Sulforaphanes for Secondary Chemoprevention of Non-Muscle-Invasive Bladder Cancer
by Nagi B. Kumar1,2*, Mark Alexandrow3
1Cancer Epidemiology Program Moffitt Cancer Center and Research Institute, Tampa, FL 33612, USA
2Genitourinary Oncology Moffitt Cancer Center and Research Institute, Tampa, FL 33612, USA
3Molecular Biology Moffitt Cancer Center and Research Institute, Tampa, FL 33612, USA
*Corresponding author: Nagi B Kumar, Cancer Epidemiology Program Moffitt Cancer Center and Research Institute, Tampa, FL 33612, USA
Received Date: 13 October 2025
Accepted Date: 20 October 2025
Published Date: 22 October 2025
Citation: Kumar NB, Alexandrow M (2025) Sulforaphanes for Secondary Chemoprevention of Non-Muscle-Invasive Bladder Cancer. J Urol Ren Dis 10: 1438. https://doi.org/10.29011/2575-7903.001438
Abstract
In 2025, it is estimated that in the United States about 84,870 new cases of Bladder Cancer (BC) will be diagnosed and 17,420 deaths will result from BC. Among the newly diagnosed cases, 70-75% will be Non-Muscle Invasive Bladder Cancers (NMIBC), typically treated with cystoscopic Transurethral Resection (TUR) combined with intravesical therapy and placed on surveillance. However, NMIBC continues to be characterized by significant patient burden due to numerous recurrences and disease progression, requiring frequent cystoscopies, intravesical drug therapies and/or surgery. With the potential to optimize local exposure of promising agents to the bladder urothelium and the availability of BC cells obtained from periodical cystoscopies during surveillance that can be used to evaluate efficacy of interventions, these closely monitored patients represent an ideal cohort for the evaluation of chemopreventive agents. Currently, other than smoking cessation, there is a paucity of research that systematically examines specific agents relevant for secondary chemoprevention of BC. More recently, several epidemiological, in vitro, preclinical and early phase trials, including our preliminary studies, have shown that Sulforaphanes (SFN) are potent inhibitors of BC carcinogenesis. The goal of the current review is to provide a comprehensive review of these early findings that establishes the rationale to evaluate sulforaphanes as promising agents for secondary chemoprevention for non-muscle invasive bladder cancer.
Introduction
Bladder Cance
In 2025, it is estimated that in the United States about 84,870 new cases of Bladder Cancer (BC) will be diagnosed and 17,420 deaths will result from BC [1]. Among the newly diagnosed cases, 7075% will be Non-Muscle Invasive Bladder Cancers (NMIBC), typically treated with cystoscopic Transurethral Resection (TUR) combined with intravesical therapy and placed on surveillance [1-5]. NMIBC is typically treated with endoscopic Transurethral Resection (TUR), combined with intra-vesical therapy with Bacillus Calmette-Guerin (BCG) or chemotherapeutic agents (e.g., mitomycin C), delivered via a urethral catheter, to prevent or delay recurrence after TUR [3,4-7]. Risk of recurrence is 5060% for grades 1 and 2 tumors and 80% for grade 3 tumors, with a median time to first recurrence of 2.7 years [5]. A dramatic decline in survival is reported as cancer progresses from NMIBC to MIBC [6-8]. Given the high risk of recurrence and disease progression specifically in T0 and T1 NMIBC, careful surveillance with periodic cystoscopy is currently the standard clinical practice. With the potential to optimize local exposure of promising agents to the bladder urothelium and the availability of BC cells obtained from periodical cystoscopies during surveillance that can be used to evaluate efficacy of interventions, these closely monitored patients represent an ideal cohort for the evaluation of chemopreventive agents. Currently, other than smoking cessation, there is a paucity of research that systematically examines specific agents relevant for secondary chemoprevention of BC [9-11]. Secondary Chemoprevention is a prevention strategy that focuses on individuals who have been diagnosed with cancer that may progress to invasive cancer. This strategy aims to limit the development and progression of malignant lesions to metastatic cancer [12].
Current Strategies for BC Chemoprevention
Primary and secondary chemoprevention strategies in BC have primarily relied upon smoking cessation since cigarette smoking (mainly exposure to aromatic amines) accounts for 50% of BCs [13]. Non-tobacco related occupational exposure to amines, 4-aminobiphenyl & anilines (10% of all cases), as well as phenacetin derived analgesics have also been known to contribute to the etiology of BC [13]. Others have focused on vitamin and minerals including selenium, vitamin C, Vitamin B6 and Vitamin E. However, the majority of these studies failed to identify promising agents for primary or secondary chemoprevention of BC. Other chemopreventive efforts have explored the role of selective COX2 inhibitors suggesting a potential correlation between COX-2 expression and prognosis [14,15]. In subgroup analysis of a phase III clinical trial in NMIBC patients treated with standard therapy and celecoxib suggested that time to recurrence was longer in pT1 NMIBC patients treated with celecoxib compared with those receiving placebo. However, the increased risk of cardiovascular events has limited clinical translation [14]. Additionally, research is being conducted with erlotinib, the highly selective, reversible inhibitor of epidermal growth factor receptor (HER1/EGFR) tyrosine kinase which is overexpressed in more than 75% of BCs [16]. A phase II clinical trial involving neoadjuvant administration of erlotinib in patients before undergoing radical cystectomy showed a complete response rate in 25% of patients. However, substantial skin toxicity was noted, especially in patients who experienced complete response [17]. A phase IIa randomized multi-institutional trial (NCT02169284) is ongoing to investigate the role of erlotinib as well as with genistein [18] in pre-surgical Radical Cystectomy (RC) or TURBT patients. However, to date, there is paucity of evidence of any one agent that has been found to be effective for primary or secondary chemoprevention of BC. The goal of the current review is to provide the rationale and report early findings that establishes the need to evaluate sulforaphanes as promising agents for secondary chemoprevention for non-muscle invasive bladder cancer [19-22].
