A Confirmatory Randomized Controlled Trial Evaluating a Micro-Implantable Pulse Generator for the Treatment of Peripheral Neuropathic Pain: 3- and 6-Month Results from the COMFORT 2 Study
By Mitchell Engle1, Genaro Gutierrez2, Alexander Hersel3, Christopher Netzel4, Vishal Khemlani5, Leonardo Kapural6, Efrain Cubillo7, John Hatheway8, Gregory Moore9, Ali Valimahomed10, Khurram Khan11, Marwan Shuayto12, Ariel Majjhoo13, Dawood Sayed14, Usman Latif14, Drew Trainor15, Reginald Ajakwe16, Peter Staats17, James Makous18, Patrick Martin19*, Shilpa Kottalgi19, Mehul J Desai20, COMFORT 2 Study Group
1Institute of Precision Pain Medicine, Corpus Christi, TX, USA
2Pain Specialists of America, Austin, TX, USA
3Pain Management and Injury Relief, Thousand Oaks, CA, USA
4Coastal Health Research, Jacksonville, FL, USA
5Columbia Pain Management, Portland, OR, USA
6Center for Clinical Research, Carolinas Pain Institute, Winston-Salem, NC, USA
7Pain Institute of Southern Arizona, Tucson, AZ, USA
8Virginia Mason Franciscan Health, WA, USA
9Pacific Sports and Spine, Eugene, OR, USA
10Advanced Orthopedics and Sports Medicine Institute, NJ, USA
11Insight Research Institute, Flint, MI, USA
12Michigan Neurology and Spine Center, Port Huron, MI, USA
13Neurointerventional Pain Management, Monroe, MI, USA
14University of Kansas Medical Center, Kansas City, KS, USA
15Denver Spine and Pain Institute, Denver, CO, USA
16 Spine & Pain, Burbank, CA, USA
17Premier Pain Centers, Shrewsbury, NJ, USA
18Makous Research, LLC, Carlsbad, CA, USA
19Nalu Medical, Inc., Carlsbad, CA, USA
20International Spine Pain & Performance Center, Washington, DC, USA
*Corresponding author: Patrick Martin, Nalu Medical, Inc., 2320 Faraday Ave, Carlsbad, CA 92008, USA
Received Date: 12 July 2025
Accepted Date: 21 July 2025
Published Date: 25 July 2025
Citation: Engle M, Gutierrez G, Hersel A, Netzel C, Khemlani V (2025) A Confirmatory Randomized Controlled Trial Evaluating a Micro-Implantable Pulse Generator for the Treatment of Peripheral Neuropathic Pain: 3- and 6-Month Results from the COMFORT 2 Study. Chron Pain Manag 9: 171. DOI: https://doi.org/10.29011/2576-957X.1000171
Abstract
Purpose: We report the results of COMFORT 2, a confirmatory randomized controlled trial for peripheral nerve stimulation evaluating a non-integrated, micro-implantable pulse generator for the treatment of chronic pain. The protocol for this study is identical to its sister study (COMFORT RCT) to allow for pooling data from both trials. Methods: Eligible subjects were randomized to either the Active Arm, receiving peripheral nerve stimulation and conventional medical management, or the Control Arm, receiving conventional medical management alone in a 2:1 ratio. Areas treated were limited to the shoulder, lower back, knee or the foot/ankle. Results: At 3 months there was an 80% (67/84) responder rate with an average pain reduction of 66% for the COMFORT 2 Active Arm (p<0.001) compared to a 4% (2/55) responder rate and 3% pain reduction for the Control Arm. At 6 months the combined (COMFORT and COMFORT 2 randomized controlled trials) Active Arm data showed an 82% (101/123) responder rate with an average pain reduction of 66% (p<0.001). Conclusions: The stand-alone COMFORT 2 outcomes are consistent with published results from COMFORT. The pooled data allow for a larger sample size and show robust and consistent improvement in all outcome metrics (p<0.001). This represents the first Level 1 evidence for PNS in the treatment of chronic pain of peripheral nerve origin.
Keywords: Peripheral nerve stimulation; Chronic pain; Pain management; Implantable neurostimulators; Micro-implantable neurostimulation; Neuropathic pain
Introduction
Peripheral nerve stimulation (PNS) is a well-established treatment for chronic pain, typically used only after other therapies have been exhausted. The COMFORT Group previously reported results from the COMFORT RCT (Clinical Study of a Micro-Implantable Pulse Generator FOR the Treatment of Peripheral Neuropathic Pain), which showed strong and statistically significant improvement in pain, functionality, quality of life, pain interference, depression, global impression of change, and general satisfaction.
We now report the initial outcomes of COMFORT 2, a second RCT that uses an identical protocol to COMFORT, with the same non-integrated micro-IPG system (Nalu Neurostimulation System, Nalu Medical, Inc., Carlsbad, CA; FDA product code GZF) that was previously studied in COMFORT. This represents the first Level 1 evidence for a permanently implanted PNS device.
The use of identical protocols for COMFORT and COMFORT 2 was intentional to allow pooling of data from both studies. Each study incorporated a pragmatic design to mimic standard clinical practice as much as practical, to ensure applicability of the results outside of the study setting.
