Psychopharmacology of Brazilian Native Plants Focusing on Mood: A Bibliographical Survey Followed by a Systematic Review
by by Allan Falconi-Souto1*, Gabriela Morales-Lima1, Yutaka Kuroki2, Fúlvio Rieli Mendes1
1Center for Natural and Human Sciences, Federal University of ABC, Alameda da Universidade, SN, São Bernardo do Campo, 09606-045, São Paulo, Brazil
2Delightex Pte. Ltd., 230 Victoria Street #15-01, Bugis Junction Towers, 188024, Singapore
*Corresponding author: Allan Falconi-Souto, Center for Natural and Human Sciences, Federal University of ABC, Alameda da Universidade, SN, São Bernardo do Campo, 09606-045, São Paulo, Brazil.
Received Date: 16 January 2025
Accepted Date: 23 January 2025
Published Date: 27 January 2025
Citation: Falconi-Souto A, Morales-Lima G, Kuroki Y, Mendes FR (2025) Psychopharmacology of Brazilian Native Plants Focusing on Mood: A Bibliographical Survey Followed by a Systematic Review. Curr Res Cmpl Alt Med 9: 262. https://doi.org/10.29011/2577-2201.100262
Abstract
Given the difficulty of treating some psychiatric and neurodegenerative conditions, Brazil’s rich biodiversity provides an optimal scenario for exploring therapeutic alternatives based on bioactive compounds from natural products. 30 non-scientific books were consulted to identify plants popularly used for any purposes related to feelings of well-being. Native species with four or more citations in different books were considered as most popular species, and underwent a systematic review in PubMed, LILACS and Periódicos CAPES data bases, including articles in the area of psychopharmacology published up to December 2023, written in Portuguese, English or Spanish.The major use of the 27 native species identified in the survey was as stimulant or calmative, mainly through aqueous extractions of leaves and barks. The systematic review includes 568 articles on the psychopharmacological effects of 23 of the original 27 native species identified, from which 28 articles corresponds to clinical stage.Although most of the studies reviewed support the popular use attributed to some species, it is noteworthy that the vast majority relate to preliminary studies, especially on in vitro antioxidant activity, and that clinical studies are still very scarce. In this sense, this review may serve not only as a guide to the effects popularly and scientifically attributed to Brazilian flora products, but also to encourage future research and the development of alternative therapies against psychological stress.
Keywords: Brazilian flora; Non-scientific literature, Adaptogens; Psychopharmacology; Stress; Systematic review.
Introduction
Brazil is one of the most biodiverse countries in the world, as it is estimated that thousands of plant species are distributed along its six phytogeographic domains, being almost half of them endemic (Brazil Flora Group, 2022). This high level of biodiversity makes Brazilian flora an optimal scenario for the investigation of bioactive natural products. However, most plant-derived medicines currently approved focus on other disorders than the ones affecting the central nervous system. For instance, from 71 species in the List of Medicinal Plants of Interest to the Unified Health System in Brazil, only three are indicated for central nervous system disorders, all of them for anxiety (Silva et al., 2022).
It is estimated that one in eight people have some mental disorder in the worldwide population, with anxiety and depressive disorders being the most common problems (World Health Organization, 2022). Mental disorders are caused, among other factors, by physical and mental stress, and mechanisms that counteract some kind of stress are among the main focus of search for their therapies. Adaptogens are natural substances that provide organic resistance through an increase in its capacity to respond to stress by interacting with biomarkers such as corticosteroids and antioxidant enzymes (Mendes and Carlini, 2007). Adaptogens can improve not only physical resistance, but also mental performance, by counteracting stress with an nonspecific pharmacological mechanism (Panossian et al., 2021).
Since psychological stress affects emotional well-being and triggers neuroinflammatory and neuroendocrine responses, which can result in neuropsychiatric conditions (Salim, 2016), the psychological effects of adaptogens are an important matter of study, in respect of subjective feelings of well-being, such as happiness, tranquility, mood and motivation. This study focused on a literature review of Brazilian plants with popular use for general well-being and delightful moments, aiming to find the most common species used for this purpose and scientific studies that could provide support for these folk use and possible mechanisms involved.
Methodology
Survey about popular use
Non-scientific literature was consulted in order to find out plants popularly used for any purposes related to feelings of well-being, classified into six categories defined in Table 1. The list of related terms was updated every time that a new term and use were found during the survey. The literature consulted was composed of a total of 30 books, from which 27 were written in Portuguese, two in English and one in Spanish (Supplementary data 1).
The species with relevant use were included in a data base designed for this study, containing its part, recommendation, preparation, posology and the source of the information. Spelling errors on books were identified and corrected manually, while synonyms were grouped under the scientific names currently accepted, according to Rio de Janeiro Botanical Garden (2023), Missouri Botanical Garden (2023), and IPNI (2023) data bases. Species with four or more citations in different books were maintained as most popular species, and their origin and phytogeographic domains were determined according to the Rio de Janeiro Botanical Garden (2023) data base. Threatened species were considered according to the Official List of Threatened Brazilian Flora Species (Ministério do Meio Ambiente, 2022). Only native or endemic species were selected for the systematic review of scientific literature.
|
Category |
Description |
Terms |
|
Energy |
Provide physical and mental boosts, enhance attention, mood and motivation; Physiological activation and sensory stimulation; arousal |
Energy, energizer, restorative, vitalizing, rejuvenator, anti-ageing, brain stimulant, memory booster, exciting, uplifting, revitalizing Increases concentration, focus and cognitive function Prevents exhaustion, tiredness, weakness, asthenia, atonia, fatigue, lethargy, somnolence, drowsiness, indisposition and debility Helps with lack of attention, memory or energy Biomarkers: adrenaline, adenosine, ATP, acetylcholine/cholinergic transmission |
|
Relaxatio n |
Release the stress and anxiety accumulated in their hectic day to day lives; |
Relaxing, calmative, tranquilizing, soothing, easeful, relaxation, sedative |
|
emotional state of low tension, absence of arousal |
Possess restful properties and acts as a stress reliever, avoid anxiety, boredom, hysteria, nervousness, inquietude, uneasiness, insomnia and nervous breakdown Unwind, peaceful mind, restful sleep, central nervous system depression Biomarkers: cortisol/corticosterone |
|
|
Happiness |
Feelings of calm, satisfaction and contentment; In tune with the world around us, including pleasure, meaning and commitment |
Pleasant, delightful, enjoyable, brings happiness Avoids loneliness and unhappiness, antidepressant Biomarkers: serotonin, positive mood |
|
Euphoria/ Pleasure |
Initiate a feeling or state of intense excitement and happiness; Associated with reward, containing liking and wanting |
Pleasure and reward system Euphoria, excitement, joyousness, ecstasy, delight Biomarkers: dopamine, endorphins |
|
Love/ Social bonding |
Sensing a feeling of bonding and coherence; Familiar love, self-love, selfless love, enduring love; Involvement/attachment to families, other people, and activities, commitment to social norms and institutions |
Love, helps with insight, introspection and selfexamination Self-love, warmth, bonding Biomarkers: oxytocin |
|
Arousal |
Arousal, sensory alertness, desire, sexual wellness, romance |
Aphrodisiac, sexual wellness, impotence Biomarkers: androgens, testosterone |
Table 1: Mood states categories and possible psychopharmacological effects related.
Scientific evidence
Scientific literature was searched in PubMed, CAPES and LILACS data bases. The search process was done using the scientific binomial name and identified synonyms of each plant selected previously as key-words, and we included studies published up to December 2023. The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guideline was followed for the systematic review of the identified articles (Page et al., 2021).
Relevant studies were selected based on its title and abstract, when supporting the popular use or with potential central nervous system effect related to the present project. Studies were excluded if written in a language other than English, Spanish or Portuguese, or if the full text could not be obtained. Only experimental studies with plant extracts investigating its popular use were included: reviews and case-control studies or experiments with isolated compounds or plant mixtures were excluded from this review. Articles in which the scientific names of the species were not explicitly mentioned were also excluded.
The articles selected were fully read in order to confirm its validity to this review’s purposes, which resulted in an inclusion of 568 studies (Figure 1).
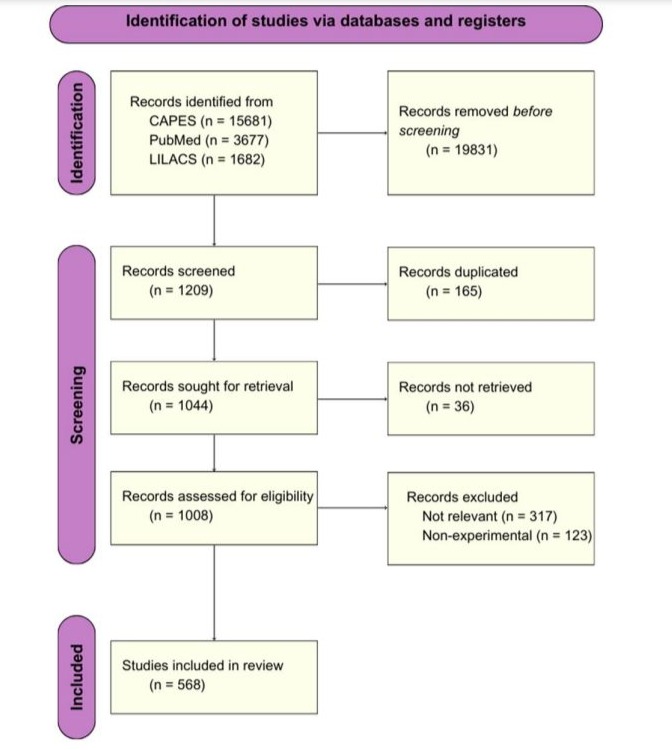
Figure 1: PRISMA flow diagram for new reviews which included searches of data bases.
Results
The non-scientific literature survey identified 115 species cited in at least four books, being twenty-seven species native to Brazil (Table 2). Most species were cited in the books by their correct botanical name, but there are several cases of synonyms or invalid names, in which we had to change for the most accepted name.
An example is the species “casca-de-anta”, cited as Drimys granadensis, that we considered as Drimys brasiliensis subsp. Brasiliensis, according to the Rio de Janeiro Botanical Garden (2023). Another particular case is one of the species known as “catuaba” and cited as Erythroxylum catuaba in the survey, which was renamed to Protium catuaba, according to the synonym available in IPNI (2023).
From the 27 native species, five are endemic in Brazil (Rio de Janeiro Botanical Garden, 2023): Aniba canelilla (Kunth) Mez (Lauraceae), Drimys brasiliensis Miers subsp. brasiliensis (Winteraceae), Himatanthus bracteatus (A. DC.) Woodson (Apocynaceae), Passiflora alata Curtis (Passifloraceae) and Protium catuaba (Soares da Cunha) Daly & P.Fine (Burseraceae). Among the selected species, two are threatened: Anemopaegma arvense (Vell.) Stellfeld ex de Souza is considered endangered, while P. catuaba is considered vulnerable (Ministério do Meio Ambiente, 2022).
We did not find experimental studies with D. brasiliensis subsp. brasiliensis, Himatanthus bracteatus, Protium catuaba and Pluchea sagittalis related to psychopharmacological effects. The other 23 species resulted in at least one study investigating such effects, which added up to 568 relevant studies for the systematic review. In some cases, however, we only found studies when searching by the species synonyms, as occurred to three of them: A. arvense, found by Anemopaegma mirandum; Aristolochia labiata, by Aristolochia brasiliensis; and P. catuaba, by Erythroxylum catuaba. The 540 pre-clinical studies reviewed are shown in Table 3, while the 28 clinical trials are better presented in Table 4.
|
Scientific name (family) |
Synony ms |
Popular name |
Phytogeogr aphic domains |
Parts |
Mode of use |
Popular use |
|
Anacardiu m occidentale L. (Anacardia ceae) |
Cajueiro , cajuzeiro |
Amazon Rainforest, Caatinga, Central Brazilian Savanna, Atlantic Rainforest, Pampa, Pantanal |
Bark, flowers, fruits, leaves, nuts |
Cooking, decoction, juice, maceration |
Aphrodisiac (Almeida, 1993; Alzugaray andAlzugaray, 2000; Bontempo, 1992; Cruz, 1965, Stasi andHiruma-Lima, 2002; Moreira, 1978) |
|
|
Bark, peduncle |
Cooking, decoction |
Asthenia (Alzugaray and Alzugaray, 2000; Bontempo, 1992; Corrêa et al., 1998; Cruz, 1965, Stasi andHiruma-Lima, 2002; Moreira, 1978) |
||||
|
Bark |
Cooking, decoction |
Weakness (Alzugaray and Alzugaray, 2000; Bontempo, 1992; Cruz, 1965) |
||||
|
Flowers, fruits, leaves, nuts |
Juice, maceration |
Exciting, stimulant (Almeida, 1993; Di Stasi and Hiruma-Lima, 2002) |
||||
|
Bark, flowers, fruits, leaves |
Cooking, decoction |
Atonia (Cruz, 1965, Stasi and Hiruma-Lima, 2002) |
||||
|
Peduncle |
Central nervous system depressant (Corrêa et al., 1998) |
|||||
|
Nuts |
Juice |
Memory fortifying (Almeida, 1993) |
||||
|
Bark |
Cooking |
Impotence (Cruz, 1965) |
||||
|
Anemopae gma arvense (Vell.) Stellfeld ex de Souza |
Anemopa egma mirandu m, Bignonia arvensis, |
Catuaba, tatuaba, catuabaverdadei ra, verga- |
Amazon Rainforest, Central Brazilian Savanna, Atlantic |
Aerial parts, bark, leaves, rhizome, roots |
Alcoholic preparations, decoction, tea, tincture, wine |
Aphrodisiac (Albuquerque et al., 2018; Almeida, 1993; Almeida et al., 1998; Alzugaray and Alzugaray, 2000; Corrêa et al., 1998; Lorenzi and Matos, 2002; Moreira, 1978) |
|
(Bignoniac eae) |
Bignonia miranda |
teso |
Rainforest |
Aerial parts, bark, roots |
Alcoholic preparations, decoction, tea, tincture, wine |
Memory (Albuquerque et al., 2018; Almeida, 1993; Alzugaray and Alzugaray, 2000; Lorenzi and Matos, 2002) |
|
Aerial parts, bark, roots |
Alcoholic preparations, decoction, tea, tincture, wine |
Stimulant (Albuquerque et al., 2018; Almeida, 1993; Lorenz i and Matos, 2002; Moreira, 1978) |
||||
|
Bark, rhizome, roots |
Decoction, tea, wine |
Asthenia (Alzugaray and Alzugaray, 2000; Corrêa et al., 1998; Lorenzi and Matos, 2002) |
||||
|
Bark, roots |
Decoction, tea, tincture, wine |
Energetic (Almeida, 1993; Alzugaray and Alzugaray, 2000; Lorenzi and Matos, 2002) |
||||
|
Bark, roots |
Decoction, tea, wine |
Impotence (Matos, 1999, Balbachas, 1956; Lorenzi and Matos, 2002) |
||||
|
Bark, roots |
Decoction, tea, tincture, wine |
Nervousness (Almeida, 1993; Alzugaray and Alzugaray, 2000; Lorenzi and Matos, 2002) |
||||
|
Bark, rhizome, roots |
Tea, wine |
Anxiety (Corrêa et al,. 1998; Lorenzi and Matos, 2002) |
||||
|
Bark, roots |
Decoction, tea, wine |
Insomnia (Alzugaray and Alzugaray, 2000; Lorenzi and Matos, 2002) |
||||
|
Bark, roots |
Decoction, tincture |
Neurasthenia (Almeida, 1993; Lorenzi and Matos, 2002) |
||||
|
Bark |
Decoction, tincture |
Agitated sleep, intimate weakness (Almeida, 1993) |
||||
|
Bark, roots |
Decoction |
To raise up the nervous system (Balbach, 1969) |
||||
|
Bark |
Weakness (Matos, 1999) |
|||||
|
Aniba canelilla (Kunth) Mez (Lauraceae ) |
|
Casca preciosa, folha preciosa |
Amazon Rainforest, Central Brazilian Savanna, Atlantic Rainforest |
Bark, leaves |
Infusion, tea |
Exciting, stimulant (Almeida, 1993; Balbach, 1969; Berg, 1982, Lorenzi and Matos, 2002) |
|
Bark, leaves |
Tea |
Nervous exhaustion (Berg, 1982, Lorenzi and Matos, 2002) |
||||
|
Bark, leaves, seeds |
Essential oil |
Nervous tension (Albuquerque et al., 2018) |
||||
|
Aristolochi a cymbifera Mart. & Zucc. (Aristoloch iaceae) |
|
Papo-deperu, jarrinha, milhomens |
Central Brazilian Savanna, Atlantic Rainforest |
Roots |
Decoction |
Hysteria (Almeida, 1993; Balbach, 1969; Balbachas, 1956; Cruz, 1965) |
|
Roots |
Decoction |
Sedative (Almeida, 1993; Balbach, 1969; Balbachas, 1956; Lorenzi and Matos, 2002) |
||||
|
Weakness (Bontempo, 1992; Cruz, 1965) |
||||||
|
Stimulant (Cruz, 1965) |
|
Aristolochi a labiata Willd. (Aristoloch iaceae) |
Aristoloc hia brasiliens is |
Papo-deperu, angelicó, jarrinha, milhomens |
Caatinga, Central Brazilian Savanna, Atlantic Rainforest |
Roots |
Decoction |
Sedative (Almeida, 1993; Balbach, 1969; Lorenzi and Matos, 2002) |
|
Roots |
Decoction |
Hysteria (Almeida, 1993; Balbach, 1969) |
||||
|
Leaves, roots, stems |
Anxiety (Corrêa et al., 1998) |
|||||
|
Drimys brasiliensis Miers (Winterace ae) |
Drimys chilensis, Drimys winteri |
Cascade-anta, paratudo, cascad'anta |
Caatinga, Central Brazilian Savanna, Atlantic Rainforest |
Bark |
Bath, decoction |
Stimulant (Almeida, 1993; Balbach, 1969; Balmé, 1982) |
|
Bark |
Decoction |
General weakness (Almeida, 1993; Balbach, 1969) |
||||
|
Bark |
Bath |
Tiredness (Balmé, 1982) |
||||
|
Drimys brasiliensis Miers subsp. brasiliensis (Winterace ae) |
Drymis granaden sis1 |
Cascade-anta, paratudo, cascad'anta |
Central Brazilian Savanna |
Bark |
Decoction |
General weakness (Balbachas, 1956; Bontempo, 1992; Cruz, 1965) |
|
Bark, leaves |
Stimulant (Saint-Hilaire, 2009) |
|||||
|
Erythrina crista-galli L. (Fabaceae) |
|
Mulung u, florde-coral |
Central Brazilian Savanna, Atlantic Rainforest, Pampa, Pantanal |
Bark |
Bath, decoction |
Calmative (Almeida, 1993; Alzugaray and Alzugaray, 2000; Balbach, 1969; Balbachas, 1956) |
|
Bark |
Bath |
Insomnia (Almeida, 1993) |
||||
|
Erythrina mulungu Mart. (Fabaceae) |
|
Mulung u |
Central Brazilian Savanna |
Bark |
Bath, tea |
Insomnia (Almeida, 1993; Balbach, 1969; Balbachas, 1956; Corrêa et al., 1998; Lorenzi and Matos, 2002) |
|
Bark |
Bath, tea |
Calmative (Almeida, 1993; Cruz, 1965; Lorenzi and Matos, 2002) |
||||
|
Bark |
Bath, tea |
Anxiety (Corrêa et al., 1998; Lorenzi and Matos, 2002) |
||||
|
Bark |
Bath, tea |
Psychomotor agitation (Corrêa et al., 1998; Lorenzi and Matos, 2002) |
||||
|
Bark |
Tea |
Hysteria, nervous tension, sedative (Lorenzi and Matos, 2002) |
||||
|
Nervous agitation (Cruz, 1965) |
||||||
|
Erythroxyl um coca Lam. (Erythroxy laceae) |
|
Coca |
Amazon Rainforest |
Leaves |
Elixir, infusion |
Brain stimulant (Alzugaray and Alzugaray, 2000; Moreira, 1978) |
|
Leaves |
Elixir, wine |
Exhaustion (Alzugaray and Alzugaray, 2000; Balmé, 1982) |
||||
|
Nervous manifestation, recover organic losses, tiredness, vital energy (Cruz, 1965) |
||||||
|
Antifatigue, calmative, restorative of central nervous system (Alzugaray and Alzugaray, 2000) |
||||||
|
Himatanth us |
Plumeria lancifolia |
Agoniad a |
Atlantic Rainforest |
Leaves |
Infusion |
Hysteria (Balbach, 1969; Balbachas, 1956) |
|
bracteatus (A. DC.) Woodson (Apocynac eae) |
Bark |
Anxiety (Corrêa et al, 1998) |
||||
|
Bark |
Cooking |
General weakness (Cruz, 1965) |
||||
|
Hymenaea courbaril L. (Fabaceae) |
|
Jatobá |
Amazon Rainforest, Caatinga, Central Brazilian Savanna, Atlantic Rainforest, Pantanal |
Bark, resin, sap |
Cooking, wine |
Fortifier (Bontempo, 1992; Cruz, 1965; Di-Stasi and Hiruma-Lima, 2002; Lorenzi and Matos, 2002) |
|
Bark, fruits, resin |
Cooking |
Weakness (Bontempo, 1992; Matos, 1999) |
||||
|
Nervousness (Cabral-Born, 2009) |
||||||
|
Fatigue, sedative (Lorenzi and Matos, 2002) |
||||||
|
Ilex paraguarie nsis A.St.Hil. (Aquifoliac eae) |
|
Ervamate, mate |
Caatinga, Central Brazilian Savanna, Atlantic Rainforest, Pampa |
Leaves |
Infusion |
Stimulant (Alzugaray and Alzugaray, 2000; Bontempo, 1992; Lorenzi and Matos, 2002; Simões et al., 1986) |
|
Infusion |
Vitalizing (Alzugaray and Alzugaray, 2000; Cruz, 1965) |
|||||
|
Leaves |
Infusion |
Muscular and mental fatigue (Lorenzi and Matos, 2002) |
||||
|
Provides strength and energy, fatigue resistance (Cruz, 1965) |
||||||
|
Infusion |
Tiredness (Alzugaray and Alzugaray, 2000) |
|||||
|
Indigofera suffruticos a Mill. (Fabaceae) |
Indigofer a anil |
Anil, anileiro |
Amazon Rainforest, Caatinga, Central Brazilian Savanna, Atlantic Rainforest, Pampa |
Leaves, roots |
Infusion |
Sedative (Almeida, 1993; Alzugaray and Alzugaray, 2000; Balbach, 1969; Balbachas, 1956) |
|
Lippia alba (Mill.) N.E.Br. ex Britton & P.Wilson (Verbenace ae) |
Lantana alba, Lippia citrata, Lippia geminata |
Erva- cidreira, falsamelissa, ervacidreirabrasileir a, ervacidreiradearbusto |
Amazon Rainforest, Caatinga, Central Brazilian Savanna, Atlantic Rainforest, Pampa, Pantanal |
Leaves, roots |
Alcoholic extracts, bath, compress, decoction, infusion, syrup, tea |
Sedative (Albuquerque et al., 2018; Gutiérrez et al., 2010; Haragushi and Carvalho, 2010; Lorenzi and Matos, 2002; Matos, 1999) |
|
Leaves, roots |
Alcoholic extracts, bath, boiling, compress, decoction, infusion, tea |
Calmative (Cabral-Born, 2009; Di Stasi and Hiruma-Lima, 2002; Gutiérrez et al., 2010; Junior et al., 2013; Lorenzi and Matos, 2002) |
||||
|
Leaves, roots |
Infusion |
Insomnia (Almeida, 1993; Di Stasi and Hiruma-Lima, 2002; Haragushi and Carvalho, 2010) |
||||
|
Leaves, roots |
Tea |
Hysteria (Almeida, 1993; Balbach, 1969; Balbachas, 1956) |
|
Leaves, roots |
Alcoholic extracts, bath, compress, infusion, syrup, tea |
Anxiety (Albuquerque et al., 2010; Lorenzi and Matos, 2002) |
||||
|
Leaves |
Tea |
Lethargy, strengthen brain and nerves (Balbach, 1969; Balbachas, 1956) |
||||
|
Leaves |
Tea |
Nervousness (Lorenzi and Matos, 2002; Matos, 1999) |
||||
|
Leaves, roots |
Alcoholic extracts, bath, compress, decoction, infusion, syrup |
Tranquilizer (Albuquerque et al., 2018; Gutiérrez et al., 2010) |
||||
|
Leaves |
Infusion |
Dizziness (Haragushi and Carvalho, 2010) |
||||
|
Leaves |
Inquietude (Matos, 1999) |
|||||
|
Leaves |
Tea |
Uneasiness (Lorenzi and Matos; 2002) |
||||
|
Passiflora alata Curtis (Passiflora ceae) |
Maracuj azeiro, maracujá -doce |
Amazon Rainforest, Central Brazilian Savanna, Atlantic Rainforest, Pampa |
Fruits, leaves, roots |
Decoction, infusion |
Insomnia (Corrêa et al., 1998; Junior et al., 2013; Lorenzi and Matos, 2002) |
|
|
Fruits, leaves, roots |
Infusion |
Anxiety (Corrêa et al., 1998; Junior et al., 2013) |
||||
|
Leaves |
Decoction |
Calmative (Lorenzi and Matos, 2002; Simões et al., 1986) |
||||
|
Fruits, leaves, roots |
Central nervous system depressant (Corrêa et al., 1998) |
|||||
|
Leaves |
Decoction |
Nervousness (Lorenzi and Matos, 2002) |
||||
|
Leaves |
Sedative (Simões et al., 1986) |
|||||
|
Passiflora edulis Sims (Passiflora ceae) |
Maracuj á, maracujá -azedo |
Amazon Rainforest, Caatinga, Central Brazilian Savanna, Atlantic Rainforest, Pampa, Pantanal |
Fruits, leaves, roots |
Decoction, infusion |
Insomnia (Almeida, 1993; Corrêa et al., 1998; Haragushi and Carvalho, 2010; Junior et al., 2013; Lorenzi and Matos, 2002; Matos, 1999) |
|
|
Fruits, leaves, roots |
Decoction, infusion |
Anxiety (Corrêa et al., 1998; Haragushi and Carvalho, 2010; Junior et al., 2013) |
||||
|
Fruits, leaves |
Boiling, decoction |
Nervousness (Cabral-Born, 2009; Lorenzi and Matos, 2002; Matos, 1999) |
||||
|
Leaves |
Decoction, infusion |
Calmative (Haragushi and Carvalho, 2010; Lorenzi and Matos, 2002) |
||||
|
Fruits, leaves, roots |
Central nervous system depressant (Almeida, 1993; Corrêa et al., 1998) |
|
Leaves |
Hysteria, neurasthenia, sedative (Almeida, 1993) |
|||||
|
Passiflora quadrangu laris L. (Passiflora ceae) |
Maracuj á-açu |
Amazon Rainforest, Caatinga, Central Brazilian Savanna, Atlantic Rainforest, Pampa, Pantanal |
Leaves |
Infusion |
Insomnia (Alzugaray and Alzugaray, 2000; Balbach, 1969; Balbachas, 1956; Cruz, 1965) |
|
|
Leaves |
Infusion |
Neurasthenia (Balbach, 1969; Balbachas, 1956; Cruz, 1965) |
||||
|
Leaves |
Infusion |
Calmative (Alzugaray and Alzugaray, 2000; Balbachas, 1956) |
||||
|
Leaves |
Infusion |
Nervous breakdown (Balbach, 1969; Balbachas, 1956) |
||||
|
Leaves |
Infusion |
Sedative (Alzugaray and Alzugaray, 2000; Cruz, 1965) |
||||
|
Anaphrodisiac, depression (Balbach, 1969) |
||||||
|
Paullinia cupana Kunth (Sapindace ae) |
Paulinia cupana var. sorbilis, Paullinia sorbilis |
Guaraná, guaranaz eiro |
Amazon Rainforest |
Seeds |
Capsule, infusion, powder, syrup, tea |
Stimulant (Almeida, 1993; Alzugaray and Alzugaray, 2000; Berg, 1982; Haraguchi and Carvalho, 2010; Junior et al., 2013; Lorenzi and Matos, 2002; Simões et al., 1986) |
|
Seeds |
Asthenia (Almeida, 1993; Corrêa et al., 1998; Cruz, 1965) |
|||||
|
Seeds |
Capsule, powder, syrup, tea |
Aphrodisiac (Berg, 1982; Junior et al., 2013) |
||||
|
Seeds |
Infusion |
Depression (Almeida, 1993; Alzugaray and Alzugaray, 2000) |
||||
|
Capsule, syrup, tea |
Fatigue (Junior et al., 2013; Lorenzi and Matos, 2002) |
|||||
|
Seeds |
Indisposition (Corrêa et al., 1998; Matos, 1999) |
|||||
|
Seeds |
Capsule, syrup, tea |
Physical and mental exhaustion (Corrêa et al., 1998; Junior et al., 2013) |
||||
|
Seeds |
Weakness (Bontempo, 1992; Matos, 1999) |
|||||
|
Atonia, to favor intellectual activity (Almeida, 1993) |
||||||
|
Calmative, general well-being (Cruz, 1965) |
||||||
|
Seeds |
Drowsiness (Matos, 1999) |
|||||
|
Early aging, fatigue (Lorenzi and Matos, 2002) |
||||||
|
Invigorating (Bontempo, 1992) |
||||||
|
Capsule, syrup, tea |
Somnolence (Junior et al., 2013) |
|||||
|
Pluchea sagittalis |
Pluchea quitoc |
Quitoco |
Amazon Rainforest, |
Leaves, stems |
Bath |
Hysteria (Balbach, 1969; Balbachas, 1956; Cruz, 1965; |
|
(Lam.) Cabrera (Asteracea e) |
Caatinga, Central Brazilian Savanna, Atlantic Rainforest, Pampa |
Lorenzi and Matos, 2002) |
||||
|
Leaves, stems |
Bath |
Stimulant (Balbach, 1969; Balbachas, 1956) |
||||
|
Leaves, stems |
Anxiety, insomnia (Corrêa et al., 1998) |
|||||
|
Protium catuaba (Soares da Cunha) Daly & P.Fine (Burserace ae) |
Erythrox ylum catuaba2 |
Catuaba, tatuaba |
Atlantic Rainforest |
Bark |
Decoction, tincture |
Aphrodisiac, stimulant (Almeida, 1993; Bontempo, 1992; Cruz, 1965) |
|
Bark |
Decoction |
Impotence (Balbachas, 1956; Bontempo, 1992; Cruz, 1965) |
||||
|
Bark |
Decoction, tincture |
Neurasthenia (Almeida, 1993; Cruz, 1965) |
||||
|
Bark |
Decoction, tincture |
Agitated sleep, energetic, intimate weakness, nervousness, memory (Almeida, 1993) |
||||
|
Insomnia, nervous affections, to strengthen the nervous system (Cruz, 1965) |
||||||
|
Bark |
Decoction |
To raise up the nervous system (Balbach, 1969) |
||||
|
Ptychopeta lum olacoides Benth. (Olacaceae ) |
|
Marapua ma, muirapu ama |
Amazon Rainforest |
Branches, roots, stems |
Tea |
Impotence (Almeida, 1993; Balbach, 1969; Balbachas, 1956; Lorenzi and Matos, 2002) |
|
Branches, roots, stems |
Asthenia (Almeida, 1993; Balbach, 1969; Corrêa et al., 1998) |
|||||
|
Branches, roots, stems |
Weakness (Almeida, 1993; Balbach, 1969; Balbachas, 1956) |
|||||
|
Roots |
Alcoholic preparations, dried and ground, tea |
Aphrodisiac (Albuquerque et al., 2018; Lorenzi and Matos, 2002) |
||||
|
Roots |
Alcoholic preparations, dried and ground, tea |
Antifatigue, debility, improve cognitive function, neurasthenia (Albuquerque et al., 2018) |
||||
|
Roots, stems |
Indisposition (Corrêa et al., 1998) |
|||||
|
Schinus terebinthif olia Raddi (Anacardia ceae) |
Schinus antiarthri tica, Schinus terebinthi folius, Schinus terebinthi folius var. selloana |
Aroeira, aroeiramansa, aroeiravermelh a |
Caatinga, Central Brazilian Savanna, Atlantic Rainforest, Pampa |
Atonia (Balbach, 1969) |
||
|
Calmative, impotence (Cruz, 1965) |
||||||
|
Stimulant (Di Stasi and HirumaLima, 2002) |
||||||
|
Sonchus oleraceus L. (Asteracea e) |
Sonchus laevis |
Serralha, chicóriabrava |
Caatinga, Central Brazilian Savanna, Atlantic Rainforest |
Leaves |
Cooking, decoction |
To strengthen nerve/nervous system (Balbach, 1969; Bontempo, 1992; Cruz, 1965) |
|
Whole plant |
Asthenia (Corrêa et al., 1998; Lorenzi and Matos, 2002) |
|||||
|
Theobroma cacao L. (Malvacea e) |
Cacau, cacaueir o |
Amazon Rainforest, Atlantic Rainforest |
Seeds |
Weakness (Alzugaray and Alzugaray, 2000; Matos, 1999) |
||
|
Seeds |
Drowsiness (Matos, 1999) |
|||||
|
Seeds |
Energetic, stimulant (Lorenzi and Matos, 2002) |
|||||
|
Excitatory of brain functions, exhaustion (Alzugaray and Alzugaray, 2000) |
||||||
|
Nervous exciting (Balmé, 1982) |
||||||
|
Turnera diffusa Willd. ex Schult. (Turnerace ae) |
Turnera aphrodisi aca |
Damiana |
Caatinga, Central Brazilian Savanna, Atlantic Rainforest |
Tincture |
Aphrodisiac (Alzugaray and Alzugaray, 2000; Moreira, 1978; Thomson, 1978) |
|
|
Tincture |
Stimulant (Alzugaray and Alzugaray, 2000; Balbach, 1969; Moreira, 1978) |
|||||
|
Leaves |
Infusion |
Impotence (Balbach 1969; Morgan, 1979) |
||||
|
Debility, exhaustion, nervousness (Thomson, 1978) |
||||||
|
Neurasthenia (Moreira, 1978) |
||||||
|
Xylopia aromatica (Lam.) Mart. (Annonace ae) |
Pimentadosnegros, pimentademacaco |
Amazon Rainforest, Central Brazilian Savanna |
Bark, seeds |
Pulverized roasted seeds, tincture |
Exciting, stimulant (Almeida, 1993; Balbach, 1969; Berg, 1982; Lorenzi and Matos, 2002) |
|
|
Bark, seeds |
Pulverized roasted seeds, tincture |
Aphrodisiac (Berg, 1982; Lorenzi and Matos, 2002) |
||||
|
1 Uncertain scientific identification, as considered a misapplied name by the used data base (Rio de Janeiro Botanical Garden, 2023). 2 Although an unaccepted scientific name, it was considered a synonym due to the high frequency of its application in popular literature. |
||||||
Table 2: List of popular plants from non-scientific literature.
