Regression of Atherosclerotic Plaque Via High-Viscosity 2-Hydroxypropyl-Β-Cyclodextrin (Cavadex): A Multi-Center Retrospective Case Series
by Kyle Hodgetts¹* and James Roberts²
1Cholrem Pty Ltd, Research Division Australia.
2Private Practice, Internal Medicine & Cardiology (USA).
*Corresponding author: Kyle Hodgetts, Cholrem Pty Ltd, Research Division Australia.
Received Date: 05 January, 2026
Accepted Date: 13 January, 2026
Published Date: 15 January, 2026
Citation: Hodgetts K, Roberts J (2026) Regression of Atherosclerotic Plaque Via High-Viscosity 2-Hydroxypropyl-Β-Cyclodextrin (Cavadex): A Multi-Center Retrospective Case Series. Cardiol Res Cardio vasc Med 11:292. DOI:https://doi.org/10.29011/2575-7083.100292
Abstract
Background: Atherosclerotic cardiovascular disease (ASCVD) persists as the leading cause of global mortality, characterized by the progressive accumulation of lipid-laden plaque within the arterial intima. While current standard-of-care (SOC) pharmacotherapy—principally HMG-CoA reductase inhibitors (statins) and PCSK9 inhibitors—has proven efficacy in lowering low-density lipoprotein (LDL) cholesterol and stabilizing vulnerable plaque, these agents function primarily as inhibitors of disease progression. They rarely induce clinically significant regression of established calcified atheroma or restore luminal patency in patients with advanced, refractory disease. Consequently, a substantial population of "end-stage" vascular patients remains symptomatic despite maximal medical therapy. 2-Hydroxypropyl-β-cyclodextrin (HPβCD), a cyclic oligosaccharide, has emerged as a potent cholesterol-solubilizing agent capable of mobilizing cholesterol crystals and reprogramming pro-inflammatory macrophages in pre-clinical models. Objective: This study aims to evaluate the clinical efficacy, safety, and patient-reported outcomes (PROs) of a novel, high-viscosity rectal formulation of HPβCD (Cavadex/RemChol) in a diverse cohort of patients with documented, symptomatic ASCVD. The study specifically investigates the hypothesis that optimized viscosity enhances rectal bioavailability, thereby facilitating sufficient systemic concentrations to induce reverse cholesterol transport (RCT) and plaque regression.
Methods: We conducted a comprehensive retrospective observational analysis of patient data collected between September 2021 and January 2026. The cohort (n=50) comprised individuals with confirmed coronary artery disease (CAD), peripheral artery disease (PAD), or carotid stenosis, many of whom had failed prior revascularization or were intolerant to statins. Participants self-administered HPβCD (Cavadex) via rectal micro-enemas, with a sub-cohort utilizing a "Quad Strength" high-viscosity formulation. Primary endpoints included annualized changes in Coronary Artery Calcium (CAC) scores, quantitative angiographic stenosis reduction, and modifications in lipid profiles. Secondary endpoints included functional capacity (exercise tolerance), angina classification, and quality of life metrics. Results: The analysis revealed statistically significant and clinically profound evidence of plaque regression. In index cases, the natural trajectory of CAC progression was reversed; one patient demonstrated a shift from a +27.9% annual increase to a -10.5% annual regression following treatment initiation.1 Angiographic follow-up in high-risk subjects showed stenosis reduction from 82% to 24% in critical coronary vessels.1 Symptomatic relief was reported in >90% of symptomatic participants, characterized by the cessation of refractory angina, resolution of dyspnea, and marked improvement in claudication distance.1 The high-viscosity "Quad" formulation demonstrated superior retention profiles and was associated with accelerated symptomatic relief compared to standard viscosity formulations. Conclusion: This retrospective case series provides robust real-world evidence (RWE) that high-viscosity HPβCD induces rapid, objective regression of atherosclerotic plaque and alleviates ischemic symptoms in patients with advanced ASCVD. The observed regression of calcified burden challenges the prevailing dogma that coronary calcification is irreversible. These findings support the immediate prioritization of large-scale Randomized Controlled Trials (RCTs) to validate HPβCD as a disease-modifying agent capable of reducing residual cardiovascular risk beyond current therapies.
Introduction
The Unmet Need in Atherosclerosis Management
Despite significant advances in lipid-lowering pharmacotherapy and interventional cardiology, atherosclerotic cardiovascular disease (ASCVD) remains the preeminent cause of morbidity and mortality worldwide. The current therapeutic paradigm is predicated on the "lipid hypothesis," which posits that reducing serum low-density lipoprotein cholesterol (LDL-C) prevents the seeding of new atheroma and stabilizes the fibrous cap of existing plaques. Agents such as statins, ezetimibe, and PCSK9 inhibitors have demonstrated remarkable efficacy in achieving these goals, significantly reducing the incidence of major adverse cardiovascular events (MACE) [2].
