Long-Term Pain Relief Delivered by Micro-Implantable Pulse Generator: Findings from a Large-Scale, Real-World Data Peripheral Nerve Stimulation Patient Registry
by John A Hatheway1*, Tim Ratino2, AR Swain3, Thomas Ratino2, Usman Latif4, Sailesh Arulkumar5, Mehul J Desai6,7
1Interventional Pain Medicine Physician, Gig Harbor, WA, USA
2Ratino Interventional Pain Management, Fort Worth, TX, USA
3Hocking Valley Community Hospital, Logan, OH, USA
4University of Kansas Health System, Kansas City, KS, USA
5SSM Health Neurosciences, Shawnee, OK, USA
6Spine Pain & Performance Center, Washington, DC, USA
7Monument Research Institute, Washington, DC, USA
*Corresponding author: John A Hatheway, Interventional Pain Medicine, Gig Harbor, Washington, USA
Received Date: 15 April 2025
Accepted Date: 24 April 2025
Published Date: 29 April 2025
Citation: Hatheway JA, Ratino T, Swain AR, Ratino T, Latif U, et al. (2025) Long-Term Pain Relief Delivered by MicroImplantable Pulse Generator: Findings from a Large-Scale, Real-World Data Peripheral Nerve Stimulation Patient Registry. Chron Pain Manag 9: 169. https://doi.org/10.29011/2576-957X.100069
Abstract
Introduction: Peripheral Nerve Stimulation (PNS) is an established modality for treating chronic peripheral neuralgia/ neuropathy. Recent data from the COMFORT RCT demonstrated significant, sustained long-term improvements. Real-world evidence may confirm these outcomes, provide clinical practice insights, and enable comparison with RCT data. This work represents the largest, long-term report of Real-World Data (RWD) on patients with a permanent PNS implant. Methods: Anonymized records were reviewed from a national real-world, Institutional Review Board (IRB) approved registry of patients implanted between 12/8/2021 and 3/06/2024. Data consisted of standardized Patient Global Impression of Change (PGIC) responses. Surveys were completed following permanent implantation of a micro-IPG PNS system. A responder was defined as those achieving the Minimal Clinically Important Difference (MCID) for PGIC. Results: A total of 2,273 patients were included in this analysis. Major anatomic regions treated were head/neck 7% (158/2,273), trunk 42.8% (973/2,273), upper extremity 14.1% (321/2,273), and lower extremity 36.1% (821/2,273). Sixty primary nerve targets and/or nerve combinations were treated. The responder rate was 94% (2,137/2,273) for all patients. Sixty-five percent reported “Very Much Improved” or “Much Improved”, 29% reported “Minimally Improved”, 4% reported “No Change” and 1% reported any worsening. Response rates were consistent across all anatomic targets. Conclusions: These real-world results confirm the COMFORT PNS RCT findings of long-term improvements and demonstrate generalizability of the data from that RCT. Additional data from this registry will be reported as it becomes available.
Keywords: Peripheral Nerve Stimulation; Neuromodulation; Chronic Pain; Real-World Data; Patient Global Impression of Change (PGIC); micro-IPG; Pain Management
Introduction
Chronic pain affects approximately 20% of adults in the United States, impacting over 50 million individuals and creating an enormous economic burden [1,2]. Beyond economic costs of at least $560 to $635 billion annually [3], chronic pain takes a significant, multifaceted toll on patients’ quality of life, interfering with daily activities and straining personal relationships [4].
Peripheral Nerve Stimulation (PNS) is a well-established and effective option for chronic pain management that has been used clinically for over 50 years [5-7]. Recent advancements in PNS technology, including the development of miniaturized, permanently implanted devices with advanced programming capabilities such as the micro implantable pulse generator (µ-IPG), have led to substantial and lasting pain relief, achieving levels similar to those previously only seen with Spinal Cord Stimulation (SCS) [8,9].
As PNS technology has evolved, so too has the approach to evaluating its effectiveness. Real- World Data (RWD) is now recognized as a valid source of reliable and practical data [10] and has increasingly been incorporated into the milieu of clinical research [11] to complement findings from RCTs.
Here we report RWD from the largest, long-term real-world patient registry on patients with a permanent PNS implant. This study aims to expand the evidence base by evaluating real-world patient outcomes, documenting the effectiveness of PNS therapy in a broader population, and providing confirmatory data for the sponsor’s PNS RCT.
