Transcranial Direct Current Stimulation (tDCS) to Improve Cognitive Function and Reduce Cognitive Fatigue and Fatigability in Parkinson’s Disease: A Randomized Controlled Trial
by Jessica N Keller1, Asenath XA Huether1,2, Kelly Reishus3, Hannah Rathgeber3, Jau-Shin Lou1,3*
1Department of Neurology, Sanford Health, Fargo, ND, USA
2Department of Psychology, Minnesota State Community and Technical College, Moorhead, MN, USA
3Department of Neurology, University of North Dakota School of Medicine and Health Sciences, Grand Forks, ND, USA
*Corresponding author: Jau-Shin Lou, Department of Neurology, Sanford Health, Fargo, and University of North Dakota School of Medicine and Health Sciences, Grand Forks, ND, USA.
Received Date: 12 September, 2025
Accepted Date: 22 September, 2025
Published Date: 26 September, 2025
Citation: Keller JN, Huether AXA, Reishus K, Rathgeber H, Lou JS (2025 Transcranial Direct Current Stimulation (tDCS) to Improve Cognitive Function and Reduce Cognitive Fatigue and Fatigability in Parkinson’s Disease: A Randomized Controlled Trial. Int J Geriatr Gerontol 9:211. https://doi.org/10.29011/2577-0748.100211
Abstract
Introduction: Parkinson’s Disease (PD) presents complex challenges beyond motor symptoms, including cognitive fatigue and dysfunction, for which effective treatments remain scarce. This study investigated whether anodal transcranial direct current stimulation (tDCS) could improve cognitive function and reduce cognitive fatigue and fatigability in PD patients. Methods: Participants (n = 39) were assessed at baseline, post-intervention (Day 5), and at 1- and 2-week follow-ups for either tDCS stimulation or sham stimulation. The stimulation group received 2 mA current for 20 minutes to the left dorsolateral prefrontal cortex for five daily consecutive sessions. Primary outcome measure of the treatment’s efficacy was performance on a visual search computer task to evaluate fatigability. Secondary outcome measures included an assessment battery to evaluate subjective fatigue, depression, quality of life, working memory, and attention. Results: Compared to the sham group, the stimulation group showed significant improvements in working memory, as measured by the Digit Span Forward and Backward tasks, with sustained effects observed at two weeks. No significant changes were found in cognitive fatigue, fatigability, or other cognitive domains. Discussion: Targeted tDCS may enhance specific aspects of cognitive function in PD, particularly working memory, though limitations were noted in broader applications. Future research should explore synergistic approaches integrating tDCS with complementary therapies to optimize cognitive outcomes in PD populations. Clinical Trial Registration: https://clinicaltrials.gov/, identifier [NCT03191916].
Keywords: Parkinson’s disease, transcranial direct current stimulation, attention, memory, fatigue, fatigability
Introduction
Parkinson’s Disease (PD) is the second most common and fastest growing neurological condition. It affects roughly one million people in the United States and 10 million people worldwide [1,2]. PD is associated with cardinal motor symptoms — bradykinesia, tremor, rigidity, and postural instability — in addition to nonmotor symptoms [3]. These non-motor symptoms, such as cognitive impairment, fatigue, and depression, can significantly impact a patient’s quality of life but are overshadowed by the motor symptoms [4]. The lack of standard treatments for nonmotor symptoms, like cognitive impairment and fatigue, further complicates the management of non-motor symptoms. The motivation to advance knowledge in non-motor symptoms of PD is evident.
In the last decade, there has been exponential growth in examining the use of tDCS for the improvement of PD motor and non-motor symptoms. Transcranial direct current stimulation is a noninvasive brain stimulation technique that modulates cortical excitability. Anodal tDCS (atDCS, referenced as tDCS throughout the manuscript), often used in studies evaluating its effectiveness on cognitive function, increases the likelihood of an action potential occurring by depolarizing neurons. Recently, [5] identified that healthy adults showed an increase in extracellular dopamine following a single tDCS session. This effect of tDCS on dopamine levels emphasizes the use of tDCS as a potential therapy for the disruption of the dopaminergic system, which has been suggested to be involved in cognitive impairments [6] and is present in the pathology of PD. Further, an advantage of tDCS is that it has minimal adverse effects, with patients occasionally reporting skin irritation or headache at the site of stimulation [7,8]. Additionally, atDCS influences cortical excitability by facilitating long-term potentiation and reducing long-term depression. Thus, multisession tDCS has the potential of inducing more lasting changes in the brain [9].
Dopaminergic dysfunction of the prefrontal-basal ganglia-thalamocortical loops contribute to PD-related declines in attention, memory, and executive function [10-12]. The current study evaluated the effect of tDCS to improve attention and working memory. Attention is necessary for the filtering of information in one’s environment and can be automatic (simple) or effortful (complex; [11]. Memory, particularly working memory, drives our ability to hold information for a short period of time and to be able to manipulate that information [13].
Multiple studies have evaluated the effects of tDCS on attention and memory [14]. administered four 20-minutes sessions using 1.5 mA tDCS to the left dorsolateral prefrontal cortex (l-DLPFC) and showed improved complex attention (STROOP) and working memory (paragraph recall). It is important to note that Lawrence et al. tested tDCS alone and paired with cognitive training programs, in which the latter resulted in greater cognitive effects [15]. administered sixteen 20-minute sessions using 2 mA tDCS to the l-DLPFC four days a week for four weeks and found a trend for improved working memory at 16-weeks. However, short-term improvements in working memory (immediate memory index and story learning) were not found and decrements in executive attention (written coding test) declined at four weeks. [16] administered 2 mA at the l-DLPFC in a single session and found improved working memory (three-back letter task).