Materials and Methods
The COMFORT 2 Study was Institutional Review Board approved (April 5, 2023) and conducted in compliance with local regulations and standards for good clinical practice. The study is registered on ClinicalTrials.gov (NCT05870124).
COMFORT 2 (ongoing) is being conducted at 18 US clinical sites. Subjects were enrolled between April 27, 2023, and September 9, 2024. The COMFORT 2 protocol is identical to the previously published COMFORT protocol [1], with the exception that COMFORT 2 did not allow the introduction of any new Conventional Medical Management (CMM) to a subject’s regimen. Subjects could continue using their existing CMM regimen, representative of standard clinical practice, that they had when enrolled unless it was to avoid harm to the patient’s wellbeing or safety.
In brief, eligible patients were between the ages of 18 and 80 years and were diagnosed with chronic pain (≥6 months) of peripheral nerve origin, in the knee, shoulder, low back, or foot/ankle. Postsurgical/post-traumatic, peripheral neuralgia including pain due to nerve injury, postsurgical scar formation, nerve entrapment, mononeuropathy and osteoarthritic pain were the included etiologies of chronic pain. Patients needed to follow the prescribed CMM treatment prior to enrollment.
Major exclusion criteria included: diagnosis of complex regional pain syndrome; peripheral neuralgia of metabolic origin or postherpetic neuralgia; ≥ 90 morphine milligram equivalents (MME) per day; failure to pass psychological evaluation; and intolerance of, or inability to wear or use the micro-IPG system. Full inclusion/exclusion criteria are available on ClinicalTrials. gov.
The primary efficacy endpoint was measured using the Numeric Rating Scale (NRS) pain score recorded in the Brief Pain Inventory (question 5; BPI-Q5) from the target area of chronic pain. Secondary outcome measures included the following Patient Reported Outcomes (PRO): Patient Global Impression of Change
(PGIC), Brief Pain Inventory Short Form (BPI-SF), Quality-ofLife metric (EQ-5D-5L), Beck Depression Inventory (BDI), Oswestry Disability Index (ODI). Additional secondary outcomes included patient safety, satisfaction, device comfort, usability, and subject compliance with the therapy.
Eligible subjects were randomized to CMM + PNS (Active Arm) vs CMM alone (Control Arm) in a 2:1 ratio. Those randomized to the Control Arm could cross over to the Active Arm at 3 months following randomization. There were no experimental devices or off-label procedures as part of the study. Allocation was concealed prior to randomization, which was performed using a random permuted block design (block size of 3) with a 2:1 allocation ratio (Active vs Control). Randomization was stratified by investigational site and was assigned via a centralized electronic system. Randomization sequence was generated by SASTM (version 9.4, SAS Institute Inc, Cary, NC).
Subjects randomized to the Active Arm followed the standard clinical practice for PNS including a temporary trial lead placement at the target nerve. Subjects needed to demonstrate a ≥ 50% decrease in pain during the trial period to be eligible for a permanent implant; failure to do so was considered a trial failure and those subjects exited the study.
The micro-IPGs were programmed by qualified industry clinical specialists under the direction of the study physician following usual clinical practice. Advanced programming options included paresthesia and sub-paresthesia, multiple cathodes and/or anodes, multiple areas, current steering across leads, pulse widths of up to 1000 µs and frequencies of up to 1500 Hz.
Patient Reported Outcomes (PROs) were collected at 1, 3, 6 months. Subjects will be followed out to 36 months as part of this study. The study was initially designed out to 12 months and later amended to follow subjects out to 36-months to allow for long term data collection. In addition, the sample size (from up to 100 subjects to up to 200 subjects) was updated to allow for a larger study. The primary outcome was Numeric Rating Scale (NRS) as captured in the Brief Pain Inventory-Question 5 (BPI-Q5); secondary endpoints are listed in Table 2. This report focuses on the 3- and 6-month efficacy and safety data, while the study is ongoing. In addition to reporting outcomes from this ongoing study, data was pooled from the COMFORT [2] and COMFORT 2 RCTs and presented here. This includes pooled data from the Active Arm at 1, 3 and 6-months and from the Control Arm at 1 and 3-months.
Statistical Analysis
The modified-intention-to-treat (mITT) analysis was carried out as prespecified in the Statistical Analysis Plan. The mITT population was defined as “all randomized subjects, excluding subjects who failed the trial period”. The primary effectiveness endpoint was the percentage of responders (defined as ≥ 50% reduction in pain relative to baseline) at 3 months.
The sample size for the study is based on power requirements for the primary effectiveness endpoint. A sample size of up to 200 randomized subjects was calculated based on a two-sample exact binomial test with a two-sided 0.05 alpha level, providing 90% power for a difference in responder rate between the arms.
Comparisons were made between randomized arms. Missing data were assumed to be missing at random; no imputation for missing data was used. Results were reported as mean ± Standard Deviation (SD) for continuous variables and as a percentage (count) for categorical variables, unless otherwise noted. Comparisons of responder rates between the Active Arm and Control Arm were conducted by a two-sample t-test; within-arm comparisons were conducted using a two-sample t-test. For all outcomes, percent reduction is calculated as a paired analysis within each subject and reported as mean ± SD. An analysis was undertaken by a third party to confirm poolability of data from both studies.
