2-Hydroxypropyl-Β-Cyclodextrin Reduces Atherosclerotic Plaques in Human Coronary Artery
by Kyle Hodgetts1, Mark Demian2, Yu-Yan Fang1, Zan-Min Song3*
1Cholrem Pty Ltd, Gold Coast, Queensland, Australia
2Carnegie Central Medical Clinic, Carnegie, VIC 3163, Australia
3Associate Professor, School of Medicine and Dentistry, Griffith University, Gold Coast, Queensland, Australia
*Corresponding author: Zan-Min Song, School of Medicine and Dentistry, Griffith University, Gold Coast, Queensland, Australia.
Received Date: 12 September, 2024
Accepted Date: 02 October, 2024
Published Date: 04 October, 2024
Citation: Hodgetts K, Demian M, Fang YY, Song ZM (2024) 2-Hydroxypropyl-Β-Cyclodextrin Reduces Atherosclerotic Plaques in Human Coronary Artery. Cardiol Res Cardio vasc Med 9:262. https://doi.org/10.29011/2575-7083.100262
Abstract
Cardiovascular and cerebral arterial disease has become the number 1 killer of human being. The main pathology is atherosclerotic plaque, characterised by the build-up of cholesterol in the inner lining of the arteries, which blocks blood flow. 2-hydroxypropylβ-cyclodextrin (HPβCD) has been shown to encapsulate cholesterol to cause a regression of plaques in animals. However, there was no research on its effects in human subjects. This study is unique in that it was designed and performed by the first author (KH). He had severe coronary arterial atherosclerosis treated with classical statins and multiple stents but had been declared as having a life expectancy of 0.5-2.5 years in 2019. HPβCD was infused intravenously at different doses for a period of 36 days. Several significant results have been discovered. Firstly, the treatment led to a significant reduction of plaques in the right coronary artery revealed by coronary angiography before and after the treatment regimen. Secondly, the treatment reduced the level of cholesterol and triglyceride in the blood. Thirdly, the elevated urine albumin and albumin/creatinine ratio prior to the treatment was reduced to normal level. Lastly, no significant adverse effects were observed in liver function and hearing. This is the first clinical trial to show the efficacy of HPβCD in removing atherosclerotic plaques from coronary arteries.
Key words: Atherosclerosis, Cyclodextrins, Cardiovascular disease, Coronary vessels
Introduction
Cardiovascular diseases (CVDs) are the leading cause of death and become a globe burden globally. An estimated 18 million people died from CVDs in 2019, 20.5 million deaths in 2021, representing 1/3 of all global deaths [1,2]. Of these deaths, 85% were due to heart attack and stroke [3]. The mainstay of pharmacological treatment and prevention of CVD includes anti-hypertensive medication and lipid lowering therapy. Statins, HMG-CoA reductase inhibitors, can lower blood cholesterol levels by blocking the action of a liver enzyme that helps produce cholesterol and are used as first line therapy to treat hyperlipidaemia in the primary and secondary prevention of coronary heart disease [4,5].
The blockage of coronary, cerebral and other arteries in the body is due to atherosclerosis, the buildup of plaque from deposited cholesterol, fat, and other substances into the inner lining of the arteries. The plaque narrows arterial lumen and restrict or block blood flow, and even rupture the arterial wall, leading to a blood clot. Although statin can reduce plaque progression by lowering concentration of cholesterol in the blood [6], it cannot remove cholesterol from already formed plaques. Endarterectomy is the only way to remove the plaque and stenting, also known as percutaneous coronary intervention (PCI) is the only option to widen narrowed artery and prevent high risked plaque rupture [7]. Because of the wide spread nature of atherosclerotic plaques in multiple arteries (e.g. coronary arteries, carotid arteries, aorta and arteries in the limbs) in an individual, the targeted invasive treatment is often insufficient. Therefore, novel treatment paradigms are urgently needed to reduce or remove all the plaques formed in different arteries.
