2-Hydroxypropyl-β-Cyclodextrin Induces Rapid Regression of Atherosclerotic plaque and Reduces Hyperlipidaemia in Adult with Cardiovascular Disease
by Kyle Hodgetts1, Laurence Howes2,3, Yu-Yan Fang1, Zan-Min Song4*
1Cholrem Pty Ltd, Gold Coast, Queensland, Australia
2Professor, Clinical Pharmacologist and Toxicologist, School of Medicine and Dentistry, Griffith University, Gold Coast, Queensland, Australia
3Pre-eminent Consultant Physician in Cardiology and Internal Medicine, Gold Coast University Hospital, Queensland, Australia
4Associate Professor, School of Medicine and Dentistry, Griffith University, Gold Coast, Queensland, Australia
*Corresponding author: Zan-Min Song, Associate Professor, School of Medicine and Dentistry, Griffith University, Gold Coast, Queensland, Australia.
Received Date: 08 September, 2024
Accepted Date: 13 September, 2024
Published Date: 26 September, 2024
Citation: Hodgetts K, Howes L, Fang YY, Song ZM (2024) 2-Hydroxypropyl-β-Cyclodextrin Induces Rapid Regression of Atherosclerotic plaque and Reduces Hyperlipidaemia in Adult with Cardiovascular Disease. Cardiol Res Cardio vasc Med 9:261. https://doi.org/10.29011/2575-7083.100261
Abstract
Cardiovascular and cerebral arterial diseases have become the leading cause of human mortality worldwide. The primary pathology is atherosclerotic plaque, characterized by the accumulation of cholesterol within the inner lining of the arteries, which obstructs blood flow. 2-hydroxypropyl-β-cyclodextrin (HPβCD) has been shown to encapsulate cholesterol, leading to plaque regression in animal models, and has been used to treat elevated cholesterol in patients with Niemann-Pick type C disease. However, its effects on humans with atherosclerosis and hypercholesterolemia had not been reported before. This pioneering study demonstrated that intravenous infusion of HPβCD significantly: (1) reduced the volume of plaques in the carotid bulb, (2) decreased blood cholesterol and triglyceride levels, (3) improved hepatic function, and (4) reduced blood uric acid levels in a human subject with severe ischemic heart disease. This new discovery may herald a new era for the treatment of atherosclerotic cardiovascular diseases and metabolic syndrome. We appreciate that further randomised clinical trials are needed to consolidate these findings and establish HPβCD as a therapeutic agent.
Key words: Atherosclerosis, Cyclodextrins, Cardiovascular disease, Carotid artery, Doppler ultrasound
Introduction
Cardiovascular diseases (CVDs) are the leading cause of death worldwide and have become a significant global burden. An estimated 18 million people died from CVDs in 2019, and this number increased to 20.5 million in 2021, accounting for one-third of all global deaths [1,2]. Of these deaths, 85% were due to heart attacks and strokes [3]. The current annual incidence of stroke in Europe and the USA is approximately 200 per 100,000 population, with 80% of strokes being ischemic [4,5]. Atherosclerosis is the build-up of atherosclerotic plaque (atheroma) from deposited cholesterol, fat, calcium, and other substances within the inner lining of the arteries, leading to hardened arterial walls and narrowed lumens that limit blood flow. When unstable plaque ruptures, platelets and other blood components are recruited to form thrombosis, which can partially or fully block arteries, leading to acute ischemic heart disease [6] or ischemic stroke in the brain [7].
The mainstay of pharmacological treatment and prevention of cardiovascular diseases (CVD) includes antihypertensive medications, antiplatelet agents, and lipid-lowering therapies. Statins reduce blood cholesterol levels by inhibiting HMG-CoA reductase, an enzyme involved in cholesterol production, and have become the first-line therapy for treating hyperlipidaemia and slowing the progression of arterial plaque [8-10] to prevent coronary heart disease. However, once plaques have formed, they do not regress and can only be removed through invasive procedures such as stenting or endarterectomy.
Cyclodextrins, particularly 2-hydroxypropyl-β-cyclodextrin (HPβCD), are cyclic structures with a hydrophilic outer surface and a lipophilic central cavity that can accommodate a cholesterol molecule. HPβCD has been used in the therapeutic delivery of lipophilic pharmaceutical agents [11,12]. In vitro study showed that HPβCD spontaneously extracted cholesterol from model membrane on a nanosecond time scale [13]. Furthermore, HPβCD has been shown to increase cholesterol solubility thereby aiding in regression of atherosclerosis in vitro and in vivo in murine models [14-16].
As a therapeutic agent, HPβCD has been used to treat NiemannPick type C disease (NPC) in humans, a rare genetic disorder characterized by the accumulation of cholesterol in tissues due to impaired intracellular transport, ultimately leading to neurodegeneration. Both intrathecal and intravenous (iv) administration of HPβCD have been shown to slow the progression of the disease in NPC patients [17,18]. These studies also demonstrated good tolerability of HPβCD and lack of major side effects in humans, with oral doses up to 24 grams daily, although higher doses were associated with diarrhea [11]. Most importantly, intravenous infusion showed no adverse events over the course of treatment at doses up to 2500 mg/kg [19].