Methods
Device Description
The micro-IPG device (Nalu Neurostimulation System, Carlsbad, CA, USA) is a non-integrated system with a volume of less than 1.5 cc and is FDA-cleared for both PNS and spinal cord stimulation (SCS) with an 18-year service life and MRI-conditional labelling for full body (510(k) K201618, K232415) (Figure 1). The system is powered by a small, externally worn rechargeable battery known as a Therapy Disc (TD). The device has advanced complex programming capabilities including stable high energy delivery, pulse widths up to 2K µs, frequencies up to 1.499kHz, multi-electrode configurations, multi-area treatments, scheduling, current steering and a proprietary waveform. The device can be operated remotely by a smartphone application (iOS or Android). The system can hold up to 8 unique programs that are tailored to the patient’s preferences. Patients can select which program they prefer to use, or can rely on the independent scheduling feature to cycle through the pre-installed programs.

Figure 1: Micro-implantable pulse generator device.
All patients who received micro-IPG for PNS therapy were offered the opportunity to participate in a real-world patient registry. The registry, sponsored by the device manufacturer, received IRB approval from WCG IRB (Puyallup, WA; Reference No. 20221779). Patients who completed the registry questionnaire received a modest remuneration. This analysis reviewed anonymized Patient Global Impression of Change (PGIC) data from patients who received a PNS implant between December 8, 2021 and March 6, 2024.
No investigational devices or procedures were used. Following the standard of care pathway, all patients underwent a successful temporary trial procedure before receiving a permanent implant. A successful trial was defined as achieving ≥50% reduction in pain. Those who met this criterion proceeded to a permanent implant. The implant procedure followed standard clinical practice at each center or hospital. After the surgical wound healed, the micro-IPG device was activated and programmed to optimize pain relief based on patient preferences. As part of routine care, reprogramming was performed as needed.
After a period of 3 to 6 months, patients were asked to complete a post-permanent-implant survey, which included a standardized PGIC questionnaire [12].
In exchange for completing the survey, patients received a modest remuneration consisting of disposable wearable components for the TD. The survey was then completed approximately every 6 months.
A responder was defined as a patient who met the Minimum Clinically Important Difference (MCID) for the PGIC. The MCID for the PGIC was defined as “minimally improved, moderately improved or very much improved” [13] and was consistent with other published data related to this micro-IPG system [14,15].
Statistics
There was no formal hypothesis or hypothesis testing. Since the data were from a real-world registry, the sample size was determined by the number of patients implanted with the Nalu PNS device who enrolled in the registry and who had complete data. Summary statistical analysis was performed.
Results
A total of 2,273 patients with a micro-IPG implant for PNS met the eligibility criteria and were included in the analysis. Of these, 60% were female and the average age was 68.3 years (range, 19.298.5). The permanent implants were performed at a total of 154 clinical sites across the United States. All patients underwent the requisite successful trial prior to receiving the permanent implant. Data were obtained an average of 6.6 months post permanent implant (range 1 to 31 months) (Table 1).
Since beginning treatment with your stimulator, how would describe the change in ACTIVITY LIMITATIONS, SYMPTOMS, EMOTIONS, and OVERALL QUALITY OF LIFE related to your pain condition?
Very much improved
Much improved
Minimally improved
No change
Minimally worse
Much worse
Very much worse
Table 1: Patient Global Impression of Change survey.
To align with the formatting used by private insurance payors, the areas of the body where the permanent devices were implanted were grouped into four anatomic regions: head/neck, 7% (158/2,273); trunk, 42.8% (973/2,273); upper extremity, 14.1% (321/2,273); and lower extremity, 36.1% (821/2,273). The areas of pain within those anatomic regions are listed in Table 2. Although the system is not indicated for use in the head and neck region, this data is reported here to represent real-world experience. There were 15 areas of pain (regions targeted to treat the pain) ranging from the head to the feet, and a total of 31 primary nerves were targeted (Table 3).