Results
The number of subjects consented/enrolled in COMFORT 2 was 315. Of those, 121 were randomized to the Active Arm and 64 were randomized to the Control Arm. Of those, 12 exited the study due to failed trials and were excluded from the mITT population. The COMFORT 2 subject disposition is shown in Figure 1. The final COMFORT 2 mITT population included 109 active and 64 control subjects for a total of 173 subjects. The demographics of subject population are outlined in Table 1. The distribution of targeted areas of pain was as follows: low back (45.1%, 78/173), knee (22.5 %, 39/173), shoulder (14.5%, 25/173), foot/ankle (17.9%, 31/173).
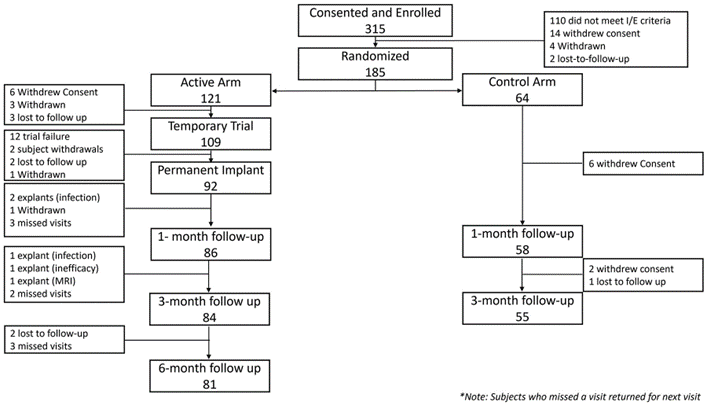
Figure 1. Subject disposition from consent to 6-month follow endpoint.
|
Characteristics |
Mean ± SD (N) [Min, Max] or % (N) |
||
|
Total mITT Population |
Active Arm (PNS+CMM) |
Control Arm (CMM Only) |
|
|
Age (in years) |
56.1 ± 12.5 (173) [19,79] |
56.6 ± 13.2 (109) [19,79] |
55.5 ± 11.3 (64) [34,78] |
|
Female |
62% (108/173) |
62% (68/109) |
62.5% (40/64) |
|
Male |
38% (65/173) |
38% (41/109) |
37.5% (24/64) |
|
Body Mass Index (BMI) |
31.3 ± 7.4 (169) [18.8, 63.9] |
31.2 ± 6.9 (106) [18.8, 53.1] |
31.5 ± 8.3 (63) [19.1, 63.9] |
|
Years since Diagnosis |
8.07 ± 9.7 (173) [0.6, 44.8] |
7.7 ± 9.3 (109) [0.6, 44.6] |
8.7 ± 10.3 (64) [0.6, 44.8] |
|
Areas of Pain |
|||
|
Low Back |
45.1% (78/173) |
46.8% (51/109) |
42% (27/64) |
|
Knee |
22.5% (39/173) |
21.1% (23/109) |
25% (16/64) |
|
Shoulder |
14.5% (25/173) |
16.5% (18/109) |
11% (7/64) |
|
Foot/Ankle |
17.9% (31/173) |
15.6% (17/109) |
22% (14/64) |
|
Opioid Usage |
|||
|
Opiates at Screening |
56% (97/173) |
55% (60/109) |
58% (37/64) |
|
Morphine Milligram Equivalents (MME)# |
15.3 ± 19.7 (173) [0, 90] |
15.0 ± 19.7 (109) [0, 90] |
16.0 ± 19.7 (64) [0,72] |
|
Conventional Medical Management* |
|||
|
Oral Medications |
99% (171/173) |
99% (108/109) |
98% (63/64) |
|
Topical Medications |
40% (70/173) |
43% (47/109) |
36% (23/64) |
|
Physical Therapy |
58% (101/173) |
57% (62/109) |
61% (39/64) |
|
Psychological Therapy |
10% (18/173) |
11% (12/109) |
9% (6/64) |
|
Acupressure/Acupuncture |
12% (21/173) |
14% (15/109) |
9% (6/64) |
|
Nerve Blocks |
46% (79/173) |
49% (53/109) |
41% (26/64) |
|
Epidural Steroid Injections |
39% (67/173) |
39% (43/109) |
38% (24/64) |
|
Others (Chiropractic, Bracing, RFA, trigger point injections, TENS, ice/heat, Massage) |
42% (73/173) |
41% (45/109) |
44% (28/64) |
|
*Subjects used 1 or more of the CMM’s listed at the time of screening. #No statistical difference was detected between the groups at baseline. |
|||
Table 1: Subject demographics and baseline characteristics. Radio Frequency ablation (RFA), Trans-Cutaneous Nerve Stimulation (TENS).