The aforementioned studies suggested the great potential of HPβCD in removing atherosclerotic plaques from arterial walls in humans. Our prior research demonstrated that iv administration of HPβCD at a dosage of 1000 mg/kg/day reduced plaque sizes in the right coronary artery, as revealed by coronary angiography, and lowered blood cholesterol and triglyceride levels [20]. This study aims to determine whether a lower dose of HPβCD (150-225 mg/ kg/day), administered via iv infusion, can reduce plaque size in carotid arteries as assessed by duplex ultrasound, and to further delineate its effects on the levels of blood lipids, uric acid and liver and renal function.
Case presentation
The patient (the first author of this paper) was a 58-year-old male with a history of ischemic heart disease, for which he had received five stents. He also had hypertension, hyperlipidaemia, and steatohepatitis (fatty liver). He began smoking at the age of 14 and accumulated a total of 84 pack-years. For 23 years, he consumed 11 standard drinks daily (equivalent to 110 grams of alcohol). He had a strong family history of arterial disease and hyperlipidaemia; his father died of heart disease at the age of 47, his mother died of heart failure at 75, and he had two brothers with hyperlipidaemia. His current medications included daily doses of aspirin (100 mg), amlodipine (10 mg), and atenolol (50 mg).
Diagnostic evaluation
Cardiovascular examination
The patient’s body mass index (BMI) was 26 kg/m², which was within the overweight range but otherwise unremarkable. His average blood pressure was 180/100 mmHg without antihypertensive medication, but it remained around 140/90 mmHg with regular antihypertensive treatment. His pulse was regular at 88 beats per minute.
Volume of the plaques
Carotid duplex ultrasound (DUS) imaging was utilized to measure the size of plaques and estimate their volumes (length × width × thickness in mm³) in both the right and left carotid bulbs, as shown in the schematic diagram (Figure 1). The DUS results revealed a mixed calcific and hyperechoic plaque at the right carotid bulb, with the anterior wall plaque measuring 5.9 × 4.5 × 2.2 mm³ and the posterior wall plaque measuring 6.7 × 5.0 × 2.0 mm³ (Figure 2A1, A2, C1, C2). There was a homogeneous hypoechoic soft atheroma at the left carotid bulb, with the anterior wall plaque measuring up to 8.0 × 1.3 mm (Figure 3A). The right carotid intima-media thickness (CIMT) was 0.7 mm, while the left CIMT was 0.6 mm.
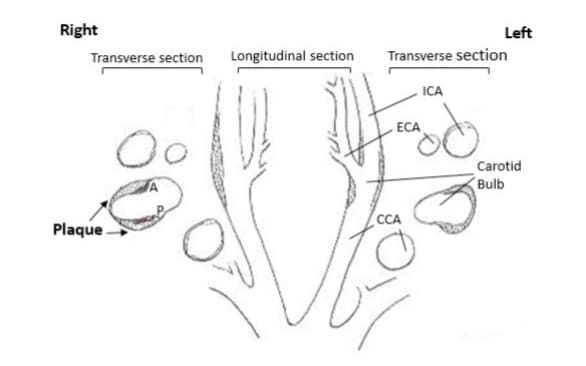
Figure 1: Schematic representation of the atherosclerotic plaques detected with duplex ultrasound imaging in the left and right carotid arteries of the subject. Hatched areas indicate plaques; CCA: common carotid artery; ICA: internal carotid artery; ECA: external carotid artery; A: anterior; P: posterior.
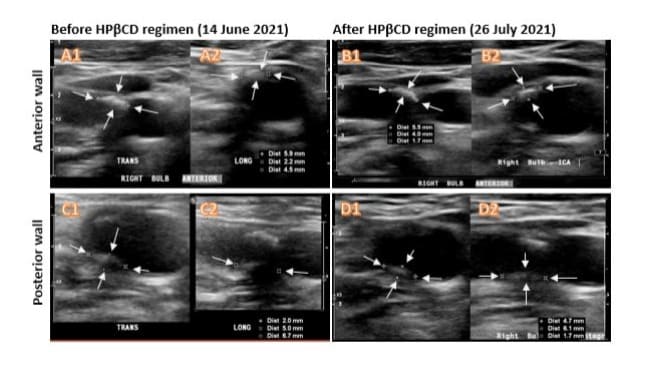
Figure 2: Ultrasound Doppler assessment of the plaques in the right carotid bulb before and after HPβCD regimen. The left panels were taken before treatment and the right panels were taken after treatment. Mixed calcific and hyperechoic carotid plaques (arrows) were located at the anterior (A1, A2, B1 and B2) and posterior (C1, C2, D1 and D2) walls of the right carotid bulb. Each plaque was imaged at both transverse plane (TRANS in A1, B1, C1 and D1) and longitudinal plane (LONG in A2, B2, C2 and D2). The sizes of the plaques were measured in mm and indicated in three dimensions as Dist. Note that each image has its individual scale bar so not all the images in this figure are on the same scale.
