, Margot-Duclot A, Attal N (2013) Psychiatric co-morbidities in patients with chronic peripheral neuropathic pain: a multicentre cohort study. Eur J Pain 17: 1547-1557.
- Strand N, D’Souza RS, Hagedorn JM, Pritzlaff S, Sayed D, et al. (2022) Evidence-based clinical guidelines from the American Society of Pain and Neuroscience for the use of implantable peripheral nerve stimulation in the treatment of chronic pain. J Pain Res 15: 2483-2504.
- Manchikanti L, Sanapati MR, Soin A, Kaye AD, Kaye AM, et al. (2024) Comprehensive evidence-based guidelines for implantable peripheral nerve stimulation (PNS) in the management of chronic pain: from the American Society of Interventional Pain Physicians (ASIPP). Pain Physician 27: S115-S191.
- Mao Z, Lv J, Sun Y, Shen J, Gao Y, et al. (2024) Peripheral nerve stimulation for neuropathic pain management: a narrative review. Pain Ther 13: 1387-1406.
- Deer T, Pope J, Benyamin R, Vallejo R, Friedman A, et al. (2016) Prospective, multicenter, randomized, double-blinded, partial crossover study to assess the safety and efficacy of the novel neuromodulation system in the treatment of patients with chronic pain of peripheral nerve origin. Neuromodulation 19: 91-100.
- Gilmore CA, Ilfeld BM, Rosenow JM, Li S, Desai MJ, et al. (2020) Percutaneous 60-day peripheral nerve stimulation implant provides sustained relief of chronic pain following amputation: 12-month followup of a randomized, double-blind, placebo-controlled trial. Reg Anesth Pain Med 45: 44.
- Hatheway J, Hersel A, Engle M, Gutierrez G, Khemlani V, et al. (2024) Clinical study of a micro-implantable pulse generator for the treatment of peripheral neuropathic pain: 12-month results from the COMFORTrandomized controlled trial. Reg Anesth Pain Med rapm-2024-106099.
- Hatheway JA, Ratino T, Swain AR, Ratino T, Latif U, et al. (2025) LongTerm Pain Relief Delivered by MicroImplantable Pulse Generator: Findings from a Large-Scale, Real-World Data Peripheral Nerve Stimulation Patient Registry. Chron Pain Manag 9: 169.
- Sdrulla AD, Guan Y, Raja SN (2018) Spinal cord stimulation: clinical efficacy and potential mechanisms. Pain Pract 18: 1048-1067.
- Ong Sio LC, Hom B, Garg S, Abd-Elsayed A (2023) Mechanism of action of peripheral nerve stimulation for chronic pain: a narrative review. Int J Mol Sci 24: 4540.
- Mishra LN, Kulkarni G, Gadgil M (2022) Modeling the impact of the variation in peripheral nerve anatomy on stimulation. J Pain Res 15: 4097-4111.
- Kalia H, Pritzlaff S, Li AH, Ottestad E, Gulati A, et al. (2022) Application of the novel Nalu Neurostimulation System for peripheral nerve stimulation. Pain Manag 12: 795-804.
- Abrecht CR, Gabriel RA, Dutton RP, Kaye AD, Michna E, et al. (2015) National perioperative outcomes for intrathecal pump, spinal cord stimulator, and peripheral nerve stimulator procedures. Pain Physician 18: 547-554.
- Gilmore C, Ilfeld B, Rosenow J, Li S, Desai M, et al. (2019) Percutaneous peripheral nerve stimulation for the treatment of chronic neuropathic postamputation pain: a multicenter, randomized, placebocontrolled trial. Reg Anesth Pain Med 44: 637-645.
- Deer TR, Russo MA, Sayed D, Pope JE, Grider JS, et al. (2024) The Neurostimulation Appropriateness Consensus Committee (NACC)®: recommendations for the mitigation of complications of neurostimulation. Neuromodulation 27: 977-1007.
- Schlaeppi JA, Schreen R, Seidel K, Pollo C (2023) Intraoperative neurophysiological monitoring during spinal cord stimulation surgery: a systematic review. Neuromodulation 26: 1319-1327.
- Falowski SM, Sharan A, McInerney J, Jacobs D, Venkatesan L, et al. (2019) Nonawake vs awake placement of spinal cord stimulators: a prospective, multicenter study comparing safety and efficacy. Neurosurgery 84: 198-205.
- Shils JL, Arle JE (2018) Neuromonitoring for spinal cord stimulation lead placement under general anesthesia. J Clin Neurol 14: 444-453.
- Kurian RJ, Falowski S (2025) Spinal cord stimulation: neuromodulation for intraoperative neuromonitoring personnel. Neurodiagn J 1-23.
- Sohn HM, Ryu JH (2016) Monitored anesthesia care in and outside the operating room. Korean J Anesthesiol 69: 319-326.
- Bhananker SM, Posner KL, Cheney FW, Caplan RA, Lee LA, et al. (2006) Injury and liability associated with monitored anesthesia care: a closed claims analysis. Anesthesiology 104: 228-234.
- Hasoon J, Urits I, Viswanath O, Varrassi G, Simopoulos TT, et al. (2021) Percutaneous spinal cord stimulation lead placement under deep sedation and general anesthesia. Pain Ther 10: 1719-1730.