Cyclodextrins, particularly 2-hydroxypropyl-β-cyclodextrin (HPβCD), is playing a pivot role at anti-plaque therapy. HPβCD are cyclic structures, containing a hydrophilic outer surface and a lipophilic central cavity, that cater a molecule cholesterol. HPβCD has been used to its advantage in therapeutic delivery of lipophilic pharmaceutical agents [8,9]. Furthermore, HPβCD has been shown to increase cholesterol solubility thereby aiding in regression of atherosclerosis in vitro and in vivo in murine models [10-12].
HPβCD has been used in humans with Niemann Pick type C disease (NPC), which is a rare genetically inherited disease resulting in accumulation of cholesterol in tissues due to inability of intracellular transport, ultimately resulting in neurodegeneration. Studies have shown that intrathecal and intravenous (iv) application of HPβCD slows progress of disease in NPC patients [13,14]. In vitro study showed that HPβCD spontaneously extracted cholesterol from model membrane on a nanosecond time scale [15]. This raised the possibility of removing atherosclerotic plaques in arterial walls.
There have been many clinical studies using HPβCD in humans which have shown good tolerability with oral doses up to 24 grams and only diarrhea as a side effect at higher doses [8]. Other routes such as iv administration have shown tolerability at higher doses of up to 2.5g/kg with no observed adverse events over the course of treatment [16].
This pioneering study aimed to find out whether HPβCD could solubilize cholesterol and remove atherosclerotic plaques in a CVD patient with severely blocked coronary arteries that failed to improve with statin and repetitive stents. It also aimed to find out whether higher doses with iv administration would cause any adverse effect. The findings may lead to revolutions in the treatment of CVD caused by arterial atherosclerosis.
Case presentation
The patient was a 56-year-old male. He had history of ischemic heart disease with 3 stents and hypertension. He started smoking since 14 years old with 84 pack years. He had daily consumption of 11 standard drinks (110 grams alcohol) for 23 years. He had strong family history of arterial disease and hyperlipidaemia with father died of heart disease at 47 years old and mother died of heart failure at 75 years old and two brothers with hyperlipidaemia.
Diagnostic evaluation
Cardiovascular examination showed a body mass index (BMI) of 26 kg/m2 but was otherwise unremarkable. His average blood pressure was 180/100 mmHg without any anti-hypertension medication while blood pressure remained around 140/90 mmHg after anti-hypertension medication. His pulse was 88 bps regular and in sinus rhythm. His lipid profile showed a total cholesterol of 7.5mmol/L, with triglyceride of 9.3 mmol/L and high-density lipoprotein (HDL) of 1.3 mmol/L. His glucose level was at normal range and the percentage of HbA1c was 5.2% (Reference Range <6.5%). Angiogram showed a patent stent in the left anterior descending artery but severe in-stents stenosis in the mid right coronary artery (RCA) stent, as well as severe distal RCA stenosis that required intervention. He was on daily aspirin 100mg, amlodipine 10mg, and atenolol 50mg.
Treatment and management
The patient was started on a HPβCD (FUJIFILM Wako Chemicals or CAVASOL HP W7) prepared in Sodium Chloride 0.9% and administered intravenously through a 0.22 micrometre filter (Sartorius). HPβCD was formulated as a 20% solution and diluted in saline either 500ml or 1000ml based on doses, which was infused as average 500ml/hour. Vital sign and blood sugar level were observed closely. HPβCD commencement day was counted as Day 1. The HPβCD dose was commenced (Figure 1) at 100mg/kg on days 1 and 2, and gradually increased to 200mg/kg, 300mg/kg and 500mg/kg from day 3 to day 5. After two days’ washout on days 6 and 7, HPβCD dose was increased to 1000mg/kg on day 8. On day 10, HPβCD was switched to another source (CAVASOL) at dose of 1000mg/kg but the intravenous infusion had to cease when patient felt a pin and needle in his chest, lasting approximately 2 hours. Otherwise, patient felt no other adverse event. Vital signs were stable as blood pressure ranging 125-136/72-92mmHg, and heart rate at 76-89 per minute. A dose of 700mg/kg was administered on day 10. After 5 days’ washout from D11-15, HPβCD was switched back to FUJIFILM brand for the rest of treatment as 750 mg/kg was administered on Day 16, 1000mg/kg on day 17 and 300mg/kg on day 19. HPβCD with 300mg/kg was repeated for 8 doses with interval days between days 22-36.