|
Anatomic Region (n) |
Area of pain (n) |
|
Head/Neck (158) |
Head (85) |
|
Neck (73) |
|
|
Upper Extremity (321) |
Shoulder (258) |
|
Upper Arm (39) |
|
|
Lower Arm (13) |
|
|
Hand (11) |
|
|
Trunk (973) |
Abdomen (25) |
|
Upper Back (32) |
|
|
Lower Back (896) |
|
|
Pelvis (20) |
|
|
Lower Extremity (821) |
Hip (62) |
|
Upper Leg (91) |
|
|
Lower Leg (100) |
|
|
Knee (364) |
|
|
Foot (146) |
Table 2: Distribution of PNS Stimulation.
|
Axillary (48) |
Medial Branch Cervical (83) |
Sacral (17) |
|
Brachial Plexus (20) |
Medial Branch Lumbar (215) |
Saphenous (49) |
|
Cluneal (561) |
Medial Branch Thoracic (311) |
Sciatic (114) |
|
Dorsal Scapula (1) |
Median (2) |
Superior Genicular (289) |
|
Femoral (21) |
Obturator (2) |
Suprascapular (221) |
|
Greater Auricular (2) |
Occipital (88) |
Sural (8) |
|
Iliohypogastric (3) |
Peroneal, Common (41) |
Tibial (46) |
|
Ilioinguinal (18) |
Peroneal, Deep (9) |
Trigeminal (4) |
|
Inferior Genicular (46) |
Peroneal, Superficial (7) |
Ulnar (6) |
|
Intercostal (15) |
Pudendal (9) |
Not specified (2) |
|
Lateral Femoral Cutaneous (11) |
Radial (4) |
Table 3: Primary Nerves Targeted.
For all 2,273 patients, 94% (2137/2273) achieved MCID for PGIC, with 19% (441/2273) reporting “Very Much Improved”, 46% (1046/2273) reporting “Much Improved”, 29% (650/2273) reporting “Minimally Improved”, 4% (102/2273) reporting “No Change” and 1% (34/2273) reported any worsening (Figure 2). Sixty-five percent (1487/2273) reported “Very Much Improved” or “Much Improved”.
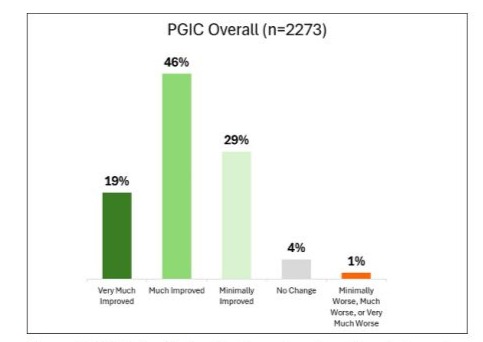
Figure 2: PGIC distribution for the entire cohort. Data indicate that most patients experienced notable improvement, with the majority reporting either “Very Much Improved” or “Much Improved” outcomes.
The outcomes were consistent regardless of the anatomic region, with MCID achieved in 97% of head/neck patients, 95% of upper extremity patients, 93% of trunk patients and 94% of lower extremity patients (Figure 3). Only 5% or less of patients reported “No Change” in any anatomic region. And no more than 2% of patients in any anatomic region reported a worse change. This data is consistent with the sponsor’s PNS RCT in which 95% of subjects met the MCID for PGIC [8].
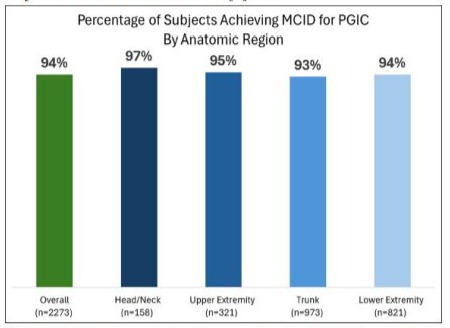
Figure 3: Comparison of subjects achieving MCID by anatomic region.
The consistency of outcomes across regions indicates that no single region skewed the data. Each region’s outcomes compared favourably to the overall assessment in all patients (Figure 4).
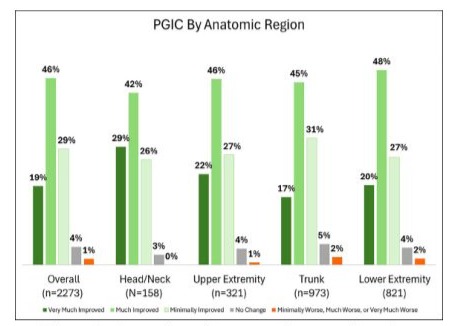
Figure 4: Comparison of outcomes by anatomic region.