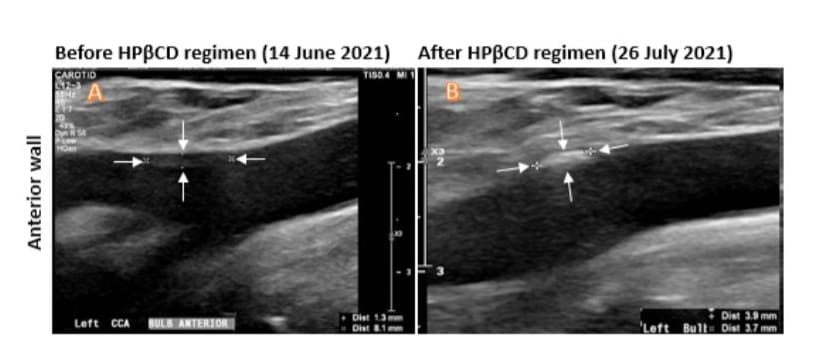
Figure 3: Ultrasound Doppler assessment of the plaques in the anterior wall of the left carotid bulb. A: Image taken before the HPβCD regimen. B. Image taken after the HPβCD regimen. The sizes of the plaques were indicated by arrows and measured in mm for each image (Dist at the lower right corners). The increased density was noticeable after HPβCD regimen (B).
Velocity of blood flow: Carotid doppler was used to assess the peak systolic velocity (PSV) and end diastolic velocity (EDV) of the right (Figure 4A, C) and left CCA and ICA. On the right ICA, the PSV was 54.9 cm/s, the EDV was 15 cm/s and the PSV ratio (ICA/ CCA) was 0.73. On the left ICA, the PSV was 58.4 cm/s, the EDV was 20.9 cm/s and PSV ratio (ICA/CCA) was 0.83 (Table 1). All these measurements indicated the ICAs had normal or less than 50% stenosis [21,22]. The detailed measurements of other parameters in CCA, ECA and vertebral arteries are shown in (Table 1).
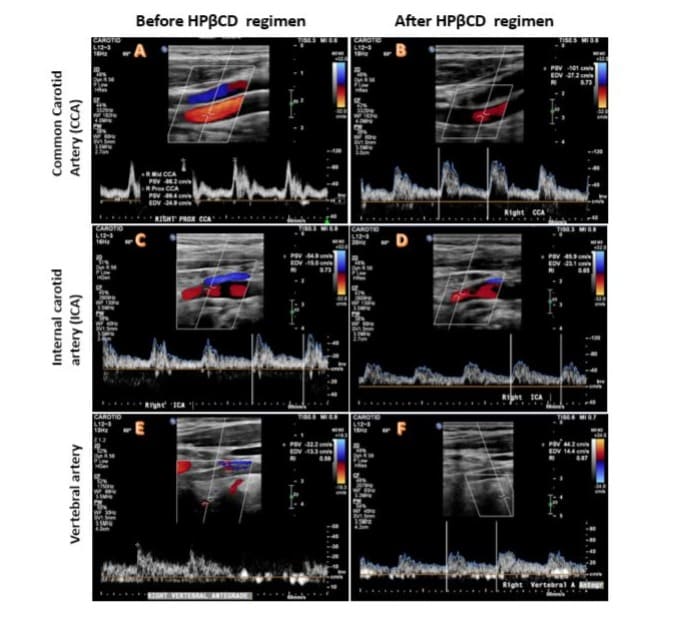
Figure 4: Blood flow velocity and spectrum measurement based on Colour Doppler Ultrasound. Left panels (A, C and E) were imaged before HPβCD treatment for the common carotid artery (CCA), internal carotid artery (ICA) and vertebral artery (vertebral A). Right panels (B, D and F) were imaged after HPβCD treatment for the same arteries. The blood flow velocity was measured as peak systolic velocity (PSV) and end-diastolic velocity (EDV). No significant changes were observed before and after HPβCD treatment in any of these arteries, indicating no detectable stenosis before the treatment. Colour Doppler Velocity mode: Blood flow away from the probe is blue while flow towards the probe is red (BART).
