Reiki (Laying on of Hands) Improves Behavioral Changes and Modulates Hippocampus Gene Expression Related to Inflammation and Oxidative Markers in Stressed Mice
by Daniele Cristina Moreira1, Victor Hugo Dantas Guimarães1, Emisael Stênio Batista Gomes1, Eduardo Pinheiro dos Santos1, Lucyana Conceição Farias1, Alfredo Maurício Batista De-paula1, André Luiz Sena Guimaraes1, Sérgio Henrique Sousa Santos1,2*
1Laboratory of Health Science, Graduate Program in Health Sciences, Montes Claros State University (Unimontes), Montes Claros, MG, Brazil
2Institute of Agricultural Science (ICA). Food Engineering, Federal University of Minas Gerais (UFMG), Montes Claros, MG, Brazil
*Corresponding author: Sérgio Henrique Sousa Santos, Institute of Agricultural Sciences. Food Engineering, Universidade Federal de Minas Gerais (UFMG); Avenida Universitária, 1.000 – Universitário, 39.404-547, Montes Claros, MG, Brazil.
Received Date: 7 November 2024
Accepted Date: 14 November 2024
Published Date: 18 November 2024
Citation: Moreira DC, Guimarães VHD, Gomes ESB, dos Santos EP, Farias LC, et al. (2024) Reiki (Laying on of Hands) Improves Behavioral Changes and Modulates Hippocampus Gene Expression Related to Inflammation and Oxidative Markers in Stressed Mice. Curr Res Cmpl Alt Med 8: 259. https://doi.org/10.29011/2577-2201.100259
Abstract
Introduction: Reiki (rei means universal, and ki, vital energy) is a complementary/ alternative therapy practiced through the laying on of hands and was introduced by Mikao Usui based on Sanskrit texts at the end of the nineteenth century. Reiki principles state that everything in the universe consists of energy, including the human body, and changes in this energy can lead mankind to diseases. Nowadays anxiety and stress are highly prevalent in the world and cause changes in concentration, memory, and cognition. Objective: The present study aimed to evaluate the effect of reiki on induced-stress animals by paw shocks, investigating its role in behavioral and brain inflammatory actions. Method: 12-week-old male Swiss mice were divided into five groups: control, reiki control, stress, stress + glove control, and stress + reiki. Data analysis was blinded. Results: Mice treated with reiki had behavioral improvement, modulating mobility, and decreasing stress compared to untreated mice. These findings were associated with the hippocampal gene expression of the brain inflammasome pathway, in which the Nod-like receptor protein 3 (NRLP3) genes and the antioxidant catalase were altered. Conclusion: The present study indicates that stressed mice by paw shocks treated with reiki present improved locomotor activity. In addition, reiki modulated inflammation-related gene expression by inhibiting the NLRP3 inflammasome and reducing the action of catalase.
Keywords: Reiki; Alternative therapies; Stress; Hippocampus; NLRP3 inflammasome; Catalase
Introduction
Chronic psychological stress and depression have become a major health problem worldwide [1] being predicted which may affect about 300 million people worldwide[2]. Brain and humor disorders are exponentially increasing every year becoming more frequent [3].
Clinical and animal research has proven that stress is an important risk cause or hindering factor for numerous pathologies, including diabetes, cardiovascular disease, bone loss, neurodegenerative diseases, and cancer [4-8]. Stress might cause psychological and physiological changes involving the activation of the hypothalamicpituitary-adrenal axis (HPA) and the sympathetic nervous system, which may influence the patient mood, behavior, and health [9].
Stressful or traumatic situations cause continuous changes in behavior through two different actions of knowledge [1012]: One of them is Pavlovian conditioning, which happens when a previously neutral stimulus is related to intimidation and, in this way, reaches the experience of remembering fear. The second mechanism is sensitization, which is a generalized and unassociated change in sentimental responsiveness [13].
The immune system may be associated with important actions of the psychiatric disorders caused by stressful conditions, such as major depressive disorder (MDD). In regular situations, the HPA axis monitors and modulates immune regulation through numerous hormones, such as glucocorticoids [14-18]. However, stressful events cause hyperactivity of the HPA axis, and repeated stress leads to resistance to corticosteroids, causing an exaggerated inflammatory response [14,19,20]. Unraveling the strong, but still poorly understood, link between stress and depression is essential for the preparation of more effective conventional treatments [21,22]. It has long been known that the hippocampus adjusts the HPA axis [23,24] and hippocampal neurons are important for the normal activation of endocrine and behavioral stress response elements and emotional behavior [25-27].
In this context, different approaches to dealing with stress, promoting well-being, and improving quality of life are a growing research area in the Integrative and Complementary Practices (ICP) application [28]. Although energy medicine or human biofield therapies are only a part of integrative medicine, a current survey of cancer patients has found that these individuals report the greatest benefit from energy medicine over other complementary therapies [29]. Some of these energetic approaches include qigong, therapeutic touch, homeopathy, and reiki [30,31].
Reiki (laying on of hands) (rei means universal, and ki, vital energy) was introduced by Mikao Usui based on Sanskrit texts at the end of the 19th century and was later put into practice by Hawayo Takata [32-35]. Reiki considers that everything in the universe consists of energy, including the human body, and disturbed changes in this energy can lead to pathologies [36,37].
Although the beneficial effects of reiki have been reported in the literature, few experimental studies have addressed stress-related behavioral and brain factors. Thus, the present study aimed to evaluate the effect of reiki on the stressed mice induced by paw shocks examining the possible alterations in the gene expression of the Nod-like receptor protein 3 (NLRP3) and catalase (CAT) inflammasome along with stress tolerance.