|
Scientific name (family) |
Properties |
Experimental investigation |
Reference |
|
Anacardium occidentale L. (Anacardiaceae) |
Antioxidant |
ABTS, DPPH, NO and cationic scavenging activity; β-carotene bleaching assay; FIC; ORAC; MDA and ROS levels reduction; increased antioxidant enzymatic activity; reducing power |
(Abas et al., 2006; Ajileye et al., Ayodele, 2022; Andrade et al., 2023, 2011; Anyaegbu et al., 2017; Bini et al., 2023; Broinizi et al., 2007, 2008; Chaikhong et al., 2022; Chaves et al., 2010; Chotphruethipong et al., 2017; Cruz Reina et al., 2022; R. A. Da |
|
et al., 2019, 2021; Elekofehinti et al., 2016; Encarnação et al., 2016; Gomes et al., 2013; Gordon et al., 2012; Indirayati et al., 2020; Junsathian et al., 2018; Kamath and Rajini, 2007; Kongkachuichai et al., 2015; Lopes et al., 2012; Melo Cavalcante et al., 2003; Melo et al., 2008; Moo-Huchin et al., 2015; Oloruntola, 2021; Oyedemi et al., 2017; Pereira et al., 2015; Porto-Luz et al., 2020; Queiroz et al., 2010, 2011; Quesado Junior et al., 2017; Razab and Abdul-Aziz, 2010; Razali et al., 2008; Ribeiro et al., 2021; Santana Andrade et al., 2022; J. A. S. Santoset al., 2018; Sija et al., 2019; Silva et al., 2021; Soares et al., 2013; Souza et al., 2017; Srichomphu et al., 2022; Sulbarán et al., 2013; Trevisan et al., 2006; Vieira et al., 2011; Zielinski et al., 2014b) |
|||
|
Neuroprotective |
Inhibited Aβ1-42 aggregation; protected SK-N-SH cells against H2O2 |
||
|
Protected HT22 and Neuro-2a cells against glutamate and H2O2induced toxicity |
|||
|
Inhibited nitric oxide production in stimulated BV-2 cells |
|||
|
Protected HT22 cells against hemozoin-induced damage and hCMED/D3 cells against increased permeability |
|||
|
Neuromodulatio n |
AChE and BChE inhibition |
||
|
MAO inhibition |
|||
|
Reduced GABA-transaminase activity |
|||
|
Increased neurite length |
|||
|
Increased tyrosine hydroxylase positive neuronal density in nucleus accumbens |
|||
|
Anti-ageing |
Increased C. elegans pharynx pumping rate and lifespan |
||
|
Memory enhancer |
Reduced escape latency time and increased retention time on Morris water maze |
||
|
Sedative |
Reduced spontaneous locomotion |
||
|
Hypnotic |
Reduced latency and increased time of pentobarbital-induced sleep |
|
No effect on pentobarbital-induced sleep |
|||
|
Anxiolytic |
Increased time spent and entries on elevated plus maze open arms |
||
|
Antidepressant |
No effect on forced swim test |
||
|
Aphrodisiac |
Reverted stress-induced sexual behavior impairment; increased testosterone serum |
||
|
Antistress |
Reduced corticosterone levels |
||
|
Anemopaegma arvense (Vell.) Stellfeld ex de Souza (Bignoniaceae) |
Neuroprotective |
Protected SH-SY5Y against rotenone-induced toxicity |
|
|
Aniba canelilla (Kunth) Mez (Lauraceae) |
Antioxidant |
ABTS and DPPH scavenging activity |
(Cruz et al., 2023; J. K. R. Da Silva et al., 2007; Martins et al., 2016) |
|
Neuromodulatio n |
AChE inhibition |
||
|
Memory enhancing |
Reverted scopolamine-induced damage on Morris water maze |
||
|
Aristolochia cymbifera Mart. & Zucc. (Aristolochiacea e) |
Antioxidant |
DPPH scavenging activity |
|
|
Aristolochia labiata Willd. (Aristolochiacea e) |
Antioxidant |
DPPH scavenging activity; inhibition of linoleic acid oxidation; reducing power |
|
|
Drimys brasiliensis Miers (Winteraceae) |
Antioxidant |
ABTS and DPPH scavenging activity; reducing power; ORAC |
(Barrientos et al., 2023; Bridi et al., 2019; Fonseca Gomes et al., 2013) |
|
Neuromodulator |
AChE and BChE inhibition |
||
|
Erythrina cristagalli L. (Fabaceae) |
Antioxidant |
DPPH scavenging activity |
|
|
Erythrina mulungu Mart. (Fabaceae) |
Neuromodulator |
Nicotinic receptor blockade |
|
|
Sedative |
Reduced entries on elevated plus maze arms and locomotor activity on open field test; no effects on rotarod test |
||
|
Anxiolytic |
Reduced avoidance latency on elevated T-maze |
||
|
Increased time spent in the light compartment on light-dark transition |
|||
|
No effect on elevated plus maze |
|||
|
Antidepressant |
No effect in immobility time on forced swim test |
||
|
Erythroxylum |
Antioxidant |
DPPH scavenging activity; ORAC |
|
Neuromodulatio n |
Effect upon AChE dependent on brain region |
(Bortoli et al., 2018; C. Branco et al., 2013; M. L. Machado et al., 2021; |
|
|
No effect on MAO inhibition |
|||
|
Antidepressant effect dependent on NMDA receptors and nitric oxide |
|||
|
Reduced normetanephrine levels increased by stress |
|||
|
Neuroprotection |
Inhibition of amyloid β-structures aggregation |
||
|
Reduced lipid peroxides in telencephalon, and increased in midbrain and cerebellum |
|||
|
Protected hippocampal and cortical slices against glutamate-induced toxicity |
|||
|
Increased myelination, gray matter count and density in brain of lung adenocarcinoma-bearing mice; no effects on brain lipid peroxides |
(Cittadini et al., 2019a, 2019b) |
||
|
Prevented dopaminergic neuronal death and increased axonal length and branching |
|||
|
Reduced stress-induced cellular damage in cortex, hippocampus and striatum |
|||
|
Locomotion |
Reduced MPTP-induced hypolocomotion and reserpine- induced catalepsy |
||
|
Reverted locomotor alterations induced by methylmalonic and malonic acids exposure |
|||
|
Delayed Aβ1-42-induced paralysis |
|||
|
Prevented motor alteration in stressed rats on open field test |
|||
|
Memory enhancer |
Aqueous extract prevented scopolamine-induced memory impairment on step-down avoidance task, but hydroethanolic extract induced impairment |
||
|
Reduced haloperidol-induced memory dysfunction on Morris water maze |
|||
|
Decreased investigation time on social recognition test; increased latency on step-down inhibitory avoidance task; increased latency on Morris water maze only with |
|
high doses (250 mg/kg) |
|||
|
Anxiolytic |
Reduced burying behavior on marble burying test; increased time and entries on elevated plus maze open arms |
||
|
Increased time spent and number of entries on elevated plus maze open arms; increased number of entries into open field central zone |
|||
|
No effect on elevated plus maze parameters |
|||
|
Increased number of entries on elevated plus maze closed arms, while reducing time spent |
|||
|
Antidepressant |
Reduced immobility time on forced swim test |
||
|
Reduced immobility time on tail suspension test |
|||
|
Anti-ageing |
Extended lifespan |
||
|
Reduced lifespan in high doses (50 mg/mL) |
|||
|
Energetic |
No effect on physical performance during exercise |
||
|
Increased energy expenditure by VCO2/VCO2 ratio |
|||
|
Antistress |
Increased survival against environmental stresses induced by starvation, paraquat and desiccation |
||
|
Indigofera suffruticosa Mill. (Fabaceae) |
Antioxidant |
DPPH scavenging activity |
|
|
Neuromodulatio n |
Increased glycine and reduced glutamic acid concentration in serum, without affecting tyrosine, phenylalanine or tryptophan |
||
|
Lippia alba (Mill.) N.E.Br. ex Britton & P.Wilson (Verbenaceae)
|
Antioxidant |
ABTS, DPPH and cationic scavenging activity; ORAC; inhibition of linoleic acid oxidation; MDA levels reduction; increased antioxidant enzymatic activity; β-carotene bleaching assay; reducing power |
(Azambuja et al., 2011; Barros et al., 2022; Celis et al., 2007; Chies et al., 2013; Da Silva Port’s et al., 2013; Farias et al., 2019; Finamor et al., 2023; Hay et al., 2018; JaramilloColorado et al., 2020; Joshi et al., 2018; L. et al., 2017; Morais et al., 2013, 2009; Nunes et al., 2018; Parodi et al., 2012; Puertas‐Mejía et al., 2002; Ramos et al.,2003; RodríguezSevilla et al., 2014a, 2014b; Santos Filho et al., 2023; Stashenko et al., 2014, 2004; Teixeira De Oliveira et al., 2018; Varón et al., 2007) |
|
Neuromodulatio |
AChE inhibition |
|
n |
No effect on AChE levels |
||
|
Anxiolytic activity dependent of GABAA and 5-HT3a/b |
|||
|
Dendritogenesis |
|||
|
Diazepam potentiation dependent of GABAergic system |
|||
|
Sedative |
Decreased time spent and increased number of falls on rotarod |
||
|
Reduced rearing on open field test, number of entries and time on elevated plus maze open arms; reduced time on rotarod |
|||
|
Reduced locomotion |
|||
|
Induced immobilization in Drosophila melanogaster |
|||
|
Induced deep anesthesia and death in Neohelice granulata |
|||
|
Anesthesia induction |
|||
|
Hypnotic |
Increased time and reduced latency on pentobarbital-induced sleep |
||
|
Increased ketamine-induced sleeping time |
|||
|
Anxiolytic |
Increased permanence in the light compartment on light-dark test |
||
|
Increased time of entries on elevated plus maze open arms |
|||
|
Reduced avoidance latency on elevated T-maze |
|||
|
Potentiate diazepam reduction of anesthesia time induction |
|||
|
Increased number of entries in the upper zone on novel tank test |
|||
|
Antistress |
Reduced cortisol levels |
||
|
Increased cortisol levels |
|||
|
No effect on cortisol levels |
|||
|
Passiflora alata Curtis (Passifloraceae) |
Antioxidant |
ABTS and DPPH scavenging activity; β-carotene bleaching assay; ORAC; MDA levels reduction; reducing power |
(Colomeu et al., 2014; Doyama et al., 2005; Lugato et al., 2014; Muniz et al., 2023; Ożarowski et al., 2019; Reis et al., 2020; Rudnicki et al., 2007; |
|
Memory enhancer |
No effect on memory acquisition in step-down inhibitory avoidance task |
||
|
Sedative |
Reduced entries on elevated plus |
|
Increased GABA levels on brain tissue |
|||
|
Antidepressant effect dependent on DA/5-HT transmission |
|||
|
Neuroprotection |
Increased Aβ1-40 and reduced Aβ1-42 levels and AlCl3 hippocampal neurodegeneration |
||
|
Reduced neurodegeneration induced by acetylcholine and glutamate |
|||
|
Locomotion |
No effect on rotarod |
||
|
No effect on wire or chimney test |
|||
|
Memory enhancing |
No effect on memory acquisition in step-down inhibitory avoidance task |
||
|
Reverted AlCl3-induced impairment on Morris water maze |
|||
|
Reduced number of errors and increased visit score and time in illuminated arm on arm radial maze |
|||
|
Inhibited chemotaxic memory loss induced by Aβ1-42 expression |
|||
|
Sedative |
Reduced head-dipping and rearing on elevated plus maze |
||
|
Reduced locomotor activity on open field test |
(Ayres et al., 2015, p. 201; Deng et al., 2010; Figueiredo et al., 2016; Klein et al., 2014) |
||
|
Hypnotic |
Increased pentobarbital sleepingtime |
||
|
No effect on thiopental-induced sleep |
|||
|
Reduced latency and increased duration on ethyl ether-induced sleep |
|||
|
Anxiolytic |
Increased entries in elevated plus maze open arms |
(Ayres et al., 2015; Barbosa et al., 2008; Coleta et al., 2006; Deng et al., 2010; Li et al., 2011; Otify et al., 2015; Petry et al., 2001) |
|
|
Increased time spent in elevated plus maze open arms |
|||
|
Reduced burying behavior on marble burying test |
|||
|
Increased number of transitions and time in the light compartment on light-dark procedure |
|||
|
Antidepressant |
Reduced immobility time on forced |
|
swim test |
|||
|
Reduced immobility time on tail suspension test |
|||
|
Anti-ageing |
Extended lifespan |
||
|
Antistress |
Reduced AlCl3-induced aggressiveness |
||
|
Passiflora quadrangularis L. (Passifloraceae) |
Antioxidant |
DPPH scavenging activity; reducing power |
|
|
Hypnotic |
Increased ethyl ether-induced hypnosis |
||
|
Anxiolytic |
Increased time spent on elevated plus maze open arms, reduced freezing time on open field test and number and time of head dips on holeboard test |
||
|
Paullinia cupana Kunth (Sapindaceae) |
Antioxidant |
ABTS, DPPH scavenging activity; FIC; TRAP; xanthine oxidase system’s inhibition; increased antioxidant enzymatic activity; MDA and ROS levels reduction; reducing power |
(Bittencourt et al., 2014; Boasquívis et al., 2018; Da Silva Bittencourt et al., 2020; Dabulici et al., 2020; Ferrari, 2002; K. N. Machado et al., 2021; Majhenič et al., 2007; Martins, 2010; Mattei et al., 1998; Mingori et al., 2017; Peixoto et al., 2017; Portella et al., 2013; Roggia et al., 2020; Sereia et al., 2019; Veloso et al., 2017; |
|
Neuroprotection |
Protected SH-SY5Y cells against rotenone-induced toxicity |
||
|
Protected neural cells against vincristine-induced damage |
|||
|
Protected BV-2 cells against H2O2induced toxicity |
|||
|
Reduced methylmercury-induced mortality on SH-SY5Y cells |
|||
|
Increased lifespan |
|||
|
Protected SH-SY5Y cells against glyoxal, methylglyoxal and acrolein-induced toxicity |
|||
|
Reduced Aβ1-42 aggregation |
|||
|
Prevented ASH neurons against polyQ-induced death |
|||
|
Neuromodulatio n |
AChE inhibition |
(Ruchel et al., 2017; Sereia et al., 2019; Zamberlan et al., 2020) |
|
|
Anxiolytic effect dependent on 5HT/DA/Glu systems |
|||
|
Antidepressant effect partially |
|
dependent on adenosinergic transmission |
|||
|
Panicolytic effect dependent on DA/5-HT transmission |
|||
|
Locomotion |
Reverted acrolein-induced reduction of exploratory behavior on open field test |
||
|
Only high doses (100 mg/kg) increased locomotion frequency on open field test |
|||
|
No effect on reverting locomotor impairments by aging |
|||
|
Reduced Aβ1-42-induced motor impairments in C. elegans |
|||
|
No effect on spontaneous locomotor activity |
|||
|
Memory enhancer |
Reverted recognition index impairment in hyperlipidemic rats on object recognition task |
||
|
Reduced espace latency in normal and in scopolamine-induced amnesic rats on Morris water maze |
|||
|
No effect on discrimination index in novel object recognition task |
|||
|
Reverted scopolamine-induced amnesia on passive avoidance |
|||
|
No effect on number of errors or time on Lashley III maze, or on active avoidance test |
|||
|
Hypnotic |
No effect on pentobarbital-induced sleep |
||
|
Anxiolytic |
No effect on elevated plus maze |
||
|
Increased espace latency on elevated T-maze |
|||
|
Decreased inhibitory avoidance latency on elevated T-maze |
|||
|
Reverted acrolein-induced anxiety on open field test |
|||
|
Antidepressant |
Reduced immobility time on forced swim test |
||
|
Anti-ageing |
Extended lifespan |
||
|
No effect on lifespan |
|||
|
Aphrodisiac |
Increased testosterone levels in serum |
||
|
Energetic |
Increased time in water on forced swim test |
||
|
Reduced methylmercury-induced |
|
sleepiness and increased daily activity |
|||
|
Ptychopetalum olacoides Benth. (Olacaceae) |
Antioxidant |
ABTS, DPPH and superoxide scavenging activity; MDA levels reduction; increased antioxidant enzymatic activity |
|
|
Neuroprotective |
Reverted oxygen and glucose deprivation damage in hippocampus |
||
|
Reverted Aβ1-42 deposition and hippocampal neuronal death, and reduced astrocytosis without affecting BDNF levels |
|||
|
Neuromodulator |
AChE inhibition in frontal cortex, hippocampus and striatum |
||
|
Memory effects dependent of 5- HT1a |
|||
|
Antidepressant activity mediated by α2-adrenoceptor |
|||
|
Antidepressant activity mediated by D1r/B-NAr, but not 5-HT |
|||
|
Locomotion |
Restored locomotor impairment induced by reserpine on open field test |
||
|
No effect on rotarod |
|||
|
Memory enhancer |
Increased latency on step-down inhibitory avoidance |
(A. L. Da Silva et al., 2007; Da Silva et al., 2009, 2008, 2004; Figueiró et |
|
|
Reverted amnesia induced by scopolamine and MK-801 on stepdown inhibitory avoidance |
|||
|
Increased time spent in the light compartment on light-dark procedure in unpredictable chronic mild stress model |
|||
|
Increased latency on step-down inhibitory avoidance and recognition index after 24h on object recognition test |
|||
|
Sedative |
Decreased locomotion frequency on open field test |
||
|
Anxiolytic |
Reduced head dips number and increased latency on holeboard test |
||
|
Antidepressant |
Reduced immobility time on forced swim test and tail suspension test |
||
|
Antistress |
Restore corticosterone levels in unpredictable chronic mild stress model |
||
|
Schinus terebinthifolia |
Antioxidant |
ABTS, DPPH and cationic scavenging activity; β-carotene |
(Belhoussaine et al., 2022; Bendaoud et al., 2010; Bernardes et al., 2014, |
|
Raddi (Anacardiaceae) |
bleaching assay; ORAC; MDA levels reduction; increased antioxidant enzymatic activity; reducing power; increased survival against H2O2 |
2011; Carneiro et al., 2023, 2016; Corradi I. et al., 2018; Costa et al., 2013; Da Silva Dannenberg et al., 2016; Da Silva Nascimento et al., 2023; De Oliveira et al., 2020; Dedvisitsakul and Watla-iad, 2022; Dos Santos Da Rocha et al., 2019; El- Massry et al., 2009; Ennigrou et al., 2018, 2017; Feriani et al., 2021; Guimarães et al., 2023; Horozić et al., 2022; Labre Da Silva et al., 2019; Miranda Dos Santos et al., 2023; Rebelatto et al., 2020; Ribeiro et al., 2015; Rocha et al., 2018; Sassi et al., 2020; Scheid et al., 2018; Sereniki et al., 2016; Silva et al., 2017; Todirascu-Ciornea et al., 2019; Uliana et al., 2016; Velázquez et al., 2003) |
|
|
Neuromodulatio n |
Reduced AChE levels |
||
|
Locomotion |
Reverted rotenone-induced impairment in locomotor activity on open field test and motor performance on rotarod |
||
|
Memory enhancer |
Increased arms entries and spontaneous alternation on Y-maze |
||
|
Anxiolytic |
Increased time spent and reduced latency to top on novel tank diving test |
||
|
No effect on elevated plus maze |
|||
|
Antidepressant |
Reduced immobility time on forced swim test |
||
|
Sonchus oleraceus L. (Asteraceae) |
Antioxidant |
ABTS, DPPH and cationic scavenging activity; ORAC; MDA and ROS levels reduction; reducing power; increased antioxidant enzymatic activity |
(Aissani et al., 2022; Al Juhaimi et al., 2017; Alpinar et al., 2009; Disciglio et al., 2017; Elshikh et al., 2023; Liet al., 2015, 2018; Mawalagedera et al., 2016; McDowell et al., 2011; Nouidha et al., 2023; Ou et al., 2014, 2012; Salim et al., 2023; Schaffer et al., 2005; Sergio et al., 2020; Teugwa et al., 2013; Vecchia et al., 2022; Xia et al., 2011; Yin et al., 2007) |
|
Neuromodulatio n |
AChE inhibition |
||
|
Anti-ageing |
Increased longevity, increased expression of anti-ageing genes and decreased expression of senescence genes |
||
|
Locomotion |
Reduced freezing time, and increased travel distance and center zone entries on open field test |
||
|
Memory enhancer |
Reduced latency and path length and increased target crossing in Morris water maze |
|
ischemic oxidative damage |
|||
|
Neuromodulator |
Reduced hippocampal vacuoles and increased synaptic connections |
||
|
Anxiolytic |
No effect on elevated plus maze |
||
|
Aphrodisiac |
Increased testosterone and estradiol levels in male Carassius auratus, with no effect on females |
||
|
Turnera diffusa Willd. ex Schult. (Turneraceae) |
Antioxidant |
ABTS, DPPH and superoxide scavenging activity; FIC; inhibition of linoleic acid oxidation; MDA and ROS levels reduction; reducing power |
(Ahmad et al., 2017; Bernardo et al., 2022, 2021, 2017; Bezerra et al., 2011; Garza-Juárez et al., 2011; Ivanišová et al., 2018; Kim et al., 2022; Lucio-Gutiérrez et al., 2012; Palma-Wong et al., 2023; Pérez- Meseguer et al., 2010; Reyes-Becerril et al., 2020; Urbizu-González et al., |
|
Neuroprotective |
Reduced glutamate toxicity against SH-SY5Y cells |
||
|
No effect on 6-OHDA toxicity against SH-SY5Y cells |
|||
|
No effects on hippocampal neuronal death rates in aged rats |
|||
|
High doses (1 mg/mL) have cytotoxic activity on astrocytes |
|||
|
Neuromodulator |
Sexual behavior mediated by nitric oxide |
||
|
AChE, BChE and MAO-A inhibition |
|||
|
Antistress |
No effect on ACTH or corticosterone levels |
||
|
Aphrodisiac |
Enhanced sexual behavior |
||
|
Enhanced sexual behavior in impotent rats |
|||
|
Increased testosterone levels |
|||
|
Antidepressant |
Reduced immobility time on forced swim test |
||
|
Anxiolytic |
Increased time and entries on elevated plus maze open arms |
||
|
Reduced burying behavior on marble burying test |
|||
|
Hypnotic |
No effect on latency or total pentobarbital-induced sleeping time |
||
|
Memory enhancer |
No effect on scopolamine-induced amnesia on passive avoidance test |
||
|
Locomotion |
No effect on locomotor activity by ambulation or motor coordination |
||
|
by rotarod performance |
|||
|
No effect on inverted screen test |
|||
|
Xylopia aromatica (Lam.) Mart. (Annonaceae) |
Antioxidant |
ABTS and DPPH scavenging activity |
|
|
5-HT: 5-hydroxi-triptamine; ABTS:2,2’-azinobis-(3-ethylbenzothiazoline-6-sulfonate); AChE: acetylcholinesterase; BChE: butyrylcholinesterase; DPPH: 2,2-diphenyl-1-picrylhydrazyl; DA: dopamine; FIC: ferrous iron chelating assay;MDA: malondialdehyde; ORAC: oxygen radical absorbance capacity ;ROS: reactive oxygen species. |
|||
Table 3. Pre-clinical studies on psychopharmacological effects of plant extracts.