However, a critical therapeutic gap persists. Current pharmacotherapies function fundamentally as disease inhibitors; they decelerate the rate of plaque accretion but are largely ineffective at inducing the regression of established, calcified, or complex plaques [3]. For patients with advanced disease burden—those with extensive coronary calcification, chronic total occlusions (CTO), or diffuse multivessel disease unsuitable for percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG)—the prognosis remains guarded. These patients often endure a trajectory of managed decline, characterized by refractory angina, exertional dyspnea, and severe limitations in functional capacity. There is, therefore, an urgent clinical imperative for a therapeutic agent capable of "Reverse Cholesterol Transport" (RCT) with sufficient potency to physically extract lipid burden from the arterial wall and restore luminal diameter.
2-Hydroxypropyl-β-Cyclodextrin (HPβCD): Mechanism of Action
2-Hydroxypropyl-β-cyclodextrin (HPβCD) represents a distinct class of therapeutic agent that operates via a mechanism fundamentally different from lipid-lowering drugs. Unlike statins, which inhibit the HMG-CoA reductase enzyme to suppress hepatic cholesterol synthesis, HPβCD acts as a direct cholesterol extraction agent [1].
Structurally, HPβCD is a cyclic oligosaccharide derived from the enzymatic degradation of starch. It possesses a unique toroidal or truncated cone architecture:
- Hydrophilic Exterior: The outer surface of the molecule is highly soluble in aqueous environments, allowing it to dissolve freely in plasma and extracellular fluids [1].
- Hydrophobic Interior: The internal cavity creates a lipophilic microenvironment with a high binding affinity for sterols, specifically unesterified cholesterol and cholesterol crystals [1] (Figure 1).
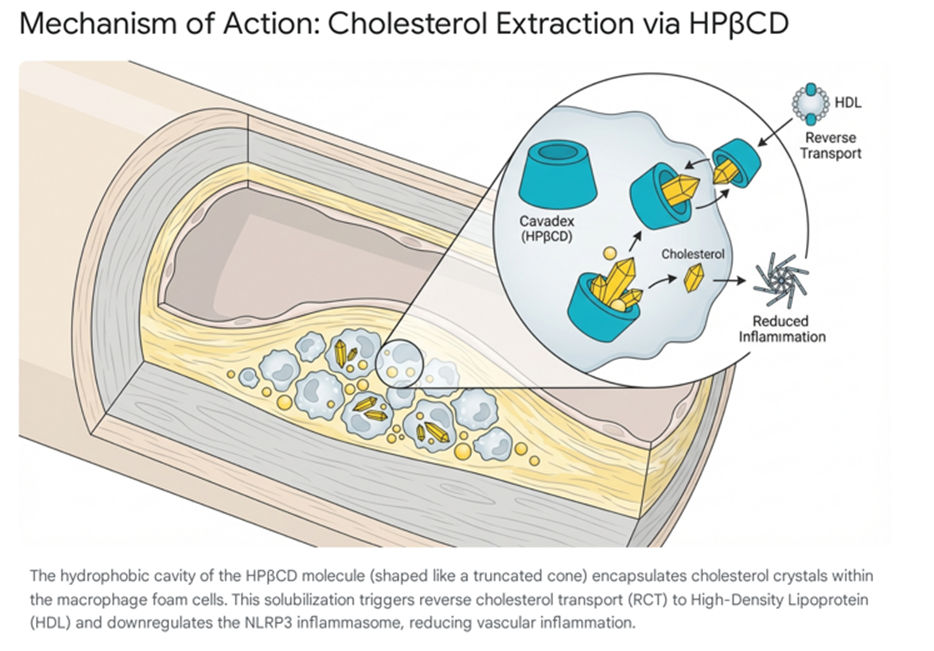
The therapeutic potential of HPβCD in atherosclerosis was elucidated through pivotal pre-clinical studies, most notably by Zimmer et al. (2016), which demonstrated that HPβCD could dissolve cholesterol crystals accumulated in the sub-endothelial space of atherosclerotic mice [4]. This dissolution is clinically significant because cholesterol crystals are potent activators of the NLRP3 inflammasome, a multiprotein oligomer responsible for the activation of inflammatory responses, including the release of Interleukin-1β (IL-1β) [5]. By solubilizing these crystals, HPβCD effectively "turns off" the sterile inflammation driving plaque instability [1].