Methods
Animals
Male Swiss mice at 12 weeks of age (N = 40) were divided into 5 groups (n = 8 each) (Table 1) and kept in the vivarium of the State University of Montes Claros (UNIMONTES), Minas Gerais (MG), under standard temperature [22 ± 2ºC], air humidity between 60 ± 5%, 12h light/dark cycle, low sound levels (below 40 dB) with a balanced diet (50.3% carbohydrates, 41.9% protein and 7.8% fat (2.18 kcal/g Purina-Labine ®) and filtered tap water ad libitum [38]. The mice were packed in polypropylene boxes measuring 414 x 344 x 168 mm with galvanized steel lids. All procedures performed involving animals complied with the institution’s ethical standards. The analyzed data was blinded, being sent without identification to a third researcher to plot and evaluate the significance.
|
Group Description |
Group ID |
|
ST (Standard) |
G1 |
|
ST + Reiki |
G2 |
|
STR (stress) |
G3 |
|
STR + Glove |
G4 |
|
STR + Reiki |
G5 |
Table 1: Experimental Groups.
Induction of stress by shock to the paws
This study’s conditioned fear stress methodology has been previously described [39,40]. Briefly, starting on the day after pulp exposure, the rats were subjected to conditioning fear stress sessions for 33 consecutive days and after starting treatment for another 51 days in a conditioning fear stress chamber (37 cm x 25 cm x 21 cm, Skinner Box, ELT-02, Eltrones, Joinville, SC, Brazil, Brazil. BR). In each stress section, the animals were presented with a neutral conditioned stimulus (sound lasting two seconds) before the shock occurred (five seconds of 1.10 mA). During the stress section, six shocks were applied, and the interval between each shock was 25 s. Considering the procedures, each stress section lasted 185 seconds. The animals in the control group were also placed alone in the chamber and induced to the same experimental conditions, but the sound they heard was only the stimulus without shocks [39-41]. The tests were carried out in an experimental room with one animal at a time, to prevent other animals from hearing the noises emitted by the animal subjected to the experiment. The equipment was sanitized with 70% ethanol before and after each mouse remained.
Application of Reiki
To apply the technique, the researcher took the Reiki level 1 course, with a professional master’s in Reiki based on the Usui and Tibetan Reiki method of natural healing, certifying aptitude in the technique application. Level 1 training consists ofknowledge about the concepts of reiki, the energy body, knowledge of treatment/symbols, practice and meditation. During this period, it is possible to apply it to other people, animals, and plants.
In the Control-Glove group, the following technique was performed: for 15 minutes, during 51 consecutive days, a pair of gloves connected to a portable battery, heated, filled with cotton, attached to a mannequin about one meter away, were placed on each of the boxes of the animals in this group.
The Reiki group received the following treatment: for 15 minutes, during 51 consecutive days, the same person laid his hands directly on each box of the animals in this group, without direct physical contact with them [42] (Figure S1 Supplementary material).
During the Reiki application, the animals remained in their boxes freely, while the researcher’s hands were imposed. During this period, the qualitative mice behavior was noticeable regarding the decrease in agitation, and approximation between the animals, representing aspects of tranquility and calmness.
Open field test
Locomotor activity was assessed using open field tests to quantify the stress of fear conditioning by animals. The test was carried out in the box of an open square field (1m²) that had its floor divided into 25 equal areas (20 cm²) [39,40]. The mice were placed individually in the central area and allowed to freely explore an area for five minutes. The animal’s trajectory was quantified in centimeters traveled using the Image J program (Wayne Rasband, National Institutes of Health, Bethesda, MD). The field was cleaned with 70% ethanol after each run, and the rats were then returned to the appropriate cages. The experimenter was unaware of the grouping of the mice. Open field tests were carried out before and during stress induction and before animal sacrifice.
At the end of the experiment, the animals were kept fasting overnight (12 h) and euthanized by decapitation. The samples were collected, weighed, and stored immediately in liquid nitrogen at -80 °C for investigation. The brain was removed and separated from the hippocampus, hypothalamus and cerebellum, the heart, and large and small intestines. The material was placed in properly labeled containers and fixed in 10% formaldehyde solution.
Histology
Samples from the brain’s hippocampus were kept in formaldehyde before being transferred to 70% ethyl alcohol solution for later inclusion in paraffin. Sections of 7μm thickness were obtained in a specific microtome followed by assembly on glass slides previously prepared and treated with HE for analysis of adipocyte size. The size was evaluated using a FSX100 fluorescence microscope (Olympus, Center Valley, PA, USA) [43].
mRNA levels by Real-Time RT-PCR
Tissue samples from the cerebral hippocampus were treated with Trizol (Invitrogen Corp.VR, San Diego, CA, USA) and DNAse (Invitrogen Corp.VR). Reverse transcription was performed with Moloney Murine Leukemia Virus (MMLV) (Invitrogen Corp. VR). mRNA levels of genes of interest (Table 2) were determined by Real-Time RT-qPCR (SYBR GREEN reagent) in Applied Biosystems® QuantStudio™ 6 Flex Real-Time PCR System. Gene expression was quantified using the comparative relative method 2-∆∆CT (cycle thresholds) using GAPDH as endogenous control [44]. The primer selection tool used was the Primer-BLAST a tool for finding specific primers.
|
Gene |
Primer sequence |
|
NLRP3 |
Forward: 5. TCCAGTGAGGTGGTGTGAAAGG-3 Reverse: 5. TCAGTGGCTAAGAGGCACCTTG-3 |
|
CAT |
Forward: 5. TCCCGAGTCTCTCCATCAGGTTTC-3 Reverse: 5. TAGCCATTCATGTGCCGGTGAC-3 |
|
GAPDH |
Forward: 5. AAGAAGGTGGTGAAGCAGGCATC-3 Reverse: 5. CGAAGGTGGAAGAGTGGGAGTTG-3 |
|
NLRP3: Nod-like receptor protein 3; CAT: catalase e GAPDH; Glyceraldehyde 3 phosphate dehydrogenase. |
|
Table 2: Primer gene sequences used for real-time PCR analysis.