Sulforaphanes as a Promising Agent for Chemoprevention of BC
Several epidemiological, in vitro, preclinical, and early phase trials have shown that the phytochemicals, isothiocyanates (ITCs), specifically allyl isothiocyanate and SFN present in Brassicaceae or- “cruciferous” vegetables as their precursor glucosinolates – sinigrin, glucotropaeolin, gluconasturtiin and glucoraphanin [2-23] respectively may have specific effects at different stages of tumor progression. SFN,(-)-1-isothiocyanato-(4R)- (methylsulfinyl) butane CH3-SO-(CH2)4-NCS is an isothiocyanate found in high concentrations in broccoli sprouts, first isolated and shown as a potent anti-carcinogenic agent in 1992 by Zhang, et al [24]. Broccoli accumulates significant amounts of the phytonutrient glucoraphanin (4-methylsulfinylbutyl glucosinolate), which is metabolized in vivo to the biologically active SFN. This conversion requires myrosinanse, which is present in the plant as well as the gastrointestinal tract [21].
Evidence from Epidemiological studies
Epidemiological studies have shown the potential role of increased fluid intake and consumption of cruciferous vegetables, particularly broccoli in reducing risk of BC [25-27]. In a large prospective study, 39% reduction in BC risk was observed with an intake of 2 servings or more of broccoli compared to <1 serving per week (p=0.0009) [26]. In a prospective population study of nearly
50,000 men, cruciferous vegetable intake was shown to reduce BC risk [27-29]. In a case control study, a significant inverse association was observed between mortality from BC and broccoli intake. Additionally, a significant reduction of disease specific death (57% reduction) and overall mortality (43% reduction) was also observed [23]. In another case control study, [22] a reduced risk of BC was observed when raw cruciferous vegetables were consumed. In a meta-analysis of population studies reported to date, cruciferous vegetable intake has been demonstrated to be chemopreventive for BC [30]. However, other studies have failed to observe these protective effects. In an epidemiological study with a 10 year follow up, no association between cruciferous vegetable intake and BC was observed. However, the result was based on a one-time data collection [19] at baseline and a 10 year follow up. In another pooled analysis, higher intakes of total and non-starchy vegetable was associated with reduced risk of bladder cancer for women but not in men [31]. In a meta-analysis [32], the highest cruciferous vegetables intake was not significantly associated with a lower risk of bladder cancer, compared with the lowest cruciferous vegetables intake category. Taken together, the epidemiological evidence pertaining to consumption of CV and BC risk is mixed due to significant variations in research designs, methods used to quantify CV consumption, including CV storage and preparation methods. Abbaoui et al notes that the method of consumption of CV can significantly change the amount of isothiocyonates and other bioactives an individual is exposed to [33-39]. For example, quantities of isothiocyanates in vegetables are significantly reduced by cooking and storage processes [29,30]. Therefore, the amount of isothiocyanates an individual consumes through CV intake via food intake may not be enough to ever reach therapeutic levels to protect against BC. On the other hand, standardized, stable formulations of SFN-rich BSE may be ideal to use in early phase chemoprevention trials to determine this association.
Evidence from in vitro studies
In vitro studies in BC, [28,30-33] breast [31] lung, [35] prostate, [36,37] colorectal [38] and leukemia cells lines [39] have shown SFN to be a potent inhibitor of carcinogenesis through several molecular mechanisms [20]. We recently reported the identification of the first inhibitors (called CMGi/MCMi) of the CMG helicase (Cdc45-MCM-GINS) that block ATPase function of the MCM core and cause loss of the MCM/CMG proteins from DNA/chromatin [33]. The CMG is required for DNA replication, recovery from replicative stresses, and maintaining genome stability [34]. Loss of CMGs from chromatin by CMGi causes increased DNA damage, apoptosis, and loss of viability selectively in tumor cells driven by mutant-Ras (K- or H-Ras) [33]. Mutant Ras alleles are known to shorten G1 length (via Cyclin E elevation downstream), which prevents complete loading of all required MCM/CMG complexes onto DNA necessary for a healthy S-phase [35], creating a CMG helicase vulnerability in tumor cells relative to non-tumor cells [34]. This is compounded by the fact that loss of p53 function (in tumor cells) is synthetically lethal if MCMs are simultaneously reduced on chromatin [34]. Thus, our results indicate that compounds/drugs that further reduce MCM/CMG function (presence on DNA) in tumor cells will cause selective loss of tumor viability, a novel anti-cancer approach in the future clinic [33].