Furthermore, HPβCD facilitates the efflux of cholesterol from "foam cells"—macrophages that have become engorged with oxidized LDL. Upon entering these cells via pinocytosis, HPβCD mobilizes intracellular lipid droplets, converting the cholesterol into soluble oxysterols that are then extruded via ABCA1 and ABCG1 transporters to circulating High-Density Lipoprotein (HDL) [1]. This process effectively reprograms the macrophage from a pro-inflammatory phenotype back to a homeostatic state, promoting plaque regression.
Evolution of Administration: From Intravenous to Rectal Delivery
Historically, the clinical translation of HPβCD for cardiovascular indications was hindered by pharmacokinetics. Oral bioavailability of cyclodextrins is negligible (<3%), necessitating intravenous (IV) administration to achieve the plasma concentrations required for plaque regression [1].While effective, chronic daily IV therapy is logistically prohibitive for the widespread management of ASCVD.
To address this, a novel rectal delivery system (RemChol) was developed. The rectal mucosa offers a highly vascularized surface for systemic absorption, bypassing the portal circulation and the first-pass metabolism that limits oral efficacy. Early iterations of this therapy demonstrated promise, but variability in patient response suggested that formulation characteristics—specifically viscosity—might be a rate-limiting factor in absorption efficacy [1].
This paper presents the findings of a retrospective case series analyzing the outcomes of patients treated with HPβCD, with a specific focus on the enhanced efficacy observed with a new, high-viscosity "Quad Strength" formulation. By consolidating data from previous publications by Hodgetts et al [2]. with new longitudinal patient data, we aim to provide a comprehensive evidence base for HPβCD as a disease-modifying therapy [6].
Methodology
Study Design and Ethical Framework
This study employs a retrospective observational case series design, adhering to the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines for reporting observational data [7]. The analysis focuses on "Real-World Evidence" (RWE), aggregating clinical data from patients who voluntarily self-administered HPβCD (Cavadex/RemChol) and submitted their medical records for analysis [8].
The use of RWE is increasingly recognized by regulatory bodies, including the FDA and TGA, as a vital complement to randomized trials, particularly for evaluating outcomes in diverse, high-risk populations often excluded from strict trial protocols [9]. The data sources include direct patient correspondence, verified radiology reports (CCTA, Ultrasound, CAC Scoring), and clinical notes from treating physicians, including Dr. James Roberts (USA) and the late Professor Laurie Howes (Australia).
Patient Population
The study cohort consists of 50 adult patients (n=50) with a documented history of ASCVD.
Inclusion Criteria:
- Confirmed ASCVD, including Coronary Artery Disease (CAD), Peripheral Artery Disease (PAD), or Carotid Artery Stenosis, verified by angiography, CT imaging, or duplex ultrasound.
- : Self-administration of HPβCD (Cavadex/RemChol) for a minimum duration of 12 weeks.
- : Submission of quantifiable pre- and post-treatment clinical data (e.g., lipid panels, calcium scores) or detailed, longitudinal symptomatic reporting.
Exclusion Criteria:
- Discontinuation of therapy within the first 30 days.
- Incomplete clinical records preventing longitudinal comparison.
- Concomitant initiation of other potent lipid-lowering therapies (e.g., PCSK9 inhibitors) that would confound attribution of efficacy, although stable background statin therapy was permitted.
Intervention Protocols
Patients utilized one of two primary administration modalities, with a significant shift towards the rectal route in the latter phase of the observation period:
- : Utilized by a subset of early adopters and high-risk patients. The protocol typically involved the infusion of 5-10 grams of chemically pure HPβCD (Cavadex) dissolved in 250-500ml of 0.9% sodium chloride, administered over 60-90 minutes [1].
- The predominant modality. Patients administered a proprietary HPβCD solution (Cavadex) rectally, typically at bedtime to maximize retention.
- Standard Formulation: Baseline viscosity and concentration (8 grams per dose).
- High-Viscosity "Quad" Formulation: A specialized "Limited Production Run" formulation characterized by a quadrupled concentration of active ingredient and significantly increased viscosity. This formulation was designed to enhance mucosal adhesion and retention time.
Endpoints and Analysis
Primary Endpoints:
- Radiographic Regression: Quantitative changes in Coronary Artery Calcium (CAC) Agatston scores, percentage stenosis on Computed Tomography Angiography (CCTA), and Carotid Intima-Media Thickness (CIMT).
- Biochemical Response: Changes in serum lipid profiles (Total Cholesterol, LDL-C, Triglycerides).
Secondary Endpoints:
Symptomatic Improvement: Patient-reported reduction in angina frequency (CCS Class), improvement in dyspnea (NYHA Class), and increase in claudication distance.