Statistical analyses
Data were analyzed using GraphPad Prism version 8.0 and analyzed with means ± standard error of the mean (SEM). Multiple comparisons were performed using unidirectional ANOVA or bidirectional ANOVA followed by the Bonferroni post-test. Complementary analyses were performed using student’s t-test. A 95% confidence interval was considered for statistical significance and the p-value was set at p <0.05.
The delta variation graph between the end and the beginning of the reiki treatment was calculated and the statistical analyses were performed.
Ethical Guidelines
The research followed national and international guidelines regarding animal research, submitted and approved by Comitê de Ética em Experimentação e Bem-Estar Animal (CEEBEA) under number 176/2018 from (CEEBEA - State University of Montes Claros – Annex A). All applicable institutional and/or national guidelines for the care and use of animals were followed.
Results
Behavioral changes with chronic stress due to paw shock
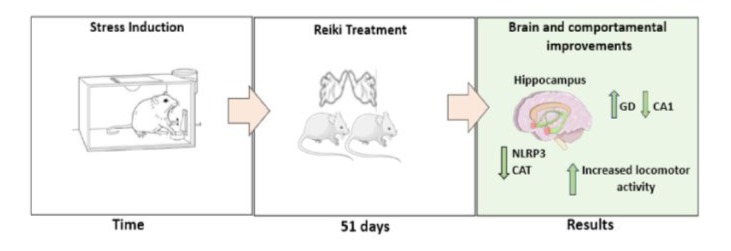
Overall locomotor activity quantified the stress of conditioned fear. It has been shown that the stress of conditioned fear decreases the overall locomotor activity of the animal (39, 40) In the present study, it was observed that stress reduced the distance covered (p < 0.001), while in the group treated with reiki, there was a recovery (Figure 1).
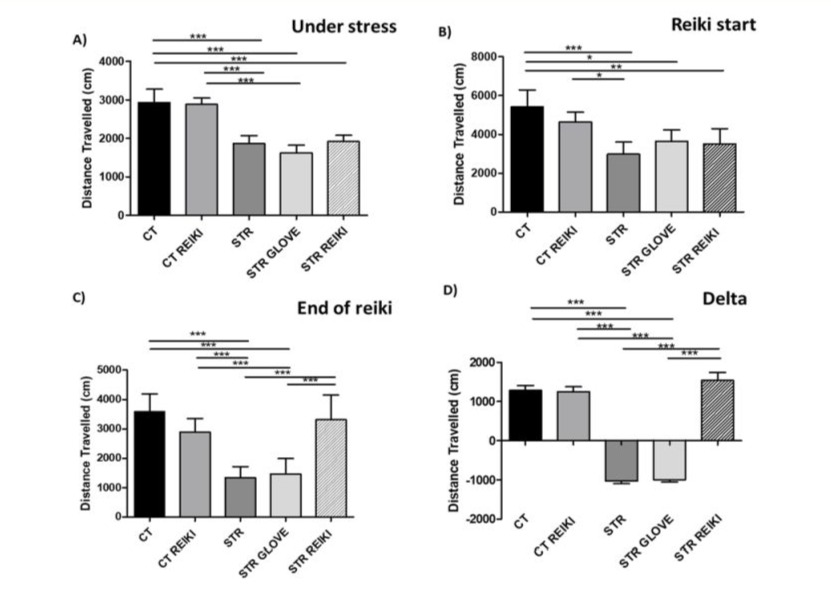
Figure 1: Representation of the open field test after stress by shocks to the paws of mice a) stressed animals after 33 days, b) beginning of reiki treatment, c) end of reiki treatment after 51 days, d) delta variation chart between the end and beginning of treatment. Data were expressed in mean ± SEM. ANOVA test followed by Bonferroni multiple comparations *p < 0.05; **p < 0.01; **p < 0.001.
Gene expression after Reiki treatment in the cerebral hippocampus after stress.
Catalase (CAT) showed increased expression in STR GLOVE (13.86 ± 4.303) p<0.0001, while STR REIKI showed decreased expression and NLRP3 (Nod-like receptor protein 3) was significantly reduced in STR REIKI (0.4783 ± 4.617) p 0.009, in STR GLOVE it showed increased expression (5.109 ± 1.102) p 0.001 compared to the stress group (Figure 2).
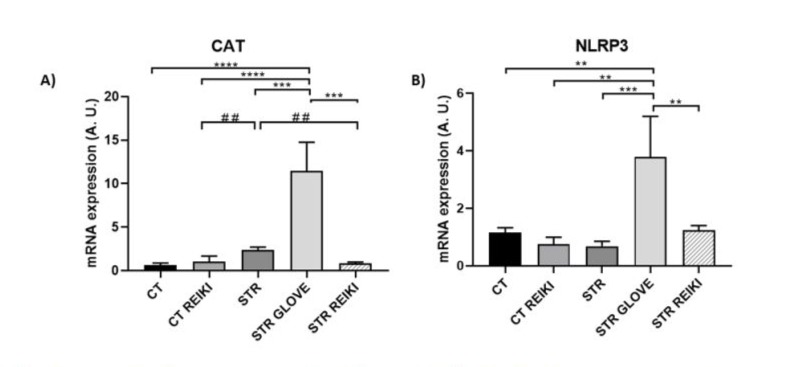
Figure 2: mRNA levels in cerebral hippocampus in mice A) CAT: catalase, B) NLRP3: Nod-like receptor protein 3. Data were expressed in mean ± SEM. ANOVA test followed by Bonferroni multiple comparations *p < 0.05; **p < 0.01; **p < 0.001. # Teste T *p < 0.05.