Currently, there are no treatments for fatigue in PD although roughly half of the PD population experience this non-motor symptom [17,18]. Fatigue is defined as “the subjective and general sensation of tiredness and difficulty initiating or engaging in activities” [19]. Fatigue can be divided into mental and physical domains. Mental fatigue refers to the extra effort required to pay attention. Physical fatigue refers to the extra effort required to initiate or complete an activity. Cognitive fatigability is “the deterioration in the performance of attention tasks over an extended period of time.” A key difference between fatigue and fatigability is the subjective versus objective nature of the two, respectively.
Studies in other neurological diseases, such as Multiple Sclerosis (MS), have found short- and long-term improvements in fatigue symptoms following five consecutive 20-minutes tDCS sessions at 2 mA to the l-DLPFC [20] and with 1.5 mA to the motor area for 15 minutes a day for five consecutive days [21]. One study in PD patients found reduced subjective fatigue following 2 mA stimulation for 20 minutes to the DLPFC for 8 total sessions [22].
The first aim of this study was to examine whether anodal tDCS to the left dorsolateral prefrontal cortex for five consecutive days improved cognitive function and reduced cognitive fatigue and fatigability in PD patients. We predicted that compared to the sham control group, the experimental group would show improved performance in complex attention, as measured by the STROOP task, and increased working memory, as measured by the Digit Span Test (Forward and Backward). We predicted that the treatment group would also show less fatigue, as measured by the Multidimensional Fatigue Inventory (MFI). We also predicted that the treatment group would show less fatigability compared to the sham group, as measured by performance across a challenging computer task (i.e., treatment group would show less worsening of reaction time or accuracy).
The second aim was to assess the short-term and long-term efficacy of the tDCS intervention. We assessed short-term effects by comparing the baseline measurements to those taken immediately after the final tDCS session on the 5th day (i.e., post-test). We assessed long-term effects by comparing baseline measurements to those taken at 1-week and 2-week follow-ups (i.e., 7 and 14 days after last tDCS session, respectively). We predicted that 5 consecutive sessions of atDCS targeting the l-DLPFC would induce both immediate and sustained improvements in cognitive function, as well as reductions in cognitive fatigue and fatigability in PD patients.
Materials and Methods Participants
We recruited PD patients from the Movement Disorders Clinic at the Sanford Brain and Spine Center in Fargo, North Dakota. All patients spoke fluent English and met clinical diagnosis of PD with at least two of the four diagnostic criteria for PD: tremor, rigidity, bradykinesia, and postural instability. All participants were responsive to levodopa and maintained their treatment regimen for the duration of the study. The following exclusion criteria were applied: (1) patients with dementia (MOCA score < 21), (2) psychosis, (3) presence of other neurological conditions such as multiple sclerosis, stroke, epilepsy, (4) internal electric stimulators such as deep brain stimulator, pacemaker, spinal cord stimulator, or (5) severe medical illnesses such as COPD, congestive heart failure, or renal failure. The study initially required participants to meet criteria for mild cognitive impairment (21 < MOCA < 26). The eligibility criterion pertaining to cognitive status was modified to include individuals with normal cognition in order to evaluate the effectiveness of tDCS on individuals who may be experiencing minimal or early cognitive changes.
This study was performed in accordance with the Declaration of Helsinki. This study was approved by the Sanford Health IRB, and we obtained informed consent from all participants. Participants were paid $300 for their participation in the study. The study is registered at the United States Registry of Clinical Trials with the reference ID NCT03191916.
Procedure
The study was a randomized, single-blind, placebo-controlled experiment to compare the two groups of PD participants. Research personnel randomly assigned participants to either the stimulation (“stim”) group or control (“sham”) group using a virtual coin flip simulator. The research team only was aware of the intervention assignment. Following a screening visit to determine eligibility, all consented participants completed the primary and secondary outcome measures and the first tDCS session on a Monday. Stimulation was administered for five consecutive days (MondayFriday). On the fifth day (Friday), and at the 1-week and 2-week follow-up visits, participants again completed all primary and secondary outcome measures. See Figure 1 for a flowchart of the study.
Figure 1: Study flow diagram
Transcranial Direct Current Stimulation
Transcranial direct current stimulation (MagStim HDCkit tDCS) was administered daily for five consecutive days. The current intensity was 2 mA and was applied for 20 minutes with a 30-second ramp up and ramp down period. The anode electrode was 25 cm2 and the cathode electrode was 51 cm2 and were placed at the left dorsolateral prefrontal cortex (F5 according to the 1020 international system for EEG electrode placement) and the right supraorbital area, respectively. The pads that surrounded the electrodes were soaked in a saline solution before each session. The sham group did not receive stimulation during the 20-minute session but had the same electrode placement and ramp-up and ramp-down period.