NMIBC are often driven by mutant H-Ras, acquire p53 mutations/LOF as BC progresses [3], and SFN exposure to tumor cells (including BC) is known to cause loss of viability and DNA damage [33-35]. Intriguingly, the target of SFN, Keap1, is known to bind not only NRF2 (discussed below) but also to the MCM3 subunit of the CMG helicase 55 . Binding of Keap1 to the CMG does not degrade the CMG but instead appears to be involved in proper CMG function in some unknown manner [36]. Based on these observations, we predict that the CMG might be a vulnerability in BC [33-35], and that SFN might (at least indirectly) target the CMG and further reduce its function in some way in BC treated with SFN. Such SFN effects on the CMG/MCM could derive from SFN-Keap1 interactions, which may abrogate an important function of Keap1 in maintaining/allowing CMG function on DNA, as suggested by others [38]. Clearly, such a mechanism for potential SFN inhibitory effects on the CMG will require further validation. SFN-Keap1 interaction brings up another question: what is the fate of NRF2 in BC treated with SFN? To date, there are no reports of the action of SFN on Nrf2 in BC cells. Keap1 normally ubiquitinylates NRF2, leading to Nrf2 degradation in cells.
SFN-Keap1 interactions abrogate this, allowing NRF2 accumulation and activation of NRF2 transcriptional programs involved suppressing oxidative damage. SFN/NRF2 are potent inducers of phase I/II detox enzymes (AKR1B10, NQO1) and antioxidant proteins (GPX2). As such, SFN treatment typically causes NRF2 gain and cytoprotection (protection against carcinogenesis, mutagenesis, and other forms of toxicity of electrophiles and reactive forms of oxygen [39-41]. Studies have shown that the Nrf2 activation by SFN in the bladder occurred primarily in the epithelium, which is the primary area for BC development [42]. Thus, activating Nrf2 by SFN might be a means to prevent cancer initiation in non-tumor tissue [43]. However, in other cancer models, SFN activated Nrf2 in normal cells, but not in cancer cells [44,45]. Such opposing results, taken together, suggest SFN effects on precancerous or non-cancerous cell types would be cytoprotective against tumor formation, while SFN effects on tumor cells would not be cytoprotective due to poor NRF2 induction by SFN.As another anti-BC mechanism, SFN has been shown to prevent BC progression by downregulating NF-kB, resulting in induction of cell cycle arrest and apoptosis, [38] while selectively targeting abnormal/malignant cells [46,47] compared to normal bladder cells [28] for these NF-kB effects. It has been shown that the NF-κB pathway is critical in ROS related pro-tumorigenic effects in BC [48,49], and NF-κB signaling correlates with aggressive BC behavior and poor clinical outcomes [50]. BC is also highly associated with inflammation where hydrogen peroxide, cytokines (Il 1-alpha, Il-6 and TNF-alpha), pro-inflammatory factors (Il 8, Il18, Cox-2, PGE2), as well as some chemokines, accumulate during bladder carcinogenesis [51,52]. SFN has been shown to inhibit such inflammatory responses, including downregulation of Cox-2 and reduction of PGE2 levels [51,52]. In a preliminary pilot study, we identified 20 high-grade NMIBC patients who had progressed from NMIBC and those who remained stable during surveillance, admitted and treated at our Cancer Center and performed RNA sequencing of the BC tissue to contrast differentially expressed genes (DEGs) between the two groups. RNA sequencing revealed a higher expression of genes associated with TNF-α signaling via NF-κB in the recurrent tumors compared to non-recurrent tumors [53-74]. The ability of SFN to suppress NF-kB and inflammatory responses, which promote BC aggressiveness, supports SFN use as a novel therapeutic agent in BC intervention in future trials.
Evidence from Preclinical studies
Preclinical evidence using animal models have demonstrated bioavailability of SFN with metabolites distributed to all tissues, including the bladder, suggesting the potential for systemic benefits [39-53,54]. Administration of a freeze-dried aqueous extract of broccoli sprouts to Sprague-Dawley rats significantly and dosedependently inhibited BC development induced by N-butyl-N-(4hydroxybutyl) nitrosamine [54]. The incidence, multiplicity, size, and progression of BC were all inhibited by the extract, while the extract itself caused no histologic changes in the bladder. Moreover, the inhibition of bladder carcinogenesis by the extract was associated with significant induction of phase II detoxification enzymes in the bladder. Over 70% of the isothiocyanates present in the extract were excreted in the urine as isothiocyanate equivalents (isothiocyanates + dithiocarbamates) in 12 h after a single p.o. dose, indicating high bioavailability and rapid urinary excretion. Urinary concentrations in extract-treated rats were 2 to 3 orders of magnitude higher than those in plasma, indicating that the bladder epithelium, the major site of bladder carcinogenesis, is most exposed to p.o. dosed isothiocyanate.
In a murine UMUC3 xenograft model, semi purified diets containing 4% broccoli sprouts, or 2% broccoli sprout isothiocyanate extract or gavaged pure SFN or erucin (each at 295 μmol/kg, similar to dietary exposure) produced tumor weight reduction of 42% (p = 0.02), 42% (p = 0.04), 33% (p = 0.04), and 58% (p < 0.0001), respectively. SFN and erucin metabolites were present in mouse plasma (micromolar range) and tumor tissue, with N-acetylcysteine conjugates as the most abundant [39]. Other preclinical trials have demonstrated positive results using murine models with SFN for breast, [55] skin, [56] and pancreatic [57] cancers. In preclinical models, SFN metabolites were detected systemically in tissues including the small intestine, prostate, kidney and lung, bladder, as well as, in bladder tumor tissue [39,53,57]. Evidence from preclinical studies demonstrates that SFN delivered orally are selectively delivered to bladder via urinary excretion, in contrast to currently available intravesical agents that are delivered via urethral catheter. Additionally, sustained urinary storage in the bladder in humans (unlike in rodents that are prone to frequent urination) may facilitate the disassociation of SFN metabolites and increase the exposure of BC cells to the SFN in urine [28]. These preclinical data provide robust evidence of organ-specific bioavailability and effectiveness of SFN in modulating bladder carcinogenesis and carcinogenesis at other sites, ideal to be tested for BC chemoprevention.