|
Scientific name |
Preparation |
Type of study (sample) |
Intervention |
Main results |
Reference |
|
Erythrina mulungu |
Not specified |
Randomized, placebo- controlled, tripleblind, and parallel clinical trial (N=200) |
Erythrina mulungu (500 mg), Passiflora incarnata (500 mg), or Midazolam (15 mg) were administered orally to volunteers who underwent third molar extraction. Anxiety was evaluated through physiological parameters (heart rate, blood pressure, and oxygen saturation) and questionnaire assessment. |
Unlike Passiflora incarnata and Midazolam, Erythrina mulungu showed no significant anxiolytic effect. |
|
|
Ilex paraguariensis |
Instant mate tea dissolved in water |
Interventional trial (N=15) |
Healthy women received mate tea after an overnight fasting. They had their blood samples collected at baseline, after 1 hour of the first intake, and after 7 days of mate tea intake (5 g of mate tea diluted |
Mate tea intake decreased MDA levels after the first administration, and this reduction was maintained after the 7-day protocol. Total antioxidant status and the expression of |
|
in 500 ml of water daily). Plasma lipid peroxidation (MDA assay), kinetics of the diene conjugation formation, total antioxidant status, and expression of the antioxidant enzymes SOD, CAT, and GPx were evaluated. |
antioxidant enzyme genes were observed after the prolonged administration. |
||||
|
Aqueous extract |
Placebo- controlled trial (N=20) |
Participants received Ilex paraguariensis aqueous extract (50 g for each 250 ml of water, 'terere' preparation') or water and performed running tests. Participants had their baseline performance evaluated and received 250 ml of the extract or placebo 60 minutes before the test and the remaining 250 ml 30 minutes before the test. |
Ilex paraguariensis extract decreased the execution time that volunteers spent on running the test, an effect that was not seen in the control group. |
||
|
Soluble yerba mate |
Double-blind, randomized, placebo- controlled, crossover study (N=89) |
Patients living with HIV/AIDS under antiretroviral therapy received chocolate, placebo chocolate, yerba mate tea, or placebo tea to be consumed for 15 days. After 15 days, participants crossed over to the other experimental arm. The protocol consisted of 4 phases separated |
No differences between the treatments (chocolate, yerba mate tea, or placebos) were observed regarding the lipidic and oxidative profile of individuals. |
|
by 15 days washout period each, so the participants underwent the 4 possibilities of intervention. Exercise practice, lipid profile, and lipid oxidation (MDA assay) were evaluated. |
|||||
|
Yerba mate infusion |
Bioavailability study (N=17) |
Healthy volunteers had blood samples collected (baseline) and then received yerba mate infusion or water. New blood samples were collected 20, 40, 50, 60, 80, 100, and 120 minutes after intake. The following parameters were assessed in plasma: total polyphenols concentration, antioxidant capacity (FRAP and ABTS methods), uric acid levels, and total protein levels. |
Yerba mate increased plasmatic total polyphenols concentration and antioxidant capacity (FRAP and ABTS assays), while no differences were observed in protein and uric acid levels. |
||
|
Soluble yerba mate |
Crossover, placebo- controlled study (N=9) |
Healthy male volunteers received a placebo (500 ml of water) and after a 7-day washout period crossed over to the experimental arm and received yerba mate (5g in 500 ml of water). After 1 hour of ingesting a placebo or yerba mate, the resting energy expenditure was |
Acute yerba mate intake induced an increased energy expenditure. |
|
assessed through indirect calorimetry for 30 minutes. |
|||||
|
Instant mate tea dissolved in water |
Randomized, crossover study (N=12) |
Male volunteers were randomized and received mate tea (5 mg/ml, 3 times/day) or water for 11 days. On the 8th day, subjects performed eccentric elbow flexion exercises, and maximal isometric elbow flexion was assessed before and after 0, 24, 48, and 72 hours of training. Blood samples were taken 24, 48, and 72 hours after exercise, and the following parameters were evaluated: total phenolics, GSH, GSSG, GSH: GSSG ratio, and lipid hydroperoxides. After a seventeen-day washout, the volunteers crossed to the other experimental arm. |
After eccentric exercise, muscle strength decreased in both groups, but mate tea improved the rate of strength recovery 24 hours after exercise. Mate tea improved plasmatic total phenolic content, although this level decreased 72 hours after exercise. GSH blood levels decreased in the control group 48 and 72 hours after exercise, an effect not seen in the mate tea group. GSSG, GSH: GSSH and lipid hydroperoxides levels were unaffected by mate intake. |
||
|
Encapsulated ground green yerba mate leaves |
Double-blind repeatedmeasures crossover placebo- controlled study (N=12) |
Healthy women underwent a three-session repeated measure protocol. In the first session, a baseline assessment was performed, including evaluating individual performance and body composition. Before session 2, |
It was shown an increase of fatty acid oxidation during exercise in the yerba mate group compared to placebo. Scores for hunger, prospective eating, and desire to eat were reduced in the yerba mate group, whereas measures of |
|
the volunteers were randomized to receive yerba mate (4 x 500 mg in capsules) or placebo and after 120 minutes of resting, they were subjected to a cycle ergometer for 30 minutes. On session 3 the volunteers crossed over to the other experimental arm and the protocol was repeated. The following parameters were assessed: fatty acid oxidation, profile of mood state score; and appetite and satiety by a visual analogue scale. |
focus, energy, and concentration increased. |
||||
|
Powder (leaves mixed with stems) |
Double-blind, crossover design (N=11) |
Male cyclists received yerba mate (5 g, daily) or placebo for five days and after 1 hour underwent ergometer-based assessments. Before and during tests, blood and respiratory gas samples were taken. Adrenaline concentration and fat utilization were evaluated in plasma. |
Yerba mate increased plasmatic adrenaline concentration and fat utilization during a short simulated cycling trial, while respiratory exchange ratios were not impacted. |
||
|
Spray-dried aqueous yerba mate extract |
Interventional trial (N=14) |
Healthy volunteers received capsules of spray-dried yerba mate extract (3 capsules, 3 times/day) and were evaluated at baseline and after |
The extract consumption improved the antioxidant capacity after 7 and 60 days. The yerba mate intake also decreased the antioxidant |
|
7, 30, and 60 days regarding electrocardiogra m, and hematological, urinary, and biochemical tests. |
enzyme activity of GSH (after 7 and 60 days), SOD (after 7, 30, and 60 days), CAT (after 7 and 30 days), and paraoxonase-1 (after 7 days). A decrease in lipid hydroperoxides (after 30 and 60 days) and MDA levels (after 7 and 30 days) were observed, while no differences in GPx activity were detected. |
||||
|
Instant mate tea dissolved in water |
Randomized, crossover study (N=12) |
Volunteers received mate tea (5 mg/ml, 3 times/day) or water for 11 days. Starting day 8, three sets of 20 maximal isokinetic eccentric for elbow flexors with one arm were performed and the rate of torque development at 0-50, 0-100, 0- 200, and 100-200 ms were evaluated on the following time points: before and at 0, 24, 48, and 72 hours after exercise. Moreover, blood samples were taken before and after 24, 48, and 72 hours of exercise, and creatine kinase, aldolase, total phenols, and GSH:GSSG ratio were measured. After a 17-day washout period, the participants |
Mate tea improved the rate of torque development > 50 ms after 48 and 72 hours of eccentric exercise. Mate tea also increased aldolase and total phenols levels, as well as GSH: GSSG ratio, independently of exercise. |
|
crossed over to the other experimental arm. |
|||||
|
Tea |
Observational study (N=8) |
Healthy volunteers consumed a single preparation of mate tea prepared with 4 or 8 g of mate and had their plasmatic antioxidant capacity assessed after 1 and 2 hours (chemiluminesce nt method based on an ABAPluminol system). |
There was an increase in the antioxidant capacity of the blood plasma of volunteers who received the tea made of 8 g of yerba mate when compared to the baseline evaluation. |
||
|
Soluble mate tea |
Crossover, pilot clinical trial (N=10) |
Men volunteers were divided into two groups and received yerba mate or placebo. Then, they were submitted to a one-repetition maximum test after one hour on the bench press and leg press. Muscle strength was evaluated. |
Yerba mate did not affect muscle strength in the leg press or bench press exercise when compared to placebo. |
||
|
Kombucha |
Sensory descriptive study with consumers (N=105) |
Volunteers received green tea, black tea, and yerba-mate kombuchas subsequently in a balanced presentation order. Evoked emotions were evaluated through a checkall-that-apply questionnaire, which consists of a 39-word list with emotion terms. |
Awareness that the beverage was yerba-mate kombucha evoked the peaceful, loving, and quiet feelings. |
(Dartora et al., 2023) |
|
|
Lippia alba |
Essential oil from leaves |
Randomized, placebo- controlled clinical trial |
Academic stress levels were measured before and after the |
Lippia alba essential oil significantly decreased |
|
(N=38) |
evaluation instrument implementation along with essential oil inhalation (30 minutes of dispersion). |
academic stress levels assessed through the academic stress inventory. |
|||
|
Essential oil from leaves |
Randomised, controlled, experimental trial (N=95) |
Participants had their anxiety assessed (STAI) before and after the intervention protocol. The protocol consisted of a daily inhalation of the essential oil for 4 weeks. |
Lippia alba essential oil intervention decreased the STAI scores in the posttest assessment. |
||
|
Passiflora edulis |
Aqueous extract |
Cross-over, double-blind study (N=9) |
Volunteers received passiflora (lyophilized 10% tea) or a placebo at night and were evaluated for serum biochemical assays, electrocardiogra m and electroencephalo gram on the following morning. After a 1-week washout period, the volunteers crossed over to the other experimental arm. Hypnotic effects were assessed through a self-report questionnaire. |
No significant differences were detected on electrocardiogra m or hypnotic effects assessment. Five volunteers presented 'drug rhythm' on electroencephalo gram assessment, of which two volunteers presented this pattern also after placebo. |
|
|
Paullinia cupana |
Powder |
Double-blind, placebo- controlled study (N=30) |
Guarana (2 x 500mg/day) was administered orally to healthy volunteers in a four-day protocol and cognitive, sleep, and anxiety (STAI) scales parameters were assessed. |
It was not observed significant alterations in cognition, sleep, and anxiety upon guarana administration. |
|
|
Powder |
Randomized, |
Guarana (2 x |
It was not |
|
double-blind, placebo- controlled study (N=45) |
500mg/day) was administered orally to healthy elderly volunteers in a 5month protocol and cognitive, sleep, and anxiety (STAI) scales parameters. |
observed any alteration in cognitive, sleep, and anxiety aspects upon guarana administration after 3 and 5 months of guarana intake. |
|||
|
Dried ethanolic extracts of guarana, Panax ginseng, and guarana/ginseng extracts combination |
Double-blind, counterbalanced, placebo- controlled study (N=28) |
Participants received a placebo, guarana (75 mg), ginseng (200 mg), and a guarana/ginseng combination (75/200 mg) at different moments, and had their cognitive and mood effects assessed. Patients were evaluated during 5 sessions with a 7-day washout period between them. Cognitive performance and mood were evaluated before and after 1, 2.5, 4, and 6 hours after the treatment (COGDRAS, serial subtraction tasks, and BLVAS). |
Guarana, ginseng, or guarana/ginseng combination improved task performance in the evaluated time points when compared to placebo. Guarana group presented improvements, particularly in attention and sentence verification tasks. Moreover, the ginseng and guarana/ginseng combination improved the speed of attention task performance and the speed of memory task performance, although led to a decrease in accuracy. Finally, guarana and the guaranaginseng combination improved the performance on the serial subtraction task. |
||
|
Extract - not specified |
Randomized, double-blind, crossover study (N=36) |
Breast cancer patients were randomized to receive guarana (75 mg, daily, orally) or placebo during the radiation therapy (28 sessions). Before |
No differences were observed between or within the groups at any assessment. |
|
the 14th session, the patients crossed over to the other experimental arm. Fatigue and depression were assessed (BDI, BFI and CFQ) before the 1st, 14th, and 28th radiation session. |
|||||
|
Guarana powder |
Longitudinal and Intervention Study (N=12) |
Healthy volunteers with overweight had blood samples collected in a 12hour fast and 1h after guarana powder drink consumption (3 g of powder in 300 ml of water). After 15 days of the drink's daily consumption, samples were collected again after a 12-hour fast and after the first hour of drink ingestion. The following parameters were evaluated: resistance of LDL to ex-vivo oxidation, total antioxidant status, ORAC, DNA damage in lymphocytes, and the activity of the antioxidant enzymes SOD, CAT, and GPx. |
The lag time of LDL oxidation increased after 1 hour of the drink consumption on day 1 and day 15 of intervention. CAT and GPx increased after guarana ingestion on days 1 and 15 and also increased after the 12-hour fast on day 15 compared to day 1. SOD and total antioxidant status levels were not altered. An ORAC increase was observed 1 h after the drink ingestion on day 1 and day 15, while oxidative damage to DNA was also acutely influenced. |
||
|
Dry guarana extract |
Phase II randomized, double-blind, placebo- controlled crossover trial (N=75) |
Breast cancer patients with fatigue after their first cycle of chemotherapy were randomized into guarana (50 mg, twice a day) or placebo groups for 21 days. The patients underwent a 7- |
Guarana improved FACT- F, FACT-ES, and BFI index on days 21 and 49, while improved CFQ on day 21 but not on day 49. |
|
day washout period and then crossed over to the other experimental arm. After 21 days, they were evaluated again regarding fatigue, sleep quality, anxiety, and quality of life questionnaires (CFQ, FACT-F, FACT-ES, BFI, PSQI, and HADS). |
|||||
|
Standardized dry purified Paullinia cupana extract (PC-18) |
Phase II, initially uncontrolled, open study (N=40) |
The participants were adults diagnosed with cancer under chemotherapy and screened for fatigue (BFI). They received the PC-18 extract twice a day (37.5 mg) for three weeks. After this phase, the participants who showed improvement or stabilization of the BFI index (N = 36) were selected and randomized. The following phase consisted of the administration of a placebo or PC- 18 (37.5 mg, twice a day) for 3 additional weeks. Then, the participants were evaluated through BFI and two additional fatigue scales (FACT-F and CFQ), as well as anxiety (HADS) and sleep quality questionnaires (PSQI). |
After the initial phase, 36 patients showed an increase or stabilization of BFI scores upon PC-18 intervention. However, three weeks after randomization, no differences were observed between PC-18 or placebo groups in the assessed parameters. |
||
|
Commercial |
Prospective, |
Volunteers were |
Guarana showed |
|
preparation |
randomized, single-blind, placebo- controlled, crossover study (N=27) |
submitted to guarana intake (360 mg x 3) or cornstarch (placebo) in random order for 5 days. Wellbeing, anxiety, and mood were assessed (PWB, SAS and BL- VAS) at four different time points: pretreatment, treatment 1, washout, and treatment 2. |
no significant difference in comparison with placebo. |
||
|
Dry guarana extract |
Randomized, double-blind, placebo- controlled phase II study (N=60) |
Patients with advanced neck and head cancer with an indication for chemoradiothera py were randomized and assigned to the guarana or placebo group. Those in the guarana group received guarana (50 mg, twice a day) during the six weeks of chemoradiothera py treatment. Patients' fatigue and quality of life were evaluated on days 1, 21, 42, and 63 (FACT-F, FACT-HN, EORTC QLQ- 30, EORTC QLQ H&N35 |
Guarana showed improvement in pain, social eating, swallowing, coughing, and weight loss parameters after the first cycle of chemoradiothera py by FACT- HN35. However, these parameters worsened after the end of the cycles. |
||
|
Standardized dry purified Paullinia cupana extract (PC-18) |
Results of two Double-Blind, Randomised Clinical Trials (N=32, study 1; and N=72, study 2) |
In both studies, volunteers were early breast cancer patients who presented an increase in fatigue scores after the first cycle of chemotherapy. Fatigue was assessed using |
In both studies, PC-18 did not show a decrease in fatigue scores. |
|
two questionnaires (CFQ and BFI). Study 1: Patients received a placebo or PC-18 (37.5 mg, twice daily) during 3 weeks. Then, the patients had a one-week washout period before crossover to the other experimental arm. Fatigue assessments were performed before and after the first, second, and third cycles of chemotherapy. Study 2: Patients received PC-18 (7.5 and 12.5 mg) or placebo orally twice a day and had the fatigue assessed before the first and second chemotherapy cycles and after 21 days of randomization. |
|||||
|
Powder |
Randomized, double-blind, crossover trial (N=27) |
Guarana supplementation (125 mg/kg, orally) was administered to volunteers submitted to a maximal- intensity cycling task. Cognitive performance was evaluated before and after exercise (simple reaction time, choice reaction time, immediate word recall test, and BL-VAS), as well as maximal oxygen consumption measurement. |
Guarana decreased choice reaction time before and after exercise when compared to placebo, although no significant differences were observed in the other parameters. |
||
|
ABTS: 2,2’-azinobis-(3-ethylbenzothiazoline-6-sulfonate); CAT: catalase; FRAP: ferric reducing antioxidant |
|||||
power; GPx: glutathione peroxidase; GSH: glutathione; GSSG: glutathione disulfide; MDA: malondialdehyde; ORAC: oxygen radical absorbance capacity; SOD: superoxide dismutase. Inventories and questionnaires: BDI: Beck depression inventory; BFI: brief fatigue inventory; BL-VAS: Bond-Lader visual analogue scales; CFQ: Chalder fatigue scale; COGDRAS: cognitive drug research computerized assessment system; EORTC QLQ30: european organization for research and treatment of cancer quality of life questionnaire core 30; EORTC QLQ H&N35: european organization for research and treatment of cancer quality of life questionnaire head and neck module; FACT-ES: functional assessment of chronic illness therapy-endocrine symptoms; FACT-F: functional assessment of chronic illness therapy-fatigue; FACT-HN: functional assessment of cancer therapy - head and neck; HADS: hospital anxiety and depression scale; PSQI: Pittsburgh sleep quality index; PWB: psychological well-being scale; SAS: self-rating anxiety state scale; STAI: state-trait anxiety inventory. |
Table 4: Clinical studies on psychopharmacological effects of plant extracts.
Discussion
The non-scientific survey about plants popularly used for general well-being and delightful moments identified 115 species cited in at least four books, being 27 natives from Brazil. These species belong to 18 families, the most common was Fabaceae, with four species, followed by Passifloraceae, with three species, and Anacardiaceae, Aristolochiaceae, Asteraceae, Winteraceae, two species each. The plant parts most used were leaves and barks, and the main forms of preparation were aqueous extracts, which comprehends infusions, decoctions, juices and baths. Among the popular uses, the most common were either as a stimulant, to counteract weakness and fatigue, or as calmative, against anxiety, nervousness, and insomnia as well.
Search in scientific data bases revealed 568 studies after the inclusion and exclusion criteria. The most studied effect was the antioxidant activity, found in all but two species reviewed – A. arvense and E. mulungu –, with positive results observed in a great variety of methods, mainly radical scavenging and stimulation of antioxidant enzymes, such as superoxide dismutase, catalase and glutathione peroxidase. Antioxidant capacity is usually attributed to secondary metabolites, such as flavonoids, present in many vegetal species that act as reactive oxygen species scavengers (Dias et al., 2021). Different degrees of antioxidant effect were found for the same plant depending on the parts used and the extraction method. Antioxidant potential is a property very exploited in plants with economic importance, especially in agriculture and food industries, which may explain the considerable volume of studies with some particular species – such as A. occidentale, T. cacao, P. cupana, I. paraguariensis, and Passiflora spp. –, which comprehends up to 78 % of articles in this present review. Although an antioxidant effect regarding botanical compounds is highly unspecific, the capacity of neutralizing reactive oxygen species could be used to prevent the onset of some neuropsychiatric disorders, in particular neurodegenerative ones, as oxidative stress causes cellular death by mitochondrial damage and DNA mutation (Guo et al., 2013).
We also found studies evaluating the effects of extracts in different neurotransmitter systems. The cholinergic system was the most studied, with half of the mentioned plants investigated as their anticholinesterase activity.
It is known that anticholinesterase drugs are used for the treatment of Alzheimer’s disease and other dementias, promoting cognitive and mood improvement (Balázs et al., 2021). In fact, several species found in the survey are used for memory deficits and the scientific search confirmed the potential of Brazilian flora specimens on learning and memory enhancement. A. occidentale, I. paraguariensis, P. cupana, P. olacoides and S. oleraceus administration improved rodents’ performance on Morris water maze or inhibitory step-down avoidance. A. canelilla and P. edulis extracts reverted memory impairment caused by scopolamine and AlCl3, respectively. I. paraguariensis and P. cupana improved memory in both healthy and amnesic conditions.
Other disorders were also objects of studies, such as anxiety and depression, evaluated for P. olacoides, L. alba and P. edulis, for instance, attributing serotonergic and dopaminergic regulatory effects to these species. The studies indirectly supported some uses observed in the survey, as relaxing, energetic and arousal mood improvement. For example, a calmative activity attributed to L. alba was supported by the hypnotic effect and cortisol reduction after extractadministration; sedative effect was also found in Passiflora spp. and E. mulungu, species used for insomnia treatment, along with L. alba.
The species I. paraguariensis and P. cupana are used for their stimulant and energetic properties and were evaluated in correlate tests. Studies showed that T. diffusa and A. occidentale not only improved, but also reverted impairments in sexual behavior, reproducing the aphrodisiac effects alleged by population. P. cupana and T. cacao could also present some aphrodisiac effects, as its administration increased seric testosterone levels.
On the other hand, some popular uses could not be supported by scientific studies due to its difficulty in translating into pharmacological properties. Aristolochia spp., for example, had an indication for hysterical affections, and no study investigating this kind of property was found. Although hysteria is an outdated term, sedative and anxiolytic effects addressed by popular use are enough to justify studies seeking a relaxing activity to these plants.
Considering all the studies reviewed, only a small part of them focused on mood, most of them using animal models to investigate depression and anxiety. Accordingly, the survey and the systematic review couldn’t find results for most of the relevant terms related to delightful moments, such as mood states related to love and social bonding or more subjective emotions of nervousness, happiness or joy, for example. One study addressed emotional wellbeing after the use of I. paraguariensis: participants reported peaceful, loving and quiet emotions after mateconsumption, but the individuals were told the beverage’s composition, which may have influenced on the individuals feedback because this plant is often used in their daily life, above any evoked delightful emotions (Dartora et al., 2023).
We identified 28 clinical studies with the selected species. The search initially retrieved 31 clinical studies; however one was excluded due to methodological issues and two proved to be different publications related to the same studies. Thus, the potential of five species were evaluated in clinical studies: E. mulungu, I. paraguariensis, L. alba, P. edulis, and P. cupana. Withal, the species with more clinical studies reported are I. paraguariensis and P. cupana. Focusing on mood, I. paraguariensis showed improvement performance on exercise recovery (Panza et al., 2016), while P. cupana presented conflicting results regarding fatigue scores in oncological patients and cognitive performance tasks on attention and memory (Galduróz and Carlini, 1994, 1996; Kennedy et al., 2004). Two studies using L. alba supported its anxiolytic (Alvarado Garcia et al., 2021) and antistress uses (Soto Vásquez, 2019). The anxiolytic and hypnotic effects of E. mulungu (Da Cunha et al., 2021)and P. edulis (Maluf et al., 1991)were also evaluated, but these clinical studies did not confirm the investigated effects. Thus, except for these two species with negative results, the clinical trials corroborate the pre-clinical results and the effects attributed in folk medicine.
Overall, although a few studies showed a therapeutic or mood effect in human volunteers, due to conflicting and scarce evidence, more double-blind, randomized, placebo controlled trials with representative sample sizes are required, because only six studies followed robust clinical design. It is worth highlighting that only studies that followed the criteria of inclusion were discussed in this review.
There are some limitations of this study which must be discussed. We observed some divergence about the geographical distribution and accepted scientific names depending on the source consulted. We adopted the Rio de Janeiro Botanical Garden (2023) data base as the main reference to classify a species as native and endemic, but the occurrence and current correct name are not always the same in all data bases.
One of the major limitations in this study was correctly attributing the names described in the non-scientific literature to the actual scientific nomenclature. As already mentioned, P. catuaba and Drymis spp. were species reviewed by names other than the ones found in the survey – E. catuaba, D. winteri and D. granadensis. Although its synonyms were also included on the scientific review, it’s possible that the botanical material employed in some studies does not correspond to the species cited. Unfortunately, the incorrect identification of species and the use of incorrect botanical material are issues that are still present.
Another common problem observed in the survey was the existence of two or more species known by the same popular name; sometimes species belonging to the same family and genus, but also observed for species very distant taxonomically. The survey found two species of Erythrina with popular name of “mulungu”, E. cristagalli and E. mulungu, both used as anxiolytics; however a different species is usually employed in experimental studies, E. velutina, which have studies exploring anticholinesterase (Santos et al., 2012), hypnotic (Ozawa et al., 2008)and anxiolytic (Raupp et al., 2008)activities. It is worth noting that E. velutina is the species normally sold in herbal preparations.
This review tried to avoid such complications by making the lack of scientific name an exclusion criterion, but a major impact of dubious identification still persists, as reflected in the results from Protium catuaba studies. The proposed synonym that retrieved results, Erythroxylum catuaba, is believed to be a nomen nudum, originating from an incorrect identification of Trichilia sp. (Ducke, 1966). There is, indeed, a great number of species known as “catuaba”, such as Erythroxylum vaccinifolium and Anemopaegma arvense, this one recognised as true “catuaba”. For this reason, the incorrect identification of E. catuaba could be easily replicated along phytotherapeutic products and non-scientific publications, as its popular use converged to aphrodisiac effects and was often intertwined between species due to ecological distribution and lack of quality control in pharmaceutical process (Marques, 1998). Thus, taxonomic studies are as important as pharmacological investigation, as its support and application is extremely necessary to solve some of these limitations.
Conclusion
The biodiversity of the Brazilian flora provides an enormous opportunity to study alternative therapeutics, by the effects of natural products on psychiatric and neurodegenerative disorders. In this context, this survey identified 27 native species with popular uses for general well-being and delightful moments and several indications were supported by scientific studies. It is noticeable, though, that the major part of these results only explored an antioxidant activity, and just a small fraction of the studies investigated an effect in a clinical trial. In this sense, the present review not only presents the actual popular and scientific evidence of psychopharmacological effects in Brazilian flora, but may serve as an indicator of where future investigations in psychological stress therapeutics must focus, on both pre-clinical and, specially, clinical research.
Acknowledgements: This work was supported by Delightex Pte. Ltd.
Ethical considerations: Not applicable
Conflict of interest: YK is the chief scientific officer at Delightex Pte. Ltd., which financially supported this work. AF and GM received a scholarship grant from Delightex Pte. Ltd. for their contributions.
Authors’ contributions: All authors reviewed the manuscript and agreed to its final format. Specific contributions are described as follows: AF: Investigation; visualization; writing. GM: Investigation; visualization. YK: Conceptualization; funding acquisition. FRM: Conceptualization; funding acquisition; methodology; supervision; writing.
References
- Abas F, Lajis NH, Israf DA, Khozirah S, Umi Kalsom Y (2006) Antioxidant and nitric oxide inhibition activities of selected Malay traditional vegetables. Food Chem 95:566-573.
- Afoakwa E, Ofosu-Ansah E, Budu A, Mensah-Brown H, Takrama J, et al. (2015) Roasting effects on phenolic content and free-radical scavenging activities of pulp preconditioned and fermented cocoa (Theobroma cacao) beans. Afr J Food Agric Nutr Dev 15:9635-9650.
- Aguillón Osma J, Maldonado ME, Loango Chamorro N, Arango Varela SS, Landázuri P (2013) Antioxidant and antiproliferative activity of ethanolic and aqueous extracts from leaves and fruits juice Passiflora edulis. Perspect. En Nutr Humana 15:13-25.
- Aguillón‐Osma J, Luzardo‐Ocampo I, Cuellar‐Nuñez ML, Maldonado‐Celis ME, Loango‐Chamorro N, et al. (2019) Impact of in vitro gastrointestinal digestion on the bioaccessibility and antioxidant capacity of bioactive compounds from Passion fruit (Passiflora edulis) leaves and juice extracts. J Food Biochem 43.
- Ahmad S, Rehman T, Abbasi W, Zaman M (2017) Analysis of antioxidant activity and total phenolic content of some homoeopathic mother tinctures. Indian J Res Homoeopathy 11:21.
- Airton JM, Annik IDS, Renan SP, Wilson RM, Maricelma DSS, et al. (2022) Evaluation of the anxiolytic effects of acute administration ofPassiflora alata extract in wistar rats submitted to swimming. J Med Plants Res 16:44-51.
- Aissani F, Grara N, Bensouici C, Bousbia A, Ayed H, et al. (2022) Algerian Sonchus oleraceus L.: a comparison of different extraction solvent on phytochemical composition, antioxidant properties and anticholinesterase activity. Adv Tradit Med 22:383-394.
- Ajileye OO, Obuotor EM, Akinkunmi EO, Aderogba MA (2015) Isolation and characterization of antioxidant and antimicrobial compounds from Anacardium occidentale L. (Anacardiaceae) leaf extract. J King Saud Univ - Sci 27:244-252.
- Akbarmehr A, Peighambardoust SH, Soltanzadeh M, Jafari SM, Sarabandi K (2023) Microencapsulation of Yerba mate extract: The efficacy of polysaccharide/protein hydrocolloids on physical, microstructural, functional, and antioxidant properties. Int J Biol Macromol 234:123678.
- Akoa SP, Kouam JCD, Ondobo ML, Ndjaga JM, Djocgoue PF, et al. (2021) Identification of Methylxanthines and Phenolic Compounds by UPLC-DAD-ESI-MS OTOF and Antioxidant Capacities of Beans and Dark Chocolate Bars from Three Trinitario×Forastero Cocoa (Theobroma cacao L.) Hybrids. J Food Res 10:32.
- Akomolafe SF, Asowata-Ayodele AM (2022) Roasted cashew (Anacardium occidentale L.) nut-enhanced diet forestalls cisplatin-initiated brain harm in rats. Heliyon 8: e11066.
- Al Juhaimi F, Ghafoor K, Ahmed IAM, Babiker EE, Özcan MM (2017) Comparative study of mineral and oxidative status of Sonchus oleraceus, Moringa oleifera and Moringa peregrina leaves. J Food Meas Charact 11:1745-1751.
- Al Khoury A, El Khoury A, Rocher O, Hindieh P, Puel O, et al. (2022) Inhibition of Aflatoxin B1 Synthesis in Aspergillus flavus by Mate (Ilex paraguariensis), Rosemary (Rosmarinus officinalis) and Green Tea (Camellia sinensis) Extracts: Relation with Extract Antioxidant Capacity and Fungal Oxidative Stress Response Modulation. Molecules 27:8550.
- Algarve TD, Assmann CE, Cadoná FC, Machado AK, Manica-Cattani MF, et al. (2019) Guarana improves behavior and inflammatory alterations triggered by methylmercury exposure: an in vivo fruit fly and in vitro neural cells study. Environ Sci Pollut Res 26: 15069-15083.
- Al-Khalaifah HS, Amer SA, Al-Sadek DMM, Khalil AA, Zaki EM, et al. (2020) Optimizing the Growth,Health, Reproductive Performance, and Gonadal Histology of Broodstock Fantail Goldfish (Carassius auratus, L.) by Dietary Cacao Bean Meal. Animals 10:1808.
- Alkhatib A, Atcheson R (2017) Yerba Maté (Ilex paraguariensis) Metabolic, Satiety, and Mood State Effects at Rest and during Prolonged Exercise. Nutrients 9:882.
- Alpinar K, Özyürek M, Kolak U, Güçlü K, Aras Ç, et al. (2009) Antioxidant Capacities of Some Food Plants Wildly Grown in Ayvalik of Turkey. Food Sci Technol Res 15:59-64.
- Alvarado Garcia PAA, Soto Vasquez MR, Rosales Cerquin LE, Alfaro-Ttito BM, RodrigoVillanueva EM (2021) Anxiolytic-like Effect of Essential Oils Extracted from Lippia alba and Lippia citriodora. Pharmacogn J 13:1377-1383.