Risks of tDCS
Transcranial direct current stimulation is a safe procedure. It has been tested in thousands of subjects worldwide with no evidence of adverse effects to date [8]. Hundreds of studies have used tDCS in different clinical conditions without causing any adverse effects. To further examine the safety of tDCS specifically, [23] retrospectively reviewed adverse effects in 77 healthy subjects and 25 patients who underwent a total of 567 tDCS sessions at 1mA. They found that adverse effects caused by tDCS are not different from those of placebo stimulation, which include mild tingling sensations (75%), light itching sensations (30%), moderate fatigue (35%), and headache (11.8%). A recent article by [24] investigated the adverse events associated with repeated sessions of tDCS and found adverse events did not increase with higher levels of tDCS exposure. A comprehensive review of over 33,000 tDCS sessions found no evidence of serious adverse events [7]. Of the 20 participants in our study that received the tDCS intervention, 2 (10%) experienced a “slightly painful poking sensation” at the location of the anode electrode placement during the tDCS stimulation, 1 (5%) experienced a mild headache after the tDCS, and 15 (75%) felt mild tingling sensations during the tDCS. No noted effects were found in the sham group. The lab director and medical expert reviewed all events of reported adverse reactions.
All participants had the option to withdraw from the study.
Outcome Measurements Primary Outcome Measure: Computer Task
To investigate cognitive fatigability, we used a visual search computer task. The primary outcome measures were Reaction Time (RT) and Accuracy (hits minus false alarms; HFA). Stimuli were presented on a 15-inch monitor set to a refresh rate of 60 Hz. Stimuli were presented and performance was recorded using Presentation Software (Version 18.1, Neurobehavioral Systems, Inc., Berkley, CA). All stimuli were presented in black on a white background. Participants were seated 34 cm from the computer monitor, with the distance held constant with the use of a chin rest. Participants were instructed to respond by pressing designated buttons on a button box.
Figure 2 illustrates the basic trial sequence. A trial began with a black central fixation dot. After 500 ms, the dot is replaced by a search array consisting of 11 L’s in various orientations and 1 T. Participants located the “T” in the array of L’s. They then indicated the target orientation by pressing a key. If the vertical line of the “T” was on the left, participants were instructed to press the left button. Conversely, if the vertical line of the “T” was on the right, participants were instructed to press the right button. Participants were instructed to answer as quickly as possible, while limiting mistakes. The trial ends after the participant responds, or, if the participant fails to respond within 8,000 ms, the trial is terminated. Before the next trial begins, a white screen is presented for 1,000 ms.
Figure 2: Schematic of the visual search task. A blank screen is presented for 500 ms. A search array is presented in which the participant must discriminate the horizontal orientation of one “T’ amongst 11 “L’s” at different orientations. The trial ends upon participant response or after 8,000 ms and is followed by a 1,000 ms intertrial interval.
Each participant completed a practice block with 16 trials, and feedback was provided following each trial. During practice, the researcher monitored and provided additional instructions, if needed. Following the practice block, there were 40 test blocks with 24 trials per block. Each block was followed by a screen stating that participants could take an optional break; pressing the central button would initiate the start of the next block. Reaction time and error rate for each block were recorded, with cognitive fatigue measured as deterioration of reaction time (RT) or Accuracy (HFA) over the completion of the task (evaluated over 5 epochs of 8 blocks per epoch).
Secondary Outcome Measures
The secondary outcome measures included the Multidimensional Fatigue Inventory (MFI), Center for Epidemiological Studies Depression Scale (CES-D), Single Item McGill Quality of Life (QOL) Scale, Stroop Test A, B & C, and the Digit Span Test (Forward and Backward).
The Multidimensional Fatigue Inventory (MFI) is a 20-item self–report instrument that measures five dimensions of fatigue independently: general fatigue, physical fatigue, mental fatigue, reduced motivation, and reduced activity [25]. Each dimension score ranges from 4 to 20 (most severe). Our study examined the mental and physical fatigue domains.
The Center for Epidemiological Studies Depression Scale (CES-D) is a 20-question self-assessment that reports a general score of depression [26]. A score greater than 16 is indicative of a diagnosis of depression. Depression and fatigue have similar symptomology—lack of energy, tiredness, difficulty initiating and completing tasks—which highlights the necessity to evaluate possible comparative variables.
The Single Item McGill Quality of Life (QOL) Scale is a questionnaire that assesses a person’s subjective quality of life in consideration to the following areas of their life: physical, emotional, social, spiritual, and financial [27]. The scale ranges 1-10 with 10 indicating the highest QOL.
The Stroop Test A, B & C, is a measure of processing speed, attention, and inhibition [28]. For each sub-test, the participant reads the words aloud as quickly and accurately as possible for a 45-second duration. In Stroop A, the participant is required to read color words (Red, Green, or Blue) printed in black ink. In Stroop B, the participant is required to name ink colors out loud (text consists of the letter “x”). In Stroop C, the participants are required to name the color ink of opposing color words (e.g., the word Blue written in Red ink, with the correct response being “Red”). The scores are then standardized based on the participant’s age and education level. Ultimately, an Interference score is calculated to reflect cognitive flexibility and inhibitory control by effectively ignoring distracting information. A score of 40 or greater would demonstrate statistically normal cognitive flexibility and inhibitory control.
The Forward and Backward Digit Span Test assesses multiple areas of cognitive function. The Forward Digit Span is a measure of simple attention [29]. Participants repeat progressively longer series of numbers until the participant can no longer complete the task. The Backward Digit Span test is a measure of complex attention and working memory. The participant again repeats a series of numbers, except in reverse order from what the researcher says. Scores reflect the total points earned (one point for each correct line; ranges from 0-14) and the longest string of digits (LDS) completed successfully.