|
Carotid A |
Right |
Left |
||||||
|
Measurement (cm/s) |
PSV Before HPβCD |
PSV After HPβCD |
EDV Before HPβCD |
EDV After HPβCD |
PSV Before HPβCD |
PSV After HPβCD |
EDV Before HPβCD |
EDV After HPβCD |
|
CCA |
99.4 |
101 |
24.9 |
27.2 |
77.6 |
96.9 |
26.0 |
25.5 |
|
ICA |
54.9 |
65.9 |
15 |
23.1 |
58.4 |
80.0 |
20.9 |
29.8 |
|
ICA/CCA |
0.73 |
0.65 |
- |
- |
0.61 |
0.83 |
- |
- |
|
ECA |
96 |
100 |
25.2 |
24.2 |
67.4 |
109.0 |
14.1 |
20.4 |
|
Vertebral A |
32.2 |
44.2 |
13.3 |
14.4 |
36.4 |
39.1 |
13.7 |
15.4 |
|
CCA: common carotid artery, ICA: internal carotid artery, ECA: external carotid artery, PSV: peak systolic velocity, EDV: end-diastolic velocity, Vertebral A: Vertebral Artery |
||||||||
Table 1: Measurement of blood flow velocity in carotid artery and vertebral artery
Lipid profile: The lipid profile showed markedly elevated triglyceride (20 mmol/L), total cholesterol (10.6 mmol/L), non-HDL (9.5 mmol/L), and normal high-density lipoprotein (HDL, 1.1 mmol/L) as indicated in (Figure 5) baseline values.
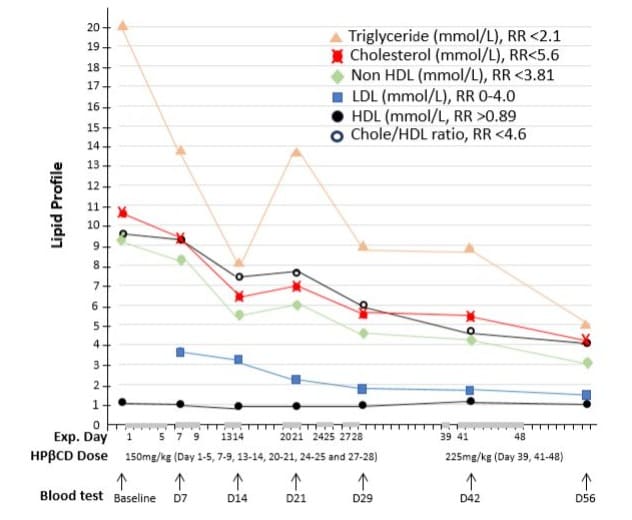
Figure 5: Effects of intravenous infusion of HPβCD on lipid profile. The horizontal axis represents the days of the experiment (Exp. Day), the doses of HPβCD in mg per kilograms of body weight per day, and venous blood samples collected before (baseline) and during HPβCD treatment regimen. The grey bars on the horizonal axis highlight the days with HPβCD administration and the gaps between these bars were drug-free days. The levels of blood triglyceride, cholesterol, non-HDL, LDL, HDL were measured in mmol/L with normal reference range (RR) indicated. It clearly showed that HPβCD treatment induced 60-75% decrease in the levels of different lipids and cholesterol/HDL ratio, while HDL levels remained stable.
Liver function and other blood tests: The results indicated slightly elevated liver enzymes in serum, with alanine transaminase (ALT) at 56 U/L, aspartate transaminase (AST) at 32 U/L, and gamma-glutamyl transferase (GGT) at 101 U/L. However, alkaline phosphatase (ALP), albumin, and bilirubin levels were within the normal range (Figure 6 baseline values). The patient’s urate level was 0.506 mmol/L, slightly above the normal range of 0.2-0.5 mmol/L. In addition, both blood glucose levels and estimated glomerular filtration rate (eGFR) were within normal limits.
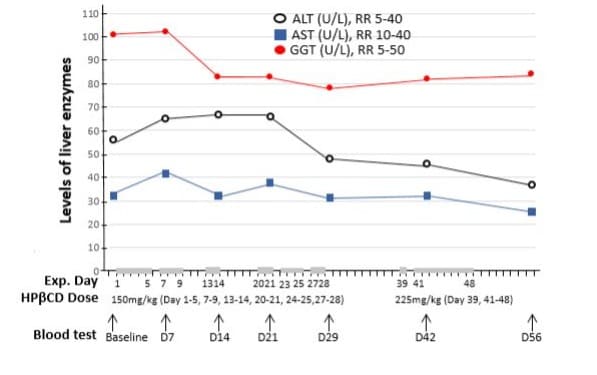
Figure 6: Effects of intravenous infusion of HPβCD on liver function. The horizontal axis represents the days of the experiment (Exp. Day), the doses of HPβCD in mg per kilograms of body weight per day, and venous blood samples collected before (baseline) and during HPβCD treatment regimen. The grey bars on the horizonal axis highlight the days with HPβCD administration and the gaps between these bars were drug-free days. The levels of blood alanine transaminase (ALT), aspartate aminotransferase (AST) and gamma glutamyl transferase (GGT) were measured in U/L with normal reference range (RR) indicated. HPβCD administration induced a slight decrease in mildly elevated ALT and GGT, while AST remained within normal range throughout the treatment period.
Urine test: The urine albumin was 51 mg/L (high), urine creatinine was 5.9 mmol/L (normal), and urine albumin-creatinine ratio was 8.6 (high) as shown in (Figure 7) baseline value.