- Alves JSF, Marques JI, Demarque DP, Costa LRF, Amaral JG, et al. (2020) Involvement of Isoorientin in the Antidepressant Bioactivity of a Flavonoid-Rich Extract from Passiflora edulis f. flavicarpa Leaves. Rev Bras Farmacogn 30:240-250.
- Alves JFS, Silva AMDS, Da Silva RM, Tiago PRF, De Carvalho TG, et al. (2020) In Vivo Antidepressant Effect of Passiflora edulis f. flavicarpa into Cationic Nanoparticles: Improving Bioactivity and Safety. Pharmaceutics 12:383.
- Alviano WS, Alviano DS, Diniz CG, Antoniolli AR, Alviano CS, et al. (2008) In vitro antioxidant potential of medicinal plant extracts and their activities against oral bacteria based on Brazilian folk medicine. Arch Oral Biol 53:545-552.
- Ana María DB, Rosa María VV, Lilian MN, Lucía MM, Oscar GP, et al. (2019) Neurobehavioral and toxicological effects of an aqueous extract of Turnera diffusa Willd (Turneraceae) in mice. J Ethnopharmacol 236:50-62.
- Andrade RAMDS, Da Silva DC, Souza MMBD,De Oliveira RL, Maciel MIS, et al. (2023) Microencapsulation of phenolic compounds from cashew apple (Anacardium occidentale L.) agro-food waste: Physicochemical characterization, antioxidant activity, biodisponibility and stability. Food Chem Adv 3:100364.
- Andrade TDJADS, Araújo BQ, Citó AMDGL, Da Silva J, Saffi J, et al. (2011) Antioxidant properties and chemical composition of technical Cashew Nut ShellLiquid (tCNSL). Food Chem 126:1044-1048.
- Anesini C, Ferraro G, Filip R (2006) Peroxidase-like activity of Ilex paraguariensis. Food Chem 97:459464.
- Anyaegbu OC, Ajayi AM, Adedapo ADA (2017) Hypolipidemic and antioxidant effects of the Methanolic stem bark extract of Anacardium occidentale Linn. In triton-X 100 induced hyperlipidemic rats. Orient Pharm Exp Med 17:211-221.
- Arantes LP, Machado ML, Zamberlan DC, Da Silveira TL, Da Silva TC, et al. (2018) Mechanisms involved in anti-aging effects of guarana (Paullinia cupana) in Caenorhabditis elegans. Braz J Med Biol Res 51: e7552.
- Areta JL, Austarheim I, Wangensteen H, Capelli C (2018) Metabolic and Performance Effects of Yerba Mate on Well-trained Cyclists. Med Sci Sports Exerc 50:817-826.
- Ariza-Ortega JA, García EA, Rodríguez-Meléndez CT (2021) Proximal chemical evaluation, antioxidant content and fatty acids in fermented and dried cocoa beans, roasted cocoa beans and cocoa pulp bar (Theobroma cacao L. Criollo cultivar). Rev Chil Nutr 48:500-506.
- Arletti R, Benelli A, Cavazzuti E, Scarpetta G, Bertolini A (1999) Stimulating property of Turnera diffusa and Pfaffia paniculata extracts on the sexual behavior of male rats. Psychopharmacology (Berl.) 143:15-19.
- Arlorio M, Coïsson JD, Travaglia F, Varsaldi F, Miglio G, et al. (2005) Antioxidant and biological activity of phenolic pigments from Theobroma cacao hulls extracted with supercritical CO2. Food Res Int 38:10091014.
- Arlorio M, Locatelli M, Travaglia F, Coïsson JD, Grosso ED, et al. (2008) Roasting impact on the contents of clovamide (N-caffeoyl-L-DOPA) and the antioxidant activity of cocoa beans (Theobroma cacao L.). Food Chem 106:967-975.
- Arriaga AMC, Lemos TLG, Santiago GMP, Andrade-Neto M, Braga MA, et al. (2013) Chemical composition and antioxidant activity of Indigofera suffruticosa. Chem Nat Compd 49:150-151.
- Ayres ASFSJ, De Araújo LLS, Soares TC, Costa GM, Reginatto FH, et al. (2015) Comparative central effects of the aqueous leaf extract of two populations of Passiflora edulis. Rev Bras Farmacogn 25:499-505.
- Ayres ASFSJ, Santos WB, Junqueira-Ayres DD, Costa GM, Ramos FA, et al. (2017) Monoaminergic neurotransmission is mediating the antidepressant-like effects of Passiflora edulis Sims fo. edulis. Neurosci Lett 660:79-85.
- Azambuja CR, Mattiazzi J, Riffel APK, Finamor IA, Garcia LDO, et al. (2011) Effect of the essential oil of Lippia alba on oxidative stress parameters in silver catfish (Rhamdia quelen) subjected to transport. Aquaculture 319:156-161.
- Azizah O, Abbe MMJ, Kong KW, Amin I, Nawalyah AG, et al. (2010) Epicatechin content and antioxidant capacity of cocoa beans from four different countries. Afr J Biotechnol 9:1052-1059.
- Baeza G, Sarriá B, Bravo L, Mateos R (2018) Polyphenol content, in vitro bioaccessibility and antioxidant capacity of widely consumed beverages. J Sci Food Agric 98:1397-1406.
- Baharum Z, Akim A, Taufiq-Yap Y, Hamid R, Kasran R (2014) In VitroAntioxidant and Antiproliferative Activities of Methanolic Plant Part Extracts of Theobroma cacao. Molecules 19:18317-18331.
- Balázs N, Bereczki D, Kovács T (2021) Cholinesterase inhibitors and memantine for the treatment of Alzheimer and non-Alzheimer dementias. Ideggyógy Szle 74:379-387.
- Baldera Ocampo JF, Granda Santos MS, Chavez Quintana SG (2021) Capacidad antioxidante y polifenoles totales de infusión de cascarilla de cacao (Theobroma cacao) y macambo (Theobroma bicolor). Rev. Investig. Agroproducción Sustentable 5:13.
- Barbosa PR, Valvassori SS, Bordignon CL, Kappel VD, Martins MR, et al. (2008) The Aqueous Extracts of Passiflora alata and Passiflora edulis Reduce Anxiety-Related Behaviors Without Affecting Memory Process in Rats. J Med Food 11:282-288.
- Barbosa Santos T, De Araujo FP, Neto AF, De Freitas ST, De Souza Araújo J, et al. (2021) Phytochemical Compounds and Antioxidant Activity of the Pulp of Two Brazilian Passion Fruit Species: Passiflora Cincinnata Mast. And Passiflora Edulis Sims. Int J Fruit Sci 21:255-269.
- Barrientos RE,Romero-Parra J, Cifuentes F, Palacios J, Romero-Jola NJ, et al. (2023) Chemical Fingerprinting, Aorta Endothelium Relaxation Effect, and Enzymatic Inhibition of Canelo (Drimys winteri J. R. Forst. & G. Forst, (D.C) A. Gray, Family Winteraceae) Fruits. Foods 12:2580.
- Barros LDSP, Santos Da Cruz EDN, De Araújo Guimarães B, Setzer WN, Veras Mourão RH, et al. (2022) Chemometric analysis of the seasonal variation in the essential oil composition and antioxidant activity of a new geraniol chemotype of Lippia alba (Mill.) N.E.Br. ex Britton & P. Wilson from the Brazilian Amazon. Biochem Syst Ecol 105: 104503.
- Baseggio AM, Kido LA, Viganó J, Carneiro MJ, Lamas CDA, et al. (2022) Systemic antioxidant and anti‐inflammatory effects of yellow passion fruit bagasse extract during prostate cancer progression. J. Food Biochem 46.
- Bassani DC, Nunes DS, Granato D (2014) Optimization of Phenolics and Flavonoids Extraction Conditions and Antioxidant Activity of Roasted Yerba-Mate Leaves (Ilex paraguariensis A. St.-Hil., Aquifoliaceae) using Response Surface Methodology. An Acad Bras Ciênc 86:923-934.
- Bastos D, Saldanha L, Catharino R, Sawaya A, Cunha I, et al. (2007) Phenolic Antioxidants Identified by ESI-MS from Yerba Maté (Ilex paraguariensis) and Green Tea (Camelia sinensis) Extracts. Molecules 12:423-432.
- Bastos DHM, Ishimoto EY, Ortiz M, Fernando Ferri A, Torres EAFS (2006) Essential oil and antioxidant activity of green mate and mate tea (Ilex paraguariensis) infusions. J Food Compos Anal 19:538-543.
- Becker AG, Parodi TV, Zeppenfeld CC, Salbego J, Cunha MA, et al. (2016) Pre-sedation and transport of Rhamdia quelen in water containing essential oil of Lippia alba: metabolic and physiological responses. Fish Physiol Biochem 42:73-81.
- Becker AM, Cunha HP, Lindenberg AC, De Andrade F, De Carvalho T, et al. (2019) Spray-Dried Yerba Mate Extract Capsules: Clinical Evaluation and Antioxidant Potential in Healthy Individuals. Plant Foods Hum Nutr 74:495-500.
- Belhoussaine O, El Kourchi C, Harhar H, Bouyahya A, El Yadini A, et al. (2022) Chemical Composition, Antioxidant, Insecticidal Activity, and Comparative Analysis of EssentialOils of Leaves and Fruits of Schinus molle and Schinus terebinthifolius. Evid Based Complement Alternat Med 2022:1-12.
- Belščak A, Komes D, Horžić D, Ganić KK, Karlović D (2009) Comparative study of commercially available cocoa products in terms of their bioactive composition. Food Res Int 42:707-716.
- Bendaoud H, Romdhane M, Souchard JP, Cazaux S, Bouajila J (2010) Chemical Composition and Anticancer and Antioxidant Activities of Schinus Molle L. and Schin us Terebinthifolius Raddi Berries Essential Oils. J Food Sci 75: C466-C472.
- Benítez-Correa E, Bastías-Montes JM, Acuña-Nelson S, Muñoz-Fariña O (2023) Effect of choline chloridebased deep eutectic solvents on polyphenols extraction fromcocoa (Theobroma cacao L.) bean shells and antioxidant activity of extracts. Curr Res Food Sci 7:100614.
- Bernardes NR, Glória LL, Nunes CR, Pessanha FF, Muzitano MF, et al. (2011) Quantificaçãodos Teores de Taninos e Fenóis Totais e Avaliação da Atividade Antioxidante dos Frutos de Aroeira. Rev Vértices 13:117-128.
- Bernardes NR, Heggdorne-Araújo M,Borges IFJC, Almeida FM, Amaral EP, et al. (2014) Nitric oxide production, inhibitory, antioxidant and antimycobacterial activities of the fruits extract and flavonoid content of Schinus terebinthifolius. Rev Bras Farmacogn 24:644-650.
- Bernardi A, Ballestero P, Schenk M, Ferrario M, Gómez G, et al. (2019) Yerba mate (Ilex paraguariensis) favors survival and growth of dopaminergic neurons in culture. Mov Disord 34:920-922.
- Bernardo J, Ferreres F, Gil-Izquierdo Á, Valentão P, Andrade PB (2017) Medicinal species as MTDLs: Turnera diffusa Willd. Ex Schult inhibits CNS enzymes and delays glutamate excitotoxicity in SH-SY5Y cells via oxidative damage. Food Chem Toxicol 106:466-476.
- Bernardo J, Malheiro I, Videira RA, Valentão P, Santos AC, et al. (2021) Trichilia catigua and Turnera diffusa extracts: In vitro inhibition of tyrosinase, antiglycation activity and effects on enzymes and pathways engaged in the neuroinflammatory process. J Ethnopharmacol 271:113865.
- Bernardo J, Santos AC, Videira RA, Valentão P, Veiga F, et al. (2022) Trichilia catigua and Turnera diffusa phyto-phospholipid nanostructures: Physicochemical characterization and bioactivity in cellular models of induced neuroinflammation and neurotoxicity. Int J Pharm 620:121774.
- Berté KAS, Beux MR, Spada PKWDS, Salvador M, Hoffmann-Ribani R (2011) ChemicalComposition and Antioxidant Activity of Yerba-Mate (Ilex paraguariensis A.St.-Hil., Aquifoliaceae) Extract as Obtained by Spray Drying. J Agric Food Chem 59:5523-5527.
- Bezerra AG, Mendes FR, Tabach R, Carlini EA (2011) Effects of a hydroalcoholic extract of Turnera diffusa Willd. Ex Schult., Turneraceae, in tests for adaptogenic activity. Rev Bras Farmacogn 21.
- Bezerra AG, Negri G, Duarte-Almeida JM, Smaili SS, Carlini EA (2016) Phytochemical analysis of hydroethanolic extract of Turnera diffusa Willd and evaluation of its effects on astrocyte cell death. Einstein São Paulo 14:56-63.
- Bezerra AG, Smaili SS, Lopes GS, Carlini EA (2013) Effects of Panax ginseng, Turnera diffusa and Heteropterys tomentosa extracts on hippocampal apoptosis of aged rats. Einstein São Paulo 11:163-167.
- Bezerra GP, Góis RWDS, Brito TSD, Lima FJBD, Bandeira MAM, et al. (2013) Phytochemical study guided by the myorelaxant activity of the crude extract, fractions and constituent from stem bark of Hymenaea courbaril L. J Ethnopharmacol 149:62-69.
- Biapa P, Tammo F, Tekumu J, Nkwikeu P, Yembeau N, et al. (2019) In vitro Antisickling properties, Free radicals quenching potential, protective properties against oxidative mediated ion toxicity of combinations of Theobroma cocoa beans (sterculaceae). Algerian J Nat Prod 7.
- Bini KKN, Kobenan KC, Kouakou M, Kouadio IS, Zengin G, et al. (2023) Phytochemical profiling, antioxidant activities, enzymatic activities and insecticidal potential of aqueous extracts of four plants on the larvae of Helicoverpa armigera (Lepidoptera: Noctuidae), the main pest of cotton plant in Ivory Coast. Arch. Insect BiochemPhysiol 113: e22017.
- Bittencourt LDS, Zeidán‐Chuliá F, Yatsu FKJ, Schnorr CE, Moresco KS, et al. (2014) Guarana ( Paullinia cupana Mart.) Prevents β‐Amyloid Aggregation, Generation ofAdvanced Glycation‐end Products (AGEs), and Acrolein‐Induced Cytotoxicity on Human Neuronal‐Like Cells. Phytother Res 28:1615-1624.
- Bixby M, Spieler L, Menini T, Gugliucci A (2005) Ilex paraguariensis extracts are potent inhibitors of nitrosative stress: A comparative study with green tea and wines using a protein nitration model and mammalian cell cytotoxicity. Life Sci 77:345-358.
- Boasquívis PF, Silva GMM, Paiva FA, Cavalcanti RM, Nunez CV, et al. (2018) Guarana ( Paullinia cupana ) Extract Protects Caenorhabditis elegans Models for Alzheimer Disease and Huntington Disease throughActivation of Antioxidant and Protein Degradation Pathways. Oxid Med Cell Longev 2018:1-16.
- Bordiga M, Locatelli M, Travaglia F, Coïsson JD, Mazza G, et al. (2015) Evaluation of the effect of processing on cocoa polyphenols: antiradical activity, anthocyanins and procyanidins profiling from raw beans to chocolate. Int J Food Sci Technol 50:840-848.
- Borja Fajardo JG, Horta Tellez HB, Peñaloza Atuesta GC, Sandoval Aldana AP, Mendez Arteaga JJ (2022) Antioxidant activity, total polyphenol content and methylxantine ratio in four materials of Theobroma cacao L. from Tolima, Colombia. Heliyon 8: e09402.
- Bortoli PM, Alves C, Costa E, Vanin AP, Sofiatti JR, et al.(2018) Ilex paraguariensis: Potential antioxidant on aluminium toxicity, in an experimental model of Alzheimer’s disease. J Inorg Biochem 181:104-110.
- Botella-Martínez C, Lucas-Gonzalez R, Ballester-Costa C, Pérez-Álvarez JÁ, Fernández-López J, et al. (2021) Ghanaian Cocoa (Theobroma cacao L.) Bean Shells Coproducts: Effect of Particle Size on Chemical Composition, Bioactive Compound Content and Antioxidant Activity. Agronomy 11:401.
- Bracesco N, Dell M, Rocha A, Behtash S, Menini T, et al. (2003) Antioxidant Activity of a Botanical Extract Preparation of Ilex paraguariensis: Prevention of DNA Double-Strand Breaks in Saccharomyces cerevisiae and Human Low-Density Lipoprotein Oxidation. J Altern Complement Med 9:379-387.
- Branco C, Scola G, Rodrigues A, Cesio V, Heinzen H, et al. (2013) Organic and Conventional Yerba Mate (Ilex paraguariensis A. St. Hil) Improves Metabolic Redox Status of Liver and Serum in Wistar Rats. Antioxidants 2:100-109.
- Branco CDS, Scola G, Rodrigues AD, Cesio V, Laprovitera M, et al. (2013) Anticonvulsant, neuroprotective and behavioral effects of organic and conventional yerba mate (Ilex paraguariensis St. Hil.) on pentylenetetrazol-induced seizures in Wistar rats. Brain Res Bull 92:60-68.
- Bravo L, Goya L, Lecumberri E (2007) LC/MS characterization of phenolic constituents of mate (Ilex paraguariensis, St. Hil.) and its antioxidant activity compared tocommonly consumed beverages. Food Res Int 40:393-405.
- Bravo L, Mateos R, Sarriá B, Baeza G, Lecumberri E, et al. (2014) Hypocholesterolaemic and antioxidant effects of yerba mate (Ilex paraguariensis) in high-cholesterol fed rats. Fitoterapia 92:219-229.
- Bridi R, Giordano A, Peñailillo MF, Montenegro G (2019) Antioxidant Effect of Extracts from Native Chilean Plants on the Lipoperoxidation and Protein Oxidation of Bovine Muscle. Molecules 24:3264.
- Broinizi PRB, Andrade-Wartha ERSD, Silva AMDOE, Novoa AJV, Torres RP, et al. (2007) Avaliação da atividade antioxidante dos compostos fenólicos naturalmente presentes em subprodutos do pseudofruto de caju (Anacardium occidentale L.). Ciênc E Tecnol Aliment 27:902-908.
- Broinizi PRB, Andrade-Wartha ERSD, Silva AMDOE, Torres RP, Azeredo HMC, et al. (2008) Propriedades antioxidantes em subproduto do pedúnculo de caju (Anacardium occidentale L.): efeito sobre a lipoperoxidação e o perfil de ácidos graxos poliinsaturados em ratos. Rev Bras Ciênc Farm 44:773-781.
- Bruna C, Eichhikz I, Rohn S, Kroh LW, Huyskens-Kell S (2010) Bioactive compounds and antioxidant activity of cocoa hulls (Theobroma cacao L.) from different origins. J Appl Bot Food Qual 83.
- Bu Wong M, Rodríguez NS, Alejo JLP, Pérez MF (1999) Actividad de la Indigofera suffruticosa Millen la epilepsia crónica experimental y su relación con aminoácidos neurotransmisores. Rev Cuba Plantas Med 4:18-21.
- Budaraga IK, Susanti E, Asnurita A, Nurdin E, Ramaiyulis R (2019) The Antioxidant Characteristics of The Liquid Smoke of Cocoa Shell (Theobroma cacao, l) In Different Water Content Variations. J Appl Agric Sci Technol 3:226-238.
- Cádiz-Gurrea M, Borrás-Linares I, Lozano-Sánchez J, Joven J, Fernández-Arroyo S, et al. (2017) Cocoa and Grape Seed Byproducts as a Source of Antioxidant and Anti-Inflammatory Proanthocyanidins. Int J Mol Sci 18:376.
- Cahyanti RE, Hala Y, Mu’nisa A (2021) Determination of total phenolic content and antioxidant activity of fruit mix from Malino, Gowa Regency, South Sulawesi. IOP Conf Ser Earth Environ Sci 911:012085.
- Campos AM, Escobar J, Lissi EA (1996) The Total Reactive Antioxidant Potential (TRAP) and Total Antioxidant Reactivity (TAR) of Ilex paraguayensis Extracts and Red Wine. J Braz Chem Soc 7:43-49.
- Campos AR, Barros AIS, Albuquerque FAA, Leal LKAM, Rao VSN (2005) Acute effects of guarana (Paullinia cupana Mart.) on mouse behaviour in forced swimming and open field tests. Phytother Res 19:441-443.
- Cao S, Aman Y, Fang EF, Tencomnao T (2022) P. edulis Extract Protects Against Amyloid-β Toxicity in Alzheimer’s Disease Models Through Maintenance of Mitochondrial Homeostasis via the FOXO3/DAF-16 Pathway. Mol Neurobiol 59:5612-5629.
- Caporaso N, Whitworth MB, Fowler MS, Fisk ID (2018) Hyperspectral imaging for non-destructive prediction of fermentation index, polyphenol content and antioxidant activity in single cocoa beans. Food Chem 258:343-351.
- Cardoso Vilela F, Soncini R, Giusti-Paiva A (2009) Anxiolytic-like effect of Sonchus oleraceus L. in mice. J Ethnopharmacol 124:325-327.
- Carini M, Maffei Facino R, Aldini G, Calloni M, Colombo L (1999) Characterization of phenolic antioxidants from Maté (Ilex Paraguayensis) by liquid chromatography/mass spectrometry and liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 12:1813-1819.
- Carneiro MJ, López BGC, Lancellotti M, Franchi GC, Nowill AE, et al. (2016) Evaluation of the chemical composition and biological activity of extracts of Tetragoniscaangustula propolis and Schinus terebinthifolius Raddi (Anacardiaceae). J Apic Res 55:315-323.
- Carneiro MJ, Pinheiro GP, Baseggio AM, Maróstica-Júnior MR, Sawaya ACHF (2023) Chemical Composition and Antioxidant Activity of Essential Oil from Male and Female Schinus terebinthifolius. Pharmacogn Res 15:484-491.
- Carrillo LC, Londoño-Londoño J, Gil A (2014) Comparison of polyphenol, methylxanthines and antioxidant activity in Theobroma cacao beans from different cocoa-growing areas in Colombia. Food Res Int 60:273-280.
- Cassol LA, Almeida Filho ESD, Oliveira ACSD (2019) Performance Comparison Between a Natural and a Commercial Antioxidant on Smoked Pacu (Piaractus mesopotamicus). J Agric Sci 11:225.
- Cazarin, C.B.B., Silva, J.K.D., Colomeu, T.C., Zollner, R.D.L., Maróstica Junior, M.R., 2014. Capacidade antioxidante e composição química da casca de maracujá (Passiflora edulis). Ciênc Rural 44:1699-1704.
- 100. Celis CN, Rivero PE, Isaza JH, Stashenko E, Martínez JR (2007) Estudio comparativo de la composición y actividad biológica de los aceites esenciales extraídos de Lippia alba, Lippia origanoides y Phyla dulces, especies de la família Verbenaceae. Sci Tech XIII, 103-105.
- 101. Chaikhong K, Chumpolphant S, Rangsinth P, Sillapachaiyaporn C, Chuchawankul S, et al. (2022) Antioxidant and Anti-Skin Aging Potential of Selected Thai Plants: In Vitro Evaluation and In Silico Target Prediction. Plants 12:65.
- 102. Chandra S, Gonzalez De Mejia E (2004) Polyphenolic Compounds, Antioxidant Capacity, and Quinone Reductase Activity of an Aqueous Extract of Ardisia compressa in Comparison to Mate ( Ilex paraguariensis ) and Green ( Camellia sinensis ) Teas. J Agric Food Chem 52:3583-3589.
- 103. Chaves MH, Antônia Maria Das Graças Lopes C, Lopes JAD, Costa DAD, Oliveira CAAD, et al. (2010) Fenóis totais, atividade antioxidante e constituintes químicos de extratos de Anacardium occidentale L., Anacardiaceae. Rev. Bras. Farmacogn. 20:106-112.
- 104. Chen XM, Kitts DD, Ma Z (2017) Demonstrating the relationship between the phytochemical profile of different teas with relative antioxidant and anti-inflammatory capacities.Funct. Foods Health Dis 7:375.
- 105. Chies C, Branco C, Scola G, Agostini F, Gower A, et al. (2013) Antioxidant Effect of Lippia alba (Miller) N. E. Brown. Antioxidants 2:194-205.
- 106. Chin E, Miller KB, Payne MJ, Hurst WJ, Stuart DA (2013) Comparison of antioxidant activity and flavanol content of cacao beans processed by modern and traditional Mesoamerican methods. Herit Sci 1:9.
- 107. Choi MS, Park HJ, Kim SR, Kim DY, Jung UJ (2017) Long-Term Dietary Supplementation with Yerba Mate Ameliorates Diet-Induced Obesity and Metabolic Disorders in Mice by Regulating Energy Expenditure and Lipid Metabolism. J Med Food 20:1168-1175.
- 108. Chotphruethipong L, Benjakul S, Kijroongrojana K (2017) Optimization of extraction of antioxidative phenolic compounds from cashew (Anacardium occidentale L.) leaves using response surface methodology. J. Food Biochem. 41: e12379.
- 109. Cittadini MC, Albrecht C, Miranda AR, Mazzuduli GM, Soria EA, et al. (2019a) Neuroprotective Effect of Ilex Paraguariensis Intake on Brain Myelin of Lung Adenocarcinoma-Bearing Male Balb/c Mice. Nutr Cancer 71:629-633.
- 110. Cittadini MC, Canalis AM,Albrecht C, Soria EA (2015) Effects of oral phytoextract intake on phenolic concentration and redox homeostasis in murine encephalic regions. Nutr Neurosci 18:316-322.
- 111. Cittadini MC, Repossi G, Albrecht C, Di Paola Naranjo R, Miranda AR, et al. (2019b) Effects of bioavailable phenolic compounds from Ilex paraguariensis on the brain of mice with lung adenocarcinoma. Phytother Res 33:1142-1149.
- 112. Cogoi L, Marrassini C, Saint Martin EM, Alonso MR, Filip R, et al. (2023) Inhibition of Glycation End Products Formation and Antioxidant Activities of Ilex paraguariensis: comparative study of fruit and leaves extracts. J Pharmacopuncture 26:338-347.
- 113. Coleta M, Batista MT, Campos MG, Carvalho R, Cotrim MD, et al. (2006) Neuropharmacological evaluation of the putative anxiolytic effects ofPassiflora edulis Sims, its sub-fractions and flavonoid constituents. Phytother Res 20:1067-1073.
- 114. Colomeu TC, Figueiredo D, Cazarin CBB, Schumacher NSG, Maróstica MR, et al. (2014) Antioxidant and anti-diabetic potential of Passiflora alata Curtis aqueous leaves extract in type 1 diabetes mellitus (NODmice). Int Immunopharmacol 18:106-115.
- 115. Colpo AC, De Lima ME, Maya-López M, Rosa H, Márquez-Curiel C, et al. (2017) Compounds from Ilex paraguariensis extracts have antioxidant effects in the brains of rats subjected to chronic immobilization stress. Appl Physiol Nutr Metab 42: 1172-1178.
- 116. Colpo AC, Rosa H, Lima ME, Pazzini CEF, De Camargo VB, et al. (2016) Yerba mate (Ilex paraguariensis St. Hill.)-based beverages: How successive extraction influences the extract composition and its capacity to chelate iron and scavenge free radicals. Food Chem 209:185-195.
- 117. Colpo G, Trevisol F, Teixeira AM, Fachinetto R, Pereira RP, et al. (2007) Ilex paraguariensis has antioxidant potential and attenuates haloperidol-induced orofacial dyskinesia and memory dysfunction in rats. Neurotox Res 12:171-180.
- 118. Contreras-Esquivel JC, Cano-González CN, Ascacio-Valdes J, Aguirre-Joya JA, Aguillón-Gutierrez D, et al. (2022) Polyphenolic-rich extracts from the leaves of Ilex paraguariensis and Larrea divaricata and their antioxidant and antiCOVID-19 potential. Biotecnia 25: 61-66.
- 119.Corradi I, De Souza E, Sande D, Takahashi JA (2018) Correlation between phenolic compounds contents, anti-tyrosinase and antioxidant activities of plant extracts. Chem Eng Trans 64:109-114.
- 120. Correa VG, De Sá-Nakanishi AB, Gonçalves GDA, Barros L, Ferreira ICFR, et al. (2019) Yerba mate aqueous extract improves the oxidative and inflammatory states of rats with adjuvant-induced arthritis. Food Funct 10:5682-5696.
- 121. Cortez D, Quispe-Sanchez L, Mestanza M, Oliva-Cruz M, Yoplac I, et al. (2023) Changes in bioactive compounds during fermentation of cocoa (Theobroma cacao) harvested in Amazonas-Peru. Curr Res Food Sci 6:100494.
- 122. Costa COD, Ribeiro PR, De Castro RD, Fernandez LG (2013) Avaliação da atividade antioxidante em amostras comerciais de Schinus terebinthifolius (aroeira vermelha). Rev Ciênc Médicas E Biológicas 12:312.
- 123. Crozier SJ, Preston AG, Hurst JW, Payne MJ, Mann J, et al. (2011) Cacao seeds are a “Super Fruit”: A comparative analysis of various fruit powders and products. Chem Cent J 5:5.
- 124. Cruz EDN, Barros LDSP, Guimarães BDA, Mourão RHV, Maia JGS, et al. (2023) Seasonal Variation in Essential Oil Composition and Antioxidant Capacity of Aniba canelilla (Lauraceae): A Reliable Source of 1-Nitro-2-phenylethane. Molecules 28:7573.
- 125. Cruz R, Chavez SG, Fernández-Jeri AB (2021) Actividad antioxidante y ácidos grasos de aceite de semillas de siete frutas nativas de la región Amazonas, Perú. Inf Tecnológica 32:141-148.
- 126. Cruz Reina LJ, Durán-Aranguren DD, Forero-Rojas LF, Tarapuez-Viveros LF, Durán-Sequeda D, et al. (2022) Chemical composition and bioactive compounds of cashew (Anacardium occidentale) apple juice and bagasse from Colombian varieties. Heliyon 8: e09528.