Data Analysis
At the time of the protocol design, there were few studies that had studied the effect of tDCS in PD. Therefore, sample size was determined based on the number of participants in the background studies. Previous literature had single samples with 8-16 participants [15,16]. In this comparative study, we aimed for twenty participants in each test group.
All statistical tests were performed using SPSS version 29 (IBM SPSS Statistics). The test results were considered significant at α = .05. Tests for qualitative variables were compared using the chisquare test, and tests for quantitative variables used a t- or 2-way ANOVA test (first testing for normality and equality of variance). Raw scores for each outcome were submitted to a 2 (Group: tDCS, Sham) x 2 (Visit) mixed ANOVA, with Group as the betweensubjects variable and Visit as the within-subjects variable. Based on the hypotheses of this study (short and long-term effects of tDCS), three two-way ANOVA tests were performed to make specific comparisons. Visit compared 1) baseline performance to post-visit (immediately after treatment on day five), 2) baseline to 1-week follow-up, and 3) baseline to 2-week follow-up. For the purposes of this study, we did not explore changes in participant performance between the three post-visits.
Results
A total of 43 PD participants were enrolled in this study from 2015 until 2025 (interruptions in recruitment and data collection due to personnel changes). The study was registered in 2017 due to initiators of the study being unaware of the requirement for prospective registration of all interventional trials. Six participants were enrolled during this period. We registered the trial as soon as we became aware of this policy. Four participants were excluded, three prior to administration of stimulation: two were not eligible due to a MOCA < 21, indicating potential dementia, and one voluntarily withdrew participation prior to the first day of testing and stimulation. One participant was withdrawn due to being unable to complete all tDCS sessions due to adverse weather. Enrollment was ended upon obtaining the proposed sample size, based on those who initiated the intervention. A total of 39 PD participants (tDCS: 20, sham: 19) completed the study and were included in the final analysis. Participant characteristics for the study are shown in Table 1.
|
Demographics (SD) |
Overall Sample (N= 39) |
tDCS Group (N=20) |
Sham Group (N=19) |
|
Gender, (% female) |
38% |
28% |
41% |
|
Age, years |
70.03 (5.9) |
70.3 (6.5) |
69.7 (5.4) |
|
Education |
15.51 (2.4) |
16.1 (2.0) |
14.87 (2.7) |
|
LEDD |
780.87 (419.5) |
804.4 (449.8) |
756.1 (395.8) |
|
PD Duration, years |
5.53 (4.2) |
5.59 (3.7) |
5.47 (4.5) |
|
H&Y Stage |
1.56 (0.6) |
1.55 (0.6) |
1.58 (0.6) |
|
MOCA |
27.03(2.1) |
27.05 (2.4) |
27.0 (1.6) |
|
Abbreviations: H&Y- Hoehn and Yahr; LEDD- Levodopa Equivalent Daily Dosage (Tomlinson et al, 2010); MOCA- Montreal Cognitive Assessment; SD- Standard Deviation. *Denotes a significant difference between the tDCS and Sham group *p ≤ .05 |
|||
Table 1: Characteristics of tDCS and Sham Parkinson’s disease participants
Computer Task
Trials with RTs less than 200 ms were excluded from the analysis. Participant responses under 200 ms on the discrimination task were considered anticipatory responses or inattention errors. Error trials were also removed from the analysis. Data for two participants were removed due to HFA rates below 0.5 for a visit, suggesting that they were performing at the same rate as guessing for the discrimination task. An additional participant’s data was removed for the 1-week follow-up only, as the error rate was below 0.5 for the visit. The slope of median RTs across each epoch (8 blocks per epoch) was calculated and submitted to a two-way mixed ANOVA. The slope was used to evaluate the change in fatigue across the task. A negative slope reflected hastening of speed and a positive slope indicated slowing in responses in a single computer task session. A main effect of Visit was found for the baseline to post-test comparison (p = .003), and baseline to 2-week follow-up comparison (p = .002). Both comparisons identified that compared to the baseline visit, at the follow-up visit all participants responded more quickly at the end of the computer task compared to the start. None of the Group x Visit interactions were significant (p > .05).
Accuracy was analyzed as hits (correct trials) minus false alarms (correct trials within the 200 ms window). The slope of mean accuracy rates were submitted to the two-way mixed ANOVA. A positive slope indicated participants were making more errors at the end of the computer task compared to the start. A negative slope indicated that participants were making fewer errors as they completed the computer task. A main effect of Visit was found for the baseline to 2-week follow-up comparison (p = .046), indicating that all participants showed improved accuracy from start-to-finish at the follow-up compared to baseline visit. No other main effects were found nor were any interactions significant (p > .05). Reaction time and Accuracy data are presented in Table 2.