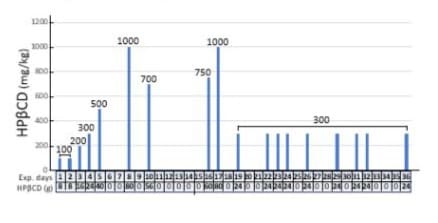
Figure 1: Regimen for iv administration of HPβCD. The days into the trial and the total weight of HPβCD are indicated on the horizontal axis with drug free days denoted are “0”. The doses of HPβCD are calculated at the body weight of 80 kg and noted in the vertical axis.
To evaluate changes in the size/volume of atherosclerotic plaques in right coronary arteries, coronary angiography was performed by cardiac physicians prior and post of HPβCD treatment regimen on 30 days before treatment and 14 days after treatment. A series of investigations were performed to monitor possible drug induced changes in organ functions. Blood samples were collected regularly to monitor full blood count, lipid profile and liver function on day 0 (baseline), D6, D12, D14, D16, and D21. Urine was tested for albumin-creatinine ratio (uACR) on day0, D6, D12, D14, and D21. Ultrasound scan of the liver was done before and in the middle of HPβCD regimen. Audiology test was performed during HPβCD treatment and post treatment follow-up.
Results
HPβCD significantly reduces atherosclerotic plaques in the right coronary artery (RCA)
The arterial lumen of the RCA was imaged one month before HPβCD administration (Figure 2A, 2C) and two weeks after HPβCD administration (Figure 2B, 2D). Prior to HPβCD administration, multiple constrictions were observed along the main branch of the RCA (Figure 2A, 2C). The formal report of angiogram stated that RCA was the dominant vessel with 60-70% in stent restenosis (ISR) in mid segment and 70% stenosis precrux. Recommendation from cardiologist was “elective percutaneous coronary intervention (PCI) to RCA”.
Apparently, the subject did not follow the recommendation of his cardiologist but started iv infusion HPβCD regimen. The angiograms taken two weeks after the final dose of HPβCD are shown in (Figure 2B,2D). There is a significant reduction in plaque size accompanied by notable enlargement of the lumen. Recommendation from cardiologist has been changed to “Continue medical management and risk factor modification”.
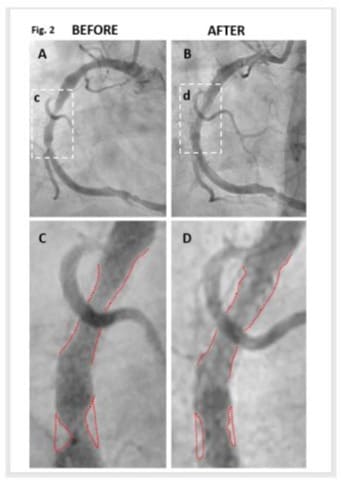
Figure 2: HPβCD Reduces Atherosclerotic Plaques in the Right Coronary Artery (RCA) in angiogram. The arterial lumen of the RCA was imaged before (Panels A and C) and after (Panels B and D) HPβCD administration. The areas enclosed in boxes in Panels A and B are magnified for detailed examination in Panels C and D, respectively. Panel A, C: Prior to drug administration, multiple constrictions were observed along the main branch of the RCA. Panel C highlights these constrictions with red outlines delineating both the artery’s boundary and the plaques. Radiologist’s report on RCA: “Large calibre & dominant vessel with 60-70% in stent restenosis (ISR) in mid segment. There is 70% stenosis precrux”. Panel B & D: Two weeks following drug treatment, there is a notable enlargement of the lumen accompanied by a significant reduction in plaque size.