- 127. Da Costa CAR, Machado GGL, Rodrigues LJ, De Barros HEA, Natarelli CVL, et al. (2023) Phenolic compounds profile and antioxidant activity of purple passion fruit’s pulp, peel and seed at different maturation stages. Sci Hortic 321:112244.
- 128. Da Costa Miranda V, Trufelli DC, Santos J, Campos MP, Nobuo M, et al. (2009) Effectiveness of Guaraná ( Paullinia cupana ) for Postradiation Fatigue and Depression: Results of a Pilot Double-Blind Randomized Study. J Altern Complement Med 15:431-433.
- 129. Da Cunha RS, Amorim KS, Gercina AC, De Oliveira ACA, Dos Santos Menezes L, et al. (2021) Herbal medicines as anxiolytics prior to third molar surgical extraction. A randomized controlled clinical trial. Clin Oral Investig 25:1579-1586.
- 130. Da Silva AL, Bardini S, Nunes DS, Elisabetsky E (2002) Anxiogenicproperties of Ptychopetalum olacoides Benth. (Marapuama). Phytother Res 16:223-226.
- 131. Da Silva AL, Ferreira JG, Da Silva Martins B, Oliveira S, Mai N, et al. (2008) Serotonin receptors contribute to the promnesic effects of P. olacoides (Marapuama). Physiol Behav 95:88-92.
- 132. Da Silva AL, Piato ÂL, Ferreira JG,Martins BS, Nunes DS, et al. (2007) Promnesic effects of Ptychopetalum olacoides in aversive and non-aversive learning paradigms. J Ethnopharmacol 109:449-457.
- 133. Da Silva AL, Piato ÂLS, Bardini S, Netto CA, Nunes DS, et al. (2004) Memory retrieval improvement by Ptychopetalum olacoides in young and aging mice. J Ethnopharmacol 95:199-203.
- 134. Da Silva AL, Silva Martins BD, Linck VDM, Herrmann AP, Mai N, et al. (2009) MK801- and scopolamine-induced amnesias are reversed by an Amazonian herbal locally used as a “brain tonic.” Psychopharmacology (Berl.) 202:165-172.
- 135. Da Silva Bittencourt L, Schnorr CE, Santos DC, Rostirolla DC, Moresco KS, et al. (2020) Chronic acrolein exposure in Wistar rats: The effects of guarana extracts. J Funct Foods 65:103733.
- 136. Da Silva Dannenberg G, Funck GD, Mattei FJ, Da Silva WP, Fiorentini ÂM (2016) Antimicrobial and antioxidant activity of essential oil from pink pepper tree (Schinus terebinthifolius Raddi) in vitro and in cheese experimentally contaminated with Listeria monocytogenes. Innov Food Sci Emerg Technol 36:120127.
- 137. Da Silva DPB, Florentino IF, Da Silva Moreira LK, Brito AF, Carvalho VV, et al. (2018) Chemical characterization and pharmacological assessment of polysaccharide free, standardized cashew gum extract (Anacardium occidentale L.). J Ethnopharmacol 213:395-402.
- 138. Da Silva JK, Cazarin CBB, Colomeu TC, Batista ÂG, Meletti LMM, et al. (2013) Antioxidant activity of aqueous extract of passion fruit (Passiflora edulis) leaves: In vitro and in vivo study. Food Res Int 53: 882890.
- 139. Da Silva JKR, Sousa PJC, Andrade EHA, Maia JGS (2007) Antioxidant Capacity and Cytotoxicity of Essential Oil and Methanol Extract of Aniba canelilla (H.B.K.) Mez. J Agric Food Chem 55:9422-9426.
- 140. Da Silva LVF, Veras Mourão RH, Manimala J, Lnenicka GA (2018) The essential oil of Lippia alba and its components affect Drosophila behavior and synaptic physiology. J Exp Biol jeb 176909.
- 141. Da Silva Nascimento M, Dos Santos PH, De Abreu FF,Shan AYKV, Amaral RG, et al. (2023) Schinus terebinthifolius Raddi (Brazilian pepper) leaves extract: in vitro and in vivo evidence of anti-inflammatory and antioxidant properties. Inflammopharmacology 31:2505-2519.
- 142.Da Silva Oliveira C, Fonseca Maciel L, Spínola Miranda M, Da Silva Bispo E (2011) Phenolic compounds, flavonoids and antioxidant activity in different cocoa samples from organic and conventional cultivation. Br Food J 113:1094-1102.
- 143. Da Silva Port’s P, Chisté RC, Godoy HT, Prado MA (2013) The phenolic compounds and the antioxidant potential of infusion of herbs from the Brazilian Amazonian region. Food Res Int 53:875-881.
- 144. Da Silva RA, Dihl RR, E Santos DN, De Abreu BRR, De Lima A, et al. (2013) Evaluation of antioxidant and mutagenic activities of honey-sweetened cashew apple nectar. Food Chem Toxicol 62:61-67.
- 145. Dabulici CM, Sârbu I, Vamanu E (2020) The Bioactive Potential of Functional Productsand Bioavailability of Phenolic Compounds. Foods 9:953.
- 146. Dartora B, Crepalde LT, Hickert LR, Fabricio MF, Ayub MAZ, et al. (2023) Kombuchas from black tea, green tea, and yerba-mate decocts: Perceived sensory map, emotions, and physicochemical parameters. Int J Gastron Food Sci 33:100789.
- 147. De Andrade DVG, Madureira De Oliveria D, Barreto G, Bertolino LA, Saraceno E, et al. (2008) Effects of the extract of Anemopaegma mirandum (Catuaba) on Rotenone-induced apoptosis in human neuroblastomas SH-SY5Y cells. Brain Res 1198:188-196.
- 148. De Campos DL, Queiroz LY, Fontes-Junior EA, Pinheiro BG, Da Silva JKR, et al. (2023) Aniba canelilla (Kunth) Mez essential oil and its primary constituent, 1-nitro-2-phenylethane, inhibits acetylcholinesterase and reverse memory impairment in rodents. J Ethnopharmacol 303:116036.
- 149. De Carvalho JM, De Figueiredo RW, De Sousa PHM, De Luna FMT, Maia GA (2018) Cashew nutoil:
- effect of kernel grade and a microwave preheating extraction step on chemical composition, oxidative stability and bioactivity. Int J Food Sci Technol 53:930-937.
- 150. De Castro PCF, Hoshino A, Silva JCD, Mendes FR (2007) Possible anxiolytic effect of two extracts of Passiflora quadrangularis L. in experimental models. Phytother Res 21:481-484.
- 151. De Freitas Souza C, Baldissera MD, Bianchini AE, Da Silva EG, Mourão RHV, et al. (2018) Citral and linalool chemotypes of Lippia alba essential oil as anesthetics for fish: a detailed physiological analysis of side effects during anesthetic recovery in silver catfish (Rhamdia quelen). Fish Physiol Biochem 44:21-34.
- 152. De Gois JS, Almeida TS, De Andrade RM, Toaldo IM, Bordignon-Luiz MT, et al. (2016) Direct solid analysis for the determination of Mn, Ni, Rb and Sr in powdered stimulant plants using high-resolution continuum source atomic absorption spectrometry followed by chemometric classification based on elemental composition, polyphenol content and antioxidant activity. Microchem J 124:283-289.
- 153. De Lima ACS, Soares DJ, Da Silva LMR, De Figueiredo RW, De Sousa PHM, et al. (2014) In vitro bioaccessibility of copper, iron, zinc and antioxidant compounds of whole cashew apple juice and cashew apple fibre (Anacardium occidentale L.) following simulated gastro-intestinal digestion. Food Chem 161:142-147.
- 154. De Lima ME, Ceolin Colpo AZ, Maya-López M, Rangel-López E, Becerril-Chávez H, et al. (2019) Comparing the Effects of Chlorogenic Acid and Ilex paraguariensis Extracts on Different Markers of Brain Alterations in Rats Subjected to Chronic Restraint Stress. Neurotox Res 35:373-386.
- 155. De Oliveira Campos MP, Riechelmann R, Martins LC, Hassan BJ, Casa FBA, et al. (2011) Guarana ( Paullinia cupana ) Improves Fatigue in Breast Cancer Patients Undergoing Systemic Chemotherapy. J Altern Complement Med 17:505-512.
- 156. De Oliveira DM, Barreto G, Galeano P, Romero JI, Holubiec MI, et al. (2011) Paullinia cupana Mart. var. Sorbilis protects human dopaminergic neuroblastoma SH-SY5Y cell line against rotenone-induced cytotoxicity. Hum Exp Toxicol 30:1382-1391.
- 157. De Oliveira JB, Das Neves JVG, Da Silva MV (2016) Phytochemical analysis and antioxidant activity of the hydroethanolic extract of Passiflora edulis f. flavicarpa redisues. Bol Cent Pesqui Process Aliment 34.
- 158. De Oliveira VS, Augusta IM, Braz MVDC, Riger CJ, Prudêncio ER, et al. (2020) Aroeira fruit (Schinus terebinthifolius Raddi) as a natural antioxidant: Chemical constituents, bioactive compounds and in vitro and in vivo antioxidant capacity. Food Chem 315:126274.
- 159. De Santana FC, De Oliveira Torres LR, Shinagawa FB, De Oliveira E Silva, AM, Yoshime LT, et al. (2017) Optimization of the antioxidant polyphenolic compounds extraction of yellow passion fruit seeds (Passiflora edulis Sims) by response surface methodology. J Food Sci Technol 54:3552-3561.
- 160. De Souza MDSS, Barbalho SM, Damasceno DC, Rudge MVC, De Campos KE,et al. (2012) Effects of Passiflora edulis (Yellow Passion) on Serum Lipids and Oxidative Stress Status of Wistar Rats. J Med Food 15:78-82.
- 161. De Vargas FS, Almeida PDO, De Boleti APA, Pereira MM, De Souza TP, et al. (2016) Antioxidant activity and peroxidase inhibition of Amazonian plants extracts traditionally used as anti-inflammatory. BMC Complement Altern Med 16:83.
- 162. Dedvisitsakul P, Watla-iad K (2022) Antioxidant activity and antidiabetic activities of Northern Thai indigenous edible plant extracts and their phytochemical constituents. Heliyon 8: e10740.
- 163.Del Giglio AB, Cubero DDIG, Lerner TG, Guariento RT, De Azevedo RGS, et al. (2013) Purified Dry Extract of Paullinia cupana (Guaraná) (PC-18) for Chemotherapy-Related Fatigue in Patients with Solid Tumors: An Early Discontinuation Study. J Diet Suppl 10:325-334.
- 164. Deng J, Zhou Y, Bai M, Li H, Li L (2010) Anxiolytic and sedative activities of Passiflora edulis f. flavicarpa. J Ethnopharmacol 128:148-153.
- 165. Deus VL, Cerqueira E Silva MBD, Maciel LF, Miranda LCR, Hirooka EY, et al. (2018) Influence of drying methods on cocoa (Theobroma cacao L.): antioxidant activity and presence of ochratoxin A. Food Sci Technol 38:278-285.
- 166. Devaki K, Sunitha M (2009) Antioxidant activity of Passiflora edulis sims leaves. Indian J Pharm Sci 71:310.
- 167. Deviani V, Hardianto A, Farabi K, Herlina T (2022) Flavanones from Erythrina crista-galli Twigs and Their Antioxidant Properties Determined through In Silico and In Vitro Studies. Molecules 27:6018.
- 168. Dias MC, Pinto DCGA, Silva AMS (2021) Plant Flavonoids: Chemical Characteristics and Biological Activity. Mol Basel Switz 26:5377.
- 169. Disciglio G, Tarantino A, Frabboni L, Gagliardi A, Giuliani MM, et al. (2017) Qualitative characterization of cultivated and wild edible plants: mineral elements, phenols content and antioxidant capacity. Ital J Agron 12:1036.
- 170. Dos Santos Da Rocha P, De Araújo Boleti AP, Do Carmo Vieira M, Carollo CA, Da Silva DB, et al. (2019) Microbiological quality, chemical profile as well as antioxidant and antidiabetic activities of Schinus terebinthifolius Raddi. Comp Biochem Physiol Part C Toxicol Pharmacol 220:36-46.
- 171. Dos Santos FAR, Xavier JA, Da Silva FC, Merlin JPJ, Goulart MOF, et al. (2022) Antidiabetic, Antiglycation, and Antioxidant Activities of Ethanolic Seed Extract of Passiflora edulis andPiceatannol In Vitro. Molecules 27:4064.
- 172. Dos Santos GJ, Defendi RO, Düsman E, Biffi MT, Berton GH, et al. (2023) Valorization of Wastes from the Juice Passion Fruit ProductionIndustry: Extraction of Bioactive Compounds from Seeds, Antioxidant, Photoprotective and Antiproliferative Activities. Waste Biomass Valorization 14:1233-1250.
- 173. Dos Santos LC, Mendiola JA, Sánchez-Camargo ADP, Álvarez-Rivera G, Viganó J, et al. (2021) Selective Extraction of Piceatannol from Passiflora edulis by-Products: Application of HSPs Strategy and Inhibition of Neurodegenerative Enzymes. Int J Mol Sci 22:6248.
- 174. Doungue HT, Kengne APN, Kuate D (2018) Neuroprotective effect and antioxidant activity of Passiflora edulis fruit flavonoid fraction, aqueous extract, and juice in aluminum chloride-induced Alzheimer’s disease rats. Nutrire 43:23.
- 175. Doyama JT, Rodrigues HG, Novelli E.B, Cereda E, Vilegas W (2005) Chemical investigation and effects of the tea of Passiflora alataon biochemical parameters in rats. J Ethnopharmacol 96:371-374.
- 176. Duangjan C, Rangsinth P, Gu X, Wink M, Tencomnao T (2019) Lifespan Extending and Oxidative Stress Resistance Properties of a Leaf Extracts from Anacardium occidentale L. in Caenorhabditis elegans. Oxid Med Cell Longev 2019:1-16.
- 177. Duangjan C, Rangsinth P, Zhang S,Wink M, Tencomnao T (2021) Anacardium Occidentale L. Leaf Extracts Protect Against Glutamate/H2O2-Induced Oxidative Toxicity and Induce Neurite Outgrowth: The Involvement of SIRT1/Nrf2 Signaling Pathway and Teneurin 4 Transmembrane Protein. Front Pharmacol 12: 627738.
- 178. Ducke A (1966) A catuaba na botânica sistemática, científica e pseudo-científica. Rev Bras Farmácia 47:267-272.
- 179. Dudonné S, Vitrac X, Coutière P, Woillez M, Mérillon J.-M (2009) Comparative Study of Antioxidant Properties and Total Phenolic Content of 30 Plant Extracts of Industrial Interest Using DPPH, ABTS, FRAP, SOD, and ORAC Assays. J Agric Food Chem 57:1768-1774.
- 180. Ebuehi OAT, Anams C, Gbenle OD, Ajagun‐Ogunleye MO (2019) Hydro‐ethanol seed extract of Theobroma cacao exhibits antioxidant activities and potential anticancer property. J Food Biochem 43: e12767.
- 181. Echeverry SM, Medina HI, Costa GM, Aragón DM (2018) Optimization of flavonoid extraction from Passiflora quadrangularis leaves with sedative activity and evaluation of its stability under stress conditions. Rev Bras Farmacogn 28:610-617.
- 182. Efing LC, Caliari TK, Nakashima T, De Freitas RJS (2009) Caracterização química e capacidade antioxidante da erva-mate (Ilex paraguariensis St. Hil.). Bol Cent Pesqui Process Aliment 27.
- 183. El‐Demerdash FM, Jebur AB, Nasr HM, Hamid HM (2019) Modulatory effect of Turnera diffusa against testicular toxicity induced by fenitrothion and/or hexavalent chromium in rats. Environ Toxicol 34:330-339.184.Elekofehinti O, Osehodion R, Adeyelu T, Ogunwa T, Olatunde I, et al. (2016) Hypoglycemic, Hypolipidemic and Antioxidant Potentials of Aqueous and Ethanolic Leaf Extracts of Anacardium occidentale in Alloxan Induced Type I Diabetic Rat Model. Br J Med Med Res 14:1-10.
- 185.El-Massry KF, El-Ghorab AH, Shaaban HA, Shibamoto T (2009) Chemical Compositions and Antioxidant/Antimicrobial Activities of Various Samples Prepared from Schinus terebinthifolius Leaves Cultivated in Egypt. J Agric Food Chem 57:5265-5270.
- 186. Elshikh AA, Elnour M, Elkamali H, Elbalola A, Garbi M (2023) In‐vitro Investigation of Anti‐Parasite, Anti‐Oxidant Activities, Cytotoxicity and GC/MS analysis of Ehanolic Extracts for Rhynchosia memnonia var. memnonia and Sonchus oleraceus used in Sudanese Ethnomedicine. ChemistrySelect 8: e202204036.
- 187. Encarnação S, De Mello-Sampayo C, Graça NAG, Catarino L, Da Silva IBM, et al. (2016) Total phenolic content, antioxidant activity and pre-clinical safety evaluation of an Anacardium occidentale stem bark Portuguese hypoglycemic traditional herbal preparation. Ind Crops Prod 82:171-178.
- 188. Ennigrou A, Casabianca H, Laarif A, Hanchi B, Hosni K (2017) Maturation-related changes in phytochemicals and biological activities of the Brazilian pepper tree (Schinus terebinthifolius Raddi) fruits. South Afr J Bot 108:407-415.
- 189. Ennigrou A, Casabianca H, Vulliet E, Hanchi B, Hosni K (2018) Assessing the fatty acid, essential oil composition, their radical scavenging and antibacterial activities of Schinus terebinthifolius Raddi leaves and twigs. J Food Sci Technol 55:1582-1590.
- 190. Erol NT, Sari F, Çalikoğlu E, Veli̇Oğlu YS (2009) Green and roasted mate: phenolic profile and antioxidant activity. Turk J Agric For 33:4.
- 191. Espinola EB, Dias RF, Mattei R, Carlini EA (1997) Pharmacological activity of Guarana (Paullinia cupana Mart.) in laboratory animals. J Ethnopharmacol 55:223-229.
- 192. Estrada-Reyes R, Carro-Juárez M, Martínez-Mota L (2013) Pro-sexual effects of Turnera diffusa Wild (Turneraceae) in male rats involves the nitric oxide pathway. J Ethnopharmacol 146:164-172.
- 193. Estrada-Reyes R, Ferreyra-Cruz OA, Jiménez-Rubio G, Hernández-Hernández OT, Martínez-Mota L (2016) Prosexual Effect of Chrysactinia mexicana A. Gray (Asteraceae), False Damiana, in a Model of Male Sexual Behavior. BioMed Res Int 2016:1-9.
- 194. Estrada-Reyes R, Ortiz-López P, Gutiérrez-Ortíz J, Martínez-Mota L (2009) Turnera diffusa Wild (Turneraceae) recovers sexual behavior in sexually exhausted males. J Ethnopharmacol 123:423-429.
- 195. Everton GO, Pereira APM, Rosa PVS, Mafra NSC, Santos Júnior PS, et al. (2021) Chemical characterization, toxicity, antioxidant and antimicrobial activity of the essential oils of Hymenaea courbaril L. and Syzygium cumini (L.) Skeels. Ciênc E Nat 43: e11.
- 196. Fantinelli J, González-Arbeláez L, Ciocci-Pardo A, Schinella G, Mosca S (2016) Comparative effects of natural products on ischemia-reperfusion injury: relation to their “in vitro” antioxidant capacity. Bol Latinoam Caribe Plantas Med Aromáticas 15:151-163.
- 197. Farias IV, Fratoni E, Theindl LC, De Campos AM, Dalmarco EM, et al. (2021) In Vitro Free Radical Scavenging Properties and Anti-Inflammatory Activity of Ilex paraguariensis (Maté) and the Ability of Its Major Chemical Markers to Inhibit the Production of Proinflammatory Mediators. Mediators Inflamm 2021:1-13.
- 198. Farias PKS, Silva JCRL, Souza CND, Fonseca FSAD, Brandi IV, et al. (2019) Antioxidant activity of essential oils from condiment plants and their effect on lactic cultures and pathogenic bacteria. Ciênc. Rural 49: e20180140.
- 199. Fauth S, Campos AR, Silveira ER, Rao VS (2002) Efeitos de óleos essenciais de plantas no tempo de sono induzido por cetamina em camundongos. Rev Bras Farmacogn 12:112-113.
- 200. Feriani A, Tir M, Mufti A, Caravaca AMG, Contreras MDM, et al. (2021) HPLC–ESI–QTOF–MS/MS profiling and therapeutic effects of Schinus terebinthifolius and Schinus molle fruits: investigation of their antioxidant, antidiabetic, anti-inflammatory and antinociceptive properties. Inflammopharmacology 29:467481.
- 201. Fernandes CEF, Kuhn F, Scapinello J, Lazarotto M, Bohn A, et al. (2016) Phytochemical profile, antioxidant and hypolipemiant potential of Ilex paraguariensis fruit extracts. Ind. Crops Prod. 81: 139-146.
- 202. Fernandes CEF, Scapinello J, Bohn A, Boligon AA, Athayde ML, et al. (2017) Phytochemical profile, antioxidant and antimicrobial activity of extractsobtained from erva-mate (Ilex paraguariensis) fruit using compressed propane and supercritical CO2. J. Food Sci. Technol 54: 98-104.
- 203. Ferrari CKB, (2002) Avaliaçäo da Capacidade Antioxidante Total (CAT) e colorimetria de vinte e um diferentes tipos de alimentos comercializados no município de São Paulo (SP), Brasil (Doutorado em Nutrição). Universidade de São Paulo, São Paulo.
- 204. Ferreira BS, De Almeida CG, Faza LP, De Almeida A, Diniz CG, et al. (2011) Comparative Properties of Amazonian Oils Obtained by Different Extraction Methods. Molecules 16: 5875-5885.
- 205. Ferreres F, Sousa C, Valentão P, Andrade PB, Seabra RM, et al. (2007) New C -Deoxyhexosyl Flavones and Antioxidant Properties of Passiflora edulis Leaf Extract. J. Agric. Food Chem 55: 10187-10193.
- 206. Feudjio AF, Biapa NP, Kodjio N, Yembeau NL, Nkwikeu NP, et al. (2019) Hydroethanolic extract of Theobroma cacao beans is non toxic and attenuates oxydative stress induced by Naphtalene in Wistar rats. J. Herb. Med. Res. 4.
- 207.Figueiredo DAF, Pordeus LCM, Paulo LL, Braga RM,Fonsêca DV, et al. (2016) Effects of bark flour of Passiflora edulis on food intake, body weight and behavioral response of rats. Rev. Bras. Farmacogn. 26: 595-600.
- 208. Figueiró M, Ilha J, Linck VM, Herrmann AP, Nardin P, et al. (2011) The Amazonian herbal Marapuama attenuates cognitive impairment and neuroglial degeneration in a mouse Alzheimer model. Phytomedicine 18: 327-333.
- 209. Figueiró M, Ilha J, Pochmann D, Porciúncula LO, Xavier LL, et al. (2010) Acetylcholinesterase inhibition in cognition-relevant brain areas of mice treated with a nootropic Amazonian herbal (Marapuama). Phytomedicine 17: 956-962.
- 210. Filip R, Lotito SB, Ferraro G, Fraga CG, (2000) Antioxidant activity of Ilex paraguariensis and related species. Nutr. Res. 20: 1437-1446.
- 211. Finamor IA, Bressan CA, Ariotti K, De Lima CL, Schmidt D, et al. (2023) The long-term transport of Potamotrygon wallacei increases lactate levels and triggers oxidative stress in its brain: The protective role of recovery and the essential oil of Lippia alba. Aquaculture 572: 739461.
- 212. Flausino Jr OA, Pereira AM, Bolzani VDS, Nunes-de-Souza RL, (2007) Effects of Erythrinian Alkaloids Isolated from Erythrina mulungu (Papilionaceae) in Mice Submitted to Animal Models of Anxiety. Biol. Pharm. Bull 30: 375-378.
- 213. Flausino O, De Ávila Santos L, Verli H, Pereira AM, Bolzani VDS, et al. (2007) Anxiolytic Effects of Erythrinian Alkaloids from Erythrina mulungu. J. Nat. Prod. 70: 48-53.
- 214. Flieger J, Franus W, Panek R, Szymańska-Chargot M, Flieger W, et al. (2021) Green Synthesis of Silver Nanoparticles Using Natural Extracts with Proven Antioxidant Activity. Molecules 26: 4986.
- 215. Fonseca Gomes MR, Schuh RS, Bemvenuti Jacques AL, Dorneles GG, Montanha J, et al. (2013) Biological assessment (antiviral and antioxidant) and acute toxicity of essential oils from Drimys angustifolia and D. brasiliensis. Rev. Bras. Farmacogn 23: 284-290.
- 216. Franco G, Cartagena J, Correa G, Rojano B, Piedrahita A, et al. (2014) Actividad antioxidante del jugo de Passiflora edulis Sims (Gulupa) durante la poscosecha. Rev. Cuba. Plantas Med 19.
- 217. Gabbay Alves TV, Silva Da Costa R, Aliakbarian B, Casazza AA, Perego P, et al. (2019) Bioactive compounds and antioxidant potential for polyphenol-rich cocoa extract obtained by agroindustrial residue. Nat. Prod. Res 33: 589-592.
- 218. Galduróz JCF, Carlini EDA, (1996) The effects of long-term administration of guarana on the cognition of normal, elderly volunteers. Sao Paulo Med. J 114: 1073-1078.
- 219. Galduróz JCF, Carlini EDA, (1994) Acute efects of the Paulinia cupana, “Guaraná” on the cognition of normal volunteers. Sao Paulo Med. J 112: 607-611.
- 220. Gálvez JQ, Sánchez RT, Ruiz YC, Molina YQ, Torre CMH de la, et al. ( 2013) Potencial de actividad antioxidante de extractos fenólicos de Theobroma cacao L. (cacao). Rev. Cuba. Plantas Med 18: 201-215.
- 221. Gamarra Ochoa V, Fuertes RC, Chavez SN, Contreras CD, Goya S E, et al. (2018) Metabolitos en las hojas de Erythroxylum coca Lam y Erithroxylumnovogranatense (Morris) Vieron y evaluacion de sus propiedades biologicas mediante bioensayos. Rev. Peru. Med. Integrativa 2: 828-834.
- 222. García-Cardona DM, Landázuri P, Ayala-Zuluaga CF, Restrepo Cortes B, (2021) Marcadores bioquímicos de estrés oxidativo en jugadoras universitarias de voleibol. Efecto del consumo de Passiflora edulis (Biochemical markers of oxidative stress in female volleyball players. Effect of consumption of Passiflora edulis). Retos 43: 603-612.
- 223. García-Cardona DM, Landázuri P, Restrepo Cortés B, Sánchez-Muñoz OE, (2021) Estrés oxidativo e ingesta de Passiflora edulis en voleibolistas hombres. Rev. Asoc. Colomb. Cienc. Biológicas 94-101.
- 224. Garza-Juárez A, De La Luz Salazar-Cavazos Ma, Salazar-Aranda R, Pérez-Meseguer J, Waksman De Torres N, et al. (2011) Correlation between Chromatographic Fingerprint and Antioxidant Activity of Turnera diffusa (Damiana). Planta Med 77: 958-963.
- 225. Gazola AC,Costa GM, Castellanos L, Ramos FA, Reginatto FH, et al. (2015) Involvement of GABAergic pathway in the sedative activity of apigenin, the main flavonoid from Passiflora quadrangularis pericarp. Rev. Bras. Farmacogn 25: 158-163.
- 226. Gazola AC, Costa GM, Zucolotto SM, Castellanos L, Ramos FA, et al. (2018) The sedative activity of flavonoids from Passiflora quadrangularis is mediated through the GABAergic pathway. Biomed.Pharmacother 100: 388-393.
- 227. Ginting B, Maulana I, Saidi N, Astryna SY, (2019) Isolation and activity antioxidant test of cocoa pod husk ethyl asetat extracts (Theobroma cacao L). J. Nat 19: 49-53.
- 228. Gomes DCV, Costa DA, Araújo EJFD, Batista PDN, Rocha MDS, et al. (2013) Evaluation of antioxidant juice cashew (Anacardium occidentale Linn) in Saccharomyces cerevisiae before and after drying spray. Periód. Tchê Quím 10: 57-64.
- 229.Gordon A, Friedrich M, Da Matta VM, Herbster Moura CF, Marx F, et al. (2012) Changes in phenolic composition, ascorbic acid and antioxidant capacity in cashew apple (Anacardium occidentale L.) during ripening. Fruits 67: 267-276.
- 230. Gorjanović S, Komes D, Pastor FT, Belščak-Cvitanović A, Pezo L, et al. (2012) Antioxidant Capacity of Teas and Herbal Infusions: Polarographic Assessment. J. Agric. Food Chem 60: 9573-9580.
- 231. Gremski LA, Coelho ALK, Santos JS, Daguer H, Molognoni L, et al. (2019) Antioxidants-rich ice cream containing herbal extracts and fructooligossaccharides: manufacture, functional and sensory properties. Food Chem 298: 125098.
- 232. Guevara M, Tejera E, Granda-Albuja MG, Iturralde G, Chisaguano-Tonato M, et al. (2019) Chemical Composition and Antioxidant Activity of the Main Fruits Consumed in the Western Coastal Region of Ecuador as a Source of Health-Promoting Compounds. Antioxidants 8: 387.
- 233. Guimarães BDA, Silva RC, Andrade EHDA, Setzer WN, Da Silva JK, et al. (2023) Seasonality, Composition, and Antioxidant Capacity of Limonene/δ-3-Carene/(E)-Caryophyllene Schinus terebinthifolia Essential Oil Chemotype from the Brazilian Amazon: A Chemometric Approach. Plants 12: 2497.
- 234. Guimarães SF, Lima IM, Modolo LV, (2020) Phenolic content and antioxidant activity of parts of Passiflora edulis as a function of plant developmental stage. Acta Bot. Bras 34: 74-82.
- 235. Gunathilake KDPP, Ranaweera KKDS, Rupasinghe HPV, (2018) Analysis of rutin, β‐carotene, and lutein content and evaluation of antioxidant activities of six edible leaves on free radicals and reactive oxygen species. J. Food Biochem 42.