Computer Task
|
Computer
Task (SD) |
Baseline |
Post-visit |
1-week
follow-up |
2-week
follow-up |
|||||||
|
|
tDCS |
Sham |
tDCS |
Sham |
η2p |
tDCS |
Sham |
η2p |
tDCS |
Sham |
η2p |
|
Reaction Time |
-38.92 (34.7) |
-22.97 (58.3) |
-7.26 (27.0) |
1.71 (46.8) |
.019 |
-11.76 (35.4) |
.98 (46.4) |
.016 |
-10.80 (35.8) |
4.57 (39.1) |
.00 |
|
Accuracy |
.005
(.01) |
.006
(.03) |
-.001
(.01) |
-.011
(.03) |
.019 |
-.001
(.00) |
-.002
(.00) |
.002 |
-.002
(.00) |
-.008
(.02) |
.014 |
|
Reaction time is reported as the median slope of
response time across the entire task (start to finish). Negative values
reflect improved performance as the task progressed, with greater improvement
as magnitude increases. Accuracy is reported as the mean slope of Hits minus
False Alarm rates across the entire task. Positive values reflect improved
performance as the task progressed, with greater improvement as magnitude
increases. Effect size ( η2p
) reported for Group x Visit (baseline to respective follow-up) interaction. *Denotes
a significant interaction between the tDCS and Sham group at the compared
visits; * p ≤ .05 |
|||||||||||
Table 2: Primary measures at baseline, post-visit, 1-week, and 2- week follow-up visits
Multidimensional Fatigue Inventory
The mental and physical dimensions of the MFI were tested in this experiment. For MFI-Mental, a main effect of Visit (p = .023) was found at the 2-week follow-up and a marginally significant effect was found at the 1-week follow-up (p = .051). For both findings, less mental fatigue was reported by all participants at the followup visit compared to the initial baseline visit. No other main effects or significant interactions were found for any of the comparisons (p > .05). For MFI-Physical, the only main effect was for Visit (p = .014), with less physical fatigue at the 2-week follow-up visit. No other main effects or significant interactions were found for any of the other immediate or long-term comparisons (p > .05).
Center for Epidemiological Studies Depression Scale
For the Center for Epidemiological Studies Depression Scale (CES-D), all participants showed immediate (p = .045) and longterm (1-week: p = .004; 2-week: p < .001) effects of reduced depression scores. There were no other main effects or significant interactions for any of the visit comparisons (p > .05).
Single Item McGill Quality of Life Scale
No main effects or significant interactions were found for the Single Item McGill Quality of Life (QOL) Scale for any of the immediate or long-term comparisons (p > .05).
STROOP
For more accurate comparisons between groups, the standardized T-scores for the Stroop Test were submitted to the mixed ANOVAs. No significant main effects or interactions were found for each of the immediate and long-term comparisons (p > .05). However, a marginal main effect was found for Visit at the immediate postvisit (p = .067), with slightly lower T-scores at the post-test.
Digit Span
The Digit Span Test produces four outcome scores: forward, backward, longest digit span forward (LDSF), and longest digit span backward (LDSB). The forward and backward scores account for performance in repeating the digit sequence as the digit length increases every other trial. The longest digit span indicates the highest number of digits the participant was able to repeat in one sequence. Figure 3 illustrates the observed patterns for the significant interactions.
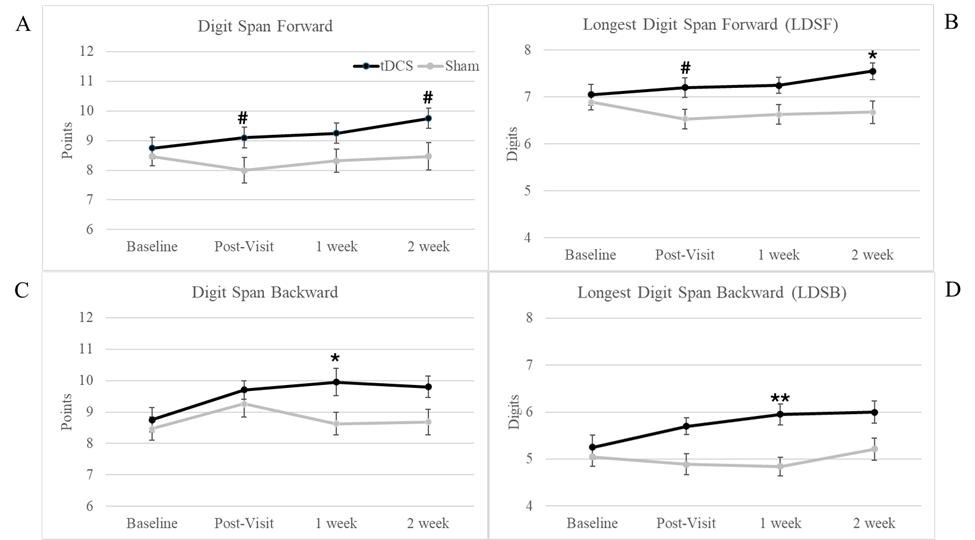
Figure 3: Digit Span Results. Significant and marginally significant Group x Visit interactions were found for the Digit Span test. (A) illustrates marginally significant interactions at the post-visit and 2-week follow-up visit, with the tDCS group. (B) illustrates the longest digit span recalled in forward order, with marginally significant interaction at the post-visit and a significant interaction at the 2-week follow-up. (C) depicts the Digit Span Backward, with a significant interaction at the 1-week follow-up. (D) depicts the Longest Digit Span Backward, which yielded a significant interaction at the 1-week follow-up visit only. For all significant and marginally significant interactions, the tDCS group showed greater improvements in performance compared to baseline than the Sham group. Error bars represent one standard error. *Denotes a significant interaction between the tDCS and Sham group at the compared visits * p ≤ .05, ** p ≤ .01, # marginal significance p ≤ .078
For the Digit Span Forward, no main effects or significant interactions were found (p > .05). However, two marginally significant Group x Visit interactions were found at the immediate and 2-week follow-up visits (both p = .076). Both interactions indicate the sham group performed the same or slightly worse at the follow-up visits whereas the tDCS group always showed improvement. For the LDSF, a significant Group x Visit interaction was found at the 2-week follow-up (p = .04) and marginally significant (p = .076) at the immediate post-visit. The same interaction pattern existed with the Digit Span Forward, with only the tDCS group showing improvement at the follow-up visits. No other main effects or interactions were significant (p > .05).
