Isolation, Anti- Biotic Sensitivity and Biochemical Characterization of Fruit Spoiling Bacteria from Different Markets of Guwahati City
Manash Pratim Sarma*, Subhankar Das, Robinson, Surjya Loying, Minakshi Bhattacharjee, Nayan Talukdar, Partha Pratim Kalita, Kundal Neog
Department of Biotechnology, Assam Down Town University, Panikhaiti, Guwahati, Assam, India
*Corresponding author: Manash Pratim Sarma, Department of Biotechnology, Assam Down Town University, Panikhaiti, Guwahati, Assam, India. Tel: +918255075275; Email: manash3268@gmail.com
Received
Date: 1 August, 2017; Accepted Date: 2 September, 2017; Published Date: 9 September, 2017
Citation: Sarma MP, Das S, Robinson, L Surjyal, B Minakshi, et al. (2017) Isolation, Anti- Biotic Sensitivity and Biochemical Characterization of Fruit Spoiling Bacteria from Different Markets of Guwahati City. Appl Clin Pharmacol Toxicol: ACPT-107.
1. Abstract
There is potentiality for a wide range of vegetables and fruit products to become contaminated with microorganisms. The range of microorganisms associated with outbreaks linked to fresh product encompasses bacteria, viruses and parasites. This study was undertaken to investigate microbial safety aspects of some fruits such as Mango (Mangifera indica), Plum (Prunus sp.), Guava (Psidium guajava), Litchi (Litchi chinensis), Grapes (Vitis vinifera), Apple (Malus domestica) and Banana (Musa acuminate).
The presence of different bacteria which were responsible for spoiling fruits was confirmed by various biochemical and microbial tests. The microorganisms isolated were characterized by VITEK analysis and identified. The results showed the presence of the bacteria like Enterobacter cloacae complex, Providencia rettgeri, Pantoea spp and Serratia plymuthica. The isolated strains were then tested against 19 different antibiotics to look into their antibiotic susceptibility. The fruits in Guwahati region are mainly spoiled by Enterobacter cloacae complex, though other organisms are also there such as Pantoea spp, Providencia rettgeri, Serratia plymuthica. Methods to control these microbes need to be framed and applied for long term storage of the fruits.
2. Keywords: Antibiotic; Fruit Spoilage Microbes; Guwahati; Vitek
3.
Introduction
Fruits are vital sources of nutrients to human beings. They give the body the necessary vitamin and minerals in the right proportion for human growth and development. Fruits are seasonal and highly perishable in nature. The fruits that are cultivated in a natural environment are exposed to microbial contamination. They begin to deteriorate shortly after harvest. The fruits that are cultivated in the nature are more susceptible to the microbial attacks. Fruits may become contaminated with harmful pathogens and spoilage microorganisms either during their growing in fields, orchards, vineyards, or greenhouses, or during harvesting, postharvest handling, and distribution.
Sometimes spoilage of fruits also depends on microbial growth depends on various factors such as temperature, pH and moisture of the particular region. Biochemical and microbial spoilage are main two causes for their spoilage. Due to the spoilage, the nutritional value and the market value of the fruits also gets deteriorated. It is estimated one-fourth of the harvested fruits are spoiled before consumption. Biochemical spoilage is caused by microorganisms like bacterial, yeast and moulds. Many microorganisms that are responsible for the spoilage of fruits are also harmful to human when consumed.
These may include respiratory failure, urinary infection, food poisoning, stomachache, fever, nausea etc. Over the years, there has been an increased demand to isolate and identify the microorganisms associated with the spoilage so that it can be controlled [1,2]. This study was undertaken to isolate and identify microorganisms that are associated or responsible for the spoilage of ripened fruits sold in Narengi and Paltan bazaar markets within Guwahati region. In addition, the study investigated the susceptibility of the microorganisms to some antibiotics. Enterobacter species are known to be associated with high resistance to most of the antimicrobial agents [3]. Numerous studies on the mutation and its association with drug resistance have been reported [4-7]. Similar experience in terms of resistance was also been experienced in the species isolated from food stuff [8-11]. The experiment was carried to estimate and identify the fruit spoiling microbes in Guwahati.