Histology
The hippocampal cell area in the CA1 region was reduced in the STR groups compared to the CT groups. In addition, there was a statistical difference between the CT and STR groups (0.5599 ± 99.76) p< 0.012, CT and STR GLOVE (8.000 ± 107.2) p<0.01 and CT and STR REIKI (1.510 ± 100.7) p< 0.004 (Figure 3).
Figure 3: Histological analysis in mice that were stressed by foot shock. Tissue sections stained with hematoxylin-eosin (HE) from the cerebral hippocampus, area of cells in the CA1 region (μm2). Data were expressed in mean ± SEM. ANOVA test followed by Bonferroni multiple comparations *p < 0.05;
The area of hippocampal cells in the dentate gyrus (GD) region was increased in the STR groups compared to the CT groups. There was no statistical difference between the CT and STR groups (Figure 4).
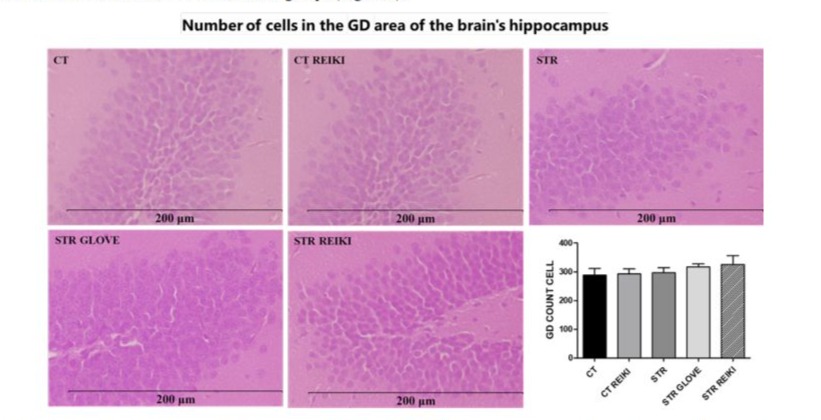
Figure 4: Histological analysis in mice that were stressed by foot shock. Tissue sections stained with hematoxylin-eosin (HE) from the cerebral hippocampus, area of cells in the GD region (μm2). Data were expressed in mean ± SEM.
Discussion
The main results of the present study identified that reiki may be an alternative therapy to treat stress and anxiety in a mice model. Animals under stress induced by paw shocks treated with reiki showed increased locomotor activity and behavioral improvement. Reiki also modulated gene expression related to inflammation and oxidative stress in the brain’s hippocampus.
The condition of stress is increasingly present in modern daily life. Since stress can change the body’s immune, neurochemical, and endocrine functions, it is understood that stress can cause damage to many pathologies [45]. In the present study, the number of freezing behaviors was more evident in the animals submitted to shocks, suggesting that fear and conditioned stress were successfully induced. An open-field test reveals changes in stress behavior that are characterized by a reduction in the distance traveled by the mice in the assessment performed through the assay, which is then used to quantify the stress of conditioned fear by the animal [46-48].
Stress has a mechanism that activates the HPA axis inducing the production of glucocorticoids (GCs) by the adrenal cortex [49]. GCs are a series of steroid hormones that establish the physiological (metabolic, cardiovascular, and immune) and behavioral (emotional, cognitive, and motor) responses to stress [50]. The primary GC in rodents is corticosterone (CORT), which acts by binding to mineralocorticoid (MR) receptors and GC receptors [51,52]. However, MR is mainly recruited under baseline and GR conditions under stress, since MR has a 10-fold higher affinity for CORT [53]. CORT has an anti-inflammatory effect that depends on its concentration and duration of exposure [54].
Thus, the neuroinflammatory response and the upregulation of proinflammatory cytokines are some of the effects linked to chronic stress [55,56]. The hippocampus is a target structure of stressinduced depression [57-60], and interleukin may be an indispensable factor that modulates the stress response and maintains the balance between neural inflammation and glucocorticoid signaling [61]. These results confirm the anti-inflammatory effects reported in animal models of depression [57,62].
The NLRP3 inflammasome appears to be a central mediator of neuroinflammation in the immune system during depression pathogenesis [63]. Microglia are dynamically influenced by stress-induced central nervous system (CNS) environmental signals, during which NLRP3 plays an important role [60]. As described in the two-step model of NLRP3 preparation and activation, the initiation step manifests in NLRP3 overexpression and the activation step is required for NLRP3 oligomerization and inflammasome assembly to allow processing of pro-IL-1β and pro-IL-18 into their mature, secreted forms [64]. In animal models of depression, the action of NLRP3 on the hippocampus correlates positively with stress- and depressive-type behaviors [65,66]. In the present research, we showed that reiki exerts its beneficial effects on stress by inhibiting the assembly of the NLRP3 inflammasome. We further found that Reiki treatment significantly reduced catalase cleavage.