- 236. Guo C, Sun L, Chen X, Zhang D, (2013) Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen. Res 8: 2003-2014.
- 237. Gurney T, Bradley N, Izquierdo D, Ronca F, (2023) Cognitive effects of guarana supplementation with maximal intensity cycling. Br. J. Nutr 130: 253-260.
- 238. Hartwig VG, Brumovsky LA, Fretes RM, Boado LS, (2012) A novel procedure to measure the antioxidant capacity of Yerba maté extracts. Food Sci. Technol 32: 126-133.
- 239. Hasanuddin A, Anwar K, Mappatoba M, Hafsah, (2019) Antibacterial And Antioxidant Activities Of Ethanol Extracts Of Cocoa Husk (Theobroma cacao L.) With Maltodextrine In Various Concentration. IOP Conf. Ser. Earth Environ. Sci 255: 012017.
- 240. Hatano VY, Torricelli AS, Giassi ACC, Coslope LA, Viana MB, et al. (2012) Anxiolytic effects of repeated treatment with an essential oil from Lippia alba and (R)- (-)-carvone in the elevated T-maze. Braz. J. Med. Biol. Res 45: 238-243.
- 241. Hay YO, Abril-Sierra MA, Sequeda-Castañeda LG, Bonnafous C, Raynaud C, et al. (2018) Evaluation of combinations of essential oils and essential oils with hydrosols on antimicrobial and antioxidant activities. J. Pharm. Pharmacogn. Res 6: 216-230.
- 242. Heldwein CG, Silva LDL, Gai EZ, Roman C, Parodi TV, et al. (2014) S- (+)-Linalool from Lippia alba: sedative and anesthetic for silver catfish (Rhamdia quelen). Vet. Anaesth. Analg 41: 621-629.
- 243. Heldwein CG, Silva LL, Reckziegel P, Barros FMC, Bürger ME, et al. (2012) Participation of the GABAergic system in the anesthetic effect of Lippia alba (Mill.) N.E. Brown essential oil. Braz. J. Med. Biol. Res 45: 436-443.
- 244. Horozić E, Sinanović D, Halilović S, Džafić I, Bajrić S, et al. (2022) In vitro antioxidant activity of methanolic extracts of the different types of commercial pepper. Rad. Šumar. Fak. Univ. U Sarajevu 52.
- 245. Huo D, Dai J, Yuan S, Cheng X, Pan Y, et al. (2023) Eco-friendly simultaneous extraction of pectins and phenolics from passion fruit (Passiflora edulis Sims) peel: Process optimization, physicochemical properties, and antioxidant activity. Int. J. Biol. Macromol 243: 125229.
- 246. Indirayati N, Nisa K, Kurang RY, Tarmo NC, Adang KTP, et al. (2020) Radical scavenging activity and total phenolic content of seven tropical plants. IOP Conf. Ser. Earth Environ. Sci. 462: 012043.
- 247. Indla E, Rajasekar K, Naveen Kumar B, Kumar SS, Chelli S, et al. (2023) Neurohistopathological Alterations Induced by Theobroma Cacao and Camellia Sinensis Extracts in Diabetic Male Wistar Rats. Cureus 15: e48492.
- 248. Indrianingsih AW, Wulanjati MP, Windarsih A, Bhattacharjya DK, Suzuki T, et al. (2021) In vitro studies of antioxidant, antidiabetic, and antibacterial activities of Theobroma cacao, Anonna muricata and Clitoria ternatea. Biocatal. Agric. Biotechnol 33: 101995.
- 249. International Plant Names Index, (2023) International Plant Names Index.
- 250. Ioannone F, Di Mattia CD, De Gregorio M, Sergi M, Serafini M, et al. (2015) Flavanols, proanthocyanidins and antioxidant activity changes during cocoa (Theobroma cacao L.) roasting as affected by temperature and time of processing. Food Chem 174: 256-262.
- 251. Ivanišová E, Farkaš A, Frančáková H,Kačániová M, (2018) Antioxidant activity and total polyphenol content of medicinal herbs with adaptogenic effect to human body. CABI Data bases 51: 119-123.252.Iwuanyanwu VU, Banjo OW, Babalola KT, Olajide OA, (2023) Neuroprotection by Alstonia boonei De Wild., Anacardium occidentale L., Azadirachta indica A.Juss. and Mangifera indica L. J. Ethnopharmacol 310: 116390.
- 253. Jaramillo-Colorado BE, Stashenko EE, Winterhalter P, (2020) Fractionation of four Colombian essential oils by countercurrent chromatography and evaluation of their antioxidant activity. J. Essent. Oil Res 32: 12-22.
- 254. Jean-Marie E, Bereau D, Poucheret P, Guzman C, Boudard F, et al. (2021) Antioxidative and Immunomodulatory Potential of the Endemic French Guiana Wild Cocoa “Guiana.” Foods 10: 522.
- 255. Jenny M, Santer E, Klein A, Ledochowski M, Schennach H, et al. (2009) Cacao extracts suppress tryptophan degradation of mitogen-stimulated peripheral blood mononuclear cells. J. Ethnopharmacol 122: 261-267.
- 256. Jonfia-Essien WA, West G, Alderson PG, Tucker G, (2008) Phenolic content and antioxidant capacity of hybrid variety cocoa beans. Food Chem 108: 1155-1159.
- 257. Jorge N, Malacrida CR, Angelo PM, Andreo D, (2009) Composição centesimal e atividade antioxidante do extrato de sementes de maracujá (Passiflora edulis) em óleo de soja. Pesqui. Agropecuária Trop. 39: 380385.
- 258. Joshi A, Prakash O, Pant AK, Kumar R, Negi MS, et al. (2018) Chemical Analysis and Antioxidant Activity of Essential Oils of Two Morphotypes of Lippia alba (Mill.) N.E. Br. Ex Britton & P. Wilson (Verbenaceae). J. Essent. Oil Bear. Plants 21: 687-700.
- 259. Junior GB, De Abreu MS, Rosa JGDSD, Pinheiro CG, Heinzmann BM, et al. (2018) Lippia alba and Aloysia triphylla essential oils are anxiolytic without inducing aversiveness in fish. Aquaculture 482: 49-56.
- 260. Junsathian P, Yordtong K, Corpuz HM, Katayama S, Nakamura S, et al. (2018) Biological and neuroprotective activity of Thai edible plant extracts. Ind. Crops Prod 124: 548-554.
- 261. Kaezer A da R, (2013) Efeito antimutagênico, antigenotóxico, antiobesidade e antioxidante da Ilex paraguariensis (erva-mate). Universidade do Estado do Rio de Janeiro.
- 262. Kamath V, Rajini PS, (2007) The efficacy of cashew nut (Anacardium occidentale L.) skin extract as a free radical scavenger. Food Chem 103: 428-433.
- 263. Kampke EH, De Souza Barroso ME, Marques FM, Fronza M, Scherer R, et al. (2018) Genotoxic effect of Lippia alba (Mill.) N. E. Brown essential oil on fish (Oreochromis niloticus) and mammal (Mus musculus). Environ. Toxicol. Pharmacol 59: 163-171.
- 264. Kennedy DO, Haskell CF, Wesnes KA, Scholey AB, (2004) Improved cognitive performancein human volunteers following administration of guarana (Paullinia cupana) extract: comparison and interaction with Panax ginseng. Pharmacol. Biochem. Behav 79: 401-411.
- 265. Kim M, Ha LK, Oh S, Fang M, Zheng S, et al. (2022) Antiphotoaging Effects of Damiana (Turnera diffusa) Leaves Extract via Regulation AP-1 and Nrf2/ARE Signaling Pathways. Plants Basel Switz 11: 1486.
- 266. Klein N, Gazola AC, De Lima TCM, Schenkel E, Nieber K, et al. (2014) Assessment of Sedative Effects of Passiflora edulis f. flavicarpa and Passiflora alata Extracts in Mice, Measured by Telemetry. Phytother. Res 28: 706-713.
- 267. Kongkachuichai R, Charoensiri R, Yakoh K, Kringkasemsee A, Insung P, et al. (2015) Nutrients value and antioxidant content of indigenous vegetables from Southern Thailand. Food Chem 173: 838-846.
- 268. Konieczynski P, Viapiana A, Wesolowski M, (2017) Comparison of Infusions from Black and Green Teas
- (Camellia sinensis L. Kuntze) and Erva-mate (Ilex paraguariensis A. St.-Hil.) Based on the Content of Essential Elements, Secondary Metabolites, and Antioxidant Activity. Food Anal. Methods 10: 3063-3070.
- 269. Kosoko AM, Olurinde OJ, Akinloye OA, (2017) Doxorubicin induced neuro- and cardiotoxicities in experimental rats: Protection against oxidative damage by Theobroma cacao Stem bark. Biochem. Biophys. Rep 10: 303-317.
- 270. Kumar S, Sharma A, (2005) Anti-anxiety activity studies of various extracts of Turnera aphrodisiaca Ward. J. Herb. Pharmacother 5: 13-21.
- 271. Kumar S, Sharma A, (2005) Anti-anxiety Activity Studies on Homoeopathic Formulations of Turnera aphrodisiaca Ward. Evid. Based Complement. Alternat. Med 2: 117-119.
- 272. Reyes-Solano L, Breksa III AP, Valdez-Torres JB, Angulo-Escalante M, Heredia JB, et al. (2017) Chemical composition and antioxidant activity of Lippia alba essential oil obtained by supercritical CO2 and hydrodistillation. Afr. J. Biotechnol 16: 962-970.
- 273. Labre DaSilva T, Sales De Oliveira V, Maria Augusta I, Monteiro Keller L, Domingues Gamallo O, et al. (2019) Aroeira (Schinus terebinthifolius Raddi) Fruit: Chemical Composition and Antioxidant Capacity. Rev. Virtual Quím 11: 1614-1624.
- 274. Lanzetti M, Pires KMP, Santos JC, Ribeiro ML, Borges RM, et al. (2013) Ready-to-drink Matte® tea (diet and regular) increased life span and pulmonary health in aged mice. Food Res. Int 54: 675-682.
- 275. Lavorgna M, Pacifico S, Nugnes R, Russo C, Orlo E, et al. (2021) Theobromacacao Criollo var. Beans: Biological Properties and Chemical Profile. Foods 10: 571.
- 276.Lecumberri E, Mateos R, Izquierdo-Pulido M, Rupérez P, Goya L, et al. (2007) Dietary fibre composition, antioxidant capacity and physico-chemical properties of a fibre-rich product from cocoa (Theobroma cacao L.). Food Chem 104: 948-954.
- 277. Leite RP, Wada RS, Monteiro JC, Predes FS, Dolder H, et al. (2011) Protective Effect of Guaraná(Paullinia cupana var. sorbilis) Pre-treatment on Cadmium-Induced Damages in Adult Wistar Testis. Biol. Trace Elem. Res 141: 262-274.
- 278. Leonard SS, Hogans VJ, Coppes‐Petricorena Z, Peer CJ, Vining TA, et al. (2010) Analysis of Free‐Radical Scavenging of Yerba Mate (Ilex paraguriensis) using Electron Spin Resonance and Radical‐Induced DNA Damage. J Food Sci 75: C14-20.
- 279. Lessa OA, Reis NDS, Leite SGF, Gutarra MLE, Souza AO, et al. (2018) Effect of the solid-state fermentation of cocoa shell on the secondary metabolites, antioxidant activity, and fatty acids. Food Sci. Biotechnol 27: 107-113.
- 280. Li H, Zhou P, Yang Q, Shen Y, Deng J, et al. (2011) Comparative studies on anxiolytic activities and flavonoid compositions of Passiflora edulis ‘edulis’ and Passiflora edulis ‘flavicarpa.’ J. Ethnopharmacol 133: 1085-1090.
- 281. Li W, Sun H, Zhou J, Zhang Y, Liu L, et al. (2015) Antibacterial activities, antioxidant contents and antioxidant properties of three traditional Chinese medicinal extracts. Bangladesh J. Pharmacol 10: 131137.
- 282. Li XY, Liu YH, Wang B, Chen CY, Zhang HM, et al. (2018) Identification of a sustainable two-plant diet that effectively prevents age-related metabolic syndrome and extends lifespan in aged mice. J. Nutr. Biochem 51: 16-26.
- 283. Lima PHB de, Chamaa ARL, (2012) The utilization of Ilex paraguariensis aqueous extract as a ergogenic resource in the aerobic exercise. Rev. Bras. Nutr. Esportiva 6: 359.
- 284. Lima DS, Duarte NBA, Barreto DLC, Oliveira GPD, Takahashi JA, et al. (2018) Passion fruit and apple: from residues to antioxidant, antimicrobial and anti-Alzheimer’s potential. Ciênc. Rural 48.
- 285. Lima M, Colpo A, Salgueiro W, Sardinha G, Ávila D, et al. (2014) Ilex paraguariensis Extract Increases Lifespan and Protects Against the Toxic Effects Caused by Paraquat in Caenorhabditis elegans. Int. J. Environ. Res. Public. Health 11: 10091-10104.
- 286. Llerena W, Samaniego I, Vallejo C, Arreaga A, Zhunio B, et al. (2023) Profile of Bioactive Components of Cocoa (Theobroma cacao L.) By-Products from Ecuador and Evaluation of Their Antioxidant Activity. Foods 12: 2583.
- 287. Lobo PCB, Da Silva DD, Pimentel GD, (2022) Acute Supplementation of Yerba Mate Extract Did Not Change Muscle Strength in Physically Active Men Following the Strength Muscle Test: A Pilot Clinical Trial. Nutrients 14: 2619.
- 288. Locatelli M, Travaglia F, Giovannelli L, Coïsson JD, Bordiga M, et al. (2013) Clovamide and phenolics from cocoa beans (Theobroma cacao L.) inhibit lipid peroxidation in liposomal systems. Food Res. Int 50: 129-134. 289.Lopes DR, Santos LO, Prentice‐Hernández C, (2021) Antioxidant and antibacterial activity of a beverage obtained by fermentation of yerba‐maté (Ilex paraguariensi) with symbiotic kombucha culture. J. Food Process. Preserv 45.
- 290. Lopes MMDA, Miranda MRAD, Moura CFH, Enéas Filho J, (2012) Bioactive compounds and total antioxidant capacity of cashew apples (Anacardium occidentale L.) during the ripening of early dwarf cashew clones. Ciênc. E Agrotecnologia 36: 325-332.
- 291. López Córdoba A, Deladino L, Martino M, (2013) Effect of starch filler on calcium-alginate hydrogels loaded with yerba mate antioxidants. Carbohydr. Polym95: 315-323.
- 292. López-Córdoba A, Deladino L, Martino M, (2014) Release of yerba mate antioxidants from corn starch– alginate capsules as affected by structure. Carbohydr. Polym 99: 150-157.
- 293. López-Vargas JH, Fernández-López J, Pérez-Álvarez JA, Viuda-Martos M, (2013) Chemical, physicochemical, technological, antibacterial and antioxidant properties of dietary fiber powder obtained from yellow passion fruit (Passiflora edulis var. flavicarpa) co-products. Food Res. Int 51: 756-763.
- 294. Lourith N, Kanlayavattanakul M, (2013) Antioxidant Activities and Phenolics of Passiflora edulis Seed Recovered from Juice Production Residue. J. Oleo Sci 62: 235-240.
- 295. Lucio-Gutiérrez JR, Garza-Juárez A, Coello J, Maspoch S, Salazar-Cavazos ML, et al. (2012) multiwavelength high-performance liquid chromatographic fingerprints and chemometrics to predict the antioxidant activity of Turnera diffusa as part of its quality control. J. Chromatogr. A 1235: 68-76.
- 296. Ludka FK, Tandler LDF, Kuminek G, Olescowicz G, Jacobsen J, et al. (2016) Ilex paraguariensis hydroalcoholic extract exerts antidepressant-like and neuroprotective effects: involvement of the NMDA receptor and the l-arginine-NO pathway. Behav. Pharmacol 27: 384-392.
- 297.Lugato D, Simão MJ, Garcia R, Mansur E, Pacheco G, et al. (2014) Determination of antioxidant activity and phenolic content of extracts from in vivo plants and in vitro materials of Passiflora alata Curtis. Plant Cell Tissue Organ Cult. PCTOC 118:339-346.
- 298. Machado KN, Paula Barbosa AD, De Freitas AA, Alvarenga LF, Pádua RMD, et al. (2021) TNF-α inhibition, antioxidant effects and chemical analysis of extracts and fraction from Brazilian guaraná seed powder. Food Chem 355: 129563.
- 299. Machado ML, Arantes LP, Da SilveiraTL, Zamberlan DC, Cordeiro LM, et al. (2021) Ilex paraguariensis extract provides increased resistance against oxidative stress and protection against Amyloid beta-induced toxicity compared to caffeine in Caenorhabditis elegans. Nutr. Neurosci 24: 697-709.
- 300. Majhenič L, Škerget M, Knez Ž, (2007) Antioxidant and antimicrobial activity of guarana seed extracts. Food Chem 104: 1258-1268.
- 301. Maluf E, Barros HMT, Frochtengarten ML, Benti R, Leite JR, et al. (1991) Assessment of the hypnotic/sedative effects and toxicity of Passiflora edulis aqueous extract in rodents and humans. Phytother. Res 5: 262-266.
- 302. Marques LC, (1998) Contribuição ao Esclarecimento da Identidade Botânica da droga Vegetal Catuaba. Rev. Racine 3: 8-11.
- 303. Martínez R, Torres P, Meneses MA, Figueroa JG, Pérez-Álvarez JA, et al. (2012) Chemical, technological and in vitro antioxidant properties of cocoa (Theobroma cacao L.) co-products. Food Res. Int 49: 39-45.
- 304. Martins CDA, (2010) Avaliação da atividade antioxidante in vitro e in vivo do guaraná (Paullinia cupana) em pó (Mestrado em Nutrição em Saúde Pública). Universidade de São Paulo, São Paulo.
- 305. Martins CFR, Salles BCC, Brigagão MRPL, Rodrigues MR, Ferreira EB, ET AL. (2015) Ethanolic extract of Passiflora edulis Sims leaves inhibits protein glycation and restores the oxidative burst in diabetic rat macrophages after Candida albicans exposure. Braz. J. Pharm. Sci 51: 869-878.
- 306. Martins FJ, Caneschi CA, VieiraJLF, Barbosa W, Raposo NRB, et al. (2016) Antioxidant activity and potential photoprotective from amazon native flora extracts. J. Photochem. Photobiol. B 161: 34-39.
- 307. Martins SPDS, Ferreira CL, Del Giglio A, (2017) Placebo-Controlled, Double-Blind, Randomized Study of a Dry Guarana Extract in Patients with Head and Neck Tumors Undergoing Chemoradiotherapy: Effects on Fatigue and Quality of Life. J. Diet. Suppl 14: 32-41.
- 308. Mateos R, Baeza G, Sarriá B, Bravo L, (2018) Improved LC-MSn characterization of hydroxycinnamic acid derivatives and flavonols in different commercial mate (Ilex paraguariensis) brands. Quantification of polyphenols, methylxanthines, and antioxidant activity. Food Chem 241: 232-241.
- 309. Mateus ARS, Crisafulli C, Vilhena M, Barros SC, Pena A, et al. (2023) The Bright and Dark Sides of Herbal Infusions: Assessment of Antioxidant Capacity and Determination of Tropane Alkaloids. Toxins 15: 245.
- 310. Matsumoto RLT, Bastos DHM, Mendonça S, Nunes VS, Bartchewsky W, et al. (2009) Effects of Maté Tea (Ilex paraguariensis) Ingestion on mRNA Expression of Antioxidant Enzymes, Lipid Peroxidation, and Total Antioxidant Status in Healthy Young Women. J. Agric. Food Chem 57: 1775-1780.
- 311. Mattei R, Dias RF, Espínola EB, Carlini EA, Barros SBM, et al. (1998) Guarana (Paullinia cupana): toxic behavioral effects in laboratory animals and antioxidant activity in vitro. J. Ethnopharmacol 60: 111-116.
- 312. Mawalagedera SMMR, Ou ZQ, McDowell A, Gould KS, (2016) Effects of boiling and in vitro
- gastrointestinal digestion on the antioxidant activity of Sonchus oleraceus leaves. Food Funct 7: 1515-1522.
- 313. Mazor Jolić S, Radojčić Redovniković I, Marković K, Ivanec Šipušić Đ, Delonga K, et al. (2011) Changes of phenolic compounds and antioxidant capacity in cocoa beans processing. Int. J. Food Sci. Technol 46: 1793-1800.
- 314. Mazzutti S, Rodrigues LGG, Mezzomo N, Venturi V, Ferreira SRS, et al. (2018) Integrated green-based processes using supercritical CO2 and pressurized ethanol applied to recover antioxidant compouds fromcocoa (Theobroma cacao) bean hulls. J. Supercrit. Fluids 135: 52-59.
- 315. McDowell A, Thompson S, Stark M, Ou Z, Gould KS, et al. (2011) Antioxidant Activity of Puha (Sonchus oleraceus L.) as Assessed by the Cellular Antioxidant Activity (CAA) Assay. Phytother. Res 25: 18761882.
- 316. Mello LD, Kubota LT, (2014) Antioxidant capacity of Ilex paraguariensis extracts by using HRP-based biosensor. Lat. Am. Appl. Res. - Int. J 44: 325-329.
- 317. Mello-Peixoto E, Figueiredo P, Silva L, Silva R, (2013) Antioxidant activity and phenol content and flavonoids total of the Hymenaea stigonocarpa Mart and Hymenaea courbaril L. Planta Med 79: s-00331352064.
- 318. Melo Cavalcante AA, Rubensam G, Picada JN, Gomes Da Silva E, Fonseca Moreira JC, et al. (2003) Mutagenicity, antioxidant potential, and antimutagenic activity against hydrogen peroxide of cashew (Anacardium occidentale) apple juice and cajuina. Environ. Mol. Mutagen 41: 360-369.
- 319. Melo EDA, Maciel MIS, Lima VLAGD, Nascimento RJD, (2008) Capacidade antioxidante de frutas. Rev. Bras. Ciênc. Farm 44: 193-201.
- 320.Melo TS, Pires TC, Engelmann JVP, Monteiro ALO, Maciel LF, et al. (2021) Evaluation of the content of bioactive compounds in cocoa beans during the fermentation process. J. Food Sci. Technol 58: 1947-1957.
- 321. Mendes FR, Carlini EA, (2007) Brazilian plants as possible adaptogens: An ethnopharmacological survey of books edited in Brazil. J. Ethnopharmacol 109: 493-500.
- 322. Menezes Filho ACPD, Oliveira Filho JGD, Castro CFDS, (2020) Avaliações antioxidante e antifúngica dos óleos essenciais de Hymenaea stigonocarpa Mart. ex Hayne e Hymenaea courbaril L. J. Biotechnol. Biodivers 8: 104-114.
- 323. Menini T, Heck C, Schulze J, De Mejia E, Gugliucci A, et al. (2007) Protective Action of Ilex paraguariensis Extract against Free Radical Inactivation of Paraoxonase-1 in High-Density Lipoprotein. Planta Med 73: 1141-1147.
- 324. Mesquita M, Santos E, Kassuya CA, Salvador MJ, (2021) Chimarrão, terere and mate-tea in legitimate technology modes of preparation and consume: A comparative study of chemical composition, antioxidant, anti-inflammatory and anti-anxiety properties of the mostly consumed beverages of Ilex paraguariensis St. Hil. J. Ethnopharmacol 279: 114401.
- 325. Mihai RA, Landazuri Abarca PA, Tinizaray Romero BA, Florescu LI, Catană R, et al. (2022) Abiotic Factors from Different Ecuadorian Regions and Their Contribution to Antioxidant, Metabolomic and Organoleptic Quality of Theobroma cacao L. Beans, Variety “Arriba Nacional.” Plants 11: 976.
- 326. Milbratz de Camargo A, Bonde H, Delwing Dal Magro D, Delwing de Lima D, De Azevedo Campanella LC, et al. (2017) Cocoa and classical music: effect on anxiety and antioxidant activity in Wistar rats / Cacao y música clásica: efecto sobre ansiedad y actividad antioxidante en ratas Wistar. Arch. Latinoam. Nutr 67: 106-115.
- 327. Milioli EM, Cologni P, Santos CC, Marcos TD, Yunes VM, et al. (2007) Effect of acute administration of hydroalcohol extract of Ilex paraguariensis St Hilaire (Aquifoliaceae) in animal models of Parkinson’s disease. Phytother. Res 21: 771-776.
- 328. Mingori MR, Heimfarth L, Ferreira CF, Gomes HM, Moresco KS, et al. (2017) Effect of Paullinia cupana Mart. Commercial Extract During the Aging of Middle Age Wistar Rats: Differential Effects on the Hippocampus and Striatum. Neurochem. Res 42: 2257-2273.
- 329. Ministério do Meio Ambiente, (2022) Portaria MMA No 148, de 7 de Junho de 2022.
- 330. Miranda AR, Cittadini MC, Albrecht C, Soria EA, (2017) Regional oxidative stressin encephalon of female mice with polyphenolic exposure from tea extracts in oral overweight plant-based treatment. Rev. Fac. Cienc. Médicas 74: 197.
- 331. Miranda Dos Santos R, LimaNogueira K, Chapla VM, (2023) Chemical Composition and antioxidant activity of Essential Oil from Schinus terebinthifolius and Siparuna guianensis Leaves. Rev. Virtual Quím 15: 295-300.
- 332. Missouri Botanical Garden, (2023) Tropicos.org.
- 333. Montoya Yepes DF, Murillo Arango W, Jiménez Rodríguez ÁA, Méndez Arteaga JJ, Aldana Porras ÁE, et al. (2021) Encapsulation of phenols of gulupa seed extract using acylated rice starch: Effect on the release and antioxidant activity. J. Funct. Foods 87: 104788.
- 334. Moo-Huchin VM, Moo-Huchin MI, Estrada-León RJ, Cuevas-Glory L, Estrada-Mota IA, et al. (2015) Antioxidant compounds, antioxidant activity and phenolic content in peel from three tropical fruits from Yucatan, Mexico. Food Chem 166: 17-22.
- 335. Morais SM, LimaKSB, Siqueira SMC, Cavalcanti ESB, Souza MST, et al. (2013) Correlação entre as atividades antiradical, antiacetilcolinesterase e teor de fenóis totais de extratos de plantas medicinais de farmácias vivas. Rev. Bras. Plantas Med 15: 575-582.
- 336. Morais SMD, Cavalcanti ESB, Costa SMO, Aguiar LA, (2009) Ação antioxidante de chás e condimentos de grande consumo no Brasil. Rev. Bras. Farmacogn 19: 315-320.
- 337. Moreno E, Ortiz BL, Restrepo LP, (2015) Contenido total de fenoles y actividad antioxidante de pulpa de seis frutas tropicales. Rev. Colomb. Quím 43: 41-48.
- 338. Mostefa N, Djebli N, Khanh PN, Ha NX, Anh HTN, et al. (2023) Anti‐Alzheimer’s Activity of
- Polyphenolic Stilbene‐Rich Acetone Fraction of the Oil‐Removed Seeds of Passiflora edulis: in Vivo and in Silico Studies. Chem. Biodivers. 20: e202201051.
- 339. Moura BM, Panza VP, Brunetta HS, Tamborindeguy AC, De Oliveira MV, et al. (2020) Effect of mate tea consumption on rapid force production aftereccentric exercise: a randomized, controlled, crossover study. Sport Sci. Health 16: 571-581.
- 340. Mudenuti NVDR, De Camargo AC, De Alencar SM, Danielski R, Shahidi F, et al. (2021) Phenolics and alkaloids of raw cocoa nibs and husk: The role of soluble and insoluble-bound antioxidants. Food Biosci 42: 101085.
- 341. Muniz BC, Falcão EL, Bastos Filho CJA, Silva FSBD, (2023) Cultivation protocol using a coir-based substrate modulates the concentration of bioactive compounds and the antioxidant activity of Passiflora alata Curtis seedlings. Ciênc. E Agrotecnologia 47: e014922.
- 342.Muntafiah A, Siahaan JH, Hardi S, Novrial D, Hernayanti H, et al. (2022) The effect of purple passion fruit juice on superoxide dismutase and malondialdehyde levels in hypercholesterolemic rats. Universa Med 41: 139-148.
- 343. Murcia Artunduaga KS, Castañeda MDR, (2022) Evaluación del contenido de fenoles totales y capacidad antioxidante de extractos etanólicos de la cáscara de cacao (Theobroma cacao L.). Rev. Investig. Agrar. Ambient 13: 53-66.
- 344. Murillo-Baca S, Ponce-Rosas F, Huamán-Murillo M, (2020) Physicochemical characteristics, bioactive compounds and minerals content in cocoa fruit (Theobroma cacao L.) shell flour. Manglar 17: 67-73.
- 345. Naranjo-Durán AM, Quintero-Quiroz J, Ciro-Gómez GL, Barona-Acevedo MJ, Contreras-Calderón JDC, et al. (2023) Characterization of the antioxidant activity, carotenoid profile by HPLC-MS of exotic colombian fruits (goldenberry and purple passion fruit) and optimization of antioxidant activity of this fruit blend. Heliyon 9: e17819.
- 346. Ngibad K, Pradana MS, Afifah J, Afiatunnisa A, (2023) Optimization of yellow passion fruit peel extraction method using methanol solvent to increase antioxidant activity. J. Pijar Mipa 18: 77-83.
- 347. Nguyen VT, Le MD, Nguyen TTT, Khong TT, Nguyen VH, et al. (2021) Microwave‐assisted extraction for optimizing saponin yield and antioxidant capacity from cacao pod husk (Theobroma cacao L.). J. Food Process. Preserv 45.
- 348. Nguyen VT, Tran NTH, Tran TG, (2022) Central composite experimental design for ultrasound‐assisted extraction optimization of alkaloid compounds and antioxidant properties from cocoa pod husk (Theobroma cacao L.). J. Food Process. Preserv 46.