4. Materials and Methods
The experiments were conducted in the Department of Biotechnology, Assam Down Town University, Panikhaiti, Guwahati, Assam and apart with identification were carried out in Assam Down Town Hospital, India. The fruits selected for the study were: Apple (Malus domestica), Mango (Mangifera indica), Banana (Musa acuminate), Litchi (Litchi chinensis), Guava (Psidium guajava), Grapes (Vitis vinifera), Plum (Prunus cerasifera) which are most frequently consumed by the people of the region.
4.1. Collection of Deteriorated Samples
All samples of fruits were collected from two markets of Guwahati region of, Narengi and Paltan Bazzar. The fruits selected were slightly spoiled ones. The samples were taken to the laboratory, washed and drained of water. A sterile blade and forceps were used to cut small section of the tissue containing both the healthy and the rotten portion. The cut portions were pounded with pistil and mortar to make paste. The pastes were kept in sterile tubes and labeled for future use. A total of 9 representative samples were tested.
4.2. Isolation of Bacteria
The pour-plate method according to Harigan and McCane (1990) [12] was adopted. Using standard Microbiological technique (serial dilution), a tenfold dilution of 1g of the sample was carried out in 9ml of sterile water (this was the aliquot). The exponential dilution was carried out to the fourth factor (10-4). 1ml of the fourth factor was aseptically transferred and plated in duplicate sets using sterile molten lukewarm nutrient agar which was amended with 1%. The plates were allowed to set and were incubated at 37ºC for 24hours. Individual colonies were chosen and the top of an individual colony each was touched lightly with a sterile loop or needle and streak on a fresh agar plate [13,14]. The various colonies observed in the plates were distinguished on the basis of their cultural characteristics such as colony size, shape, color, consistency and hemolytic characteristics. Bacterial growth was sub-cultured on NA and was preserved.
4.3. Tests for Characterization of Bacterial Colonies
The tests to characterize the bacterial colonies were Gram staining, Catalase test, Methyl red test, Indole test, citrate utilization test, fermentation of carbohydrate test, triple sugar iron test, cogulase test, temperature assays, antibiotic tests and Vitek Test. The standard procedures for its test were performed [15-18]. As the current study was on representative samples hence statistical analysis was not undertaken.
5. Results
Collection of samples (spoiled fruits) and isolation of different bacteria from spoiled fruits could be seen from (Figure 1).
The multiple tubes showing the serial dilution protocol. The microbes were cultured using pour plate and streaking methods (Figure 2 and 3).
The
results for the biochemical tests are summarized in (Table
1). Most of the bacterial strains were positive for Catalase, citrate
and coagulase tests [19]. The details of
remaining tests have been shown in (Table 1).
5.1. Temperature Sensitivity Assay of Bacteria Strains
The results for temperature sensitivity shows response at 45ºC for two out of the 10 studied strains of bacteria (Table 2 and Figure 4). The tests were found to be positive for all tested bacterial species at 30 and 40ºC. From temperature sensitivity assay [21,22] it was found that all isolated bacterial strains grow optimally at 40ºC.
5.2. Identification of Isolated Bacterial Strains by Vitek Technique
In terms concentration, the highest number of bacterial load was observed of Enterobacter species (Table 3 and Figure 5). From the Vitek Test the species were identified as six species were identified as Enterobacter cloacae complex [23,25], one each of Pantoea spp, Providencia rettgeri, Serratia plymuthica.
5.3. Antibiotic Sensitivity Assay of Bacteria Strains
The results for antibiotic tests are summarized in Table 4. From the antibiotic sensitivity test we have tested 19 antibiotics on 9 strains of bacteria that were previously isolated and seen that most of the [26] strains are resistance to some of the antibiotics.
6. Conclusion
Fruits provide our body with essential nutrients; vitamins etc. However, there are some bacteria that cause spoilage of fruits. A conclusion achieved was that the fruits in Guwahati region are mainly spoiled by Enterobacter cloacae complex, though other organisms are also there such as Pantoea spp, Providencia rettgeri, Serratia plymuthica [27]. Again, presence of antibiotic resistant strains needs to be dealt carefully. The study findings are of high importance as an appropriate method for controlling these micro organism needs to be adopted as the spoilage has economical implication.