The main mechanism against oxidative stress in the brain is the glutathione system [67]. Research has shown that mental stress can affect the functioning of this system [68,69]. In addition, stress can decrease catalase (CAT) activities in both the cerebral cortex and hippocampus, suggesting that an alteration in the endogenous antioxidant defense system is responsible for inducing depressionlike behavior in mice [70-73]. Even more, antioxidant expression has been associated with lower expression of superoxide dismutase (SOD) and CAT in the brain. It was evident that decreased levels of oxidative stress in the brain were associated with treatment [74]. In the present study, a similar result was found considering the catalase reduction which may be linked to the improvement of the antioxidant system in the brain, suggesting that reiki may work as an anti-stress and possible neuroprotective agent.
Chronic stress in mice has also been linked to a reduction in adult neurogenesis in the dentate gyrus (GD), the main region where new neurons are generated throughout life [75-77] that may be missing in the CA1 area of the hippocampus [78,79]. Some possibilities could elucidate this occurrence, such as the number of injured cells must be quantitatively higher when compared to the newly generated cells and also because the generated neurons probably do not become mature enough and thus are not neurophysiologically functional [80]. Stressed animals can induce microglial priming in GD, which is related to a hyperimmune response to stress and altered hippocampal neurogenesis [81]. In this study, there was an increase in the GD region after reiki treatment, which may support the hypothesis of an association between greater neurogenesis and lower signs of depression and stress.
Due to its capacity for neurogenesis, GD is more strongly regulated by various actions such as exercise, learning, and brain-derived neurotrophic factors, including the CA1 area [82,83]. Chronic stress negatively affects brain function and even mild chronic unpredictable stress causes atrophy of the CA1 area of mice, but not of DG which appears to be generally more resistant to stress [84-88]. Considerably, the stress induced by exposure to predators harms the CA1 area [89], but improvement of the DG [90].
Chronic unpredictable mild stress (CUMS) studies have indicated a significant reduction in the long-term potentiation of the CA1 region and the paired-pulse ratio in the hippocampus [91,92]. Differences in adult hippocampal neurogenesis were observed between genotypes during normal homeostatic conditions, with microglial deficiency [93]. The present study follows the described literature data showing a decrease in the CA1 area in the hippocampus, which may be due to these differences in the new neuron formation.
Finally, the present data show for the first time that reiki can lead to recovery in locomotor activity improve behavior in stressed animals, and even modulate gene expression related to inflammation and oxidative stress in the brain’s hippocampus. In addition, the healing effects of reiki are based on the body’s energy [37,94].
Conclusion
The present study indicates that reiki may be an alternative therapy to treat anxiety and stress. Mice stressed by paw shocks treated with reiki showed increased locomotor activity and behavioral improvement. In addition, reiki modulated inflammation-related gene expression by inhibiting the construction of the NLRP3 inflammasome and reducing the action of catalase, which may be linked to an improved antioxidant system in the hippocampus. Future studies on the mechanisms may open new perspectives for developing integrative therapies from the perspective of greater dissemination of these results and an interventional approach in the clinic.
Conflict of interest: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Author contributions: Credit authorship contribution statement: Conceptualization of study, investigation, writing- original draft D.C.M.; methodology animal experimentation E.P.S and analysis V.H.D.G and E.S.B.G; performed gene expression analysis V.H.D.G; writing original draft preparation D.C.M.; writing— review and editing D.C.M.; L. C.F.; A.M.B.P.; A. L.S.G, and S.H.S.S.; supervision S.H.S.S.; All authors read and approved the final manuscript.
Data availability: The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments: This research was partially funded by the Coordenadoria de Aperfeiçoamento do Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico (CNPQ), and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG).
References
- Menken M, Munsat TL, Toole JF (2000) The global burden of disease study: implications for neurology. Arch Neurol 57:418-420.
- Herrman H, Kieling C, McGorry P, Horton R, Sargent J, et al. (2019) Reducing the global burden of depression: a Lancet-World Psychiatric Association Commission. Lancet 393: e42-e43.
- Paykel ES (2006) Depression: a major problem for public health. Epidemiol Psichiatr Soc 15:4-10.
- Azuma K, Furuzawa M, Fujiwara S, Yamada K, Kubo KY (2015) Effects of Active Mastication on Chronic Stress-Induced Bone Loss in Mice. Int J Med Sci 12:952-957.
- Feng Z, Liu L, Zhang C, Zheng T, Wang J, et al. (2012) Chronic restraint stress attenuates p53 function and promotes tumorigenesis. Proc Natl Acad Sci U S A 109:7013-7018.
- Marin MF, Lord C, Andrews J, Juster RP, Sindi S, et al. (2011) Chronic stress, cognitive functioning, and mental health. Neurobiol Learn Mem 96:583-595.
- Sumner JA, Kubzansky LD, Elkind MS, Roberts AL, Agnew-Blais J, et al. (2015) Trauma Exposure and Posttraumatic Stress Disorder Symptoms Predict Onset of Cardiovascular Events in Women. Circulation 132:251-259.
- Hackett RA, Steptoe A (2017) Type 2 diabetes mellitus and psychological stress - a modifiable risk factor. Nat Rev Endocrinol 13:547-560.
- Wong ML, Licinio J (2001) Research and treatment approaches to depression. Nat Rev Neurosci 2:343-351.
- Rau V, DeCola JP, Fanselow MS (2005) Stress-induced enhancement of fear learning: an animal model of posttraumatic stress disorder. Neurosci Biobehav Rev 29:1207-1223.
- Seo DO, Carillo MA, Chih-Hsiung Lim S, Tanaka KF, Drew MR (2015) Adult Hippocampal Neurogenesis Modulates Fear Learning through Associative and Nonassociative Mechanisms. J Neurosci 35:1133011345.
- Siegmund A, Wotjak CT (2007) A mouse model of posttraumatic stress disorder that distinguishes between conditioned and sensitised fear. J Psychiatr Res 41:848-860.