Improvement of lipid profile
As shown in (Figure 3), lipid levels did not respond significantly on day 6 when HPβCD was administered at low doses (below 500 mg/kg) for 5 days. Once HPβCD was increased to higher doses (700 or 1000 mg/kg), all lipids (except HDL) on day 12 decreased clearly. Triglyceride decreased by 26.3% from 7.2 mmol/L on day 0 to 5.3 mmol/L on day 12; cholesterol decreased by 8.5% from 4.7 mmol/L on day 0 to 4.3 mmol/L on day 12; non-HDL decreased by 14.7% from 3.4 mmol/L on day 0 to 2.9 mmol/L on day 12; HDL levels were not significantly altered throughout the experiment, although cholesterol/HDL radio was reduced by 13.8% from 3.6 on day 0 to 3.1 on day 12.
After 5 days washout, HPβCD was re-introduced and blood was collected prior to HPβCD administration on day 16. Cholesterol was reduced by 14% from 5.0 mmol/L on day 16 to 4.3 mmol/L on day 21, non-HDL was reduced by 15.7% from 3.8 mmol/L on day 16 to 3.2 mmol/L on day 21, and cholesterol/HDL radio was reduced by 6.9% from 4.2 on day D16 to 3.9 on day 21.
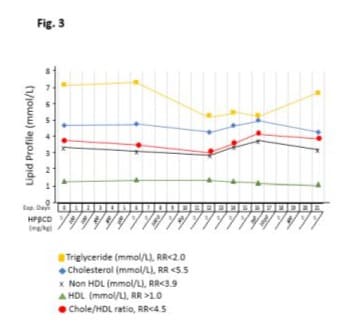
Figure 3: Effects of intravenous infusion of HPβCD on lipid profile. The horizontal axis represents the days of the experiment (Exp. Days) and the doses of HPβCD in milligrams per kilograms of body weight. After iv administration of HPβCD, venous blood samples were periodically analyzed for triglyceride, cholesterol, HDL, non-HDL, and the Cholesterol/HDL ratio. HPβCD at low doses (below 500 mg/kg) for 5 days did not significantly change lipid levels on day 6. HPβCD at higher doses (700mg/kg or 1000 mg/kg) clearly reduced all lipids (except HDL) on day 12. The effects of HPβCD administration were attenuated on days 12, 14, and 16 during 5 days drug free period. Recommencement of high doses (750mg/kg and 1000mg/kg) has lasting effects, especially on cholesterol and non-HDL levels. HDL levels were not significantly altered throughout the experiment.
Improvement on renal function
Urine albumin-creatinine ratio (uACR) was improved with HPβCD treatment (Figure 4). Albumin level on baseline was 61 mg/L and showed a transit rise in the first week to 113 mg/L with 5 low doses below 500 mg/kg of HPβCD. Albumin level declined to 68 mg/L, near baseline level, on day 12 after two higher doses (700 mg/kg or 1000 mg/kg), and its level further declined to normal range on day 21 as 20 mg/L after another high doses. No significant changes were observed for creatinine but the uACR was reduced by 50% from baseline level of 18.8 to 9.1 on day 21. Blood estimated glomerular filtration rate (eGFR) was within normal range throughout the HPβCD treatment regimen.
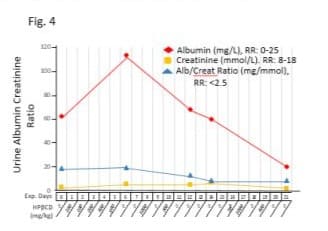
Figure 4: Effects of intravenous infusion of HPβCD on renal function. The horizontal axis represents the days of the experiment (Exp. Days) and the doses of HPβCD in milligrams per kilogram body weight. After iv administration of HPβCD, urine samples were periodically analyzed for albumin, creatinine and the albumin/creatinine ratio (uACR). Albumin level had a transient rise in the first week with 5 low doses but returned to near baseline level at higher doses. uACR was reduced by 50% from baseline level on day 21.