- 349. Nguyen VT, Tran TG, Tran NL, (2022) Phytochemical compound yield and antioxidant activity of cocoa pod husk (Theobroma cacao L.) as influenced by different dehydration conditions. Dry. Technol 40: 20212033.
- 350. Niraula P, Ghimire S, Lee H, Kim MS, (2018) Ilex paraguariensis Extends Lifespan and Increases an Ability to Resist Environmental Stresses inDrosophila. Rejuvenation Res 21: 497-505.
- 351. Nonato CDFA, De Melo EVS, Camilo CJ, Ferreira MKA, De Meneses JEA, 2023. Antibacterial Activity and Anxiolytic Effect in Adult Zebrafish of Genus LippiaL. Species. Plants 12: 1675.
- 352. Nouidha S, Selmi S, Guigonis J, Pourcher T, Chekir‐Ghedira L, et al. (2023) Metabolomics Profiling of Tunisian Sonchus oleraceus L. Extracts and Their Antioxidant Activities. Chem. Biodivers 20: e202300290. 353.Novaes P, Lopes J, Santos D, (2014) Antioxidant and phytotoxic activity in Brazilian Annonaceaeextracts. Planta Med 80: s-0034-1394931.
- 354. Nunes MR, De Souza Maguerroski Castilho M, De Lima Veeck AP, Da Rosa CG, Noronha CM, et al. (2018) Antioxidant and antimicrobial methylcellulose films containing Lippia alba extract and silver nanoparticles. Carbohydr. Polym 192: 37-43.
- 355. Oboh G, Ademosun AO, Ademiluyi AO, Omojokun OS, NwannaEE, et al. (2014) In Vitro Studies on the Antioxidant Property and Inhibition of α-Amylase, α-Glucosidase, and Angiotensin I-Converting Enzyme by Polyphenol-Rich Extracts from Cocoa (Theobroma cacao) Bean. Pathol. Res. Int 2014: 1-6.
- 356. Oga S, De Freitas P, Da Silva A, Hanada S, (1984) Pharmacological Trials of Crude Extract of Passiflora alata. Planta Med 50: 303-306.
- 357. Oh KE, Shin H, Jeon YH, Jo YH, Lee MK, et al. (2016) Optimization of pancreatic lipase inhibitory and antioxidant activities of Ilex paraguariensis by using response surface methodology. Arch. Pharm. Res 39: 946-952.
- 358. Olajide OA, Aderogba MA, Fiebich BL, (2013) Mechanisms of anti-inflammatory property of Anacardium occidentale stem bark: Inhibition of NF-κB and MAPK signalling in the microglia. J. Ethnopharmacol 145: 42-49.
- 359. Oliveira DA, Angonese M, Gomes C, Ferreira SRS, (2016) Valorization of passion fruit (Passiflora edulis sp.) by-products: Sustainable recovery and biological activities. J. Supercrit. Fluids 111: 55-62.
- 360. Oliveira EP de, Torezan GA, Gonçalves L de S, Corrente JE, McLellan KCP, et al. (2016) O consumo agudo de erva mate aumenta o gasto energético de homens jovens saudáveis: Um estudo piloto. Rev. Bras. Obesidade Nutr. E Emagrecimento 10: 242-249.
- 361. Oloruntola O, (2021) Proximate phytochemical mineral composition and antioxidant activity of Anacardium occidentale L. leaf powder. DYSONA - Life Sci 2.
- 362. Onusic GM, Nogueira RL, Pereira AMS, Flausino Júnior OA, Viana MDB, et al. (2003) Effects of Chronic Treatment with a Water-Alcohol Extract from Erythrina mulungu on Anxiety-Related Responses in Rats. Biol. Pharm. Bull 26: 1538-1542.
- 363. Onusic GM, Nogueira RL, Pereira AMS, Viana MB, (2002) Effect of acute treatment with a water-alcohol extract of Erythrina mulungu on anxiety-related responses in rats. Braz. J. Med. Biol. Res 35: 473-477.
- 364. Oracz J, Nebesny E, Żyżelewicz D, (2014) Effect of roasting conditions on the fat, tocopherol, and phytosterol content and antioxidant capacity of the lipid fraction from cocoa beans of different Theobroma cacao L. cultivars. Eur. J. Lipid Sci. Technol 116: 1002-1014.
- 365.Ordoñez E, Leon-Arevalo A, Rivera-Rojas H, Vargas E, (2019) Quantification of total polyphenols and antioxidant capacity in skins and seedsfrom cacao (Theobroma cacao L.), tuna (Opuntia ficus indica Mill), grape (Vitis Vinífera) and uvilla (Pourouma cecropiifolia). Sci. Agropecu 10: 175-183.
- 366. Ortiz SJ, Chungara M, Ibieta G, Alejo I, Tejeda L, et al. (2019) Determinación de teobromina, catequina, capacidad antioxidante total y contenido fenólico total em muestras representativas de cacao amazónico boliviano y su comparación antes y después del proceso de fermentación. Rev. Boliv. Quím 1.
- 367. Osman H, Nasarudin R, Lee SL, (2004) Extracts of cocoa (Theobroma cacao L.) leaves and their antioxidation potential. Food Chem 86: 41-46.
- 368. Osukoya O, Fadaka A, Adewale O, Oluloye O, Ojo O, et al. (2019) In vitro anthelmintic and antioxidant activities of the leaf extracts of Theobroma cacao L. AIMS Agric. Food 4: 568-577.
- 369. Otify A, George C, Elsayed A, Farag MA, (2015) Mechanistic evidence of Passiflora edulis (Passifloraceae) anxiolytic activity in relation to itsmetabolite fingerprint as revealed via LC-MS and chemometrics. Food Funct 6: 3807-3817.
- 370. Otobone FJ, Sanches AC, Nagae RL, Martins JVC, Obici S, et al. (2005) Effect of crude extract and its semi purified constituents from guaraná seeds [Paullinia cupana var. sorbilis (Mart.) lucke] on cognitive performance in Morris water maze in rats. Braz. Arch. Biol. Technol 48: 723-728.
- 371. Otobone FJ, Sanches ACC, Nagae R, Martins JVC, Sela VR, et al. (2007) Effect of lyophilized extracts from guaraná seeds [ Paullinia cupana var. sorbilis (Mart.) Ducke] on behavioral profiles in rats. Phytother. Res 21:531-535.
- 372. Ou ZQ, Schmierer DM, Rades T, Larsen L, McDowell A, et al. (2012) Application of an online postcolumn derivatization HPLC-DPPH assay to detect compoundsresponsible for antioxidant activity in Sonchus oleraceus L. leaf extracts. J. Pharm. Pharmacol 65: 271-279.
- 373. Ou ZQ, Schmierer DM, Strachan CJ, Rades T, McDowell A, et al. (2014) Influence of postharvest processing and storage conditions on key antioxidants in pūhā (S onchus oleraceus L.). J. Pharm. Pharmacol 66: 998-1008.
- 374. Oyedemi SO, Oyedemi BO, Ijeh II, Ohanyerem PE, Coopoosamy RM, et al. (2017) Alpha-Amylase Inhibition and Antioxidative Capacity of Some Antidiabetic Plants Used by the Traditional Healers in Southeastern Nigeria. Sci. World J 2017 1-11.
- 375. Ożarowski M, Pietrowiak A, Gryszczyńska A, Chaves DSDA, Krajewska-Patan A, et al. (2019) Comparison of in vitro antioxidative activities of crude methanolic extracts of three species of Passiflora from greenhouse using DPPH, ABTS and FRAP methods. Herba Pol 65: 10-21.
- 376. Ozawa M, Honda K, Nakai I, Kishida A, Ohsaki A, et al. (2008) Hypaphorine, an indole alkaloid from Erythrina velutina, induced sleep on normal mice. Bioorg. Med. Chem. Lett 18: 3992-3994.
- 377. Padilla FC, Rincon AM, Bou-Rached L, (2008) Contenido de polifenoles y actividad antioxidante de varias semillas y nueces. 58: 303-308.
- 378. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, et al. (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372: n71.
- 379. Pagliosa CM, Vieira MA, Podestá R, Maraschin M, Zeni ALB, et al. (2010) Methylxanthines, phenolic composition, and antioxidant activity of bark from residues from mate tree harvesting (Ilex paraguariensis A. St. Hil.). Food Chem 122: 173-178.
- 380. Paiva LAF, Rao VSN, Silveira ER, (1998) Effects ofPtychopetalum olacoides extract on mouse behaviour in forced swimming and open field tests. Phytother. Res 12: 294-296.
- 381. Palma-Wong M, Ascacio-Valdés JA, Ramírez-Guzmán N, Aguirre-Joya JA, Flores-Loyola E, et al. (2023) Exploration of Phenolic Content and Antioxidant Potential from Plants Used in Traditional Medicine in Viesca, Mexico. Horticulturae 9: 1252.
- 382. Panelli M, Pierine D, De Souza S, Ferron A, Garcia J, et al. (2018) Bark of Passiflora edulis Treatment Stimulates Antioxidant Capacity, and Reduces Dyslipidemia and Body Fat in db/db Mice. Antioxidants 7: 120.
- 383. Panossian AG, Efferth T, Shikov AN, Pozharitskaya ON, Kuchta K, et al. (2021) Evolution of the adaptogenic concept from traditional use to medical systems: Pharmacology of stress- and aging-related diseases. Med. Res. Rev 41: 630-703.
- 384. Panza VP, Diefenthaeler F, Tamborindeguy AC, Camargo CDQ, De Moura BM, et al. (2016) Effects of mate tea consumption on muscle strength and oxidative stress markers after eccentric exercise. Br. J. Nutr 115: 1370-1378.
- 385. Pardo-Jumbo A, Matute NL, Echavarria AP, (2017) Determinación de compuestos bioactivos y actividad antioxidante de la pulpa de maracuyá (passiflora edulis). FACSALUD-UNEMI 1: 5-11.
- 386. Parodi TV, Cunha MA, Heldwein CG, De Souza DM, Martins ÁC, et al. (2012) The anesthetic efficacy of eugenol and the essential oils of Lippia alba and Aloysia triphylla in post-larvae and sub-adults of Litopenaeus vannamei (Crustacea, Penaeidae). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol 155: 462-468.
- 387.Patil PP, Patil VS, Khanal P, Darasaguppe HR, Charla R, et al. (2022) Network pharmacology and in vitro testing of Theobroma cacao extract’s antioxidative activity and its effects on cancer cell survival. PLOS ONE 17: e0259757.
- 388. Peixoto H, Roxo M, Röhrig T, Richling E, Wang X, et al. (2017) Anti-Aging and Antioxidant Potential of Paullinia cupana var. sorbilis: Findings in Caenorhabditis elegans Indicate a New Utilization for Roasted Seeds of Guarana. Medicines 4: 61.
- 389. Peralta IN, Cogoi L, Filip R, Anesini C, (2013) Prevention of Hydrogen Peroxide‐Induced Red Blood Cells Lysis by Ilex paraguariensis Aqueous Extract: Participation of Phenolic and Xanthine Compounds. Phytother. Res 27: 192-198.
- 390. Pereira AC da S, Wurlitzer NJ, Dionisio AP, Lacerda Soares MV, Rocha Bastos M do S, et al. (2015) Synergistic, additive and antagonistic effects of fruit mixtures on total antioxidant capacities and bioactive compounds in tropical fruit juices. Arch. Latinoam. Nutr 65: 119-127.
- 391. Pereira CS, (2015) Efeitos do chá mate (llex paraguariensis) na estrutura óssea de ratas na perimenopausa. Universidade Estadual Paulista Júlio de Mesquita Filho.
- 392. Pereira MG, Maciel GM, Haminiuk CWI, Bach F, Hamerski F, et al. (2019) Effect of Extraction Process on Composition, Antioxidant and Antibacterial Activity of Oil from Yellow Passion Fruit (Passiflora edulis Var. Flavicarpa) Seeds. Waste Biomass Valorization 10: 2611-2625.
- 393. Pereira Santos AC, Martins Alves A, Veloso Naves MM, Reis Silva M, (2020) Nutritional profile, bioactive compounds and antioxidant capacity of jatobá-da-mata (Hymenaea courbaril, var. stilbocarpa) by product. Rev. Chil. Nutr 47: 366-371.
- 394. Pérez-Meseguer J, Garza-Juárez A, Salazar-Aranda R, Salazar-Cavazos ML, de la Torre Rodríguez YC, et al. (2010) Development and validation of an HPLC-DAD analytical procedure for quality control of damiana (Turnera diffusa), using an antioxidant marker isolated from the plant. J. AOAC Int 93: 11611168.
- 395. Petry RD, Reginatto F, de‐Paris F, Gosmann G, Salgueiro JB, et al. (2001) Comparative pharmacological study of hydroethanol extracts of Passiflora alata and Passiflora edulis leaves†. Phytother. Res 15: 162-164.
- 396. Pham TN, Nhan LTH, Cang MH, Lam TD, Thu DNA, et al. (2020) Evaluation of polyphenol, anthocyanin content and antioxidant capacity of some Vietnamese fruits. IOP Conf. Ser. Mater. Sci. Eng 736: 062016.
- 397. Piato ÂL, Detanico BC, Jesus JF, Lhullier FLR, Nunes DS, et al. (2008) Effects of Marapuama in the chronic mild stress model: Further indication of antidepressant properties. J. Ethnopharmacol 118: 300-304.
- 398. Piato AL, Detanico BC, Linck VM, Herrmann AP, Nunes DS, et al. (2010) Anti-stress effects of the “tonic” Ptychopetalum olacoides (Marapuama) in mice. Phytomedicine 17: 248-253.
- 399. Piato ÂL, Rizon LP, Martins BS, Nunes DS, Elisabetsky E, et al. (2009) Antidepressant profile of Ptychopetalum olacoides Bentham (Marapuama) in mice. Phytother. Res 23: 519-524.
- 400. Piccinelli AC, Santos JA, Konkiewitz EC, Oesterreich SA, Formagio ASN, et al. (2015) Antihyperalgesic and antidepressive actionsof (R)- (+)-limonene, α-phellandrene, and essential oil from Schinus terebinthifolius fruits in a neuropathic pain model. Nutr. Neurosci 18: 217-224.
- 401. Pico-Hernández SM,Murillo-Méndez CJ, López-Giraldo LJ, (2020) Extraction, separation, and evaluation of antioxidant effect of the different fractions of polyphenols from cocoa beans. Rev. Colomb. Quím 49: 19-27.
- 402. Pierre EO, Nicolas N, Pierre FOD, Martine LO, Denis ON, et al. (2015) Heritability of polyphenols, anthocyanins and antioxidant capacity of Cameroonian cocoa (Theobroma cacao L.) beans. Afr. J. Biotechnol 14: 2672-2682.
- 403. Pineli LDLDO, Rodrigues JDSQ, Costa AM, De Lima HC, Chiarello MD, et al. (2015) Antioxidants and sensory properties of the infusions of wild passiflora from Brazilian savannah: potential as functional beverages. J. Sci. Food Agric 95: 1500-1506.
- 404. Piovezan-Borges AC, Valério-Júnior C, Gonçalves IL, Mielniczki-Pereira AA, Valduga AT, et al. (2016) Antioxidant potential of yerba mate (Ilex paraguariensis St. Hil.) extracts in Saccharomyces cerevisae deficient in oxidant defense genes. Braz. J. Biol 76: 539-544.
- 405. Poblete A, López-Alarcón C, Lissi E, Campos AM, (2009) Oxygen radical antioxidant capacity (ORAC) values of herbal teas obtained employing different methodologies can provide complementary data. J. Chil. Chem. Soc 54.
- 406. Portela JL, Bianchini MC, Boligon AA, Carriço MRS, Roehrs R, et al. (2019) Ilex paraguariensis Attenuates Changes in Mortality, Behavioral and BiochemicalParameters Associated to Methyl Malonate or Malonate Exposure in Drosophila melanogaster. Neurochem. Res 44: 2202-2214.
- 407. Portella RDL, Barcelos RP, Da Rosa EJF, Ribeiro EE, Da Cruz IBM, et al. (2013) Guaraná (Paullinia cupana Kunth) effects on LDL oxidation in elderly people: an in vitro and in vivo study. Lipids Health Dis 12: 12.
- 408.Porto-Luz RGL, De Moura AJB, Da Silva B, Fett R, Da Mota Araújo MA, et al. (2020) Identification and Quantification of Antioxidant Compounds in Clarified Cashew Apple Juice ‘Cajuína.’ Curr. Nutr. Food Sci 16: 585-591.
- 409. Postay LF, Cabral DS, Heringer OA, Vieira LV, De Moraes LR, et al.(2021) The effectiveness of surfactants applied with essential oil of Lippia alba in the anesthesia of Nile tilapia (Oreochromis niloticus) and their toxicity assessment for fish and mammals. Environ. Sci. Pollut. Res 28: 10224-10233.
- 410. Prediger RDS, Fernandes MS, Rial D, Wopereis S, Pereira VS, et al. (2008) Effects of acute administration of the hydroalcoholic extract of mate tea leaves (Ilex paraguariensis) in animal models of learning and memory. J. Ethnopharmacol 120: 465-473.
- 411. Preza AM, Jaramillo ME, Puebla AM, Mateos JC, Hernández R, et al. (2010) Antitumor activity against murine lymphoma L5178Y model of proteins from cacao (Theobroma cacao L.) seeds in relation with in vitro antioxidant activity. BMC Complement. Altern. Med 10: 61.
- 412. Puertas‐Mejía M, Hillebrand S, Stashenko E, Winterhalter P, (2002) In vitro radical scavenging activity of essential oils from Columbian plants and fractions from oregano (Origanum vulgare L.) essential oil. Flavour Fragr. J 17: 380-384.
- 413. Purohit S, Barik CR, Kalita D, Sahoo L, Goud VV, et al. (2021) Exploration of nutritional, antioxidant and antibacterial properties of unutilized rind and seed of passion fruit from Northeast India. J. Food Meas. Charact 15: 3153-3167.
- 414. Queiroz C, Lopes MLM, Fialho E, Valente-Mesquita VL, (2011) Changes in bioactive compounds and antioxidant capacity of fresh-cut cashew apple. Food Res. Int 44: 1459-1462.
- 415. Queiroz C, Moreira CFF, Lavinas FC, Lopes MLM, Fialho E, et al. (2010) Effect of high hydrostatic pressure on phenolic compounds, ascorbic acid and antioxidant activity in cashew apple juice. High Press. Res 30: 507-513.
- 416. Quesado Junior S, Oliveira RLD, Marques MMM, Silva ARAD, Guedes MIF, et al. (2017) Atividade de sequestramento de radical livre dos extratos etanólicos das folhas de Anacardiaceae. Semina Ciênc. Biológicas E Saúde 38: 99-104.
- 417. Rachmawaty, Mu’nisa A, Hasri, Pagarra H, Hartati, et al. (2019) Analysis of phenolic content and antioxidant activity of cocoa pod husk (theobroma cacao l.). J. Phys. Conf. Ser 1317: 012087.
- 418. Rahayu YC, Setiawatie EM, Rahayu RP, Ramadan DE, (2023) Analysis of antioxidant and antibacterial activity of cocoa pod husk extract (Theobroma cacao L.). Dent. J. Maj. Kedokt. Gigi 56: 220-225.
- 419. Rakeli Simão Boyarski D, Rocha Ramos Barbosa D, Fernandes Santana T, Castilho Clemente R, (2020) Comparação do teor de compostos fenólicos e atividade antioxidante de extratos aquosos comerciais de Ilex paraguariensis Saint Hillaire. Rev. Cereus 12: 264-280.
- 420. Ramaiya SD, Bujang JS, Zakaria MH, (2014) Assessment of Total Phenolic, Antioxidant, and Antibacterial Activities of Passiflora Species. Sci. World J 2014: 1-10.
- 421. Ramaiya SD, Lee HH, Xiao YJ, Shahbani NS, Zakaria MH, et al. (2021) Organic cultivation practices enhanced antioxidant activities and secondary metabolites in giant granadilla (Passiflora quadrangularis L.). PLOS ONE 16: e0255059.
- 422. Ramallo IA, Salazar MO, Furlan RLE, (2015) Thin layer chromatography‐autography‐high resolution mass spectrometry analysis: accelerating the identification of acetylcholinesterase inhibitors. Phytochem. Anal 26: 404-412.
- 423. Ramírez González MB, Cely Niño VH, Ramírez SI, (2013) Actividad antioxidante de clones de cacao (Theobroma cacao L.) finos y aromáticos cultivados en el estado de Chiapas, México. Perspect. En Nutr. Humana 15: 27-40.
- 424. Ramos A, Visozo A, Piloto J, García A, Rodríguez CA, et al. (2003) Screening of antimutagenicity via antioxidant activity in Cuban medicinal plants. J. Ethnopharmacol 87: 241-246.
- 425. Rangel MP, De Mello JCP, Audi EA, (2013) Evaluation of neurotransmitters involved in the anxiolytic and panicolytic effect of the aqueous fraction of Paullinia cupana (guaraná) in elevated T maze. Rev. Bras. Farmacogn 23: 358-365.
- 426. Ranilla LG, Kwon YI, Apostolidis E, Shetty K, (2010) Phenolic compounds, antioxidant activity and in vitro inhibitory potential against key enzymes relevant for hyperglycemia and hypertension of commonly used medicinal plants, herbs and spices in Latin America. Bioresour. Technol 101: 4676-4689.
- 427. Raupp IM, Sereniki A, Virtuoso S, Ghislandi C, Cavalcanti E Silva, et al. (2008) Anxiolytic-like effect of chronic treatment with Erythrina velutina extract in the elevated plus-maze test. J. Ethnopharmacol 118: 295-299.
- 428. Razab R, Abdul-Aziz A, (2010) Antioxidants from tropical herbs. Nat. Prod. Commun 5: 441-445.
- 429. Razali N, Razab R, Junit SM, Aziz AA, (2008) Radical scavenging and reducing properties of extracts of cashew shoots (Anacardium occidentale). Food Chem 111: 38-44.
- 430.Razola-Díaz MDC, Aznar-Ramos MJ, Verardo V, Melgar-Locatelli S, Castilla-Ortega E, et al. (2023) Exploring the Nutritional Composition and Bioactive Compounds in Different Cocoa Powders. Antioxidants 12: 716.
- 431. Rebelatto EA, Rodrigues LGG,Rudke AR, Andrade KS, Ferreira SRS, et al. (2020) Sequential green-based extraction processes applied to recover antioxidant extracts from pink pepper fruits. J. Supercrit. Fluids 166: 105034.
- 432. Reis CC, Mamede AMGN, Soares A, Freitas SP, (2020) Production of lipids and natural antioxidants from passion fruit seeds. Grasas Aceites 71: 385.
- 433. Reis EDM, Neto FWS, Cattani VB, Peroza LR, Busanello A, et al. (2014) Antidepressant-Like Effect of Ilex paraguariensis in Rats. BioMed Res. Int 2014: 1-9.
- 434. Reyes-Becerril M, Ginera P, Silva-Jara J, Macias A, Velazquez-Carriles C, et al. (2020) Assessment of chemical, biological and immunological properties of “Damiana de California” Turnera diffusa Willd extracts in Longfin yellowtail (Seriola rivoliana) leukocytes. Fish Shellfish Immunol 100: 418-426.
- 435. Riachi LG, Simas DLR, Coelho GC, Marcellini PS, Ribeiro Da Silva AJ, et al. (2018) Effect of light intensity and processing conditions on bioactive compounds in maté extracted from yerba mate (Ilex paraguariensis A. St.-Hil.). Food Chem 266: 317-322.
- 436. Ribeiro MD, Onusic GM, Poltronieri SC, Viana MB, (2006) Effect of Erythrina velutina and Erythrina mulungu in rats submitted to animal models of anxiety and depression. Braz. J. Med. Biol. Res 39: 263-270.
- 437. Ribeiro NC, Lima Neto FEMD, Nobre ARDA, Silva DAD, Mayo SJ, et al. (2021) Potential antioxidant and antibacterial bioactivity of leaf and stem bark extracts in wild cashew (Anacardium occidentale L.) populations from coastal Piauí, northeastern Brazil. Feddes Repert 132: 141-157.
- 438. Ribeiro P, Fernandez L, Loureiro M, Simões R, De Castro R, et al. (2015) Phytochemical screening, antioxidant and antibacterial activities of extracts prepared from different tissues of Schinus terebinthifolius Raddi that occurs in the coast of Bahia, Brazil. Pharmacogn. Mag 11: 607.
- 439. Rio de Janeiro Botanical Garden, (2023) Flora e Funga do Brasil.
- 440. Rivelli DP, (2011) Biodisponibilidade, distribuição tecidual e atividade antioxidante do extrato hidroetanólico de Ilex paraguariensis hidrolisado e não hidrolisado (Doutorado Direto em Insumos Farmacêuticos). Universidade de São Paulo, São Paulo.
- 441. Rivelli DP, Almeida RL, Ropke CD, Barros SBM, (2011) Hydrolysis Influence on Phytochemical Composition, Antioxidant Activity, Plasma Concentration, and Tissue Distribution of Hydroethanolic Ilex paraguariensis Extract Components. J. Agric. Food Chem 59: 8901-8907.
- 442. Rocha PDSD, Campos JF, Nunes-Souza V, Vieira MDC, Boleti APDA, et al. (2018) Antioxidant and Protective Effects of Schinus terebinthifolius Raddi Against Doxorubicin-Induced Toxicity. Appl. Biochem. Biotechnol 184: 869-884.
- 443. Rodríguez-Sevilla E, Ramirez-Silva MT, Palomar-Pardavé M, Romero-Romo M, Marty JL, et al. (2014) A Novel Tyrosinase Base Biosensor for the Quantification of Antioxidant Capacity: Evaluation on Infusions of Medicinal Plants. ECS Trans 64: 49-57.
- 444. Rodríguez-Sevilla E, Ramírez-Silva MT, Romero-Romo M, Ibarra-Escutia P, Palomar-Pardavé M, et al. (2014) Electrochemical Quantification of the Antioxidant Capacity of Medicinal Plants Using Biosensors. Sensors 14: 14423-14439.
- 445. Roggia I, Dalcin AJF, De Souza D, Machado AK, De Souza DV, et al. (2020) Guarana: Stability-Indicating RP-HPLC method and safety profile using microglialcells. J. Food Compos. Anal 94: 103629.
- 446. Romana-Souza B, Pires TC, Monte-Alto-Costa A, (2015) Mate tea-mediated reduction in catecholamine synthesis improves cutaneous wound healing of chronically stressed mice. Food Res. Int 71: 32-40.
- 447. Romanini CV, Wesz Machado M, Biavatti MW, Weffort De Oliveira RM, (2006) (Artigo 8 publicado v.28 n.2) Avaliação da atividade ansiolítica e antidepressiva do extrato fluido e fração aquosa de folhas de Passiflora alata Curtis em camundongos. Acta Sci. Health Sci 28: 159-164.
- 448. Roncon C, Biesdorf De Almeida C, Klein T, Palazzo De Mello J, Audi E, et al. (2011) Anxiolytic Effects of a Semipurified Constituent of Guaraná Seeds on Rats in the Elevated T-Maze Test. Planta Med 77: 236241.
- 449. Rotili Maria Cristina C, Coutro S, Celant VM, Vorpagel JA, Barp FK, et al. (2013) Composição, atividade antioxidante e qualidade do maracujáamarelo durante armazenamento. Semina Ciênc. Agrár 34: 227-240.
- 450. Rotili Maria Cristina Copello, Vorpagel JA, Braga GC, Kuhn OJ, Salibe AB, et al. (2013) Atividade antioxidante, composiçãoquímica e conservação do maracujá-amarelo embalado com filme PVC. Rev. Bras. Frutic 35: 942-952.
- 451. Ruchel JB, Braun JBS, Adefegha SA, Guedes Manzoni A, Abdalla FH, et al. (2017) Guarana (Paullinia cupana) ameliorates memory impairment and modulates acetylcholinesterase activity in Poloxamer-407induced hyperlipidemia in rat brain. Physiol. Behav 168: 11-19.
- 452. Rudnicki M, De Oliveira MR, Veiga Pereira TD, Reginatto FH, Dal-Pizzol F, et al. (2007) Antioxidant and antiglycation properties of Passiflora alata and Passiflora edulis extracts. Food Chem 100: 719-724.
- 453.Ruth SLDC, David SM, Laan DCP, Barbara JPDS, Larissa BB, et al. (2021) Antioxidant effect of Hymenaea courbaril L (Jatob) sap on the healing of wounds on mice. J. Med. Plants Res 15 160-171.
- 454. Rząsa-Duran E, Kryczyk-Poprawa A, Drabicki D, Podkowa A, Sułkowska-Ziaja K, et al. (2022) Yerba Mate as a Source of Elements and Bioactive Compounds with Antioxidant Activity. Antioxidants 11: 371.
- 455. Salbego J, Becker AG, Gonçalves JF, Menezes CC, Heldwein CG, et al. (2014) The essential oil from Lippia alba induces biochemical stress in the silver catfish (Rhamdia quelen) after transportation. Neotropical Ichthyol 12: 811-818.
- 456. Saldanha LA, (2012) Efeitos da ingestão de cafeína, café (Coffea arabica) e chá mate (Ilex paraguariensis) sobre a atividade lipolítica do tecido adiposo e parâmetros metabólicos em ratos submetidos ao exercício físico (Doutorado em Nutrição em Saúde Pública). Universidade de São Paulo, São Paulo.
- 457. Saldanha LA, (2005) Avaliação da atividade antioxidante in vitro de extratos de erva-mate (llex paraguariensis) verde e tostada e chá verde (Camellia sinensis) (Mestrado em Nutrição). Universidade de São Paulo, São Paulo.
- 458. Salim NS, Abdel-Alim M, Said HEM, Foda MF, (2023) Phenolic Profiles, Antihyperglycemic, AntiDiabetic, and Antioxidant Properties of Egyptian Sonchus oleraceus Leaves Extract: An In Vivo Study. Molecules 28: 6389.
- 459. Salim S, (2016) Oxidative stress: a potential link between emotional wellbeing and immune response. Curr. Opin. Pharmacol 29: 70-76.
- 460. Salomão P, Barroso SK, Marcellino MC, (2011) Efeitos da Marapuama (Ptychopeatalum Olacoides Benthan) nas alterações motoras induzidas por reserpina em camundongos. Rev Salusvita Online 30.