Evidence from Clinical Trials
Several clinical trials have been conducted to evaluate the effectiveness of SFN for chemoprevention, most of which have investigated bioavailability in healthy, disease-free subjects [1859,60-66]. The method of ingestion in these clinical trials has varied from pure SFN, broccoli soups/pill forms, and broccoli as a food item. Glucoraphanin (GRR) in broccoli is converted to SFN either by plant myrosinases, or if the plant myrosinases have been denatured by cooking, by bacterial myrosinases in the human colon. SFN is passively absorbed and rapidly conjugated with glutathione by Glutathione S-Transferases (GSTs), then metabolized sequentially by γ-Glutamyl-Transpeptidase (GTP), Cysteinyl-Glycinease (GCase) and N-Acetyltransferase (NAT). The conjugates are actively transported into the systemic circulation where the merapturic acid and its precursors are urinary excretion products. Deconjugation may also occur to yield the parent isothiocyanate, SFN. The mercapturic acid and cysteine conjugate forms are the major urinary metabolites of SFN. In interventions with glucosinolate-containing brussel sprouts for 1-3 weeks, increased GST enzyme activity with increased GST-alpha induction in plasma and tissues such as rectum, liver and small intestine [41,50,61,67]. Bioavailability, as measured by urinary excretion of SFN and its metabolites (in approximately 12-hour collections after dosing), was substantially greater with the SFNrich (mean = 70%) than with GRR-rich (mean = 5%) beverages. Inter-individual variability in excretion was considerably lower with SFR than with GRR beverage [68]. These studies have also corroborated the critical role of myrosinanse in metabolizing SFN in patients taking food sources vs. extract of SFN without myrosinanse had four times elevated urinary concentration [67]. Based on the bioavailability data, early clinical trials with SFN have focused on prostate cancer [60,65,68,69] and breast cancer chemoprevention [70]. In a study evaluating 60 mg (340 μmol Prostaphane®) vs. placebo for 12 months in men with biochemical recurrence of prostate cancer, a reduction in serum PSA was observed in 8/20 (40%) of prostate cancer patients in the treatment arm with no toxicities. Targeting a similar population with 200 μmol SFN daily for 20 weeks, Alumkal et al., [68] reported that 1 of 20 patients had a 50% decline in serum PSA at 5 months. Using 400 g broccoli/week versus 400g peas/week targeting men with High-Grade Prostate Intraepithelial Neoplasia) (HGPIN) for 6 months, Traka, et al., [65] showed significant changes in TGFβ, Insulin signaling and EGF receptor pathways. In a recent randomized, placebo controlled clinical trial among 98 men scheduled for a prostate biopsy, a significantly higher level of SFN and metabolites were found in urine and plasma after being treated with broccoli sprout extract (200 μmol daily for 4-8 weeks) when compared to placebo [71]. A recent double blinded randomized trial in women scheduled for breast cancer surgery compared SFN(Glucoraphanin (30 mg GFN BroccoMax™) vs.
Placebo, [70] administered for 2-8 weeks prior to women undergoing breast biopsy, reported bioavailability (urinary metabolites and plasma) and safety. Comparing pre- and post-treatment levels within each treatment group, Ki-67 (P = 0.003) and HDAC3 (P = 0.044) levels significantly decreased in benign tissues, but not in the invasive ductal carcinoma tissue [70]. Non-resectable pancreatic ductal adenocarcinoma patients receiving palliative chemotherapy, who were randomized to receive 90 mg (508 μmol SFN) Dieers Broccoraphan® daily for up to one year, did not experience an impact on their self-care and overall abilities with the use of SFN vs placebo [72]. More recently, results of a phase II randomized clinical trial (NCT03232138) targeting former smokers showed that supplementation of sulforaphane did not demonstrate a significant impact on bronchial histopathology. However, SFN significantly reduced the Ki-67 index with a 20% decrease in the sulforaphane group and a 65% increase in the placebo (P = 0.014). The difference was even greater in high-density (3+) positive Ki67, with a 44% decrease in the sulforaphane group compared with a 71% increase in the placebo (P = 0.004). Higher bioavailability of sulforaphane was correlated with greater reduction of the Ki-67 index (P for trend = 0.019). Sulforaphane treatment had no impact on the caspase-3 or TUNEL index in bronchial biopsies. No severe adverse event was observed in the study participants [73]. The early work in other cancer patient populations are critical to establish feasibility, compliance, safety, bioavailability as well as initial effectiveness to modulate intermediate endpoint biomarkers of SFN relevant to bladder carcinogenesis. To date, there are no clinical trials targeting bladder cancer patients (Figure 1).