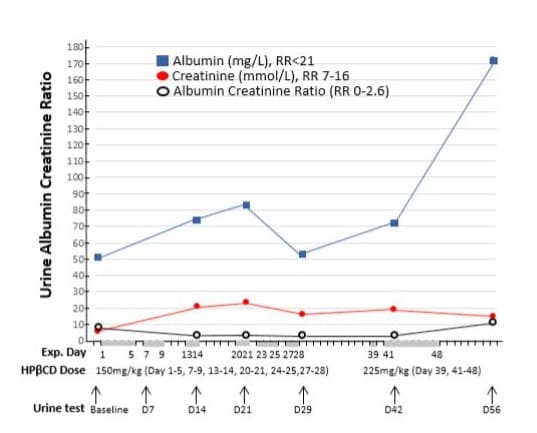
Figure 7: Effects of intravenous infusion of HPβCD on albumin and creatinine concentration in the urine. The horizontal axis represents the days of the experiment (Exp. Day), the doses of HPβCD in mg per kilograms of body weight per day, and urine samples collected before (baseline) and during HPβCD treatment regimen. The grey bars on the horizonal axis highlight the days with HPβCD administration and the gaps between these bars were drug-free days. Urine samples were tested for albumin, creatinine, and albumin/creatinine ratio (uACR). Both albumin and creatinine were increased during the treatment, although uACR was reduced.
Treatment Regimen with HPβCD and Monitoring of Organ Functions
The patient commenced treatment with HPβCD (CavadexTM, IP Australia No 2346983), prepared in 0.9% sodium chloride and administered intravenously through a 0.22-micrometre filter (Sartorius). HPβCD was formulated as a 20% stock solution and further diluted in 250 ml saline, which was infused at an average rate of 500 ml/hour. Vital signs were closely monitored throughout the treatment. The commencement day of HPβCD was designated as Day 1 (Figure 8). The initial dose of HPβCD was 150 mg/kg, administered over days 1-28 with several drug-free intervals. HPβCD was administered for a total of 17 days at 75 mg/kg twice daily (days 1-14) or 50 mg/ kg three times daily (days 20-28). After a 10 days drug-free washout period, the HPβCD dose was increased to 225 mg/kg daily (days 39-48), administered as 75 mg/kg three times daily. A diagrammatic representation of the dosing scheme is summarized in (Figure 8).
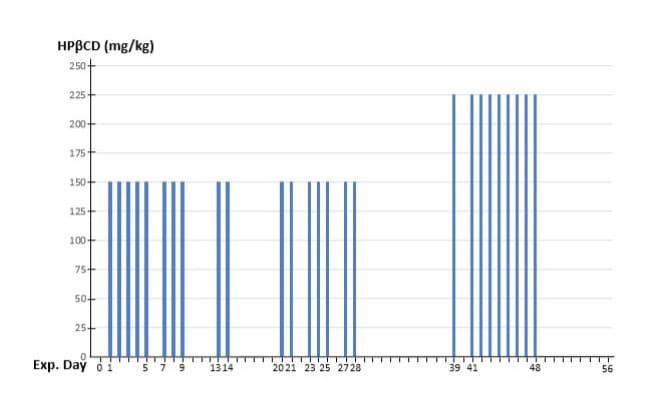
Figure 8: Regimen for iv administration of HPβCD. The horizontal axis represents the days of the experiment (Exp. Day) with a number of drug-free intervals. The vertical axis represents the doses of HPβCD in mg per kilograms of body weight (80kg) per day. Note the increased drug dose from day 39 to day 48, after 10 days drug-free washout period.
Throughout the HPβCD study, the patient continued his regular medications of antihypertensives and antiplatelets and maintained his usual lifestyle, including diet, alcohol consumption, and smoking habits as detailed above.
To evaluate changes in the size and volume of atherosclerotic plaques, carotid Doppler ultrasound was performed one week before the administration of the HPβCD regimen (on 14 June 2021) and on day 38 of the regimen (26 July 2021). A series of investigations were conducted to monitor changes in other organ functions in response to HPβCD administration. Blood samples were collected regularly to monitor full blood count, electrolytes, lipid profile, liver function, and renal function on day 0 (baseline), and on days 7, 14, 21, 29, 42, and 56. Urine was tested for the albumin-creatinine ratio (uACR). An audiology test was performed during HPβCD treatment and in post-treatment follow-up.
Results
HPβCD treatment significantly reduces plaques size in the carotid artery
Carotid duplex ultrasound (DUS) assessments were performed on day 35 of the HPβCD regimen in the same carotid bulb (Fig. 2, right panel), as described above. The percentage reduction in plaque size/volume was calculated by subtracting the posttreatment plaque size from the pre-treatment size, dividing by the pre-treatment size, and multiplying by 100. The size of the plaque on the anterior wall of the right carotid bulb was reduced to 5.5 × 4.0 × 1.7 mm³, representing a reduction of 35.9% (Figure 2. A1, A2 vs. B1, B2). The size of the plaque on the right posterior wall was reduced to 6.1 × 4.7 × 1.7 mm³, representing a reduction of 27.3% (Figure 2. C1, C2 vs. D1, D2). The size of the plaque on the anterior wall of the left carotid bulb did not significantly change but became noticeably denser after HPβCD treatment (Figure 3. A vs. B).