Transient changes in liver function
The level of liver enzymes (Figure 5) did not change significantly on day 6 with 5 consecutive days HPβCD treatment at low doses (below 500 mg/kg) for alanine transaminase (ALT), aspartate aminotransferase (AST) and gamma glutamyl transferase (GGT). The patterns of ALT, AST and GGT showed a delayed elevation on day 14 after high dose (1000 mg/kg and 700 mg/kg) of HPβCD was administrated on day 8 and day 10. Re-introduced high doses (750 mg/kg and 1000 mg/kg) on day 16 and day 17 did not further raise liver enzymes, instead, ALT,GGT and AST declined continually close to baseline. It is noted that HPβCD used on day 10 at 700 mg/kg was from CAVASOL brand and patient experienced pin and needle on the chest, therefore administration was interrupted for 5 days before switched back to FUJIFILM brand.
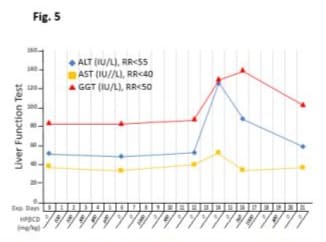
Figure 5: Effects of intravenous infusion of HPβCD on liver function tests. The horizontal axis represents the days of the experiment (Exp. Days) and the doses of HPβCD in milligrams per kilogram body weight. After iv administration of HPβCD, venous blood samples were periodically analysed for alanine transaminase (ALT), aspartate aminotransferase (AST) and gamma glutamyl transferase (GGT). RR indicates normal reference ranges. HPβCD at low doses did not change levels of liver enzymes although higher doses of different brands had different effects on liver enzymes. Details are elaborated in the text.
No adverse effect on hearing
Hearing assessment with audiometry in mid of treatment (Figure 6) indicated normal hearing for both ears. Audiology checked two years later also confirmed normal hearing in both ears with either pure tone audiometry or speech audiometry.
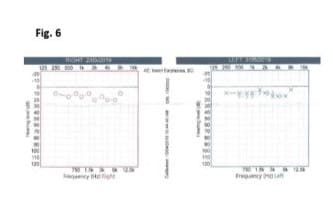
Figure 6: HPβCD had no adverse effect on hearing shown in audiometry on day 8 of HPβCD regimen. Right: Hearing (O represents air conduction, AC) was within normal limits at 250 Hz to 8 kHz. Left: Hearing was within normal limits at 250 Hz to 8 kHz (X represents AC; > represents bone conduction, BC).
Discussion
This is the first report of successful treatment for atherosclerosis using intravenous (iv) infusion of HPβCD. The patient in this study is the first author, who was advised by his cardiologist that his life span would be between 0.5 to 2 years based on the severity of the coronary arteriosclerosis. He was desperately finding ways to improve his cardiovascular condition. The trial was designed by the patient himself (first author) who was inspired by studies showing that HPβCD could reduce cholesterol in vitro and in vivo [10,12] and its safety profile with iv infusion in treating patients with Niemann–Pick type C disease [13,14,16]. This study was also conducted by himself during April and May 2019, with careful planning of HPβCD doses and close monitoring of functions of vital organs throughout the trial period. Under the US Food and Drug Administration’s (FDA) regulation, HPβCD has Individual Investigation New Drug exemptions in 2009 [17].
Efficacy of HPβCD in removing arterial plaques
To evaluate the efficacy of HPβCD in removing arterial plaques, a total of 18 doses of HPβCD were infused intravenously in 36 days. Angiography imaging showed that the plaque volume in RCA was reduced remarkably with widened lumen at in stent restenosis (ISR), which would otherwise require angioplasty to expand. This is the first direct evidence that HPβCD reduces arterial plaques in coronary arteries in human. Although this pilot study did not examine other arteries, it is logical to expect that atherosclerotic plaques in other arteries of the body, esp, carotid and cerebral arteries, aorta and lower limb arteries will also be reduced by the systematic administration of HPβCD. This urgently call for more larger scale clinical trials on patients with severe atherosclerotic plaques throughout the vascular system to further elucidate the efficacy of HPβCD in reducing/eliminating arterial plaques. This ground breaking discovery potentially revolutionise the treatment of atherosclerotic plaques in any arteries of the body and would save millions of lives in the world.