Discussion
Real-World Data (RWD) is recognized as a valid and reliable source of practical data and has been increasingly incorporated into clinical research. While it does not replace the need for data from RCTs, it is now widely accepted by US and international regulatory agencies (e.g., FDA, EMA) as evidence to support market clearance, product approvals, and post-market follow-up requirements. One of RWD’s greatest strengths is demonstrating the generalizability of RCT outcomes to the broader population [15]. In this way, it has the potential to instill confidence that a particular therapy remains appropriate for an individual patient over time [16]. Patient registries, a well-established source of RWD, provide a comprehensive view of treatment outcomes across diverse populations [17,18]. This particular registry, which had minimal eligibility criteria, offered an “all-comers” snapshot of clinical practice.
Different evaluation standards apply when comparing RWD with RCT data. RWD should be assessed with realistic expectations, acknowledging that patient adherence and variations in treatment are inherent aspects of real-world practice.
The benefits of RWD include confirming findings from controlled clinical trials, providing insights into treatment outcomes in everyday settings and offering a longitudinal perspective on disease and condition progression, thereby supporting translational research [19,20].
Additionally, since RWD reflects outcomes from a broader range of patients and clinical settings than RCTs, RWD allows clinicians to cite actual clinical use when counselling patients about potential treatments [21]. RWD may also provide data not typically available in RCTs, such as results from patients with off-label indications, comorbidities or other factors that might have excluded them from RCT participation [22].
PGIC is a well-accepted and validated endpoint in chronic pain research, providing meaningful insights into the patient’s perspective on treatment effectiveness [23]. In a review of 96 clinical trials on chronic pain, PGIC was the most commonly used end point, occurring in more than half of the studies while other endpoints were much less frequently mentioned [24]. IMMPACT (Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials) recommends PGIC for the evaluation of chronic pain [13], and the FDA considers PGIC to “represent meaningful within-patient change in the target population.” [25] Unlike pain scores alone, PGIC is preferred due to its clinical applicability, greater responsiveness to change compared to other instruments, and its ease of use by patients [26]. It assesses the overall impact of treatment on a patient’s well-being, encompassing a broader and more meaningful evaluation than traditional pain scores. PGIC captures not only pain severity but also changes in physical function and quality of life, including psychological and emotional status [27]. It is especially applicable to real-world research because it better reflects real-world clinical practice more closely than other metrics and directly captures the patient’s perception of improvement or worsening of their condition—a factor that may not be represented in other patient-reported outcome measures [28].
Differentiating PNS from PNfS, PENS, and TENS Devices
This study focused on outcomes related to the micro-IPG PNS system. Although several FDA- cleared devices exist for PNS applications, insurance coverage policies sometimes conflate different neuromodulation modalities. Understanding the distinctions between devices is essential for contextualizing the study findings. Furthermore, it is critical to note that Level 1 evidence exists for PNS therapy, while this is not the case for the other modalities [8].
PNS devices (FDA product code GZF), including the micro-IPG PNS system, are designed for permanent implantation to provide long-term stimulation. Importantly, PNS devices require a shortterm trial in which patients must demonstrate ≥50% pain reduction to qualify for permanent implantation. These devices typically have more advanced programming options than other neuromodulation devices.
In contrast, PNfS (Peripheral Nerve Field Stimulation) involves the placement of electrodes near the general area of the pain in the subcutaneous tissue, instead of directly targeting specific peripheral nerves. PNfS is less precise than PNS, as it stimulates a broader nerve field and targets the very distal nerve branches rather than specific nerves [29]. It is important to note that PNfS is considered investigational by insurance companies.
PENS (Percutaneous Electrical Nerve Stimulation) and TENS (Transcutaneous Electrical Nerve Stimulation) are both temporary methods. The PENS device (FDA product code NHI) uses percutaneous, non-permanent electrodes for short-term pain relief, but is not indicated for use as a trial device before permanent implantation of a PNS device. Again, PENS is generally considered investigational and not covered by most if not all insurance companies.