For the Digit Span Backward, a main effect of Visit was found at each immediate or long-term comparison (p < .05), with improved performance at the follow-up visit. A significant Group x Visit interaction was also found at the 1-week follow-up (p = .021), with the tDCS group showing greater improvement (1.2 average increase in score) compared to the Sham group (0.16 average increase in score). For the LDSB, a significant Group x Visit interaction was found for the 1-week follow-up (p = .006), with the Sham group worsening at the follow-up while the tDCS group improved. The only other significant finding was a main effect of Visit at the 2-week follow-up, in which all participants showed improvement in the number of digits recalled in backward order. Figure 3 illustrates the patterns observed for each of the Digit Span tests across the visits. Data for all secondary measures are presented in Table 3.
|
Assessment
(SD) |
Baseline |
Post-visit |
1-week
follow-up |
2-week
follow-up |
|||||||
|
|
tDCS |
Sham |
tDCS |
Sham |
η2p |
tDCS |
Sham |
η2p |
tDCS |
Sham |
η2p |
|
QOL |
7.6
(2.2) |
7.84
(1.7) |
8.10
(1.7) |
8.32
(1.4) |
.00 |
7.9
(1.9) |
7.79
(2.0) |
.005 |
8.4
(1.2) |
8.05
(2.3) |
.026 |
|
CESD |
11.05
(8.5) |
9.37
(9.2) |
8.65
(7.4) |
8.11
(9.0) |
.011 |
8.5
(7.7) |
6.63
(7.6) |
.00 |
8.1
(7.2) |
5.53
(6.7) |
.006 |
|
MFI Mental |
10.0
(4.0) |
9.0
(3.5) |
8.79
(3.4) |
8.79
(3.2) |
.033 |
8.63
(3.4) |
8.42
(3.7) |
.018 |
8.79
(3.2) |
8.16
(3.8) |
.005 |
|
MFI Physical |
11.42
(4.7) |
9.89
(4.0) |
11.53
(4.1) |
10.0
(3.9) |
.00 |
11.11
(3.9) |
9.47
(3.5) |
.00 |
10.0
(4.5) |
9.05
(3.7) |
.012 |
|
Digit Span Forward |
8.75
(2.3) |
8.47
(2.0) |
9.10
(2.2)# |
8.0
(2.7) |
.083 |
9.25
(2.1) |
8.32
(2.4) |
.054 |
9.75
(2.1)# |
8.47
(2.9) |
.081 |
|
LDSF |
7.05
(1.4) |
6.89
(1.0) |
7.20
(1.3)# |
6.53
(1.3) |
.083 |
7.25
(1.1) |
6.63
(1.3) |
.044 |
7.55
(1.1)* |
6.68
(1.5) |
.109 |
|
Digit Span Backward |
8.75
(2.4) |
8.47
(2.3) |
9.70
(1.8) |
9.26
(2.6) |
.002 |
9.95
(2.7)* |
8.63
(2.2) |
.135 |
9.8
(2.1) |
8.68
(2.5) |
.075 |
|
LDSB |
5.25
(1.6) |
5.05
(1.3) |
5.70
(1.1) |
4.89
(1.4) |
.068 |
5.95
(1.4)** |
4.84
(1.2) |
.186 |
6.0
(1.5) |
5.21
(1.5) |
.043 |
|
Stroop Test |
49.65
(6.6) |
48.05
(8.1) |
48.55
(4.7) |
45.58
(6.8) |
.014 |
49.50
(4.4) |
46.21
(7.6) |
.015 |
49.50
(8.3) |
48.89
(10.1) |
.004 |
|
Effect
size ( η2p ) reported for Group x Visit (baseline to
respective follow-up) interaction. Abbreviations: QOL- Single Item McGill
Quality of Life; CESD- Center for Epidemiological Studies Depression;
MFI-Multidimensional Fatigue Inventory; LDS- Longest Digit Span *Denotes
a significant interaction between the tDCS and Sham group at the compared
visits * p ≤ .05 **
p ≤ .01 #marginal
significance p ≤ .078 |
|||||||||||
Discussion
The use of tDCS for cognitive enhancements in neurological conditions has been rapidly evolving due to its ability to induce long-term neuroplastic changes. In the current study, we hypothesized that five consecutive days of tDCS at 2 mA to the l-DLPFC could lead to short- and long-term improvements in cognitive fatigue, fatigability, and cognitive function. Our findings provided support for tDCS significantly improving aspects of topdown attention, as measured using a working memory task (digit span). However, we did not find evidence of tDCS significantly reducing cognitive fatigue or fatigability nor improvement in other cognitive domains.