COMFORT 2 Outcomes
At 3 months the COMFORT 2 Active Arm responder rate (those who achieved ≥50% pain reduction) was 80% (67/84) with an average pain reduction of 66%, compared to the Control Arm responder rate of 4% (2/55) and average pain reduction of 3% (p<0.001). The high responder rate (those who achieved ≥ 80% pain reduction) was 30% (25/84) in the Active Arm compared to 0% (0/55) in the Control Arm. At 6 months the Active Arm responder rate was 79% (64/81) with an average pain reduction of 64%; the high responder rate was 30% (24/81) (Figure 2). Likewise, statistically significant improvement was achieved for all of the secondary endpoints, which included psychological and functional outcomes (Table 2). The majority of Active Arm subjects achieved Minimal Clinically Important Differences (MCID) [3-6] at 3 and 6-months for the various PROs which assess overall improvements in a clinically meaningful way rather than relying only on pain scores (Table 2).
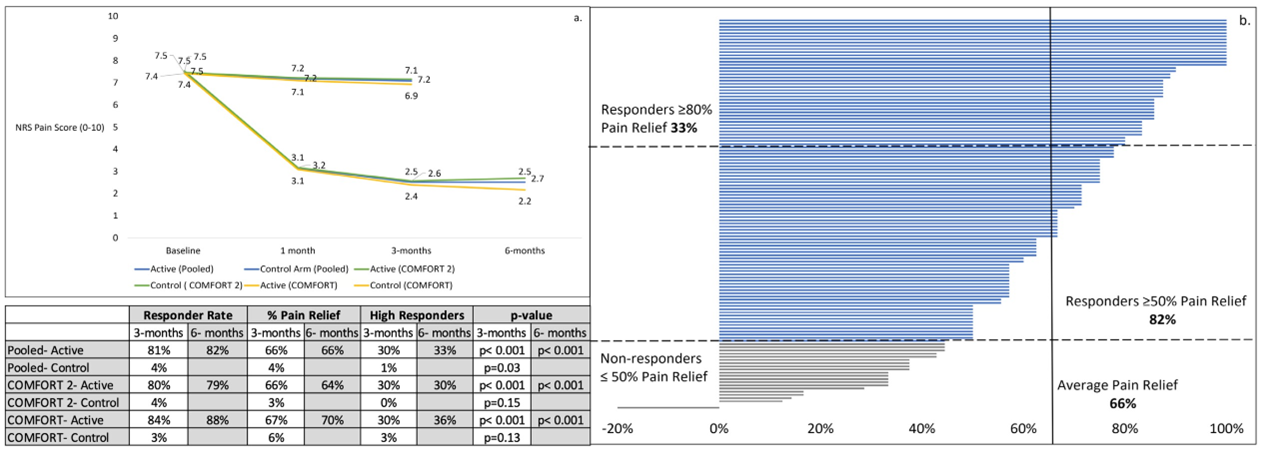
Figure 2 a. Mean NRS pain scores (BPI-Q5) and responder rates at 3 and 6 months. Pain scores captured in the office at baseline, 1, 3, and 6 months for COMFORT RCT, COMFORT 2 RCT, and pooled cohorts. Mean percent reduction in pain was statistically significant in the Active Arm compared to the Control Arm, at all timepoints in the 3 cohorts (p< 0.001). Responder rates (≥ 50% improvement) and high responder rates (≥ 80% improvement) were statistically better in the Active Arm versus the Control Arm, at p < 0.001. b. Individual subject pain relief at 6-months in the pooled cohort, Active Arm (n=123).
No Unanticipated Serious Adverse Device Effects (USADE) were reported in the study, to date. There were no Serious Adverse Events (SAE) related to the device or procedure. No reports of pocket pain have been reported to date. All non-serious adverse device effects (ADE) resolved with no sequelae. Lead migrations were reported in 3 subjects, all of which were addressed with reprogramming. Eight instances of IPG migrations, rotations, disconnections and placement issues resulted in revisions. Seven subjects reported minor infections which were resolved with antibiotics. Four additional subjects had infections that resulted in explants, with reimplantation of 1 subject upon resolution of infection. Two subjects had their devices explanted due to inefficacy and MRI compatibility issues.
Five non-device related SAEs were reported in the Active Arm at the 6-month timepoint, all resolving with no sequelae. In the Control Arm, 1 serious adverse event was reported, which was not related to the current CMM regimen. This safety profile is consistent with that reported for the COMFORT study and other studies involving the micro-IPG system [7,8].At baseline, 91% (158/173) of subjects reported the external wearable to be comfortable and easy to use. At 3-months, 79% (66/84) of Active Arm subjects reported the wearable was comfortable or very comfortable. Additionally, 90% (76/84) reported that the device was easy or very easy to use. 68% (57/84) reported typically using the device for a full day. At 6 months, 75% (61/81) of subjects continued to report the device to be very comfortable or comfortable. 88% (71/81) of subjects found the device to be very easy or easy to use and 70% (57/81) reported using it for a full day.