TENS devices (FDA product code GZJ) deliver non-invasive electrical stimulation through electrodes placed on the skin and are typically used for short-term, at-home pain management. While TENS devices are frequently used clinically, the mechanism of action is likely very different from PNS devices.
Key Differences Between Permanent and Temporary Devices
The PNS devices used in this study are designed for permanent implantation, providing consistent and ongoing stimulation to alleviate chronic pain. Unlike PENS and TENS devices, PNS devices involve a permanent Implantable Pulse Generator (IPG) and electrodes placed next to the targeted nerve(s). This distinction is important since permanent implants provide ongoing, consistent stimulation, which can result in sustained pain relief, whereas temporary devices are short-term solutions that do not allow for the same degree of long-term pain management.
Limitations
Since this study relies on real-world data, some variability in the data is inherent and expected. Additionally, the survey did not account for medication adjustments or concurrent treatments, which may have influenced patient outcomes. As previously noted, there was variability in the time period between permanent implantation and PGIC data reporting.
Conclusions
This is the first large-scale report of RWD analysis related to the micro-IPG. Findings from this cohort of 2,273 patients demonstrate that use of the micro-IPG results in clinically meaningful improvement in chronic pain conditions. The large sample size not only validates the results of the COMFORT trial, but also broadens their generalizability. Moreover, the consistent improvements observed across a large, diverse sample of realworld clinic patients treated in multiple anatomic regions and nerve combinations, emphasizes the broad applicability of PNS therapy delivered by the micro-IPG PNS system. The authors will continue to report new findings as additional data emerges.
Acknowledgments
The authors would like to thank Robyn Brook for her writing and editorial assistance; Ms. Brook was compensated for her contributions to the work by Nalu Medical, Inc.
Ethical Guidelines
This study was conducted in accordance with the ethical principles of the Declaration of Helsinki and was approved by the WCG Institutional Review Board (IRB; Puyallup, WA; Reference No. 20221779). All patients provided informed consent to participate in the registry, and data were anonymized to protect patient confidentiality. No investigational devices or procedures were used; all treatments adhered to standard clinical practice.
Funding Sources
Nalu Medical, Inc. (Carlsbad, CA) is the sponsor of the registry and supported the writing and publication fees.
Conflicts of Interest
JH is a speaker and consultant for Nalu Medical. ARS is a consultant for Nalu Medical and Stratus Medical. UL is a consultant and advisory board member for Abbott, InFormed Consent, Nalu Medical, Nevro, Saluda, Spinal Simplicity, and VIVEX Biologics; a consultant for Brixton Biosciences, Hydrocision, SPR, Stryker, and Vertex Pharmaceuticals; and receives research funding from Mainstay Medical, Nalu Medical, Spinal Simplicity, and VIVEX Biologics. SA is a consultant for Nalu Medical. MD consults for Medtronic and Nalu Medical, and owns stock options in AllaiHealth, HypreVention, SPR Therapeutics, SynerFuse, and Virdio Health. All other authors declare no conflict of interest.
Data Availability Statement
Data generated during this study are available from the corresponding author on reasonable request.
References
- Yong RJ, Mullins PM, Bhattacharyya N (2022) Prevalence of chronic pain among adults in the United States. Pain 163: e328-e332.
- Rikard SM, Strahan AE, Schmit KM, Guy GP Jr (2023) Chronic Pain Among Adults—United States, 2019–2021. MMWR Morb Mortal Wkly Rep 72: 379-385.
- Smith TJ, Hillner BE (2019) The Cost of Pain. JAMA Netw Open 2: e191532.
- Institute of Medicine (2011) Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press.
- Helm S, Shirsat N, Calodney A, Abd-Elsayed A, Kloth D, et al. (2021) Peripheral nerve stimulation for chronic pain: a systematic review of effectiveness and safety. Pain Ther 10: 985-1002.
- Wall PD, Sweet WH (1967) Temporary abolition of pain in man. Science 155: 108-109.
- Slavin KV (2011) Peripheral Nerve Stimulation. Prog Neurol Surg. Vol 24. Basel: Karger. p. 1-15.
- Hatheway J, Hersel A, Engle M, Gutierrez G, Khemlani V, et al. (2024) Clinical study of a micro-implantable pulse generator for the treatment of peripheral neuropathic pain: 12-month results from the COMFORT- randomized controlled trial. Reg Anesth Pain Med.