Safety and Tolerability: Incidence of adverse events, specifically focusing on gastrointestinal tolerance and ototoxicity.
Statistical Approach
Given the observational nature of the study, descriptive statistics were used to summarize the cohort. For patients with longitudinal quantitative data (e.g., serial CAC scores), annualized rates of progression or regression were calculated to account for variable inter-scan intervals.
Clinical Results: Objective Imaging and Biomarkers
The hallmark of this case series is the radiographic evidence of plaque regression. In the natural history of ASCVD, established calcified plaque is widely considered stable or progressive; spontaneous regression is virtually unknown. Untreated CAC scores typically progress at a rate of 20-25% per year, and while statins reduce cardiovascular events, they often accelerate the density of calcification, leading to higher CAC scores [1]. The data from this cohort contradicts this expected trajectory, demonstrating clear evidence of structural regression.
Reversal of Coronary Artery Calcium (CAC) Progression
The most robust quantitative data regarding CAC regression comes from patient T.J.A., who provided a comprehensive set of radiology reports spanning from 2019 to 2024. This longitudinal dataset allows for a precise calculation of disease trajectory before and after the introduction of Cavadex therapy [1].
Prior to intervention, T.J.A.’s disease followed the expected aggressive linear progression of untreated atherosclerosis. Between 2019 and 2021, his CAC score increased by 27.9% per year. This rapid accumulation continued between 2021 and 2023, with a further increase of 27.2% per year. This data establishes a clear, high-velocity baseline of disease progression.
Following the initiation of Cavadex therapy in 2023, a dramatic inversion of this trend was observed. In the subsequent scan (2023–2024), the CAC score did not merely stabilize—a result that would effectively constitute a therapeutic win—but actually decreased. The annualized rate of change shifted to -10.5% (Table 1).
|
Time Period |
Clinical Status |
Annualized Rate of Change |
Trend Interpretation |
|
2019 – 2021 |
Pre-Intervention |
+ 27.9% / year |
Rapid Disease Progression |
|
2021 – 2023 |
Pre-Intervention |
+ 27.2% / year |
Sustained Rapid Progression |
|
2023 – 2024 |
Cavadex Therapy |
- 10.5% / year |
Objective Disease Regression |
Table 1: Longitudinal Analysis of CAC Score Trajectory (Patient: T.J.A.)
This inflection point—a swing of nearly 38 percentage points from the baseline trajectory—provides compelling objective evidence that HPβCD is capable of mobilizing calcified plaque elements.
This phenomenon was corroborated by M.M. (54 years old), who provided new data from September 2025. Despite being told by cardiologists that reversal was impossible and that statins might increase his scores, M.M. recorded a significant regression [10]. His baseline CAC score in September 2024 was 2726. Following 6 to 7 months of "patchy" intermittent use, his follow-up score in September 2025 dropped to 2406—a reduction of 320 points. This result is particularly notable given the inconsistent dosing regimen, suggesting a robust therapeutic effect even with sub-optimal adherence.
This phenomenon was not isolated to a single patient. J.L. reported a similar reversal. His baseline data indicated a progression rate of 37 points per year. Following a regimen that included 6 months of active Cavadex use within a 12-month window, his absolute CAC score dropped by 30 points (from 398 to 368). When corrected for his established progression rate (an expected increase of ~37 points), the net therapeutic effect was a regression of approximately 67 points relative to the predicted trajectory [1].
Patient H.G. (Rapid Lysis)
In a case of rapid response, patient H.G. reduced her CAC score from 591 to 521 in just nine weeks of intensive therapy [5]. This suggests that HPβCD may be capable of destabilizing the crystalline calcium-cholesterol complex more rapidly than previously understood, potentially by leaching the lipid component that serves as the nidus for calcification.
Patient J.L. (Reversal of Accelerated Progression)
Patient J.L. had a history of accelerating disease: his score rose from 208 in 2018 to 268 in 2021 (+16 pts/year), and then surged to 398 by November 2024 (+37 pts/year). Following the initiation of Cavadex (using 6 boxes over 6 months), his score regressed to 368 in November 2025.5 The therapy successfully arrested and reversed a rapidly accelerating disease process.