- 461. Sánchez Boado L, Fretes RM, Brumovsky LA, (2015) Bioavailability and antioxidant effect of the Ilex Paraguariensis polyphenols. Nutr. Food Sci 45: 326-335.
- 462. Sánchez WF, Murillo E, Méndez JJ, (2010) Potencial antioxidante de residuos agroindustriales de tres frutas de alto consumo en el Tolima. Sci. Tech 3.
- 463. Santa-Helena E, Castro M, Victoria FN, Rodrigues JS, Gonçalves CAN, et al. (2022) Consumption of mate Ilex paraguariensis: a folk beverage with antioxidant power against myocardial ischemic injury. Acta Sci. Health Sci 44: e55845.
- 464. Santana Andrade JK, Chagas Barros RG, Gualberto NC, Santos De Oliveira C, Shanmugam S, et al. (2022) Influence of in vitro gastrointestinal digestion and probiotic fermentation on the bioaccessibility of gallic acid and on the antioxidant potential of Brazilian fruit residues. LWT 153: 112436.
- 465. Santana FCD, Mancini-Filho J, (2015) Avaliação dos compostos bioativos presentes na semente de Passiflora spp. e sua influência sobre marcadores bioquímicos, oxidativos e inflamatórios de camundongos submetidos à dieta hiperlipídica. Universidade de São Paulo, São Paulo.
- 466. Santos COD, Trindade SC, Silveira MLR, Santos RO, Sautter CK, et al. (2000) Caracterização, teor de polifenóis totais e atividadeantioxidante em diferentes tipos de erva-mate (Ilex paraguariensis St. Hill.) para chimarrão. Rev. Inst. Adolfo Lutz.
- 467. Santos ECS, Bicca MA, Blum-Silva CH, Costa APR, Dos Santos AA, et al. (2015) Anxiolytic-like, stimulant and neuroprotective effects of Ilex paraguariensis extracts in mice. Neuroscience 292: 13-21.
- 468. Santos Filho LGAD, Reis RBD, Souza ASQ, Canuto KM, Brito ESD, et al. (2023) Chemical composition and biological activities of the essential oils from Lippia alba and Lippia origanoides. An. Acad. Bras. Ciênc 95: e20220359.
- 469. Santos JAS, Sena TJO, Costa MLAD, Santos KCBS, Santos AFD, et al. (2018) Estudo do potencial antioxidante da Anacardium occidentales L. e determinação de seus compostos fenólicos. Divers. J 3: 455.
- 470. Santos JS, Deolindo CTP, Hoffmann JF, Chaves FC, Do Prado-Silva L, et al. (2018) Optimized Camellia sinensis var. sinensis, Ilex paraguariensis, and Aspalathus linearisblend presents high antioxidant and antiproliferative activities in a beverage model. Food Chem 254: 348-358.
- 471. Santos JS, Escher GB, Vieira Do Carmo M, Azevedo L, Boscacci Marques M, et al. (2020) A new analytical concept based on chemistry and toxicology for herbal extracts analysis: From phenolic composition to bioactivity. Food Res Int 132: 109090.
- 472. Santos WP, Da Silva Carvalho AC, Santos Estevam CD, Santana AEG, Marçal RM (2012) In vitroand ex vivoanticholinesterase activities of Erythrina velutinaleaf extracts. Pharm Biol 50: 919-924.
- 473. Sari F, Turkmen N, Polat G, Velioglu YS (2007) Total Polyphenol, Antioxidant and Antibacterial Activities of Black Mate Tea. Food Sci Technol Res 13: 265-269.
- 474. Sassi AB, Elayeb A, Karaman I, Marzouk B, Mastouri M (2020) Phytochemical Profile and Antiproliferative, Anti-tyrosinase, Antioxidant, and Antibacterial Potential of Schinus terebinthifoliusGrowing in Tunisia. J Herbs Spices Med Plants 26: 61-76.
- 475. Scaramussa SADL, Soares LDA, Santana LCLDA (2024) Extracts from jatobá (Hymenaea courbaril L.) peel and seeds: Antioxidant and antimicrobial activities and synergistic effect of extract combinations. Food Sci Technol Int 30: 128-136.
- 476.Schaffer S, Schmitt-Schillig S, Müller WE, Eckert GP (2005) Antioxidant properties of Mediterranean food plant extracts: geographical differences. J Physiol Pharmacol 56: 115-124.
- 477. Scheid T, Moraes MS, Henriques TP, Riffel APK, Belló-Klein A, et al. (2018) Effects of Methanol Fraction from Leaves of Schinus terebinthifoliusRaddi on Nociception and Spinal-Cord Oxidative Biomarkers in Rats with Neuropathic Pain. Evid Based Complement Alternat Med 2018: 5783412.
- 478. Schinella G, Mosca S, Cienfuegos-Jovellanos E, Pasamar MÁ, Muguerza B, et al. (2010) Antioxidant properties of polyphenol-rich cocoa products industrially processed. Food Res Int 43: 1614-1623.
- 479. Schinella GR, Troiani G, Dávila V, De Buschiazzo PM, Tournier HA (2000) Antioxidant Effects of an Aqueous Extract of Ilex paraguariensis. Biochem Biophys Res Commun 269: 357-360.
- 480. Schubert A, Pereira DF, Zanin FF, Alves SH, Beck RCR, et al. (2007) Comparison of antioxidant activities and total polyphenolic and methylxanthine contents between the unripe fruit and leaves of Ilex paraguariensisA. St. Hil. Pharmazie 62: 876-880.
- 481. Sena LM, Zucolotto SM, Reginatto FH, Schenkel EP, De Lima TCM (2009) Neuropharmacological activity of the pericarp of Passiflora edulisflavicarpa degener: putative involvement of C-glycosylflavonoids. Exp Biol Med 234: 967-975.
- 482. Sereia AL, De Oliveira MT, Baranoski A, Marques LLM, Ribeiro FM, et al. (2019) In vitroevaluation of the protective effects of plant extracts against amyloid-beta peptide-induced toxicity in human neuroblastoma SH-SY5Y cells. PLoS One 14: e0212089.
- 483. Sereniki A, Linard-Medeiros CFB, Silva SN, Silva JBR, Peixoto Sobrinho TJS, et al. (2016) Schinus terebinthifoliusadministration prevented behavioral and biochemical alterationsin a rotenone model of Parkinson’s disease. Rev Bras Farmacogn 26: 240-245.
- 484. Sergio L, Boari F, Pieralice M, Linsalata V, Cantore V, et al. (2020) Bioactive Phenolics and Antioxidant Capacity of Some Wild Edible Greens as Affected by Different Cooking Treatments. Foods 9: 1320.
- 485. Sette CVDM, Ribas De Alcântara BB, Schoueri JHM, Cruz FM, Cubero DDIG, et al. (2018) Purified Dry Paullinia cupana(PC-18) Extract for Chemotherapy-Induced Fatigue: Results of Two Double-Blind Randomized Clinical Trials. J Diet Suppl 15: 673-683.
- 486. Setti-Perdigão P, Serrano MAR, Flausino OA, Bolzani VS, Guimarães MZP, et al. (2013) Erythrina mulunguAlkaloids Are Potent Inhibitors of Neuronal Nicotinic Receptor Currents in Mammalian Cells. PLoS One 8: e82726.
- 487. Sie YY, Chen LC, Li CW, Wang CC, Li CJ,et al. (2023) Extracts and Scirpusin B from Recycled Seeds and Rinds of Passion Fruits (Passiflora edulisvar. Tainung No. 1) Exhibit Improved Functions in Scopolamine-Induced Impaired-Memory ICR Mice. Antioxidants 12: 2058.
- 488. Sija SL, Athulya AS, Mahima MR, Vidhya A (2019) Antioxidant and antimicrobial activity of different plant parts of Anacardium occidentaleL. and Mangifera indicaL.: a comparative study. Int J Pharm Sci DrugRes 11.
- 489. Silva AC da, Dias AB, Gazim ZC, Rahal IL, Laginestra B de FA, et al. (2022) Plantas com ação no sistema nervoso central que constam na relação nacional de plantas medicinais de interesse ao SUS (RENISUS). Arq. Ciênc. Saúde UNIPAR 26.
- 490. Silva SAM, Valarini MFC, Chorilli M, Venturini A, Leonardi GR (2013) Atividade Antioxidante do Extrato Seco de Cacau Orgânico (Theobroma cacao) - Estudo de Estabilidade e Teste de Aceitação de Cremes Acrescidos Deste Extrato. Rev. Ciênc. Farm. Básica E Apl 34: 493-501.
- 491. Silva END, Ramos DDC, Menezes LM, Souza AOD, Lannes SCDS, et al. (2014) Nutritional value and antioxidant capacity of “cocoa honey” (Theobroma cacao L.). Food Sci Technol Camp 34: 755-759.
- 492. Silva IDDL, Oliveira FSMD, Andrade MFD, Brito AMSS, Hallwass F, et al. (2021) Avaliação das potencialidades dos extratos vegetais de jurema preta (Mimosa tenuiflora) e cajueiro (Anacardium occidentaleL.) para uso em embalagens ativas antimicrobianas e antioxidantes. Matér. Rio Jan. 26, e12924.
- 493. Silva MMD, Iriguchi EKK, Kassuya CAL, Vieira MDC, Foglio MA, et al. (2017) Schinus terebinthifolius: phenolic constituents and in vitroantioxidant, antiproliferative and in vivoanti-inflammatory activities. Rev Bras Farmacogn 27: 445-452.
- 494. Silva NNS, Silva JRA, Alves CN, Andrade EHA, Da Silva JKR, et al. (2014) Acetylcholinesterase inhibitory activity and molecular docking study of 1-nitro-2-phenylethane, the main constituent of Aniba canelillaessential oil. Chem Biol Drug Des 84: 192-198.
- 495. Silvestrini GI, Marino F, Cosentino M (2013) Effects of a commercial product containing guaraná on psychological well-being, anxiety and mood: a single-blind, placebo-controlled study in healthy subjects. J Negat Results Biomed 12: 9.
- 496. Siow CS, Chan EWC, Wong CW, Ng CW (2022) Antioxidant and sensory evaluation of cocoa (Theobroma cacaoL.) tea formulated with cocoa bean hull of different origins. Future Foods 5: 100108.
- 497. Siqueira IR, Cimarosti H, Fochesatto C, Nunes DS, Salbego C, et al. (2004) Neuroprotective effects of Ptychopetalum olacoidesBentham (Olacaceae) on oxygen and glucose deprivation induced damage in rat hippocampal slices. Life Sci 75: 1897-1906.
- 498.Siqueira IR, Fochesatto C, Da Silva AL, Nunes DS, Battastini AM, et al. (2003) Ptychopetalum olacoides, a traditional Amazonian “nerve tonic”, possesses anticholinesterase activity. Pharmacol Biochem Behav 75: 645-650.
- 499. Siqueira IR, Fochesatto C, Torres ILS, Da Silva AL, Nunes DS, et al. (2007) Antioxidant activities of Ptychopetalum olacoides(“muirapuama”) in mice brain. Phytomedicine 14: 763-769.
- 500. Soares DJ, Do Carmo JS, Lima JDSS, Maia GA, De Souza PHM, et al. (2013) Polyphenols and antioxidant activity of cashew nuts from conventional and organic cultivation in different stages of processing. Bol Cent Pesqui Process Aliment 31.
- 501. Sotelo CL, Alvis BA, Arrázola PG (2015) Evaluación de epicatequina, teobromina y cafeína en cáscaras de cacao (Theobroma cacaoL.), determinación de su capacidad antioxidante. Rev Colomb Cienc Hortícolas 9: 124.
- 502. Sotero V, Maco M, Vela J, Merino C, Dávila É, et al. (2011) Evaluación de la actividad antioxidante y compuestos fenólicos en pulpa y semillas de cuatro frutales amazónicos de la familia Sterculiaceae. Rev Soc Quím Perú 77: 66-77.
- 503. Soto Vásquez MR (2019) Composición química y efecto del aceite esencial de las hojas de Lippia alba (Verbenaceae) en los niveles de estrés académico de estudiantes universitarios. Arnaldoa 26.
- 504. Souza AHP, Corrêa RCG, Barros L, Calhelha RC, Santos-Buelga C, et al. (2015) Phytochemicals and bioactive properties of Ilex paraguariensis: An in-vitrocomparative study between the whole plant, leaves and stems. Food Res Int 78: 286-294.
- 505. Souza CF, Lima T, Baldissera MD, Geihs MA, Maciel FE, et al. (2018) Nanoencapsulated Melaleuca alternifoliaessential oil exerts anesthetic effects in the brachyuran crab using Neohelice granulate. An Acad Bras Ciênc 90: 2855-2864.
- 506. Souza MFFD (2009) Chá mate (Ilex paraguariensis): compostos bioativos e relação com atividade biológica (Mestrado em Nutrição em Saúde Pública). Universidade de São Paulo, São Paulo.
- 507. Souza NC, De Oliveira JM, Morrone MDS, Albanus RD, Amarante MDSM, et al. (2017) Antioxidant and Anti-Inflammatory Properties of Anacardium occidentaleLeaf Extract. Evid Based Complement Alternat Med 2017: 2787308.
- 508. Souza SJD (2013) Efeito da ingestão de chocolate e erva mate no perfil lipídico e oxidativo de indivíduos com HIV/AIDS em uso de terapia antirretroviral (Mestrado em Nutrição em Saúde Pública). Universidade de São Paulo, São Paulo.
- 509. Spera KD, Figueiredo PA, Santos PCE, Barbosa FC, Alves CP, et al. (2019) Genotoxicity, anti-melanoma and antioxidant activities of Hymenaea courbaril L.seed extract. An Acad Bras Ciênc 91: e20180446.
- 510. Springer A, Ziegler H, Bach K (2023) The Influence of Antioxidant Plant Extracts on the Oxidation of O/W Emulsions. Cosmetics 10: 40.
- 511. Srichomphu P, Wattanathorn J, Thukham-Mee W, Muchimapura S (2022) Anxiety, Insomnia, and Memory Impairment in Metabolic Syndrome Rats Are Alleviated by the Novel Functional Ingredients from Anacardium occidentale. Antioxidants 11: 2203.
- 512. Stashenko EE, Jaramillo BE, Martinez JR (2004) Comparison of different extraction methods for the analysis of volatile secondary metabolites of Lippia alba (Mill.) N.E. Brown, grown in Colombia, and evaluation of its in vitroantioxidant activity. J Chromatogr A 1025: 93-103.
- 513. Stashenko EE, Martínez JR, Durán DC, Córdoba Y, Caballero D (2014) Estudio comparativo de la composición química y la actividad antioxidante de los aceites esenciales de algunas plantas del género Lippia (Verbenaceae) cultivadas en Colombia. Rev Acad Colomb Cienc Exactas Físicas Nat 38: 89.
- 514. Suazo Y, Davidov-Pardo G, Arozarena I (2014) Effect of Fermentation and Roasting on the Phenolic Concentration and Antioxidant Activity of Cocoa from Nicaragua. J Food Qual 37: 50-56.
- 515. Sukketsiri W, Daodee S, Parhira S, Malakul W, Tunsophon S, et al. (2023) Chemical characterization of Passiflora edulisextracts and their in vitroantioxidant, anti-inflammatory, anti-lipid activities, and ex-vivovasodilation effect. J King SaudUniv Sci 35: 102431.
- 516. Sulbarán B, González B, Fernández V (2013) Caracterización quimica y actividad antioxidante delpseudofruto de caujil (Anacardium occidentaleL.). Rev Fac Agron 30: 454-469.
- 517. Summa C, Raposo FC, McCourt J, Scalzo RL, Wagner KH, et al. (2006) Effect of roasting on the radical scavenging activity of cocoa beans. Eur Food Res Technol 222: 368-375.
- 518. Suzuki R, Matsushita Y, Imai T, Sakurai M, Henriques De Jesus JM, et al. (2008) Characterization and antioxidant activity of Amazonian woods. J Wood Sci 54: 174-178.
- 519. Tamagno WA, Alves C, Tessaro D, Sutorillo NT, Santin W, et al. (2022) Deferoxamine Supplementation Abolished Iron-Related Toxicity of Ilex paraguariensisExtract: Behavioral and Biochemical Evaluation in Adult Zebrafish (Danio rerio). Antioxidants 11: 1507.
- 520. Teixeira De Oliveira G, Siqueira Ferreira JM, Lima WG, Ferreira Alves L, Duarte-Almeida JM, et al. (2018) Phytochemical characterisation and bioprospection for antibacterial and antioxidant activities of Lippia albaBrown ex Britton & Wilson (Verbenaceae). Nat Prod Res 32: 723-731.
- 521.Teselkin Yu O, Babenkova IV, Kochetova AA, Osipov AN (2022) Inhibitory Effect of Aqueous Extract from Yerba Mate (Ilex paraguariensis) on the Process of Lipid Peroxidation of Liposomal Membranes. Biophysics 67: 541-548.
- 522. Teselkin Yu O, Babenkova IV, Pavlova LA, Lee A, Kochetova AA, et al. (2021) The Antioxidant Capacity of Aqueous Extracts from Yerba Mate (Ilex paraguariensis). Biophysics 66: 125-132.
- 523. Teugwa CM, Mejiato PC, Zofou D, Tchinda BT, Boyom FF (2013)Antioxidant and antidiabetic profiles of two African medicinal plants: Picralima nitida(Apocynaceae) and Sonchus oleraceus(Asteraceae). BMC Complement Altern Med 13: 175.
- 524. The Brazil Flora Group, Gomes-da-Silva J, Filardi FLR, Barbosa MRV, Baumgratz JFA, Bicudo CEM, et al. (2022) Brazilian Flora 2020: Leveraging the power of a collaborative scientific network. TAXON 71: 178-198.
- 525. Thirugnanasampandan R, Mahendran G, Bai VN (2008) Antioxidant properties of some medicinal Aristolochiaceae species. Afr J Biotechnol 7.
- 526. Tlili N, Sarikurkcu C (2020) Bioactive compounds profile, enzyme inhibitory and antioxidant activities of water extracts from five selected medicinal plants. Ind Crops Prod 151: 112448.
- 527. Todirascu-Ciornea E, El-Nashar HAS, Mostafa NM, Eldahshan OA, Boiangiu RS, et al. (2019) Schinus terebinthifoliusEssential Oil Attenuates Scopolamine-Induced Memory Deficits via Cholinergic Modulation and Antioxidant Properties in a Zebrafish Model. Evid Based Complement Alternat Med 2019: 5256781.
- 528. Toni C, Martos-Sitcha JA, Baldisserotto B, Heinzmann BM, De Lima Silva L, et al. (2015) Sedative effect of 2-phenoxyethanol and essential oil of Lippia albaon stress response in gilthead sea bream (Sparus aurata). Res Vet Sci 103: 20-27.
- 529. Tousson E, Hafez E, Zaki S, Gad A, Elgharabawy RM (2020) Evaluation of the testicular protection conferred by damiana (Turnera diffusa Willd.) against amitriptyline-induced testicular toxicity, DNA damage and apoptosis in rats. Biomed Pharmacother 132: 110819.
- 530. Trevisan MTS, Pfundstein B, Haubner R, Würtele G, Spiegelhalder B, et al. (2006) Characterization of alkyl phenols in cashew (Anacardium occidentale) products and assay of their antioxidant capacity. Food Chem Toxicol 44: 188-197.
- 531. Uliana MP, Fronza M, Da Silva AG, Vargas TS, De Andrade TU, et al. (2016) Composition and biological activity of Brazilian rose pepper (Schinus terebinthifoliusRaddi) leaves. Ind Crops Prod 83: 235-240.
- 532. Umri RJ, Maulana I, Ginting B (2019) Antioxidant and cytotoxic activity of ethyl asetat extracts of cocoa pod husk (Theobroma cacao L). IOP Conf Ser Earth Environ Sci 364: 012026.
- 533. Urbizu-González AL, Castillo-Ruiz O, Martínez-Ávila GCG, Torres-Castillo JA (2017) Natural variability of essential oil and antioxidants in the medicinal plant Turnera diffusa. Asian Pac J Trop Med 10: 121-125.
- 534. Vagula JM, Visentainer JV, Lopes AP, Maistrovicz FC, Rotta EM, et al. (2019) Content of phenolic compounds in fruit processing residues by mass spectrometry. Acta Sci Technol 41: 35043.
- 535. Valadez-Carmona L, Plazola-Jacinto CP, Hernández-Ortega M, Hernández-Navarro MD, Villarreal F, et al. (2017) Effects of microwaves, hot air and freeze-drying on the phenolic compounds, antioxidant capacity, enzyme activity and microstructure of cacao pod husks (Theobroma cacao L.). Innov Food Sci Emerg Technol 41: 378-386.
- 536. Valduga AT,Gonçalves IL, Borges ACP, Mielniczki-Pereira AA, Picolo AP (2016) Cytotoxic / antioxidant activity and sensorial acceptance of yerba-mate development by oxidation process. Acta Sci Technol 38: 115.
- 537. Vale TG, Matos FJA, De Lima TCM, Viana GSB (1999) Behavioral effects of essential oils from Lippia alba (Mill.) N.E. Brown chemotypes. J Ethnopharmacol 67: 127-133.
- 538. Vanin Dos Santos Lima M, Beloni De Melo G, Gracher Teixeira L, Grella Miranda C, Hermes De Araújo PH, et al. (2022) Green synthesis of silver nanoparticles using Ilex paraguariensisextracts: antimicrobial activity and acetilcolinesterase modulation in rat brain tissue. Green Chem Lett Rev 15: 128-138.
- 539. Vargas-Arana G, Merino-Zegarra C, Tang M, Pertino MW, Simirgiotis MJ (2022) UHPLC–MS Characterization, and Antioxidant and Nutritional Analysis of Cocoa Waste Flours from the Peruvian Amazon. Antioxidants 11: 595.
- 540. Varón E, Ospina F, Murillo E, Mendéz JJ (2007) Tamizaje fitoquimico y actividad antioxidante de extractos acuoso y orgánicos de Justicia pectoralis jacq. (amansa toros) y de volátiles y no volátiles de lippia alba mill. (pronto alivio)cultivadas en diferentes pisos térmicos. Sci Tech XIII 349-350.
- 541. Vasconcelos SMM, Macedo DS, De Melo CTV, Monteiro AP, Cunha GMA, et al. (2010) Central activity of hydroalcoholic extracts from Erythrina velutinaand Erythrina mulunguin mice. J Pharm Pharmacol 56: 389-393.
- 542. Vasic SM, Stefanovic OD, Licina BZ, Radojevic ID, Comic LR (2012) Biological activities of extracts from cultivated Granadilla Passiflora alata. EXCLI J 11: 208-218.
- 543.Vecchia CAD, Locateli G, Serpa PZ, Bianchin Gomes D, Ernetti J, et al. (2022) Sonchus oleraceusL. Promotes Gastroprotection in Rodents via Antioxidant, Anti-Inflammatory, and Antisecretory Activities. Evid Based Complement Alternat Med 2022: 7413231.
- 544. Velásquez MM, Lattig MC, Chitiva LC, Costa GM, Sutachan JJ, et al. (2023) Dendritogenic Potential of the Ethanol Extract from Lippia albaLeaves in Rat Cortical Neurons. Molecules 28: 6666.
- 545. Velázquez E, Tournier HA, Mordujovich De Buschiazzo P, Saavedra G, Schinella GR (2003) Antioxidant activity of Paraguayan plant extracts. Fitoterapia 74: 91-97.
- 546. Veloso CF, Machado AK, Cadoná FC, Azzolin VF, Cruz IBM, et al. (2017) Neuroprotective Effects of Guarana (Paullinia cupana Mart.) against Vincristine in VitroExposure. J Prev Alzheimers Dis 5: 65-70.
- 547. Vieira LM, Sousa MSB, Mancini-Filho J, Lima AD (2011) Fenólicos totais e capacidade antioxidante in vitrode polpas de frutos tropicais. Rev Bras Frutic 33: 888-897.
- 548. Vieitez I, Maceiras L, Jachmanián I, Alborés S (2018) Antioxidant and antibacterial activity of different extracts from herbs obtained by maceration or supercritical technology. J Supercrit Fluids 133: 58-64.
- 549. Viera W, Shinohara T, Samaniego I, Sanada A, Terada N, et al. (2022) Phytochemical Composition and Antioxidant Activity of Passiflora spp.Germplasm Grown in Ecuador. Plants 11: 328.
- 550. Vilela FC, De Mesquita Padilha M, Alves-Da-Silva G, Soncini R, Giusti-Paiva A (2010) AntidepressantLike Activity of Sonchus oleraceusin Mouse Models of Immobility Tests. J Med Food 13: 219-222.
- 551. Wang C, Xu FQ, Shang JH, Xiao H, Fan WW, et al. (2013) Cycloartane triterpenoid saponins from water soluble of Passiflora edulisSims and their antidepressant-like effects. J Ethnopharmacol 148: 812-817.
- 552. Wattanathorn J, Prabsattroo T, Somsapt P, Sritragool O, Thukham-mee W, et al. (2018) Sexual Enhancing Effect of Anacardium occidentalein Stress-Exposed Rats by Improving Dopaminergic and Testicular Functions. BioMed Res Int 2018: 6452965.
- 553. Weber D, Hoffmann JF, Barreto CF, Zandoná GP, Nachtigal JC, et al. (2021) Bioactive content of six passion fruit genotypes cultivated in southern Brazil. Biosci J 37: e37086.
- 554. Wong YS, Sia CM, Khoo HE, Ang YK, Chang SK, et al. (2014) Influence of extraction conditions on antioxidant properties of passion fruit (Passiflora edulis) peel. Acta Sci Pol Technol Aliment 13: 257-265.
- 555. Wong-Paz JE, Contreras-Esquivel JC, Rodríguez-Herrera R, Carrillo-Inungaray ML, López LI, et al. (2015) Total phenolic content, in vitroantioxidant activity and chemical composition of plant extracts from semiarid Mexican region. Asian Pac J Trop Med 8: 104-111.
- 556. World Health Organization (2022) World mental health report: transforming mental health for all. Executive summary.
- 557. Xia DZ, Yu XF, Zhu ZY, Zou ZD (2011) Antioxidant and antibacterial activity of six edible wild plants (Sonchus spp.) in China. Nat Prod Res 25: 1893-1901.
- 558. Xiong F, Li X, Zheng L, Hu N, Cui M, et al. (2019) Characterization and antioxidant activities of polysaccharides from Passiflora edulisSims peel under different degradation methods. Carbohydr Polym 218: 46-52.
- 559. Yahya M, Ginting B, Saidi N (2021) In-VitroScreenings for Biological and Antioxidant Activities of Water Extract from Theobroma cacao L.Pod Husk: Potential Utilization in Foods.Molecules 26: 6915.
- 560. Yamaguti-Sasaki E, Ito L, Canteli V, Ushirobira T, Ueda-Nakamura T, et al. (2007) Antioxidant capacity and in vitroprevention of dental plaque formation by extracts and condensed tannins of Paullinia cupana. Molecules 12: 1950-1963.
- 561. Yepes A, Ochoa-Bautista D, Murillo-Arango W, Quintero-Saumeth J, Bravo K, et al. (2021) Purple passion fruit seeds (Passiflora edulis F.edulis Sims) as a promising source of skin anti-aging agents: Enzymatic, antioxidant and multi-level computational studies. Arab J Chem 14: 102905.
- 562. Yin J, Kwon GJ, Wang MH (2007) The antioxidant and cytotoxic activities of Sonchus oleraceus L.extracts. Nutr Res Pract 1: 189-194.
- 563. Yonekura L, Martins CA, Sampaio GR, Monteiro MP, César LAM, et al. (2016) Bioavailability of catechins from guaraná (Paullinia cupana) and its effect on antioxidant enzymes and other oxidative stress markers in healthy human subjects. Food Funct 7: 2970-2978.
- 564. Zamberlan DC, Arantes LP, Machado ML, Da Silveira TL, Da Silva AF, et al. (2020) Guarana (Paullinia cupanaMart.) protects against amyloid-ß toxicity in Caenorhabditis elegans through heat shock protein response activation. Nutr Neurosci 23: 444-454.
- 565. Zeraik ML, Yariwake JH, Wauters JN, Tits M, Angenot L (2012) Analysis of passion fruit rinds (Passiflora edulis): isoorientin quantification by HPTLC and evaluation of antioxidant (radical scavenging) capacity. Quím. Nova 35: 541-545.
- 566. Zétola M, De Lima TCM, Sonaglio D, González-Ortega G, Limberger RP, et al. (2002) CNS activities of liquid and spray-dried extracts from Lippia alba—Verbenaceae (Brazilian false melissa). J Ethnopharmacol 82: 207-215.
- 567.Zielinski AAF, Ávila S, Ito V, Nogueira A, Wosiacki G, et al. (2014a) The Association between Chromaticity, Phenolics, Carotenoids, and In VitroAntioxidant Activity of Frozen Fruit Pulp in Brazil: An Application of Chemometrics. J Food Sci 79: C510-C516.
- 568. Zielinski AAF, Haminiuk CWI, Alberti A, Nogueira A, Demiate IM, et al. (2014b) A comparative study of the phenolic compounds and the in vitroantioxidant activity of different Brazilian teas using multivariate statistical techniques. Food Res Int 60: 246-254.
- 569. Zzaman W, Bhat R, Abedin Md Z, Yang TA (2013) Comparison between Superheated Steam and Convectional Roasting on Changes in the Phenolic Compound and Antioxidant Activity of Cocoa Beans. Food Sci Technol Res 19: 949-956.
- 570. Zzaman W, Bhat R, Yang TA (2014a) Application of Response Surface Methodology to Optimize Roasting Conditions in Cocoa Beans Subjected to Superheated Steam Treatments in Relevance to Antioxidant Compounds and Activities. Dry Technol 32: 1104-1111.
- Zzaman W, Bhat R, Yang TA (2014b) Effect of Superheated Steam Roasting on the Phenolic Antioxidant Properties of Cocoa Beans: The Phenolic Antioxidant Properties of Cocoa Beans. J Food Process Preserv 38: 1932-1938.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.