- Friedman MJ, Resick PA, Bryant RA, Brewin CR (2011) Considering PTSD for DSM-5. Depress Anxiety 28:750-769.
- Slavich GM, Irwin MR (2014) From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol Bull 140:774-815.
- Berkenbosch F, van Oers J, del Rey A, Tilders F, Besedovsky H (1987) Corticotropin-releasing factor-producing neurons in the rat activated by interleukin-1. Science 238:524-526.
- Besedovsky H, del Rey A, Sorkin E, Dinarello CA (1986) Immunoregulatory feedback between interleukin-1 and glucocorticoid hormones. Science 233:652-654.
- Sapolsky R, Rivier C, Yamamoto G, Plotsky P, Vale W (1987) Interleukin-1 stimulates the secretion of hypothalamic corticotropinreleasing factor. Science 238:522-524.
- Paraiso AF, Mendes KL, Santos SH (2013) Brain activation of SIRT1: role in neuropathology. Mol Neurobiol 48:681-689.
- Young EA, Korszun A (2002) The hypothalamic-pituitary-gonadal axis in mood disorders. Endocrinol Metab Clin North Am 31:63-78.
- Paizanis E, Hamon M, Lanfumey L (2007) Hippocampal neurogenesis, depressive disorders, and antidepressant therapy. Neural Plast 2007:73754.
- Holsboer F, Ising M (2010) Stress hormone regulation: biological role and translation into therapy. Annu Rev Psychol 61:81-109, C1-11.
- McEwen BS (2007) Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev 87:873-904.
- Roozendaal B, Phillips RG, Power AE, Brooke SM, Sapolsky RM, et al. (2001) Memory retrieval impairment induced by hippocampal CA3 lesions is blocked by adrenocortical suppression. Nat Neurosci 4:1169-1171.
- Jankord R, Herman JP (2008) Limbic regulation of hypothalamopituitary-adrenocortical function during acute and chronic stress. Ann N Y Acad Sci 1148:64-73.
- Barkus C, McHugh SB, Sprengel R, Seeburg PH, Rawlins JN, et al. (2010) Hippocampal NMDA receptors and anxiety: at the interface between cognition and emotion. Eur J Pharmacol 626:49-56.
- Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA (2011) Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature 476:458-461.
- Moraes DS, Moreira DC, Andrade JMO, Santos SHS (2020) Sirtuins, brain and cognition: A review of resveratrol effects. IBRO Rep 9:46-51.
- Kurebayashi LFS, Gnatta JR, Kuba G, Giaponesi ALL, Souza TPB, et al. (2020) Massage and Reiki to reduce stress and improve quality of life: a randomized clinical trial. Rev Esc Enferm USP 54: e03612.
- Garland SN, Valentine D, Desai K, Li S, Langer C, et al. (2013) Complementary and alternative medicine use and benefit finding among cancer patients. J Altern Complement Med 19:876-881.
- Demir Dogan M (2018) The effect of reiki on pain: A meta-analysis. Complement Ther Clin Pract 31:384-387.
- Moreira DC, Guimarães VH, de Paula AMB, Guimarães ALS, de Rezende LF, et al. (2022) Syzygium jambolanum homeopathic Formulation Improves Diabetes Modulating Adipogenic Genes in Diet-Induced Obese Mice: Comparison to the Standard Metformin Treatment. Current Traditional Medicine 8:28-38.
- Toms R (2011) Reiki therapy: a nursing intervention for critical care. Crit Care Nurs Q 34:213-217.
- Brathovde A (2006) A pilot study: Reiki for self-care of nurses and healthcare providers. Holist Nurs Pract 20:95-101.
- Vitale A (2007) An integrative review of Reiki touch therapy research. Holist Nurs Pract 21:167-179; quiz 180-1.
- McManus DE (2017) Reiki Is Better Than Placebo and Has Broad Potential as a Complementary Health Therapy. J Evid Based Complementary Altern Med 22:1051-1057.
- Pocotte SL, Salvador D (2008) Reiki as a rehabilitative nursing intervention for pain management: a case study. Rehabil Nurs 33:231232.
- Gallob R (2003) Reiki: a supportive therapy in nursing practice and self-care for nurses. J N Y State Nurses Assoc 34:9-13.
- de Pinho L, Andrade JM, Paraiso A, Filho AB, Feltenberger JD, et al. (2013) Diet composition modulates expression of sirtuins and reninangiotensin system components in adipose tissue. Obesity (Silver Spring) 21:1830-1835.
- Aguiar JC, Gomes EP, Fonseca-Silva T, Velloso NA, Vieira LT, et al. (2013) Fluoxetine reduces periodontal disease progression in a conditioned fear stress model in rats. J Periodontal Res 48:632-637.
- Gomes EP, Aguiar JC, Fonseca-Silva T, Dias LC, Moura-Boas KP, et al. (2013) Diazepam reverses the alveolar bone loss and hippocampal interleukin-1beta and interleukin-6 enhanced by conditioned fear stress in ligature-induced periodontal disease in rats. J Periodontal Res 48:151-158.
- Gomes ESB, Farias LC, Silveira LH, Jesus CI, Rocha RGD, et al. (2019) Conditioned fear stress increases bone resorption in apical periodontitislesions in Wistar male rats. Arch Oral Biol 97:35-41.
- Monezi R (2003) Avaliação de efeitos da prática de impostação de mãos sobre os sistemas hematológico e imunológico de camundongos machos. Dissertação de Mestrado, Universidade de São Paulo 2003.
- de Oliveira Santana KN, Lelis DF, Mendes KL, Lula JF, Paraiso AF, et al. (2016) Metformin Reduces Lipogenesis Markers in Obese Mice Fed a Low-Carbohydrate and High-Fat Diet. Lipids 51:1375-1384.