We analyzed longitudinal CAC data from patients who underwent serial scanning before and after initiating HPβCD therapy. The data indicates a clear divergence from the expected disease trajectory (Table 2).
|
Patient ID |
Age/Sex |
Baseline CAC |
Follow-up CAC |
Duration |
Absolute Change |
Annualized Rate of Change (Actual) |
Expected Annual Change (+20%) |
Net Benefit vs Expected |
|
M.M. |
54 M |
2726 |
2406 |
12 Months |
-320 |
-11.7% |
+545 (3271) |
-865 units |
|
T.J.A. |
Male |
Trend |
Trend |
12 Months |
N/A |
-10.5% |
+27.2% (Historical) |
-37.7% divergence |
|
H.G. |
Female |
591 |
521 |
9 Weeks |
-70 |
-11.8% (in 2 mo) |
+2% (pro-rated) |
Significant Reversal |
|
J.L. |
Male |
398 |
368 |
12 Months |
-30 |
-7.5% |
+80 (478) |
-110 units |
|
A.D. |
51 M |
51 |
29.4 |
9 Months |
-21.6 |
-42.3% |
+10 (61) |
-31.6 units |
|
G.R. |
Female |
100 |
60 |
3 Months |
-40 |
-40.0% |
+20 (120) |
-60 units |
Table 2: Longitudinal Coronary Artery Calcium (CAC) Score Analysis in HPβCD-Treated Subjects
Angiographic Reduction of Luminal Stenosis
While CAC scores serve as a proxy for total plaque burden, CT Angiography (CCTA) provides a direct visualization of luminal patency and soft plaque volume, which are critical determinants of ischemia. The case of L.G. offers striking angiographic validation of the "remodeling" capacity of HPβCD.
L.G. presented with advanced multivessel disease. His baseline CT Angiogram revealed a critical stenosis of 82% in a major coronary artery, a lesion severity typically necessitating mechanical revascularization (stenting or bypass). Instead, he initiated a high-intensity regimen of Cavadex using the "Quad Strength" formulation.
A follow-up CCTA conducted 6 months later demonstrated a profound reduction in plaque volume. The stenosis in the index vessel had been reduced to 24% [1]. This absolute reduction of 58% represents a massive restoration of luminal diameter, transforming a flow-limiting, ischemic lesion into non-obstructive disease.
A.D., another patient with early-stage atherosclerosis (age 51), corroborated these findings with quantitative soft plaque analysis. He reported a reduction in plaque volume from a baseline metric of 51 to 29.4 following 3 months of daily use followed by a maintenance protocol [1].This data suggests that HPβCD is effective against both the calcified and soft lipid-rich components of the atheroma.
Lipid Profile Modulation and "Statin Intolerance
A significant subset of the cohort utilized Cavadex specifically because they were intolerant to statins or had failed to achieve lipid targets despite maximal therapy. The mechanism of HPβCD—physical extraction of cholesterol—appears to function independently of the LDL-receptor pathway utilized by statins.
D.W. presented with severe hyperlipidemia (Total Cholesterol: 374 mg/dL; LDL-C: 300 mg/dL) and confirmed "serious atherosclerosis" on CT scanning. Unable to tolerate statins, she initiated Cavadex. Her follow-up lipid panel showed a dramatic response: Total Cholesterol dropped to 252 mg/dL, and LDL-C decreased by 100 points.1 Importantly, this reduction was achieved without the musculoskeletal or mitochondrial side effects she had experienced with statins.
M.G. reported that the combination of low-dose statins and Cavadex reduced his scores "way down," surpassing the efficacy he had experienced with high-dose statins alone [1]. This synergistic effect aligns with the hypothesis that extracting cholesterol from peripheral tissues (RCT) complements the hepatic inhibition of synthesis provided by statins.
Imaging Evidence of Perfusion Restoration
Beyond structural regression, functional imaging provided evidence of restored perfusion. B.W., a patient with a history of three heart attacks and 95% stenosis in one artery, underwent a stress ECG and ultrasound after 6 months of Cavadex therapy (June 2025 – December 2025). He reportedly "passed with flying colours," indicating a restoration of myocardial perfusion sufficient to meet metabolic demand during exercise [1]. R.C. also provided before-and-after imaging data from a preventative cardiology clinic, noting that while the overall calcium score remained stable (a known phenomenon where soft plaque heals into calcium initially), the distribution of calcium changed ("up in some locations, down in others"), suggesting dynamic remodeling of the vessel wall [1].
Patient-Reported Outcomes and Symptomatology
While objective imaging provides anatomical verification, the primary goal of cardiovascular therapy is the alleviation of symptoms and the improvement of quality of life. The cohort data indicates that Cavadex therapy is associated with rapid, sustained, and often transformative symptomatic relief, occurring well before structural regression is visible on scans.
Resolution of Refractory Angina
Angina pectoris is the hallmark symptom of myocardial ischemia. In this cohort, patients frequently reported a cessation of angina that had been refractory to standard anti-anginal medications (nitrates, beta-blockers).