Pooled Analysis (COMFORT and COMFORT 2)
Pooling the COMFORT and COMFORT 2 data yielded a sample size of 250 (Active n=155; Control n=95). At 3 months the pooled Active Arm responder rate was 81% (103/127) with an average pain reduction of 66%, compared to the pooled Control Arm responder rate of 4% (3/84) and an average pain reduction of 4% (p<0.001); the high responder rate (those who achieved ≥ 80% pain reduction) was 30% (38/127) for the pooled Active Arm compared to 1% (1/84) in the pooled Control Arm. At 6 months the pooled Active responder rate was 82% (101/123) with an average pain reduction of 66%; the high responder rate was 33% (40/123). The pooled Active Arm was statistically better than the pooled Control Arm at reducing pain (p<0.001) at 3-months (Figure 2).
Figure 2a shows the outcomes for 3 cohorts, COMFORT RCT, COMFORT 2 RCT and the pooled results. The outcomes show excellent consistency across all time points between the 3 cohorts with the responder rate, average pain reduction and the high responder rate in alignment.
In the pooled Active Arm, a statistically significant improvement was observed for each PRO when the 3- and 6-month values were compared to baseline. In the control group, the difference between PROs at baseline and 3 months was not statistically significant except for one of the PROs (Table 2).
|
Active Arm |
Control Arm |
|||||
|
Assessment |
Group |
Baseline |
3 months |
6 months |
Baseline |
3 months |
|
Mean ± SD (N) |
Mean ± SD (N) [Mean % change] {% of Subjects achieving MCID} |
Mean ± SD (N) [Mean % change] {% of Subjects achieving MCID} |
Mean ± SD (N) |
Mean ± SD (N) [Mean % change] {% of Subjects achieving MCID} |
||
|
Brief Pain Inventory- Severity |
COMFORT 2 |
6.93 ± 1.23 (109) |
2.84 ± 1.91 (84) [58%; p<0.001] {82%} |
2.97 ± 1.80 (80) [57%; p<0.001] {85%} |
6.89 ± 1.30 (63) |
6.75 ± 1.50 (53) [0.3%; p=0.53] {6%} |
|
POOLED |
6.86 ± 1.32 (155) |
2.87 ± 1.91 (126) [57%; p< 0.001] {81%} |
2.86 ± 1.68 (122) [58%; p<0.001] {87%} |
6.83 ± 1.46 (94) |
6.63 ± 1.60 (80) [1%; p=0.24] {6%} |
|
|
Brief Pain Inventory- Interference |
COMFORT 2 |
6.02 ± 1.93 (109) |
2.40 ± 2.40 (84) [60%; p<0.001] {83%} |
2.64 ± 2.16 (79) [53%; p<0.001] {89%} |
5.89 ± 2.07 (63) |
5.53 ± 2.29 (51) [1%; p=0.3] {31%} |
|
POOLED |
6.01 ± 2.0 (155) |
2.37 ± 2.28 (126) [59%; p<0.001] {85%} |
2.44 ± 2.05 (120) [57%; p< 0.001] {88%} |
5.97 ± 2.01 (94) |
5.60 ± 2.19 (78) [2%; p=0.16] {32%} |
|
|
Beck Depression Inventory |
COMFORT 2 |
10.28 ± 8.77 (109) |
5.01 ± 6.58 (84) [39%; p<0.001] {68%} |
5.33 ± 7.73 (81) [39%; p<0.001] {72%} |
9.16 ± 8.18 (64) |
9.47 ± 8.04 (55) [1%; p=0.91] {40%} |
|
POOLED |
10.75 ± 8.98 (155) |
5.48 ± 6.73 (127) [38%; p< 0.001] {68%} |
5.13 ± 7.05 (123) [42%; p<0.001) {75%} |
10.15 ± 9.22 (95) |
9.31 ± 7.93 (84) [10%; p=0.21] {43%} |
|
|
EQ-5D-5L- Quality of Life |
COMFORT 2 |
0.623 ± 0.153 (109) |
0.785 ± 0.137 (84) [34%; p<0.001] {73%} |
0.781 ± 0.135 (80) [35%; p<0.001] {65%} |
0.635 ± 0.133 (64) |
0.649 ± 0.135 (55) [5%; p=0.34] {33%} |
|
POOLED |
0.625 ± 0.153 (155) |
0.784 ± 0.134 (127) [37%; p< 0.001] {68%} |
0.785 ± 0.129 (120) [37%; p<0.001] {66%} |
0.620 ± 0.131 (95) |
0.644 ± 0.137 (84) [7%; p=0.04] {36%} |
|
|
Oswestry Disability Index |
COMFORT 2 |
43.3 ± 14.4 (109) |
23.51 ± 17.2 (83) [46%; p<0.001] {75%} |
22.61 ± 16.1 (81) [49%; p<0.001] {75%} |
41.3 ± 15.5 (64) |
39.5 ± 16.8 (55) [6%; p=0.7] {20%} |
|
POOLED |
43.15 ± 14.1 (155) |
23.24 ± 16.5 (126) [46%; p <0.001] {75%} |
22.39 ± 15.8 (123) [48%; p< 0.001] {74%} |
42.10 ± 15.7 (95) |
39.68 ± 17.1 (84) [6%; 0.12] {24%} |
|
Table 2: Patient Reported Outcomes (PRxO). Brief Pain Inventory (BPI), Quality-of-Life metric (EQ-5D-5L), Beck Depression Inventory (BDI), Oswestry Disability Index (ODI).