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402-408.
- Xu M, Sun J, Wang Q, Zhang Q, Wei C, et al. (2018) Chronic restraint stress induces excessive activation of primordial follicles in mice ovaries. PLoS One 13: e0194894.
- Choleris E, Thomas AW, Kavaliers M, Prato FS (2001) A detailed ethological analysis of the mouse open field test: effects of diazepam, chlordiazepoxide and an extremely low frequency pulsed magnetic field. Neurosci Biobehav Rev 25:235-260.
- Ramos A, Mormede P (1998) Stress and emotionality: a multidimensional and genetic approach. Neurosci Biobehav Rev 22:33-57.
- Prut L, Belzung C (2003) The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol 463:3-33.
- Herman JP, McKlveen JM, Ghosal S, Kopp B, Wulsin A, et al. (2016) Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr Physiol 6:603-621.
- Smith SM, Vale WW (2006) The role of the hypothalamic-pituitaryadrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci 8:383-395.
- de Kloet ER, Karst H, Joels M (2008) Corticosteroid hormones in the central stress response: quick-and-slow. Front Neuroendocrinol 29:268-272.
- Orchinik M, Murray TF, Moore FL (1991) A corticosteroid receptor in neuronal membranes. Science 252:1848-1851.
- Reul JM, de Kloet ER (1985) Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology 117:2505-2511.
- Sorrells SF, Munhoz CD, Manley NC, Yen S, Sapolsky RM (2014) Glucocorticoids increase excitotoxic injury and inflammation in the hippocampus of adult male rats. Neuroendocrinology 100:129-140.
- Nozari M, Nahavandi A, Zeinivand M, Eslami Gharaati M, Godarzi M, et al. (2020) Ibuprofen Protection Against Restrained Chronic Stressinduced Depression in Male Rats. Basic Clin Neurosci 11:413-422.
- Liu L, Zhao Z, Lu L, Liu J, Sun J, et al. (2019) Icariin and icaritin ameliorated hippocampus neuroinflammation via mediating HMGB1 expression in social defeat model in mice. Int Immunopharmacol 75:105799.
- Jiang N, Lv J, Wang H, Huang H, Wang Q, et al. (2020) Ginsenoside Rg1 ameliorates chronic social defeat stress-induced depressive-like behaviors and hippocampal neuroinflammation. Life Sci 252:117669.
- Xie W, Meng X, Zhai Y, Zhou P, Ye T, et al. (2018) Panax Notoginseng Saponins: A Review of Its Mechanisms of Antidepressant or Anxiolytic Effects and Network Analysis on Phytochemistry and Pharmacology. Molecules 23:940.
- Li J, Gao W, Zhao Z, Li Y, Yang L, et al. (2022) Ginsenoside Rg1 Reduced Microglial Activation and Mitochondrial Dysfunction to Alleviate Depression-Like Behaviour Via the GAS5/EZH2/SOCS3/ NRF2 Axis. Mol Neurobiol 59:2855-2873.
- Zhang YQ, Wang XB, Xue RR, Gao XX, Li W (2019) Ginsenoside Rg1 attenuates chronic unpredictable mild stress-induced depressive-like effect via regulating NF-kappaB/NLRP3 pathway in rats. Neuroreport 30:893-900.
- Yamanishi K, Doe N, Mukai K, Hashimoto T, Gamachi N, et al. (2022) Acute stress induces severe neural inflammation and overactivation of glucocorticoid signaling in interleukin-18-deficient mice. Transl Psychiatry 12:404.
- Liu B, Xu C, Wu X, Liu F, Du Y, et al. (2015) Icariin exerts an antidepressant effect in an unpredictable chronic mild stress model of depression in rats and is associated with the regulation of hippocampal neuroinflammation. Neuroscience 294:193-205.
- Adachi M, Barrot M, Autry AE, Theobald D, Monteggia LM (2008) Selective loss of brain-derived neurotrophic factor in the dentate gyrus attenuates antidepressant efficacy. Biol Psychiatry 63:642-649.
- Shi Z, Ren H, Huang Z, Peng Y, He B, et al. (2017) Fish Oil Prevents Lipopolysaccharide-Induced Depressive-Like Behavior by Inhibiting Neuroinflammation. Mol Neurobiol 54:7327-7334.
- Xin C, Kim J, Quan H, Yin M, Jeong S, et al. (2019) Ginsenoside Rg3 promotes Fc gamma receptor-mediated phagocytosis of bacteria by macrophages via an extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinase-dependent mechanism. Int Immunopharmacol 77:105945.
- Xie W, Zhu T, Dong X, Nan F, Meng X, et al. (2019) HMGB1-triggered inflammation inhibition of notoginseng leaf triterpenes against cerebral ischemia and reperfusion injury via MAPK and NF-kappaB signaling pathways. Biomolecules 9:512.
- Dringen R (2000) Metabolism and functions of glutathione in brain. Prog Neurobiol 62:649-671.
- Sahin E, Gumuslu S (2004) Alterations in brain antioxidant status, protein oxidation and lipid peroxidation in response to different stress models. Behav Brain Res 155:241-248.
- Hwang KA, Hwang YJ, Hwang IG, Song J, Jun Kim Y (2019) Low temperature-aged garlic extract suppresses psychological stress by modulation of stress hormones and oxidative stress response in brain. J Chin Med Assoc 82:191-195.
- Jindal A, Mahesh R, Bhatt S (2013) Etazolate, a phosphodiesterase 4 inhibitor reverses chronic unpredictable mild stress-induced depression-like behavior and brain oxidative damage. Pharmacol Biochem Behav 105:63-70.