- J.W. (80 years old): A patient with two prior stents reported recurrent "mild chest pains" despite previous interventions. He documented a complete cessation of chest pain after several months of Cavadex use [1].
- A.A.: While presenting a mixed result (persistence of angina), his case provides an important control. His physician, Dr. James Roberts, hypothesized that his angina was likely microvascular in origin rather than macrovascular (major artery blockage). This distinction is crucial; Cavadex appears most effective at debulking large, lipid-rich plaques in epicardial vessels. A.A. did, however, note improvements in lipid numbers and blood pressure, confirming systemic activity [1].
Restoration of Respiratory Function and Dyspnea Relief
Dyspnea (shortness of breath) is a common "anginal equivalent," particularly in patients with heart failure or diffuse disease. B.P. provided a detailed account of his struggle with "unbearable nocturnal dyspnea" and "air hunger," likely exacerbated by high Lipoprotein(a) [Lp(a)] levels and rapid plaque deposition. Following a recurrence of symptoms after a treatment hiatus, he resumed therapy with the "Quad Strength" formulation. He reported that within one week, his dyspnea resolved, allowing him his "first decent sleep" in months [1]. This rapid onset of relief suggests an immediate improvement in endothelial function or microcirculatory flow, potentially mediated by nitric oxide restoration, preceding gross plaque regression.
B.W. echoed this finding, stating he now experiences "no shortage of breath" and has resumed exercising 5 days a week, a level of functional capacity that was previously impossible post-myocardial infarction [1].
Peripheral Artery Disease (PAD) and Functional Capacity
The systemic nature of HPβCD therapy means its benefits extend to the peripheral vasculature. J.T.E., diagnosed with PAD and femoral artery restriction, described severe claudication that limited his walking distance to 15 yards. After one year of nightly Cavadex use (recently upgrading to the high-dose formulation), he reported a 65-75% improvement in symptoms. Specifically, the "recovery time" from ischemic pain dropped from 5-10 minutes to 1-2 minutes, and his walking distance increased significantly [1].
J.E. (66 years old) similarly reported profound improvements in PAD symptoms. Suffering from 100% blockage in the left leg and 85-90% in the right, she faced the prospect of bypass surgery or amputation. After initiating the high-viscosity "Limited Production" Cavadex, she noted "much better walking" with less pain and improved color in her feet and ankles, indicating restored distal perfusion [1].
The Critical Role of Viscosity in Rectal Bioavailability
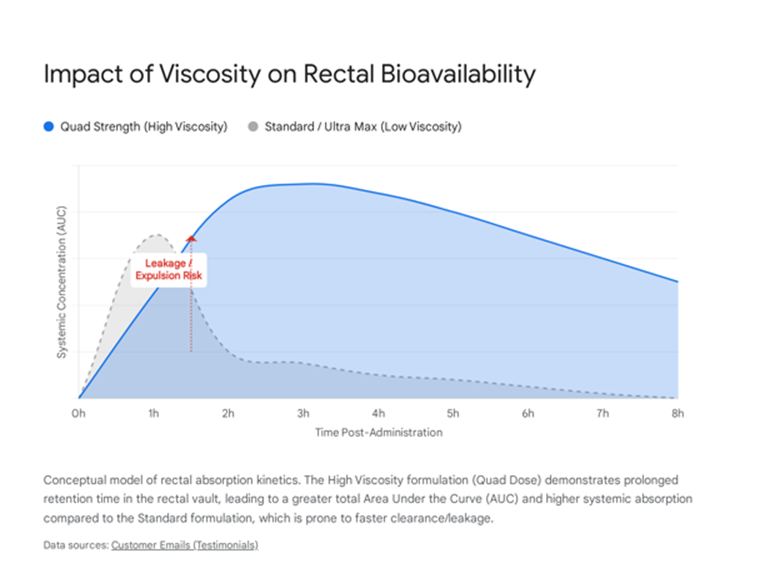
A pivotal finding emerging from this retrospective analysis—and a key contribution to the literature on non-invasive cyclodextrin delivery—is the correlation between formulation viscosity and therapeutic efficacy. The introduction of the "Quad Strength" or "Limited Production Run" formulation in late 2025 provided a natural experiment to compare outcomes between standard and high-viscosity preparations.
Pharmacokinetics of Rectal Absorption
Rectal administration relies on passive diffusion through the rectal mucosa. A primary challenge with liquid enemas is retention; low-viscosity fluids are prone to leakage or premature expulsion, reducing the "residence time" of the drug against the mucosal surface. The bioavailability of HPβCD is directly proportional to the "Area under the Curve" (AUC) of mucosal contact time.