Working memory engages several components of attention, with the requirement to selectively attend to relevant information, maintain the task-relevant items, and then retrieve the information [13]. In everyday life, working memory plays a role in learning new information, following instructions, decision making, and comprehension. Each of these functions significantly impact patient quality of life. The task used to measure working memory, the digit span, comprised of two parts. The forward span is relatively simpler and can evaluate one’s ability to hold auditory information for a short period of time. The backward span is more challenging in that it builds on the forward task and also requires manipulation of the information by reversing the order of the digits during retrieval. Our study found significant interactions, suggesting tDCS improves functioning, immediately following 5 consecutive days of tDCS and at the 2-week follow-up for the Digit Span Forward and only at the 1-week follow-up for the Digit Span Backward. This suggests that there may be limitations to the benefit of tDCS on working memory, but improvements can be observed up to two weeks. These patterns support previous evidence of long-term improvement in working memory due to tDCS [14,15].
We did not expect to find that tDCS would not improve other areas of cognition. However, [15] also reported unexpected findings, including declines in long-term memory performance in their tDCS group. One of the strongest arguments proposed by Biundo is that altered network function, resulting from Parkinson’s disease, affects the effectiveness of tDCS. Reduced intra- and inter-network connectivity has been found in many areas, including the default mode network and executive control network, which are highly involved in working memory processes [30]. In addition to the potential that tDCS is resulting in a non-uniform impact on the l-DLPFC and involved networks, our results may also be impacted by the level of difficulty of the task involved. Although we selected cognitive tasks that we felt were demanding, other studies have noted that the complexity of the task may impact how regions of the brain are engaged and therefore affect the level of excitability [31].
The effect of tDCS on PD-related cognitive fatigue has been understudied. Much of the research that has been conducted has explored the effect of tDCS in different neurological conditions, targeted physical fatigue and fatigability, and applied tDCS to the motor cortex [32]. One study has observed a significant improvement in subjective fatigue, measured using the Fatigue Severity Scale, when tDCS was applied to the DLPFC for 8 sessions during a 2-week period [22]. Benefits of tDCS were observed up to three months [33] found that ten sessions of tDCS to the DLPFC yielded minor decreases in subjective fatigue in PD patients but the effects were not significant.
Limitation
A limitation of the current study was that the sample consisted of people with PD who had relatively high cognitive function and early/mild PD. It may be noted that most of the participants had normal MOCA scores, relatively normal STROOP interference scores, and low error rates on the computer task, suggesting we may have observed a ceiling effect with little room for improvement. It would be important to consider limitations of tDCS in certain subgroups of PD patients. Additionally, we observed numerous main effects of Visit in our analyses, suggesting that all participants— both in the stimulation and sham groups—showed relative improvement on various tasks due to a learning effect or other reasons due to the design of the study (i.e., potential that improved QOL and depression scores were due to regular in-person visits).
Future Directions
Considering future directions, there is a growing body of literature showing the synergistic effect of tDCS with other therapies. [14] showed that cognitive training combined with tDCS may enhance the effects of tDCS on neuronal plasticity. A recent study [34] is one of the first studies to show a significant effect of tDCS combined with Tai Chi for improving cognitive function. This highlights the need to consider multiple therapies when researching and maximizing the benefits of utilizing tDCS to enhance cognitive function and achieve other research objectives.
Conclusion
This study was conducted to evaluate the immediate and long-term effects of tDCS on cognitive function and cognitive fatigue and fatigability in PD patients, as well as contribute to finding the best parameters for tDCS intervention. Our findings supported that five consecutive sessions of tDCS at 2 mA to the l-DLPFC can yield significant improvements to working memory up to two weeks, with limited benefits to other areas of cognition and fatigue.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author Contributions
JSL contributed to the conception and design of the study. JK, AH, KR, and HR enrolled participants. AH and JK collected data. AH and JK ran the statistical analysis and interpreted the data. JK wrote the original draft of the manuscript and AH contributed to writing and editing of the manuscript. All authors reviewed and approved the submitted version of the manuscript.
Funding
This research was supported by the Peltier Family Grant to the Parkinson’s disease Research Lab at Sanford Health in Fargo, North Dakota.
References
- Armstrong M, Okun M (2020) Diagnosis and treatment of Parkinson Disease: a review. JAMA. 323: 548-560.
- Dorsey E, Sherer T, Okun M, Bloem B (2018) The emerging evidence of the Parkinson pandemic. J Parkinsons Dis. 8: S3-S8.
- Jankovic J (2008) Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 79:368-376.
- Chaudhuri K, Prieto-Jurcynska C, Naidu Y, Mitra T, Frades-Payo B, et al. (2010) The nondeclaration of nonmotor symptoms of Parkinson’s disease to health care professionals: An international study using the nonmotor symptoms questionnaire. Mov Disord. 25:704–709.
- Fonteneau C, Redoute J, Haesebaert F, Le Bars D, Costes N, et al. (2018) Frontal transcranial direct current stimulation induces dopamine release in the ventral striatum in human. Cereb Cortex. 28: 2636-2646.
- Nieoullon A (2002) Dopamine and the regulation of cognition and attention. Prog Neurobiol. 67:53-83.
- Bikson M, Grossman P, Thomas C, Zannou AL, Jiang J, et al. (2016) Safety of transcranial direct current stimulation: evidence based update 2016. Brain Stimul. 9: 641-661.