- Crum BA, Strommen JA (2007) Peripheral nerve stimulation and monitoring during operative procedures. Muscle Nerve 35: 159-170.
- Cooper AN, Sen H, Kanjanapanang N, Saad K, Wahl G, et al. (2025) Adverse events associated with peripheral nerve stimulation: an analysis of the MAUDE data base and implications for pain and spine clinicians. Neuromodulation 28: 619-626.
- Falowski SM, Celii A, Sestokas AK, Schwartz DM, Matsumoto C, et al. (2011) Awake vs. asleep placement of spinal cord stimulators: a cohort analysis of complications associated with placement. Neuromodulation 14: 130-4; discussion 134-5.
- Al-Salahat A, Dilsaver DB, Chen YT, Sharma R, Kapoor N, et al. (2025) Trends and demographic disparities in the utilization of intraoperative neuromonitoring in the United States, 2008 to 2021. J Clin Neurophysiol.
- Hwang R, Field N, Kumar V, Paniccioli S, Grey R, et al. (2019) Intraoperative neuromonitoring in percutaneous spinal cord stimulator placement. Neuromodulation 22: 341-346.
- Shils JL, Arle JE (2012) Intraoperative neurophysiologic methods for spinal cord stimulator placement under general anesthesia. Neuromodulation 15: 560-71; discussion 571-2.
- Hagedorn JM, Deer TR, Falowski SM, Yadav A, Comer A, et al. (2020) An observational study of intraoperative neuromonitoring as a safety mechanism in placement of percutaneous dorsal root ganglion stimulation and spinal cord stimulation systems. J Pain Res 13: 33493353.
- Engle M, Gutierrez G, Hersel A, Netzel C, Khemlani V (2025) A Confirmatory Randomized Controlled Trial Evaluating a MicroImplantable Pulse Generator for the Treatment of Peripheral Neuropathic Pain: 3- and 6-Month Results from the COMFORT 2 Study. Chron Pain Manag 9: 171.
- International Organization for Standardization. ISO 14155:2020 Clinical investigation of medical devices for human subjects — Good clinical practice 2020.
- Barbour V on behalf of COPE Council (2016) Journals’ best practices for ensuring consent for publishing medical case reports: guidance from COPE. Committee on Publication Ethics (COPE).
- Agha RA, Fowler AJ, Rajmohan S, Barai I, Orgill DP (2016) Preferred reporting of case series in surgery; the PROCESS guidelines. Int J Surg 36: 319-323.
- Kopp SL, Vandermeulen E, McBane RD, Perlas A, Leffert L, et al. (2025) Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine Evidence-Based Guidelines (fifth edition). Reg Anesth Pain Med rapm-2024-105766.
- Hatheway J, Hersel A, Song J, Engle M, Gutierrez G, et al. (2025) Clinical study of a micro-implantable pulse generator for the treatment of peripheral neuropathic pain: 3-month and 6-month results from the COMFORT-randomised controlled trial. Reg Anesth Pain Med 50: 561-567.
- Telkes I, Behal A, Hadanny A, Olmsted ZT, Chitnis G, et al. (2021) Rapid Visualization tool for intraoperative dorsal column mapping triggered by spinal cord stimulation in chronic pain patients. Annu Int Conf IEEE Eng Med Biol Soc 2021: 5760-5763.
- Deer TR, Eldabe S, Falowski SM, Huntoon MA, Staats PS, et al. (2021) Peripherally induced reconditioning of the central nervous system: a proposed mechanistic theory for sustained relief of chronic pain with percutaneous peripheral nerve stimulation. J Pain Res 14: 721-736.
- Wilent WB, Ndege M-R, Doan A (2025) The future of intraoperative neuromonitoring (IONM) in spinal surgery. NASSJ. N Am Spine Soc J 24: 100777.
- Gundogdu EC, Kale A, Mercan M, Yayla V, Ozcan UE, et al. (2023) Integration of intraoperative neurophysiological monitoring into laparoscopic pelvic nerve decompression surgery: a novel technique for protecting pelvic nerves. CEOG 50: 198.
- Biscevic M, Sehic A, Krupic F (2020) Intraoperative neuromonitoring in spine deformity surgery: modalities, advantages, limitations, medicolegal issues - surgeons’ views. EFORT Open Rev 5: 9-16.
- Laratta JL, Shillingford JN, Ha A, Lombardi JM, Reddy HP, et al. (2018) Utilization of intraoperative neuromonitoring throughout the United States over a recent decade: an analysis of the nationwide inpatient sample. J Spine Surg 4: 211-219.
- Black J, Singhal I, Murphy JE, Staudt MD (2025) The Use of Intraoperative Neuromonitoring in Routine Percutaneous Spinal Cord Stimulator Surgery is Not Associated with Improved Placement, Patient Safety, or Pain Severity Outcomes. Neurosurgery 97: 13771387.
- Prescient & Strategic Intelligence. U.S. Intraoperative Neuromonitoring Market Size & Share Analysis - Emerging Trends, Growth Opportunities, Competitive Landscape, and Forecasts (2025 - 2032).
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.