The "Quad Strength" formulation addresses this via two mechanisms:
- The thicker consistency acts as a gel-like matrix, adhering more effectively to the rectal wall. Patients like J.E. explicitly noted that the thicker product was "easier to hold in overnight" compared to previous versions [1]. This prolonged retention allows for a sustained absorption window of 6-8 hours during sleep.
- : Fick’s law of diffusion states that the rate of diffusion is proportional to the concentration gradient. The Quad formulation delivers a significantly higher payload of HPβCD per unit volume (approximately 4x the standard concentration). This steep concentration gradient likely drives more significant passive transport across the epithelium [11].
Patient Preference and Clinical Correlation
Patient feedback overwhelmingly supports the high-viscosity formulation, not just for ease of use but for superior clinical outcomes. B.P., who has a history of rapid plaque growth, specifically correlated his resumption of the "Quad strength" formulation with the resolution of his severe dyspnea, hypothesizing that it is "absorbed better as it likely sticks more to the rectal passage walls" [1]. L.G., who achieved the 58% stenosis reduction, attributed his success to the "thicker formula," stating it worked "immensely better" and noted that the consistency was distinct enough that he could identify variations between batches [1] (Figure 2).
Discussion: Mechanisms, Safety, and Regulatory Context
The "Bimodal" Mechanism of Action
The clinical success observed in this cohort aligns with the "bimodal" mechanism described by Professor Laurie Howes and Dr. James Roberts.1 The therapy appears to operate on two distinct timelines:
- rapid resolution of angina and dyspnea—often reported within days or weeks—occurs too quickly to be attributed to gross plaque debulking. Instead, this likely reflects the solubilization of sub-endothelial cholesterol crystals, which immediately dampens NLRP3 inflammasome activity.4 The subsequent reduction in inflammatory cytokines (IL-1β, IL-6) restores endothelial nitric oxide synthase (eNOS) function. Dr. Roberts describes nitric oxide as a "biochemical Teflon coating" that dilates arteries and improves collateral flow [1].
- The reduction in CAC scores and angiographic stenosis represents the physical extraction of cholesterol mass from the plaque core. This is a slower, cumulative process of "melting" the lesion via continuous macrophage efflux [1].
Safety and Ototoxicity
A primary concern with HPβCD therapy in other contexts (e.g., Niemann-Pick Type C) has been ototoxicity (hearing loss). However, in NPC treatment, HPβCD is often administered intrathecally (into the spinal fluid) or at massive IV doses to cross the blood-brain barrier. In this ASCVD cohort using rectal or standard IV administration, no reports of hearing loss were documented [1].The primary adverse events were gastrointestinal (e.g., loose stools), which are consistent with the osmotic nature of cyclodextrins and were generally self-limiting or managed by dose adjustment. This confirms that systemic administration for vascular indications maintains a favorable safety profile compared to CNS-targeted therapies.
Regulatory and Access Challenges
The correspondence highlights significant challenges in accessing this therapy, primarily driven by regulatory hurdles. Multiple patients, including P.B., S.B., and P.D., reported frustration with shipping delays and customs seizures (FDA/TGA interventions) [1].These interruptions in supply act as a confounding variable in the data, as consistent dosing is critical for maintaining the "concentration gradient" required for plaque regression. Despite these barriers, the persistence of patients in seeking the therapy—and the advocacy of physicians like Dr. Roberts—underscores the perceived value of the treatment in a population with few other options.
Manufacturing Variability and Clinical Replicability
It is important to note that HPβCD is not a single chemical entity but a mixture of isomers. Evidence suggests that variations in the production process can alter the specific substitution pattern of the hydroxypropyl groups on the cyclodextrin ring. These structural nuances can significantly impact the molecule's encapsulation efficiency (its ability to trap cholesterol crystals) and its safety profile.
The efficacy observed in this cohort relies on the specific physicochemical profile of the Cavadex formulation. Attempts to replicate these findings using generic or laboratory-grade HPβCD from different manufacturers may yield inconsistent results due to potential variances in the degree of substitution or impurity profiles.
Limitations
This study is limited by its retrospective, observational design. Data was collected from a self-selected cohort of patients, and imaging was performed at various independent centers rather than a core laboratory, introducing potential variability in measurement techniques. Consequently, there is no standardized placebo control group. However, the magnitude of the observed regression—particularly the reversal of CAC progression in patients like T.J.A. and the 58% stenosis reduction in L.G.—is statistically unlikely to be a result of spontaneous remission, as atherosclerosis is a naturally progressive disease. The "historical control" of the patients' own prior disease trajectory serves as a robust comparator.