Statistically significant improvements were seen for each of the four areas treated in the pooled cohort (Figure 3a). All NRS average pain scores were above 7/10 at baseline compared to scores less than 3/10 in each of the 4 targeted pain areas, at 3 and 6 months. The responder rates ranged from 96% to 74% at 3 and 6 months, and percentage pain relief ranged from 73% to 61% over the same periods. When compared to baseline, all responder rates and pain reductions were statistically significant (p<0.001).
For the pooled cohort, each subject’s overall impression of change following treatment was assessed using Patient Global Impression of Change (PGIC) (Figure 3b). At 3-months, 97% of subjects reported improvement (very much, much and minimally improved), 2% reported no change and 2% of subjects reported worsening. In the control arm, majority of subjects (65%) reported no change.
Subjects were asked to rate their overall satisfaction with the micro-IPG PNS system using a 5-point Likert scale, (Figure 3c). At 3 months, 91% (115/127) of Active Arm subjects were satisfied with the system. At 6 months, 92% (112/122) of Active Arm subjects were satisfied, and three subjects (2%) were dissatisfied.
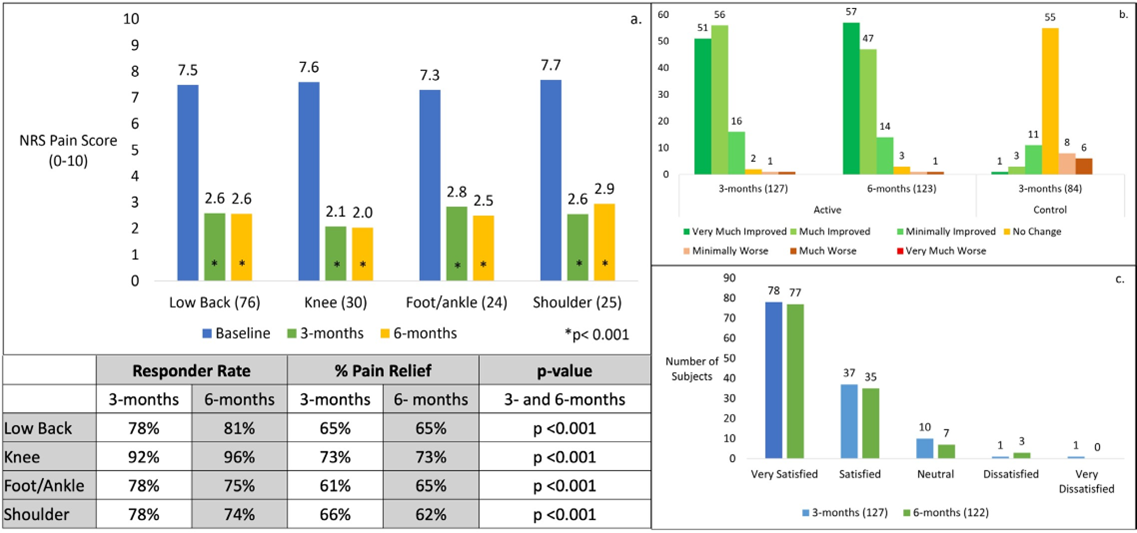
Figure 3 a. Pain relief by area of pain with mean pain score at each timepoint, in the pooled cohort, Active Arm. The improvement in pain was statistically significant in each area at both 3- and 6-months, when compared to baseline. Responder rates and mean percent pain relief shown. b. Patient Global Impression of Change (PGIC) at 3 and 6 months in the pooled cohort, Active and Control Arms. c. Satisfaction with the PNS system reported by subjects in the pooled cohort, Active Arm at 3 and 6 months.
Discussion
The COMFORT 2 study successfully met its primary endpoint. At 3 months, the Active Arm responder rate was 80% with an average pain reduction of 66%, compared to the Control Arm, which had a 4% responder rate and an average pain reduction of 3% (p<0.001). At 6-months, the responder rate for the Active Arm was 79% with an average pain reduction of 64%. At both 3 and 6-months, 30% of Active Arm subjects were high responders. For the Active Arm all end points met statistical significance and there was high patient satisfaction and excellent compliance with study requirements. The safety profile was strong with no reports of pocket pain or SAEs related to the device or procedures. The analysis of the pooled COMFORT and COMFORT 2 data (n=250) sets showed an 81% responder rate with 66% pain relief at 3 months for the Active Arm, compared to a 4% responder rate and 4% average pain relief (p<0.001) in the Control Arm; 30% of the subjects in the Active Arm were high responders. This is the first Level 1 evidence for a permanently implanted PNS device in the treatment of chronic pain.
Recently published real-world data in 2,373 PNS patients who received a micro-IPG implant showed durable long-term improvements with a 94% responder rate based on PGIC [9]. The follow-up period of residency was up to 31 months post-implant.
The basic population was similar to that in the COMFORT studies, with a majority of patients being female (60%) with an average age of 68 years.
Published healthcare economic data for this PNS device showed a 50% reduction in healthcare utilization and associated health economics burden following PNS therapy using the micro-IPG. Additionally, 31% of patients stopped using opioids following PNS therapy provided by the mirco-IPG [10].