HPβCD didn’t significantly affect blood flow
Blood flow velocities in CCA, ICA, ECA and vertebral artery on both the left and right sides were measured before and after HPβCD treatment, as summarized in (Table 1). There were no significant changes in peak systolic velocity (PSV) or end-diastolic velocity (EDV) for the right CCA (Figure 4A, 4B), ICA (Figure 4C, 4D), or vertebral artery (Figure 4E, 4F) following HPβCD treatment (Table 1). The radiologist’s report concluded “No carotid stenosis” or very early-stage diameter stenosis (<50%) based on blood flow parameters and visible plaque in the ICA, according to Doppler criteria for diagnosing ICA stenosis: normal (0%) to <50% ICA stenosis should have an ICA PSV <125 cm/s, an ICA EDV <40 cm/s, and a PSV ratio of ICA/CCA <2.021, 22. Similarly, PSVs for vertebral arteries on both sides remained within the normal range of 25-40 cm/s before and after HPβCD treatment (Table 1).
Significant improvement in lipid profile
The patient had significantly elevated levels of triglycerides, cholesterol, and non-HDL cholesterol prior to HPβCD treatment. These parameters were reduced by nearly 50% on day 14 of administering HPβCD at 150 mg/kg/day and continued to decline by day 28. Following a 10-day drug-free period, the HPβCD dose was increased to 225 mg/kg/day on day 39, resulting in further reductions in all lipid levels by day 56. Overall, HPβCD treatment reduced triglycerides by 75.5% (from 20 to 4.9 mmol/L), total cholesterol by 61.3% (from 10.6 to 4.1 mmol/L), non-HDL cholesterol by 67.3% (from 9.5 to 3.1 mmol/L), LDL cholesterol by 59.5% (from 3.7 to 1.5 mmol/L), and the cholesterol/HDL ratio by 57.3% (from 9.6 to 4.1) (Figure 5). HDL levels remained within the normal range throughout the experiment. Notably, during the 10-day drug-free period, lipid levels remained unchanged, indicating that continuous administration of HPβCD is necessary to maintain an optimal lipid profile. Detailed lipid data are summarized in (Figure 5).
Moderate improvement in liver function
The slightly elevated levels of liver enzymes (Figure 6) were reduced following HPβCD treatment. ALT decreased by 33.9%, from a baseline level of 56 U/L to 37 U/L (within the normal range) by day 56, despite a transient rise during the first three weeks. AST levels remained stable and largely within the normal range throughout the treatment period. GGT levels decreased by 17% from 101 U/L to 84 U/L within the first week and remained consistent thereafter. All other liver function parameters, including albumin, globulin, total bilirubin, and alkaline phosphatase (ALP), remained within normal limits (data not shown).
Effects on renal function
The estimated glomerular filtration rate (eGFR) in blood samples remained within the normal range throughout the HPβCD treatment regimen. However, urine albumin and creatinine concentrations were consistently above the normal range during HPβCD administration (Figure 7), with no observed improvement. Further studies are needed to determine whether this increase is related to HPβCD treatment. The urine albumin-creatinine ratio (uACR) was substantially reduced by 55.8%, although it remained above the normal range throughout the HPβCD treatment (Figure 7).
Reduction of Urate Levels in Blood
Serum urate levels were measured before and after HPβCD treatment, despite the patient having no history of gout. The treatment resulted in a 50% reduction in urate levels, from 0.506 mmol/L (reference range 0.2-0.5 mmol/L) at baseline to 0.347 mmol/L on day 29 and 0.249 mmol/L on day 56. This significant effect suggests potential applications for HPβCD in the treatment of hyperuricemia and gout.
No adverse effect on other blood tests and hearing
Full blood count and electrolytes remained normal during the treatment period (data not shown). Two hearing assessments using audiometry were conducted on day 7 of HPβCD treatment and again at the end of treatment indicated normal hearing in both ears, as evidenced by pure tone and speech audiometry results (data not shown).
Discussion
This research demonstrated for the first time that an iv administration regimen of HPβCD significantly reduced the size and volume of atherosclerotic plaques in the carotid artery. It also significantly lowered serum cholesterol, triglyceride, and uric acid levels, and moderately improved liver function. The optimal doses and treatment intervals were also established. The first author, who was also the study participant, designed the HPβCD treatment regimen based on previous publications [14,16] with drug safety profile for iv infusion documented in treating patients with Niemann–Pick type C disease [11, 17-19]. He conducted studies on himself twice in 2019 [20] and in 2021 that formed the bases in this report.