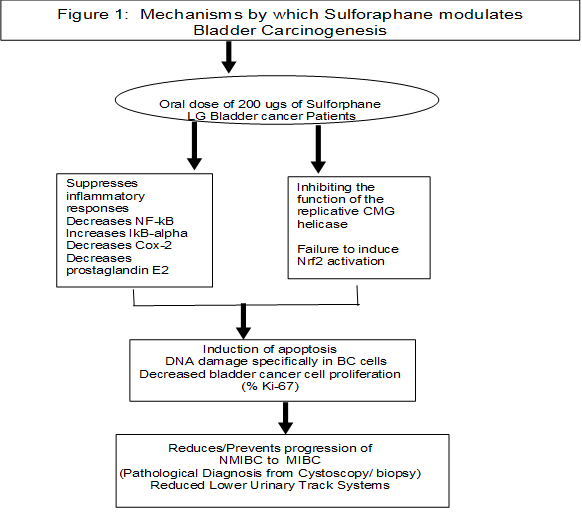
Figure 1: Mechanisams by which suloraphane modulates bladder carcinogenesis.
Discussion
Other than smoking cessation, there is a paucity of research that systematically evaluates specific agents relevant for chemoprevention of BCs. Early phase randomized phase II clinical trials should examine the safety, effectiveness and potential mechanism by which SFN perturbs the IEBs of bladder carcinogenesis and impact recurrence-free survival, in a cohort of men and women with a confirmed diagnosis of low-grade (T0T1) NMIBC. Although several mechanisms by which SFN impacts BC are identified, based on our provocative preliminary findings, it may be critical to examine if a constant dose of SFN, (vs. placebo) administered to a cohort of men and women diagnosed with To and T1, NMIBC, will lead to inhibition of the function of the replicative CMG helicase leading to reduction in markers of proliferation (%Ki-67, MCM2 (CMG subunit biomarker) increased DNA damage and apoptosis in the BC epithelial cells- relevant to BC chemoprevention. Indeed, alternate molecular mechanism to see if SFN downregulates NF-κB contributing to reduction in proliferation and increasing apoptosis of cells should be evaluated. Distinct biological factors have been identified as likely to contribute to disparate incidence rates and outcomes for male and female BC patients. Other critical covariates implied in bladder carcinogenesis, such as metabolism differences (NAT, GST polymorphisms), smoking status, Ki-67 and if patient received intravesical therapy. These covariates must be accounted for in the early phase trials. Most importantly, it may be important to evaluate the clinical outcome of recurrence-free survival, which has significant implications in disease progression and reduction of patient burden. Additionally, correlating novel intermediate endpoint biomarkers of disease progression (eg.Ki67, MCM2/GINS) with tumor recurrence will validate the potential mechanism by which SFN targets bladder carcinogenesis, as observed in previous studies. Preliminary studies provide several important caveats to inform the future clinical trial designs: (a) organ-specific bioavailability of SFN when delivered orally - selectively delivered to bladder via urinary excretion. Sustained urinary storage in the bladder in humans that may facilitate the disassociation of SFN metabolites and increase the exposure of BC cells to the SFN in the urine; (b) The dose of 120 mg (274 μmol) glucoraphanin delivering a potential daily dose of 95 μmol sulforaphane is bioavailable with no toxicities; (c) 274 µmol administered in a divided dose schedule can maximize and sustain drug levels, while providing the best chance at prolonging certain chemopreventive benefits associated with SFN consumption; (d) although a minimum duration of intervention of 2-8 weeks has been shown to be adequate to ensure bioavailability and modulation of Ki-67 in breast, the 12 months intervention may be recommended to ensure that a substantial duration of exposure will have a reasonable likelihood of perturbing intermediate endpoint biomarkers of bladder carcinogenesis, including biomarkers of proliferation(% Ki-67/ MCM2) and ultimately preventing disease recurrence; (e) evaluate response of SFN in T0T1 NMIBC tissue to determine the best timing and disease stage that may influence a cell’s response to SFN, in addition to determining the potential to prevent the field cancerization effect seen in progressive, lowgrade superficial NMIBC.
Conclusion
Several epidemiological, in vitro, preclinical and early phase trials completed by our team and others have shown that Sulforaphanes (SFN) are potent inhibitors of bladder carcinogenesis inhibiting the survival and proliferation of a wide array of animal and human BC through multiple molecular mechanisms and without toxicities at these doses, supporting further development of SFN in early phase human studies targeting BC. If the bioavailability, safety, effectiveness, and the mechanism by which SFN modulates recurrence free survival and biomarkers relevant to bladder carcinogenesis are demonstrated in early phase trials, the effects of SFN can be examined in a well-powered, phase III clinical trial of SFN to prevent bladder tumor recurrence, improve QOL, and ultimately BC progression from LG to HG, or of NMIBC to MIBC.
Reference
- Atlanta GA (2025) American Cancer Society. Accessed June. Available from:
- Burger M, Catto JW, Dalbagni G, Grossman HB, Herr H, (2013) et al. Epidemiology and risk factors of urothelial BC. Eur Urol 63: 234-241.
- Li Y, Sun L, Guo X, Mo N, Zhang J (2021) et al.Frontiers in BC Genomic Research. Front Oncol 11: 670729.
- Flaig TW, Spiess PE, Abern M, Agarwal N, Bangs R (2022) et al. NCCN Guidelines® Insights: BC, Version 2.2022. J Natl Compr Canc Netw 20: 866-878.
- Chang, SS (2024) Diagnosis and Treatment of Non-Muscle Invasive BC: AUA/SUO Guideline. J Urol 211: 533-538.
- Hilton WM, Ercole B, Parekh DJ, Sonpavde G, Ghosh R (2011) Efficacy of combined intravesical immunotherapy and chemotherapy for non-muscle invasive BC. Expert Rev Anticancer Ther 11: 949-957.