It is well established that PNS is effective for treating chronic pain. In fact, in 1977 D.M. Long concluded that “Electrical stimulation for the control of pain is now a well-accepted therapeutic modality,” and that “…implantable peripheral nerve stimulators have proved to be one of the most reliable procedures for relief of specific kinds of pain.” Furthermore, “Peripheral nerve stimulation is among the most valuable of the techniques currently available. Patients with pain of peripheral nerve injury origin can consistently obtain relief by the use of these devices [11].”
PNS has been covered by the US Medicare system since 1988, and PNS is prescribed in US Veterans Affairs (VA) and Department of Defense (DoD) medical centers. It should be noted that for a therapy to be covered in Medicare, VA, and DoD centers, it must be shown to be “…reasonable and necessary for the diagnosis or treatment of an illness or injury…made through an evidence-based process and is currently prescribed within the US Veterans Affairs and Department of Defense medical centers because it is a proven and safe therapeutic modality [12].”
After half a century of successful clinical use, there is no scientific or clinical justification to conclude that PNS is experimental or investigational. The authors agree that the safety and efficacy of a particular PNS device should be demonstrated in well-controlled clinical studies. To that end, we conclude that the COMFORT and COMFORT 2 RCTs have demonstrated that the micro-IPG system provides statistically significant and clinically meaningful improvement in the condition of treated subjects in a safe and consistent manner. Additionally, the real-world data, and published longer term outcomes from COMFORT [2] support that position. The robust and consistent clinical data, coupled with published Health Economics data, show that use of the micro-IPG improves net health outcomes.
Limitations
A limitation of this study was the lack of a blinded, sham-control. A sham treatment was not practical for technical and published clinical considerations [13]. For example, the required temporary trial would likely break the blind for subjects by exposing them to the therapy prior to randomization. To address potential bias, wellaccepted clinical research methodologies were used including a cross-over design, using the patient as their own control and the use of previously approved, efficacious therapies in the Control Arm [14].
The 3-month cross-over period could be perceived as a limitation. COMFORT 2 subjects were in chronic pain for an average of 7.9 years prior to enrollment and were seeking PNS due to a lack of CMM efficacy. Requiring patients to remain in severe pain for additional time would not add to the scientific literature nor improve the validity of the results, while violating the principle of beneficence [15].
The study did not prescribe which CMM the subjects used. However, not all CMM options were available to subjects due to prescribing practices, insurance coverage, and/or access to treatment, reflecting real-world care in the US.
Regardless of these limitations, the COMFORT and COMFORT 2 RCTs represent the first Level 1 evidence for permanently implanted PNS devices and contribute significantly to the scientific literature for chronic pain management.
Conclusions
COMFORT 2 confirms, supports, and expands upon the outcomes from the COMFORT sister study, showing that PNS therapy, delivered by the micro-IPG system, provides consistent and statistically significant improvement in all measured metrics. The excellent safety profile is consistent with other studies involving the micro-IPG system. Taken together, the statistically significant improvement in pain scores, functionality, pain interference and severity, quality of life, depression, patient global impression of change, and general patient satisfaction, shows an improvement in net health outcomes. COMFORT 2 is an ongoing study, and the authors will report additional outcomes as they become available.
Acknowledgements
We would like to thank the COMFORT 2 study subgroup and study coordinators at the following US sites for their participation and support:
S. Bainbridge¹, S. Estes¹, A. Vellore¹, M. Hogue¹, N. Patel², A. Thakral², R. Kapteyn², C. Gilmore³, J. North³, J. Priddy-Southern³, E. Liu⁴, G. Phillips⁵, R. Vora⁵, S.W. Pierce⁵, D. Parks⁵, N. Glass⁵, J. Peters⁵, R. Tatevossian⁶, B. Spiegel⁷, J. Cohen⁷, J. Caylor⁸, M. Arastu⁸, D. Russo⁹, T. Rigert⁹, A. Haroutunian¹⁰, M. Hamza¹¹, M. Greene¹¹, A. Medvedovsky¹¹, A. Paugh¹¹, K. Powell¹¹, E. Cornidez¹², C. Bailey¹², M. Duran¹², K. Farshad¹², J. Gilman¹², C. Martin¹², C. Lam¹³, B. Ittiara¹⁴, J. Scott¹⁴, K. Mender¹⁴, T. Del Guercio¹⁵, K. Rosen¹⁶, P. Geml¹⁶, J. Guertin¹⁶, C. Miller¹⁶, E. Peskin¹⁷, N. Khatri¹⁷, D. Dobritt¹⁷, A. Alkilani¹⁷, G. Kao¹⁸.
Affiliations: ¹DBPS Research LLC, CO; ²International Spine, Pain & Performance Center, D.C; ³The Center for Clinical Research, NC; ⁴Institute of Precision Pain Medicine, TX; ⁵Pacific Sports and Spine, OR; ⁶Comprehensive Spine and Pain Physicians, CA; ⁷Pain Management and Injury Relief, CA; ⁸Pain Specialists of America, TX; ⁹Columbia Pain Management PC, OR; ¹⁰Southern California Orthopedic Institute, CA; ¹¹Coastal Spine & Pain Center, FL; ¹²The Pain Institute of Southern Arizona, AZ; ¹³University of Kansas Medical Center, KS; ¹⁴NeuroInterventional Pain Management, MI; ¹⁵Advanced Orthopedics and Sports Medicine Institute, NJ; ¹⁶Michigan Neurology & Spine Center, MI; ¹⁷Insight Research Institute, MI; ¹⁸Medconsults LLC, FL.