Efficacy of HPβCD in removing arterial plaques
To evaluate the efficacy of HPβCD in removing arterial plaques, a total of 26 doses of HPβCD, starting at 150 mg/kg/day and increased to 225 mg/kg/day, were iv infused over a 48-day period. Carotid duplex ultrasound showed a significant reduction in plaque volume, with a decrease of 35.9% in the anterior plaque of the right carotid bulb and 27.3% in the posterior plaque of the right carotid bulb. This is the first direct evidence that HPβCD reduces arterial plaques in human carotid arteries, supporting our previous finding that HPβCD reduced arterial plaques in the coronary artery [20]. It is anticipated that atherosclerotic plaques in other arteries of the body, such as the aorta and lower limb arteries, would also be reduced by the systematic administration of HPβCD. If this is the case, iv infusion of HPβCD may become the preferred treatment for atherosclerotic plaques in the whole cardiovascular system.
It remains unclear which types of atherosclerotic plaques are most responsive to HPβCD treatment based on our preliminary imaging analysis using angiography and duplex ultrasound. Plaques are typically categorized as stable or unstable (vulnerable plaques), but they can be further classified into eight types using magnetic resonance imaging (MRI) according to the American Heart Association (AHA) classification system [23, 24]. The types most relevant to this study include type IV and VIII. Type IV is identified as an atheroma with a confluent extracellular lipid core surrounded by fibrous tissue, with possible calcification. Type VIII is defined as a fibrotic plaque without a lipid core, but with possible small calcifications. Previous studies [25] have described the mechanisms of plaque development and associated risk factors, such as tissue aging and dyslipidaemia, which trigger local low-grade inflammation (arterial lipidosis). These processes can lead to productive fibrous changes (stable plaques) or productive destructive inflammation (unstable plaques).
HPβCD treatment significantly reduced plaques in both the anterior and posterior walls of the right carotid bulb, as well as in the coronary arteries in our previous study. Further investigation using MRI is needed to confirm whether the observed density change in plaques after HPβCD treatment corresponds to changes in calcification. If HPβCD treatment can attract calcium into plaques, it may transform unstable plaques into stable ones. This is because calcification in carotid and coronary artery plaques could provide mechanical stability and reduces the risk of embolization for a given plaque size and degree of luminal stenosis [26,27].
The atherosclerotic plaques in this pioneering study did not significantly impact blood flow velocity within the arteries. Further double-blind clinical trials, involving subjects with severe atherosclerotic plaques, specifically those with stenosis of 50% or greater in the internal carotid artery as classified by the Radiological Society of North America [21,22] are needed to further elucidate the efficacy of HPβCD in reducing more severe arterial plaques and alleviating arterial stenosis.
HPβCD improved lipid profile and liver function
The levels of lipids, except HDL, declined significantly within 14 days of HPβCD treatment and continued to decline throughout the course of treatment. This indicates that an optimal dose of HPβCD at 150-225 mg/kg/day for 8 weeks can effectively reduce hyperlipidaemia to normal levels. More randomized double-blind clinical trials are necessary to confirm our results and establish HPβCD treatment as a standard therapy for hyperlipidaemia.
Liver steatosis was identified via ultrasound in the participant two years prior to this study, and his liver function tests, especially ALT, were consistently above the normal range. HPβCD treatment effectively reduced the elevated ALT level to normal range, accompanied by a 17% reduction in GGT. We speculate that the improvement in liver functions is related to HPβCD’s ability to reduce hyperlipidaemia.
No evidence of ototoxicity of HPβCD with iv infusion
Ototoxicity has been reported with HPβCD treatment of human Niemann Pick type C disease (NPC), especially with route of intrathecal administration [17]. In this and previous studies of our group, both independent hearing assessments before and after HPβCD iv infusion period showed normal hearing for both ears. This is consistent with previous iv infusion treatment of NPC [19]. Taken together, the current and previous research results indicate that HPβCD iv infusion does not cause ototoxicity even at high dose of 1000mg/kg/day [20].
Conclusion
The intravenous infusion regimen of HPβCD may herald a new era in combating metabolic syndrome and its serious cardiovascular sequelae, which claim more lives than any other single diseases. This regimen has been shown to be a novel and effective therapy for reducing atherosclerotic plaques in coronary and carotid arteries, lowering lipid levels, decreasing uric acid levels, and improving liver function. This novel therapy may revolutionise the treatment of arterial atherosclerosis in patients with cardiovascular diseases, the major cause of all global deaths.
Funding Information: The first author contributed to all study expenses.
Author contribution
Kyle Hodgetts (Author 1): study design and study subject. Given final approval of the version to be published
Laurence Howes (Author 2): medical care of the study subject and intellectual input for the study
Yu-Yan Fang (Author 3): data analysis and drafting manuscript
Zan-Min Song (Author 4): data evaluation and manuscript revision with intellectual input
Acknowledgments: Professor Howes passed away before writing the manuscript and he contributed greatly to the study.
Declaration of Interest Statement: The authors declare no
conflict of interest
Data availability statement: Data will be available from the corresponding author upon reasonable request.
Consent: Written informed consent has been taken from the patient.