- Hatheway J, Hersel A, Song J, Engle M, Gutierrez G, et al. (2024) Clinical study of a micro-implantable pulse generator for the treatment of peripheral neuropathic pain: 3-month and 6-month results from the COMFORT-randomised controlled trial. Reg Anesth Pain Med. rapm2023-105264.
- Simon GE, Bindman AB, Dreyer NA, Platt R, Watanabe JH, et al. (2022) When can we trust real-world data to evaluate new medical treatments? Clin Pharmacol Ther 111: 24-29.
- Dang A (2023) Real-world evidence: a primer. Pharmaceut Med 37: 25-36.
- (2015) Patient Global Impression of Change Scale. health.mil.
- Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, et al. (2005) IMMPACT. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 113: 9-19.
- Desai MJ, Raju T, Ung C, Arulkumar S, Kapural L, et al. (2024) Composite treatment response from a prospective, multi- center study (US-nPower) evaluating a miniature spinal cord stimulator for the management of chronic, intractable pain. Pain Physician 27: E881-E889.
- Blonde L, Khunti K, Harris SB, Meizinger C, Skolnik NSA (2018) Interpretation and impact of real-world clinical data for the practicing clinician. Adv Ther 35: 1763-1774.
- Dagenais S, Russo L, Madsen A, Webster J, Becnel L (2022) Use of real-world evidence to drive drug development strategy and inform clinical trial design. Clin Pharmacol Ther 111: 77-89.
- Pisa F, Arias A, Bratton E, Salas M, Sultana J (2023) Real world data for rare diseases research: the beginner’s guide to registries. Expert Opin Orphan Drugs 11: 9-15.
- Trotter JP (2002) Patient Registries: A New Gold Standard for “Real World” Research. Ochsner J 4: 211-214.
- Liu F, Panagiotakos D (2022) Real-world data: a brief review of the methods, applications, challenges, and opportunities. BMC Med Res Methodol 22: 287.
- Gliklich RE, Dreyer NA, Leavy MB, editors (2014) Registries for Evaluating Patient Outcomes: A User’s Guide. 3rd ed. Rockville (MD): Agency for Healthcare Research and Quality (US). Report No: 13(14)EHC111.
- L, Khunti K, Harris SB, Meizinger C, Skolnik NS (2018) Interpretation and impact of real- world clinical data for the practicing clinician. Adv Ther 35: 1763-1774.
- Khunti K, Almalki M, Chan JCN, Amod A (2023) The role of realworld evidence in treatment decision-making, regulatory assessment, and understanding the perspectives of people with type 2 diabetes: examples with gliclazide MR. Diabetes Ther 14: 1609-1625.
- Scott W, McCracken LM (2015) Patients’ impression of change following treatment for chronic pain: global, specific, a single dimension, or many? J Pain 16: 518-526.
- Langford DJ, Mark RP, France FO, Nishtar M, Park M, et al. (2024) Use of patient-reported global assessment measures in clinical trials of chronic pain treatments: ACTTION systematic review and considerations. Pain 165: 2445-2454.
- US Food and Drug Administration (2018) Methods To Identify What Is Important To Patients & Select, Develop Or Modify Fit-For-Purpose Clinical Outcomes Assessments, Patient- Focused Drug Development Guidance Public Workshop; 2018 Oct 15-16; Silver Spring, MD.
- Palermo T, Li R, Birnie K, Crombez G, Eccleston C, et al. (2024) Updated recommendations on measures for clinical trials in pediatric chronic pain: a multiphase approach from the Core Outcomes in Pediatric Persistent Pain (Core-OPPP) Workgroup. Pain 165: 10861100.
- Perrot S, Lantéri-Minet M (2019) Patients’ global impression of change in the management of peripheral neuropathic pain: clinical relevance and correlations in daily practice. Eur J Pain 23: 1117-1128.
- Rampakakis E, Ste-Marie PA, Sampalis JS, Karellis A, Shir Y, et al. (2015) Real-life assessment of the validity of patient global impression of change in fibromyalgia. RMD Open 1: e000146.
- Petersen EA, Slavin KV (2014) Peripheral nerve/field stimulation for chronic pain. Neurosurg Clin N Am 25: 789-797.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.