Ethical Considerations
This study involves human participants and was approved by WCG IRB Tracking Number: 20215749. Participants gave informed consent prior to taking part in the study.
Conflict of Interest
Dr. Staats is a consultant for Nalu Medical, AIS Therapeutics; he has research grants from Saluda Medical, Biotronik and Neurosalutions. He is Chief Medical Officer at electroCore and has stock or stock options from Saluda, electoCore, Nalu and Thermaquil. Shilpa Kottalgi and Patrick Martin are employed at Nalu Medical. James Makous is a consultant at Nalu Medical and owns stock in Nalu Medical. Dr. Hatheway is a speaker and consultant for Nalu Medical. Mehul J. Desai consults for Medtronic and Nalu; receives research support from Abbott, Avanos, Averitas, Mainstay, Nalu, Nature Cell, Saol, SPR Therapeutics, Vivex; and owns stock options in HyperVention, SPR Therapeutics, SynerFuse, and Virdio. Ali Valimahomed is a consultant for Nalu, SPR Therapeutics, Boston Scientific, Biotronik and Guidepoint Consulting. Mitchell Engle receives a research grant and is a speaker for Nalu Medical. Usman Latif is a consultant for Abbott, Brixton Biosciences, InFormed Consent, Nalu, Nevro, Saluda Medical, Spinal Simplicity, SPR, Stryker, Vertex Pharmaceuticals, Vertos Medical, Vivex Biologics, and WISE; an advisory board member for Abbott, InFormed Consent, Nalu, Nevro, Saluda Medical, Spinal Simplicity, Vertos Medical, and Vivex Biologics; has funded research with Mainstay Medical, Nalu, Spinal Simplicity, and Vivex Biologics; and has stock or stock options with InFormed Consent and Spinal Simplicity. All other authors report no conflicts of interest.
References
- Hatheway J, Hersel AP, Song J, Engle MP, Gutierrez G, et al. (2024) Design of a multicenter, randomized controlled trial for the treatment of peripheral neuropathic pain (COMFORT Study) with a microimplantable pulse generator. J Pain Res 17: 2891-2901.
- Hatheway J, Hersel A, Song J, Engle M, Gutierrez G, et al. (2025) Clinical study of a micro-implantable pulse generator for the treatment of peripheral neuropathic pain: 3-month and 6-month results from the COMFORT-randomised controlled trial. Reg Anesth Pain Med 50: 561-567.
- Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, et al. (2008) Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain 9: 105121.
- Ostelo RW, de Vet HC (2005) Clinically important outcomes in low back pain. Best Pract Res Clin Rheumatol 19: 593-607.
- Button KS, Kounali D, Thomas L, Wiles NJ, Peters TJ, et al. (2015) Minimal clinically important difference on the Beck Depression Inventory--II according to the patient’s perspective. Psychol Med 45: 3269-3279.
- Walters SJ, Brazier JE (2005) Comparison of the minimally important difference for two health state utility measures: EQ-5D and SF-6D. Qual Life Res 14: 1523-1532.
- Desai MJ, Raju T, Ung C, Arulkumar S, Kapural L, et al. (2023) Results From a Prospective, Clinical Study (US-nPower) Evaluating a Miniature Spinal Cord Stimulator for the Management of Chronic, Intractable Pain. Pain Physician 26: 575-584.
- Salmon J, Bates D, Du Toit N, Verrills P, Yu J, et al. (2023) Early Experience With a Novel Miniaturized Spinal Cord Stimulation System for the Management of Chronic Intractable Pain of the Back and Legs. Neuromodulation 26: 172-181.
- Hatheway JA, Ratino T, Swain AR, Ratino T, Latif U, et al. (2025) LongTerm Pain Relief Delivered by Micro-Implantable Pulse Generator: Findings from a Large-Scale, Real-World Data Peripheral Nerve Stimulation Patient Registry. Chron Pain Manag 9: 169.
- Kalia H, Thapa B, Staats P, Martin P, Stetter K, et al. (2025) Real-world healthcare utilization and costs of peripheral nerve stimulation with a micro-IPG system. Pain Manag 15: 27-36.
- Long DM (1977) Electrical stimulation for the control of pain. Arch Surg 112: 884-888.
- Centers for Medicare and Medicaid Services (2025) Medicare Coverage Determination Process. Baltimore (MD).
- Johnson S, Goebel A (2023) Sham controls in device trials for chronic pain - tricky in practice-a review article. Contemp Clin Trials Commun 35: 101203.
- Sutherland ER (2007) Sham procedure versus usual care as the control in clinical trials of devices: which is better? Proc Am Thorac Soc 4: 574-576.
- World Medical Association (2013) World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 310: 2191-2194.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.