HPβCD improved lipid profile and renal function
The levels of lipids, except HDL, declined during treatment from higher levels before treatment to low levels on day 12 treatment, but bounced back after 5 days without HPβCD on day 16. This suggest that the drug should be continuously used with gaps less than 3 days.
HPβCD usage significantly improves renal function by reducing albumin loss from urine from 61mg/L pre-treatment to 20mg/L (Reference Range 0-25) after treatment. The risk of cardiovascular mortality in low uACR could be reduced by nearly 1.5- fold, compared with high uACR [18].
Titration of HPβCD doses
To find out the optimal doses of HPβCD treatment regimen, dose at 100 mg/kg/day was commenced and was increased gradually to 1000 mg/kg/day, which was within the dose range 80-2000 mg/kg as published [16]. Dose of 700mg/kg of HPβCD was also tried from a different source on day 10 but the patient felt pin and needle in the chest, which led to 5 days drug free period and subsequent episode of raised liver enzymes.. The original HPβCD from FUFIFILM was cautiously re-introduced at 750 mg/ kg, 1000 mg/kg, and 300 mg/kg on day 16, day 17 and day 19 and liver function was checked again, whichshowed that all three biomarkers declined to near normal range on day 21. This pattern of liver enzymes from baseline to day 21 was difficult to attribute to a delayed abnormality of liver function caused by HPβCD or due to a different brand used. Nevertheless, Ory et al [13] stated that “all HPβCD formulations are equivalent with respect to either efficacy or toxicity as HPβCD is a complex mixture and the composition varies with the source”. The optimal doses are important for further exploration of alternative drug delivery route including rectal enema.
Lack of evidence of ototoxicity of HPβCD with iv infusion
Ototoxicity has been reported with HPβCD treatment, especially with route of intrathecal administration [13] but not with intravenous route [16]. In this study, two hearing assessments with audiometry in a specialised hearing laboratory during HPβCD administration and two years after the treatment were both normal for both ears. This indicates that HPβCD iv infusion is safe at the highest dose of 1000 mg/kg and over one month duration. Again, further trials in multiple centres and larger patient groups with compromised liver or renal functions need to be considered.
Conclusion
HPβCD therapy via intravenous administration significantly decreased volume of arterial plaques in the right coronary artery. The safety profile and tolerability of iv infusion of HPβCD were further demonstrated. Clinical trials in population with conditions of CAD, stroke, or peripheral artery disease (PAD) [19-21] are required to further evaluate HPβCD efficacy on reducing atherosclerosis and adverse events in terms of dosing and duration. This novel therapy may revolutionise the treatment of arterial atherosclerosis in any arteries of the body.
Funding Information: The first author contributed to all study expenses.
Data availability statement: Data will be available from the corresponding author upon reasonable request.
Declaration of Interest Statement: The authors declare no conflict of interest
Author contribution
Kyle Hodgetts (Author 1): study design and study subject. Given final approval of the version to be published
Mark Demian (Author 2): medical care of the study subject
Yu-Yan Fang (Author 3): data analysis and drafting manuscript
Zan-Min Song (Author 4): data evaluation and manuscript revision with intellectual input
ORCID: Zan-Min Song https://orcid.org/0000-0001-9971-4027
Consent: Written informed consent has been taken from the patient.
References
- Mensah GA, Fuster V, Murray CJL, Roth GA (2023) Global Burden of Cardiovascular Diseases and Risks, 1990-2022. J Am Coll Cardiol. 82:2350-2473.
- Di Cesare M, Perel P, Taylor S, Kabudula C, Bixby H, et al. (2024) The Heart of the World. Glob Heart. 19:11.
- Cardiovascular diseases (CVDs) (2021) World Health Organization.
- Stancu C, Sima A (2001) Statins: mechanism of action and effects. J Cell Mol Med. 5:378-87.