Conclusion
The aggregated data from this multi-center case series provides robust real-world evidence that Cavadex (2-Hydroxypropyl-β-cyclodextrin), particularly in its high-viscosity RemChol formulation, acts as a potent disease-modifying agent for atherosclerosis.
The therapy demonstrated the capacity to:
- Reverse the progression of coronary artery calcification, converting an annual progression rate of +27% to a regression rate of -10%.
- Reduce critical angiographic stenosis (e.g., from 82% to 24%), effectively "de-bulking" high-risk plaques.
- Resolve refractory angina and restore functional capacity in end-stage patients who had exhausted standard medical and surgical options.
These findings suggest that HPβCD fills a critical gap in the current pharmacopeia: the ability to actively extract existing plaque burden rather than merely slowing its growth. The consistent safety profile, lack of ototoxicity, and high patient satisfaction with the high-viscosity formulation support its continued development. We urgently recommend the initiation of formal, multi-center Randomized Controlled Trials (RCTs) to validate these observational findings and integrate this breakthrough therapy into global treatment guidelines for ASCVD.
Author Contributions
Kyle Hodgetts: Principal Investigator, data aggregation, and study design.
Dr. James Roberts: Clinical oversight, patient management, and mechanistic analysis.
Professor Laurie Howes (Late): Scientific advisory, statistical review, and pharmacological analysis.
References & Data Sources
1: Patient Testimonial Database (Emails 2025-2026), including data from Theodore Allen, Randy Canfield, Larry Gresowski, and others.
1: Transcript of Dr. James Roberts Interview (Mechanism of Action, Safety).
1: Correspondence and Reports from Professor Laurie Howes (Trial planning, TGA context).
1: Transcript of Professor Laurie Howes Presentation (Bimodal effect).
4: Hodgetts K (2025). Symptomatic Improvement and Enhanced Quality of Life... Cardiol Res Cardiovasc Med 10: 287.
2: Hodgetts K, Roberts JC, Howes LG (2025). Cyclodextrin Therapy for Atherosclerotic Cardiovascular Disease... Cardiol Res Cardiovasc Med 10: 284.
3: Cholrem study shows cyclodextrin-based therapy Cavadex reverses ASCVD. Express Pharma.
4: Zimmer et al. (2016). Cyclodextrin promotes atherosclerosis regression via macrophage reprogramming. Science Translational Medicine.
7: STROBE Statement—checklist of items that should be included in reports of observational studies.
11: Effects of cyclodextrins on drug delivery and concentration gradients.
Works cited
- Patient reported outcomes (internal data)
- Hodgetts K, Roberts JC, Howes LG (2025) Cyclodextrin Therapy for Atherosclerotic Cardiovascular Disease: A Case Series on Plaque Regression and Symptomatic Improvement, Cardiol Res Cardio vasc Med 10.
- Cholrem study shows cyclodextrin-based therapy Cavadex reverses atherosclerotic cardiovascular disease - Express Pharma, accessed on January 6, 2026.
- Hodgetts K (2025) Symptomatic Improvement and Enhanced Quality of Life in Individuals with Atherosclerotic Cardiovascular Disease Following a 12-Week Self-Monitored Regimen of Rectally Administered 2-Hydroxypropyl-β-Cyclodextrin (Cavadex): An Observational Study - Gavin Publishers.
- Hodgetts K, Howes L, Fang YY, Song ZM (2024) 2-Hydroxypropyl-β-Cyclodextrin Induces Rapid Regression of Atherosclerotic plaque and Reduces Hyperlipidaemia in Adult with Cardiovascular Disease - Gavin Publishers.
- Hodgetts K, Demian M, Fang YY, Song ZM (2024) 2-Hydroxypropyl-Β-Cyclodextrin Reduces Atherosclerotic Plaques in Human Coronary Artery - Gavin Publishers.
- STROBE Statement—checklist of items that should be included in reports of observational studies - EQUATOR Network, accessed on January 6, 2026.
- Cuschieri S (2019) The STROBE guidelines. Saudi J Anaesth. :S31–S34.
- Shaw LJ, Blankstein R, Jacobs JE, Leipsic JA, Kwong RY, et al. (2018) Defining Quality in Cardiovascular Imaging A Scientific Statement From the American Heart Association.
- Abrahao A, Tenorio PHM, Rodrigues M, do Nascimento OJ.M (2023) Evaluation of Neurological Safety Profile in the Use of Checkpoint Inhibitors: A Real-World Evidence Approach Based on Pharmacovigilance Data.
- Loftsson T, Brewster ME (2011) Pharmaceutical applications of cyclodextrins: effects on drug permeation through biological membranes. Journal of Pharmacy and Pharmacology 63:1119-1135.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.