- Brunoni AR, Amadera J, Berbel B, Volz MS, Rizzerio BG, et al. (2011) A systematic review on reporting and assessment of adverse effects associated with transcranial direct current stimulation. Int J Neuropsychopharmacol. 14:1133-1145.
- Liebetanz D, Nitsche MA, Tergau F, Paulus W (2002) Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain. 125: 2238-2247.
- Schönberger AR, Hagelweide K, Pelzer EA, Fink GR, Schubotz RI (2015) Motor loop dysfunction causes impaired cognitive sequencing in patients suffering from Parkinson’s disease. Neuropsychologia. 77, 409-20.
- Watson GS, Leverenz JB (2010) Profile of cognitive impairment in Parkinson’s disease. Brain Pathol. 20: 640-645.
- Zlotnik G, Vansintjan A (2019) Memory: an extended definition. Front Psychol. 7: 2523.
- Cowan N (2014) Working memory underpins cognitive development, learning, and education. Educ Psychol Rev. 26: 197-223.
- Lawrence BJ, Gasson N, Johnson AR, Booth L, Loftus AM (2018) Cognitive training and transcranial direct current stimulation for mild cognitive impairment in Parkinson’s disease: a randomized controlled trial. Parkinsons Dis. 2018:4318475.
- Biundo R, Weis L, Fiorenzato E, Gentile G, Giglio M, et al. (2015) Double-blind randomized trial of tdcs versus sham in Parkinson patients with mild cognitive impairment receiving cognitive training. Brain Stimul. 8: 1223-1225.
- Boggio PS, Ferrucci R, Rigonatti SP, Covre P, Nitsche M, et al. (2006) Effects of transcranial direct current stimulation on working memory in patients with Parkinson’s disease. J Neurol Sci. 249: 31-8.
- Friedman JH, Brown RG, Comella C, Garber CE, Krupp LB, et al. (2007) Working group on fatigue in Parkinson’s disease. Fatigue in Parkinson’s disease: a review. Mov Disord. 22: 297-308.
- Kostić VS, Tomić A, Ječmenica-Lukić M (2016) The pathophysiology of fatigue in parkinson’s disease and its pragmatic management. Mov Disord Clin Pract. 3:323-330.
- Lou JS (2009) Physical and mental fatigue in Parkinson’s disease: epidemiology, pathophysiology and treatment. Drugs Aging. 26:195-208.
- Chalah MA, Riachi N, Ahdab R, Mhalla A, Abdellaoui M, et al. (2017) Effects of left DLPFC versus right PPC tDCS on multiple sclerosis fatigue. J Neurol Sci. 372, 131-137.
- Ferrucci R, Vergari M, Cogiamanian F, Bocci T, Ciocca M, et al. (2014) Transcranial direct current stimulation (tDCS) for fatigue in multiple sclerosis. NeuroRehabilitation. 34:121-127.
- Forogh B, Rafiei M, Arbabi A, Motamed MR, Madani SP, et al. (2017) Repeated sessions of transcranial direct current stimulation evaluation on fatigue and daytime sleepiness in Parkinson’s disease. Neurol Sci. 38:249-254.
- Poreisz C, Boros K, Antal A, Paulus W (2007) Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients. Brain Res Bull. 72: 208-214.
- Nikolin S, Huggins C, Martin D, Alonzo A, Loo CK (2018) Safety of repeated sessions of transcranial direct current stimulation: A systematic review. Brain Stimul. 11: 278-288.
- Smets EM, Garssen B, Bonke B, De Haes JC (1995) The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 39: 315-325.
- Radloff LS (1977) The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1:385-401.
- Robin Cohen S, Mount BM, Strobel MG, Bui F (1995) The McGill quality of life questionnaire: a measure of quality of life appropriate for people with advanced disease. A preliminary study of validity and acceptability. J Palliat Med. 9:207-219.
- Scarpina F, Tagini S (2017) The Stroop color and word test. Front Psychol. 8:557.
- Cullum CM, Larrabee GJ (2010) WAIS-IV Use in Neuropsychological Assessment. In WAIS-IV Clinical Use and Interpretation. (pp. 167187). Elsevier Inc.
- Li J, Tan C, Zhang L, Cai S, Shen Q, et al. (2023) Neural functional network of early Parkinson’s disease based on independent component analysis. Cereb Cortex. 33:11025-11035.
- Olma MC, Dargie RA, Behrens JR, Kraft A, Irlbacher K, et al. (2013) Long-term effects of serial anodal tDCS on motion perception in subjects with occipital stroke measured in the unaffected visual hemifield. Front hum neurosci. 24: 314.
- Zaehle T (2021) Frontal transcranial direct current stimulation as a potential treatment of Parkinson’s disease-related fatigue. Brain sciences. 11: 467.
- Dobbs B, Pawlak N, Biagioni M, Agarwal S, Shaw M, et al. (2018) Generalizing remotely supervised transcranial direct current stimulation (tDCS): feasibility and benefit in Parkinson’s disease. J Neuroeng Rehabil. 15:114.
- Xu Y, Zhu J, Liu H, Qiu Z, Wu M, et al. (2023) Effects of Tai Chi combined with tDCS on cognitive function in patients with MCI: a randomized controlled trial. Front Public Health. 11:1199246.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.