- Bansal AB, Cassagnol M (2024) HMG-CoA Reductase Inhibitors.
- van Rosendael AR, van den Hoogen IJ, Gianni U, Ma X, Tantawy SW, et al. (2021) Association of Statin Treatment With Progression of Coronary Atherosclerotic Plaque Composition. JAMA Cardiol. 6:12571266.
- Lipinski MJ, Fearon WF, Froelicher VF, Vetrovec GW (2004) The current and future role of percutaneous coronary intervention in patients with coronary artery disease. J Interv Cardiol. 17:283-94.
- Gould S, Scott RC (2005) 2-Hydroxypropyl-beta-cyclodextrin (HPbeta-CD): a toxicology review. Food Chem Toxicol. 43:1451-9.
- Loftsson T, Jarho P, Másson M, Järvinen T (2005) Cyclodextrins in drug delivery. Expert Opin Drug Deliv. 2: 335-51.
- Atger VM, de la Llera Moya M, Stoudt GW, Rodrigueza WV, Phillips MC, et al. (1997) Cyclodextrins as catalysts for the removal of cholesterol from macrophage foam cells. J Clin Invest. 99:773-80.
- Liu SM, Cogny A, Kockx M, Dean RT, Gaus K, et al. (2003) Cyclodextrins differentially mobilize free and esterified cholesterol from primary human foam cell macrophages. J Lipid Res. 44:1156-66.
- Zimmer S, Grebe A, Bakke SS, Bode N, Halvorsen B, et al. (2016) Cyclodextrin promotes atherosclerosis regression via macrophage reprogramming. Sci Transl Med. 8:333ra50.
- Ory DS, Ottinger EA, Farhat NY, King KA, Jiang X, et al. (2017) Intrathecal 2-hydroxypropyl-β-cyclodextrin decreases neurological disease progression in Niemann-Pick disease, type C1: a nonrandomised, open-label, phase 1-2 trial. Lancet. 390: 1758-1768.
- Hastings C, Vieira C, Liu B, Bascon C, Gao C, et al. (2019) Expanded access with intravenous hydroxypropyl-β-cyclodextrin to treat children and young adults with Niemann-Pick disease type C1: a case report analysis. Orphanet J Rare Dis. 14:228.
- López CA, de Vries AH, Marrink SJ (2011) Molecular mechanism of cyclodextrin mediated cholesterol extraction. PLoS Comput Biol. 7:e1002020.
- Matsuo M, Togawa M, Hirabaru K, Mochinaga S, Narita A, et al. (2013) Effects of cyclodextrin in two patients with Niemann-Pick Type C disease. Mol Genet Metab. 108:76-81.
- Hastings C (2010) Request for intrathecal delivery of HPBCD for Niemann Pick Type C patients, Caroline Hastings, M.D. Principal Investigator Department of Pediatric Hematology Oncology Children’s Hospital and Research Center Oakland.
- Lin X, Song W, Zhou Y, Gao Y, Wang Y, et al. (2023) Elevated urine albumin creatinine ratio increases cardiovascular mortality in coronary artery disease patients with or without type 2 diabetes mellitus: a multicenter retrospective study. Cardiovasc Diabetol. 22:203.
- Mahjoubin-Tehran M, Kovanen PT, Xu S, Jamialahmadi T, Sahebkar A (2020) Cyclodextrins: Potential therapeutics against atherosclerosis. Pharmacol Ther. 214:107620.
- Becktel DA, Zbesko JC, Frye JB, Chung AG, Hayes M, et al. (2022) Repeated Administration of 2-Hydroxypropyl-β-Cyclodextrin (HPβCD) Attenuates the Chronic Inflammatory Response to Experimental Stroke. J Neurosci. 42:325-348.
- Qi X, Yuan Y, Xu K, Zhong H, Zhang Z, et al. (2015) (2-Hydroxypropyl)β-Cyclodextrin Is a New Angiogenic Molecule for Therapeutic Angiogenesis. PLoS One. 10: e0125323.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.