- Kobayashi H (2018) Long term follow-up in patients with initially diagnosed low grade Ta non-muscle invasive bladder tumors: tumor recurrence and worsening progression. BMC Urol 14: 5.
- Tran L, Xiao JF, Neeraj Agarwal N, Duex JE (2021) et al. Advances in BC Biology and Therapy. Nat Rev Cancer 21: 104-121.
- Johnson DC, Greene PS, Nielsen ME (2015) Surgical advances in BC: at what cost? Urol Clin North Am 42: 235-252.
- Svatek RS, Hollenbeck BK, Holmang S, Lee R, Kim SP (2014) et al.The economics of BC: costs and considerations of caring for this disease. Eur Urol 66: 253-262.
- Botteman MF, Pashos CL, Redaelli A, Laskin B, Hauser R (2003) Health economics of BC: a comprehensive review of the published literature. Pharmacoeconomics 21: 1315-1330.
- Ferlay J (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN Int J Cancer 136: E359-E386.
- Freedman ND, Silverman DT, Hollenbeck AR, Schatzkin A, Abnet CC (2011) Association between smoking and risk of BC among men and women. JAMA 306: 737-745.
- Kelly JD, Tan WS, Porta N, Mostafid H, Huddart R (2019) et al. BOXIT-A Randomised Phase III Placebo-controlled Trial Evaluating the Addition of Celecoxib to Standard Treatment of Transitional Cell Carcinoma of the Bladder (CRUK/07/004). Eur Urol 75: 593-601.
- Sabichi AL, Lee JJ, Grossman HB, Liu S, Richmond E (2011) et al. A randomized controlled trial of celecoxib to prevent recurrence of nonmuscle-invasive BC. Cancer Prev Res (Phila) 4: 1580-1589.
- Chow NH, Chan SH, Tzai TS, Ho CL, Liu HS (2001) Expression profiles of ErbB family receptors and prognosis in primary transitional cell carcinoma of the urinary bladder. Clin Cancer Res 7: 1957-1962.
- Pruthi RS, Nielsen M, Heathcote S, Wallen EM, Rathmell WK (2010) A phase II trial of neoadjuvant erlotinib in patients with muscle-invasive BC undergoing radical cystectomy: clinical and pathological results. BJU Int 106: 349-354.
- Messing E, Gee JR, Saltzstein DR, Kim K, diSant’Agnese A (2012) et al. A phase 2 cancer chemoprevention biomarker trial of isoflavone G-2535 (genistein) in presurgical BC patients. Cancer Prev Res (Phila) 5: 621-630.
- Nguyen TP, Zhang CA, Sonn GA, Eisenberg ML, Brooks JD (2021) Consumption of cruciferous vegetables and the risk of bladder cancer in a prospective US cohort: data from the NIH-AARP diet and health study. Am J Clin Exp Urol 9: 229-238.
- Fimognari C, Hrelia P (2007) Sulforaphane as a promising molecule for fighting cancer. Mutat Res 635: 90-104.
- Shapiro TA, Fahey JW, Wade KL, Stephenson KK, Talalay P (1998) Human metabolism and excretion of cancer chemoprotective glucosinolates and isothiocyanates of cruciferous vegetables. Cancer Epidemiol Biomarkers Prev 7:1091-100.
- Tang L, Zirpoli GR, Guru K, Moysich KB, Zhang Y (2008) et al. Consumption of raw cruciferous vegetables is inversely associated with BC risk. Cancer Epidemiol Biomarkers Prev 17: 938-944.
- Tang L, Zirpoli GR, Guru K, Moysich KB, Zhang Y (2010). Intake of cruciferous vegetables modifies BC survival. Cancer Epidemiol Biomarkers Prev 19: 1806-1811.
- Zhang Y, Talalay P, Cho CG, Posner GH (1992) A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc Natl Acad Sci U S A 89: 2399-2403.
- Michaud DS, Clinton SK, Rimm EB, Willett WC, Giovannucci E (2001) Risk of BC by geographic region in a U.S. cohort of male health professionals. Epidemiology 12:719-726.
- Michaud DS, Spiegelman D, Clinton SK, Rimm EB, Willett WC (2000) et al. Prospective study of dietary supplements, macronutrients, micronutrients, and risk of BC in US men. Am J Epidemiol 152: 11451153.
- Michaud DS, Spiegelman D, Clinton SK, Rimm EB, Willett WC (1999) et al. Fruit and vegetable intake and incidence of BC in a male prospective cohort. J Natl Cancer Inst 91: 605-613.
- Veeranki OL, Bhattacharya A, Tang L, Marshall JR, Zhang Y (2015) Cruciferous vegetables, isothiocyanates, and prevention of BC. Curr Pharmacol Rep 1: 272-282.
- Houghton CA, Fassett RG, Coombes JS (2013) Sulforaphane: translational research from laboratory bench to clinic. Nutr Rev 71: 709-726.
- Al-Zalabani AH, Stewart KF, Wesselius A, Schols AM, Zeegers M.P (2016) Modifiable risk factors for the prevention of BC: A systematic review of meta-analyses. Eur. J. Epidemiol 31: 811-851.
- Yu EY, Wesselius A, Mehrkanoon S, Goosens M, Brinkman M (2021). Vegetable intake and the risk of bladder cancer in the Bladder Cancer Epidemiology and Nutritional Determinants (BLEND) international study. BMC Med 19: 56.