References
- Mensah GA, Fuster V, Murray CJL, Roth GA (2023) Global Burden of Cardiovascular Diseases and Risks, 1990-2022. J Am Coll Cardiol. 82:2350-2473.
- Di Cesare M, Perel P, Taylor S, Kabudula C, Bixby H, et al. (2024) The Heart of the World. Glob Heart. 19:11.
- Cardiovascular diseases (CVDs) (2021) World Health Organization.
- Katan M, Luft A (2018) Global Burden of Stroke. Semin Neurol. 38:208-211.
- Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, et al. (2017) Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation.135:e146-e603.
- Badimon L, Padró T, Vilahur G (2012) Atherosclerosis, platelets and thrombosis in acute ischaemic heart disease. Eur Heart J Acute Cardiovasc Care.1:60-74.
- Hennerici MG (2004) The unstable plaque. Cerebrovasc Dis. 17:1722.
- Stancu C, Sima A (2001) Statins: mechanism of action and effects. J Cell Mol Med. 5:378-87.
- Bansal AB, Cassagnol M (2023) HMG-CoA Reductase Inhibitors.
- van Rosendael AR, van den Hoogen IJ, Gianni U, Ma X, Tantawy SW, et al. (2021) Association of Statin Treatment With Progression of Coronary Atherosclerotic Plaque Composition. JAMA Cardiol. 6:12571266.
- Gould S, Scott RC (2005) 2-Hydroxypropyl-beta-cyclodextrin (HPbeta-CD): a toxicology review. Food Chem Toxicol. 43:1451-9.
- Loftsson T, Jarho P, Másson M, Järvinen T (2005) Cyclodextrins in drug delivery. Expert Opin Drug Deliv. 2:335-51.
- López CA, de Vries AH, Marrink SJ (2011) Molecular mechanism of cyclodextrin mediated cholesterol extraction. PLoS Comput Biol. 7:e1002020.
- Atger VM, de la Llera Moya M, Stoudt GW, Rodrigueza WV, Phillips MC, et al. (1997) Cyclodextrins as catalysts for the removal of cholesterol from macrophage foam cells. J Clin Invest. 99:773-80.
- Liu SM, Cogny A, Kockx M, Dean RT, Gaus K, et al. (2003) Cyclodextrins differentially mobilize free and esterified cholesterol from primary human foam cell macrophages. J Lipid Res. 44:1156-66.
- Zimmer S, Grebe A, Bakke SS, Bode N, Halvorsen B, et al. Cyclodextrin promotes atherosclerosis regression via macrophage reprogramming. Sci Transl Med. 8:333ra50.
- Ory DS, Ottinger EA, Farhat NY, King KA, Jiang X, et al. (2017) Intrathecal 2-hydroxypropyl-β-cyclodextrin decreases neurological disease progression in Niemann-Pick disease, type C1: a nonrandomised, open-label, phase 1-2 trial. Lancet. 390:1758-1768.
- Hastings C, Vieira C, Liu B, Bascon C, Gao C, et al. (2019) Expanded access with intravenous hydroxypropyl-β-cyclodextrin to treat children and young adults with Niemann-Pick disease type C1: a case report analysis. Orphanet J Rare Dis.14:228.
- Matsuo M, Togawa M, Hirabaru K, Mochinaga S, Narita A, et al. (2013) Effects of cyclodextrin in two patients with Niemann-Pick Type C disease. Mol Genet Metab. 108:76-81.
- Hodgetts K, Demian M, Fang Y-Y, Song Z-M (2024) 2-hydroxypropylβ-cyclodextrin reduces atherosclerotic plaques in human coronary artery. Cardiology Research and Cardiovascular Medicine ( under review).
- Grant EG, Benson CB, Moneta GL, Alexandrov AV, Baker JD, et al. (2003) Carotid artery stenosis: gray-scale and Doppler US diagnosis-Society of Radiologists in Ultrasound Consensus Conference. Radiology. 229:340-6.
- Saba L, Loewe C, Weikert T, Williams MC, Galea N, et al. (2023) Stateof-the-art CT and MR imaging and assessment of atherosclerotic carotid artery disease: standardization of scanning protocols and measurements-a consensus document by the European Society of Cardiovascular Radiology (ESCR). Eur Radiol. 33:1063-1087.
- Stary HC, Chandler AB, Dinsmore RE , Fuster V, Glagov S, et al. (1995) A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler Thromb Vasc Biol.15:151231.
- Stary HC (2000) Natural history and histological classification of atherosclerotic lesions: an update. Arterioscler Thromb Vasc Biol 20:1177–1178.
- Gusev E, Sarapultsev A (2023) Atherosclerosis and Inflammation: Insights from the Theory of General Pathological Processes. Int J Mol Sci. 24:7910.
- Saba L, Nardi V, Cau R, Gupta A, Kamel H, et al. (2022) Carotid Artery Plaque Calcifications: Lessons From Histopathology to Diagnostic Imaging. Stroke. 53:290-297.
- Mohan J, Bhatti K, Tawney A, Zeltser R (2023) Coronary Artery Calcification.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.