- Lucca G, Comim CM, Valvassori SS, Reus GZ, Vuolo F, et al. (2009) Effects of chronic mild stress on the oxidative parameters in the rat brain. Neurochem Int 54:358-362.
- Yu Y, Bai F, Wang W, Liu Y, Yuan Q, et al. (2015) Fibroblast growth factor 21 protects mouse brain against D-galactose induced aging via suppression of oxidative stress response and advanced glycation end products formation. Pharmacol Biochem Behav 133:122-131.
- Yardim A, Gur C, Comakli S, Ozdemir S, Kucukler S, et al. (2022) Investigation of the effects of berberine on bortezomib-induced sciatic nerve and spinal cord damage in rats through pathways involved in oxidative stress and neuro-inflammation. Neurotoxicology 89:127-139.
- Dragicevic N, Copes N, O’Neal-Moffitt G, Jin J, Buzzeo R, et al. (2011) Melatonin treatment restores mitochondrial function in Alzheimer’s mice: a mitochondrial protective role of melatonin membrane receptor signaling. J Pineal Res 51:75-86.
- Goshen I, Kreisel T, Ben-Menachem-Zidon O, Licht T, Weidenfeld J, et al. (2008) Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol Psychiatry 13:717-728.
- Murray F, Smith DW, Hutson PH (2008) Chronic low dose corticosterone exposure decreased hippocampal cell proliferation, volume and induced anxiety and depression like behaviours in mice. Eur J Pharmacol 583:115-127.
- Sawada M, Sawamoto K (2013) Mechanisms of neurogenesis in the normal and injured adult brain. Keio J Med 62:13-28.
- Pawluski JL, Brummelte S, Barha CK, Crozier TM, Galea LA (2009) Effects of steroid hormones on neurogenesis in the hippocampus of the adult female rodent during the estrous cycle, pregnancy, lactation and aging. Front Neuroendocrinol 30:343-357.
- Ormerod BK, Palmer TD, Caldwell MA (2008) Neurodegeneration and cell replacement. Philos Trans R Soc Lond B Biol Sci 363:153-170.
- Scorza F A ARM, Cysneiros R M, Scorza C A, Albuquerque M D Cavalheiro, E. A (2005) Estudo qualitativo da formação hipocampal de animais hipertensos com epilepsia. Arquivos de Neuro-Psiquiatria 63:283-288.
- He H, He H, Mo L, You Z, Zhang J (2024) Priming of microglia with dysfunctional gut microbiota impairs hippocampal neurogenesis and fosters stress vulnerability of mice. Brain Behav Immun 115:280-294.
- Lee E, Son H (2009) Adult hippocampal neurogenesis and related neurotrophic factors. BMB Rep 42:239-244.
- Yamashima T, Tonchev AB, Vachkov IH, Popivanova BK, Seki T, et al. (2004) Vascular adventitia generates neuronal progenitors in the monkey hippocampus after ischemia. Hippocampus 14:861-875.
- Kiryk A, Sowodniok K, Kreiner G, Rodriguez-Parkitna J, Sonmez A, et al. (2013) Impaired rRNA synthesis triggers homeostatic responses in hippocampal neurons. Front Cell Neurosci 7:207.
- Gerges NZ, Alzoubi KH, Park CR, Diamond DM, Alkadhi KA (2004) Adverse effect of the combination of hypothyroidism and chronic psychosocial stress on hippocampus-dependent memory in rats. Behav Brain Res 155:77-84.
- Bramham CR, Southard T, Ahlers ST, Sarvey JM (1998) Acute cold stress leading to elevated corticosterone neither enhances synaptic efficacy nor impairs LTP in the dentate gyrus of freely moving rats. Brain Res 789:245-255.
- Yamada K, McEwen BS, Pavlides C (2003) Site and time dependent effects of acute stress on hippocampal long-term potentiation in freely behaving rats. Exp Brain Res 152:52-59.
- Aleisa AM, Alzoubi KH, Gerges NZ, Alkadhi KA (2006) Nicotine blocks stress-induced impairment of spatial memory and long-term potentiation of the hippocampal CA1 region. Int J Neuropsychopharmacol 9:417426.
- Vouimba RM, Munoz C, Diamond DM (2006) Differential effects of predator stress and the antidepressant tianeptine on physiological plasticity in the hippocampus and basolateral amygdala. Stress 9:2940.
- Dringenberg HC, Oliveira D, Habib D (2008) Predator (cat hair)induced enhancement of hippocampal long-term potentiation in rats: involvement of acetylcholine. Learn Mem 15:112-116.
- Lee IC, Yu TH, Liu WH, Hsu KS (2021) Social Transmission and Buffering of Hippocampal Metaplasticity after Stress in Mice. J Neurosci 41:1317-1330.
- Khoo GH, Lin YT, Tsai TC, Hsu KS (2019) Perineuronal Nets Restrict the Induction of Long-Term Depression in the Mouse Hippocampal CA1 Region. Mol Neurobiol 56:6436-6450.
- Picard K, Bisht K, Poggini S, Garofalo S, Golia MT, et al. (2021) Microglial-glucocorticoid receptor depletion alters the response of hippocampal microglia and neurons in a chronic unpredictable mild stress paradigm in female mice. Brain Behav Immun 97:423-439.
- Billot M, Daycard M, Wood C, Tchalla A (2019) Reiki therapy for pain, anxiety and quality of life. BMJ Support Palliat Care 9:434-438.
Supplementary Materials

Figure S1: Photos of the Glove Control and Reiki application technique. Source: Researcher’s personal collection.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.