- Yu P, Yu L, Lu Y (2022) Dietary consumption of cruciferous vegetables and bladder cancer risk: A systematic review and meta-analysis. Front Nutr 9: 944451
- Xiang S, Craig KC, Luo X, Welch DL, Ferreira RB (2024) Identification of ATP-Competitive Human CMG Helicase Inhibitors for Cancer Intervention that Disrupt CMG-Replisome Function. Mol Cancer Ther 23: 1568-1585.
- Xiang S, Reed DR, Alexandrow MG (2023) The CMG helicase and cancer: a tumor “engine” and weakness with missing mutations. Oncogene 42: 473-490.
- Mei L, Kedziora KM, Song EA, Purvis JE, Cook JG (2022) The consequences of differential origin licensing dynamics in distinct chromatin environments. Nucleic Acids Res 50: 9601-9620.
- Xiang S, Reed DR, Alexandrow MG (2023) The CMG helicase and cancer: a tumor “engine” and weakness with missing mutations. Oncogene 42: 473-490.
- Bryant VL, Elias RM, McCarthy SM, Yeatman TJ, Alexandrow MG (2015) Suppression of Reserve MCM Complexes Chemosensitizes to Gemcitabine and 5-Fluorouracil Mol Cancer Res (9): 1296-305.
- Wang F, Liu P, An H, Zhang Y (2020) Sulforaphane suppresses the viability and metastasis, and promotes the apoptosis of BC cells by inhibiting the expression of FAT 1. Int J Mol Med 46: 1085-1095.
- Abbaoui B, Riedl KM, Ralston RA, Thomas-Ahner JM, Schwartz SJ (2012) et al. Inhibition of BC by broccoli isothiocyanates sulforaphane and erucin: characterization, metabolism, and interconversion. Mol Nutr Food Res 56: 1675-1687.
- Choi S, Singh SV (2005) Bax and Bak are required for apoptosis induction by sulforaphane, a cruciferous vegetable-derived cancer chemopreventive agent. Cancer Res 65: 2035-2043.
- Nijhoff WA, Grubben MJ, Nagengast FM, Jansen JB, Verhagen H (1995) et al. Effects of consumption of Brussels sprouts on intestinal and lymphocytic glutathione S-transferases in humans. Carcinogenesis 16: 2125-2128.
- Rojo de la Vega M, Chapman E, Zhang DD (2018) NRF2 and the Hallmarks of Cancer. Cancer Cell 34: 21-43.
- Ding Y, Paonessa JD, Randall KL, Argoti D, Chen L (2010) et al. Sulforaphane inhibits 4-aminobiphenyl-induced DNA damage in bladder cells and tissues. Carcinogenesis 31: 1999-2003.
- Zheng K, Ma J, Wang Y, He Z, Deng K (2020) Sulforaphane Inhibits Autophagy and Induces Exosome-Mediated Paracrine Senescence via Regulating mTOR/TFE3. Mol. Nutr. Food 64: e1901231.
- Shan Y, Wu K, Wang W, Wang S, Lin N (2009) et al. Sulforaphane down-regulates COX-2 expression by activating p38 and inhibiting NFkappaB-DNA-binding activity in human bladder T24 cells. Int J Oncol 34: 1129-1134.
- Leone A, Diorio G, Sexton W, Schell M, Alexandrow M (2017) et al. Sulforaphane for the chemoprevention of bladder cancer: molecular mechanism targeted approach. Oncotarget 8: 35412-35424.
- Dang YM, Huang G, Chen YR, Dang ZF, Chen C (2014) et al. Sulforaphane inhibits the proliferation of the BIU87 bladder cancer cell line via IGFBP-3 elevation. Asian Pac J Cancer Prev 15: 1517-1520.
- Walter CEJ, Durairajan S, Periyandavan K, C GPD, G DJD (2020) et al. Bladder neoplasms and NF-κB: an unfathomed association. Expert Rev Mol Diagn 20: 497-508.
- Guan Z, Ding C, Du Y, Zhang K, Zhu JN (2014) et al. HAF drives the switch of HIF-1α to HIF-2α by activating the NF-κB pathway, leading to malignant behavior of T24 BC cells. Int J Oncol 44: 393-402.
- Kim B, Jang I, Kim K, Jung M, Lee C (2021) et al. Comprehensive Gene Expression Analyses of Immunohistochemically Defined Subgroups of Muscle-Invasive Urinary Bladder Urothelial Carcinoma. Int. J. Mol. Sci 22: 628.
- Lee YM, Cho HJ, Ponnuraj SP, Kim J, Kim JS (2011) et al. Phenethyl isothiocyanate inhibits 12-O-tetradecanoylphorbol-13-acetate-induced inflammatory responses in mouse skin. J Med Food 14: 377-385.
- Shan Y, Wu K, Wang W, Wang S, Lin N (2009) Zhao R, Cassidy A, Bao Y. Sulforaphane down-regulates COX-2 expression by activating p38 and inhibiting NF-kappaB-DNA-binding activity in human bladder T24 cells. Int J Oncol 34: 1129-1134.
- Bricker GV, Riedl KM, Ralston RA, Tober KL, Oberyszyn TM (2014) et al. Isothiocyanate metabolism, distribution, and interconversion in mice following consumption of thermally processed broccoli sprouts or purified sulforaphane. Mol Nutr Food Res 58: 1991-2000.