7. Acknowledgements
The authors acknowledge the support of all the members of Department of Biotechnology, Assam down Town University, Panikhaiti, and Guwahati, Assam.
Figure 1: Serial Dilution
Method.
Figure 2: Pour plate method.
Figure 3: Streaking Method.
Figure 4: Temperature Sensitivity Assay.
Figure 5: Graphical Representation of the No. of
Species.

Table 1: Biochemical Tests Results.
|
Sl. No. |
Bacterial strains |
AREA |
Temperature (ºC) |
|||
|---|---|---|---|---|---|---|
|
30 |
40 |
45 |
||||
|
1 |
M01 |
PALTAN BAZZAR |
Y |
Y |
Y |
|
|
2 |
L01 |
Y |
Y |
N |
||
|
3 |
G01 |
Y |
Y |
N |
||
|
4 |
GR1 |
Y |
Y |
N |
||
|
5 |
P01 |
Y |
Y |
Y |
||
|
6 |
M02 |
NARENGI |
Y |
Y |
Y |
|
|
7 |
B01 |
Y |
Y |
Y |
||
|
8 |
A01 |
Y |
Y |
Y |
||
|
9 |
P02 |
Y |
Y |
Y |
||
Table 2: Temperature sensitivity assay of bacteria strains.
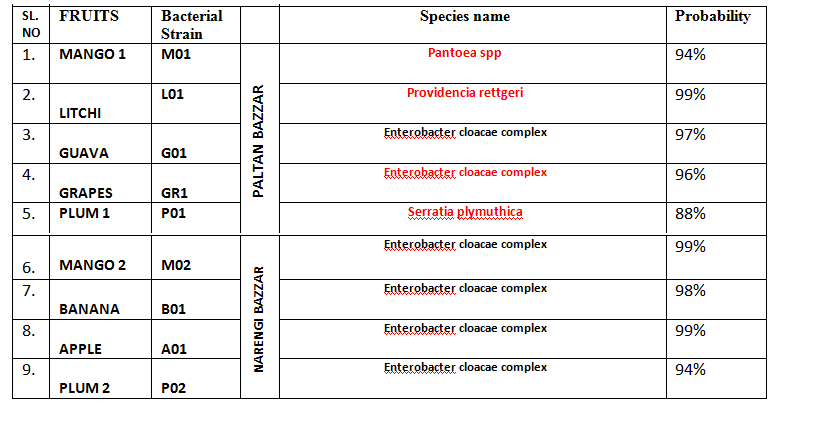
Table 3: Result of Vitek Test.
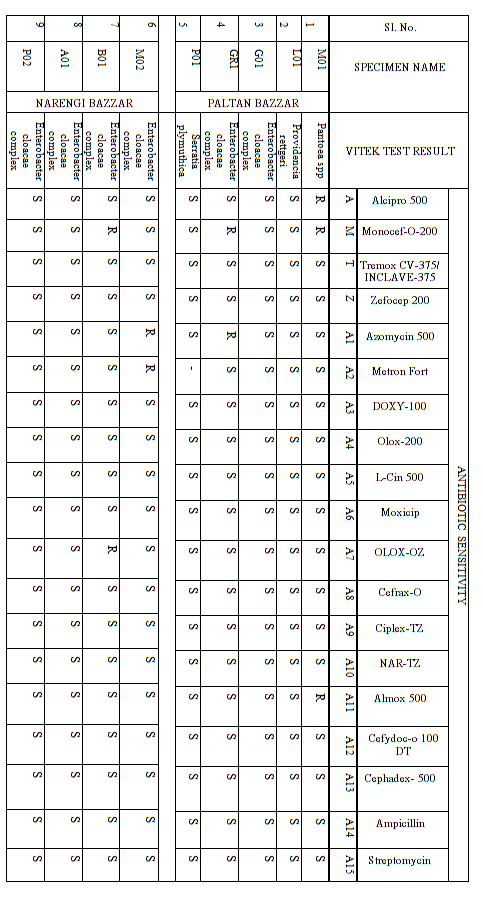
Table 4: Results for Antibiotic Tests.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.