- Munday R, Munday CM (2004) Induction of phase II detoxification enzymes in rats by plant-derived isothiocyanates: comparison of allyl isothiocyanate with sulforaphane and related compounds. J Agric Food Chem 52:1867-71.
- Cornblatt BS, Ye L, Dinkova-Kostova AT, Erb M, Fahey JW (2007) et al. Singh NK, Chen MS, Stierer T, Garrett-Mayer E, Argani P, Davidson NE, Talalay P, Kensler TW, Visvanathan K. Preclinical and clinical evaluation of sulforaphane for chemoprevention in the breast. Carcinogenesis 28: 1485-1490.
- Dickinson SE, Melton TF, Olson ER, Zhang J, Saboda K (2009) et al. Inhibition of activator protein-1 by sulforaphane involves interaction with cysteine in the cFos DNA-binding domain: implications for chemoprevention of UVB-induced skin cancer. Cancer Res 69: 71037110.
- Forster T, Rausch V, Zhang Y, Isayev O, Heilmann K (2014) et al. Sulforaphane counteracts aggressiveness of pancreatic cancer driven by dysregulated Cx43-mediated gap junctional intercellular communication. Oncotarget 5: 1621-1634
- Alumkal JJ, Slottke R, Schwartzman J, Cherala G, Munar M (2015) et al. A phase II study of sulforaphane-rich broccoli sprout extracts in men with recurrent prostate cancer. Invest New Drugs 33: 480-489.
- Kensler TW, Egner PA, Agyeman AS, Visvanathan K, Groopman JD (2013) et al. Keap1-nrf2 signaling: a target for cancer prevention by sulforaphane. Top Curr Chem 329: 163-177.
- Atwell LL, Hsu A, Wong CP, Stevens JF, Bella D (2015)et al. Absorption and chemopreventive targets of sulforaphane in humans following consumption of broccoli sprouts or a myrosinase-treated broccoli sprout extract. Mol Nutr Food Res 59: 424-433.
- Bogaards JJ, Verhagen H, Willems MI, van Poppel G, van Bladeren PJ (1994) Consumption of Brussels sprouts results in elevated alpha-class glutathione S-transferase levels in human blood plasma. Carcinogenesis 15: 1073-1075.
- Doss JF, Jonassaint JC, Garrett ME, Ashley-Koch AE, Telen MJ (2016) Phase 1 Study of a Sulforaphane-Containing Broccoli Sprout Homogenate for Sickle Cell Disease. PLoS One 11: e0152895.
- Fahey JW, Wade KL, Wehage SL, Holtzclaw WD, Liu H (2017) et al. Stabilized sulforaphane for clinical use: Phytochemical delivery efficiency. Mol Nutr Food Res 61.
- Saha S, Hollands W, Teucher B, Needs PW, Narbad A (2012) et al. Isothiocyanate concentrations and interconversion of sulforaphane to erucin in human subjects after consumption of commercial frozen broccoli compared to fresh broccoli. Mol Nutr Food Res 56: 19061916.
- Traka M, Gasper AV, Melchini A, Bacon JR, Needs PW (2008) et al. Broccoli consumption interacts with GSTM1 to perturb oncogenic signalling pathways in the prostate. PLoS One 3: e2568
- Zhang Z, Atwell LL, Farris PE, Ho E, Shannon J (2016) Associations between cruciferous vegetable intake and selected biomarkers among women scheduled for breast biopsies. Public Health Nutr 19: 12881295.
- Clarke JD, Hsu A, Riedl K, Bella D, Schwartz SJ (2011) et al. Bioavailability and inter-conversion of sulforaphane and erucin in human subjects consuming broccoli sprouts or broccoli supplement in a cross-over study design. Pharmacol Res 64: 456-643.
- Egner PA, Chen JG, Wang JB, Wu Y, Sun Y (2011) et al. Bioavailability of Sulforaphane from two broccoli sprout beverages: results of a short-term, cross-over clinical trial in Qidong, China. Cancer Prev Res (Phila) 4: 384-395.
- Cipolla BG, Mandron E, Lefort JM, Coadou Y, Della Negra E (2015) et al. Effect of Sulforaphane in Men with Biochemical Recurrence after Radical Prostatectomy. Cancer Prev Res (Phila) 8: 712-719.
- Atwell LL, Zhang Z, Mori M, Farris P, Vetto JT (2015) et al. Sulforaphane Bioavailability and Chemopreventive Activity in Women Scheduled for Breast Biopsy. Cancer Prev Res (Phila) 8: 1184-1191.
- Zhang Z, Garzotto M, Davis EW 2nd, Mori M, Stoller WA (2020) et al. Sulforaphane Bioavailability and Chemopreventive Activity in Men Presenting for Biopsy of the Prostate Gland: A Randomized Controlled Trial. Nutr Cancer 72: 74-87.
- Lozanovski VJ, Houben P, Hinz U, Hackert T, Herr I (2014) et al. Pilot study evaluating broccoli sprouts in advanced pancreatic cancer (POUDER trial) - study protocol for a randomized controlled trial. Trials 15: 204.
- Yuan JM, Kensler TW, Dacic S, Hartman DJ, Wang R (2025) et al. Randomized Phase II Clinical Trial of Sulforaphane in Former Smokers at High Risk for Lung Cancer. Cancer Prev Res (Phila) 18: 335-345.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.