Prinsepia Utilis Royle Oil Extract Improve Skin Barrier on Reconstructed Skin Model
Bo Wang 1,2* , Feifei Wang 1,2 , Han Gao 1,2
1 Shanghai Jiyan Bio-pharmaceutical Co., Ltd. Shanghai 201702, China
2 Yunnan Botanee Bio-technology Group Co., Ltd. Yunnan 650000, China
*Corresponding author: Bo Wang, Research and Development Department (R&D), Shanghai Jiyan Bio-pharmaceutical Co., Ltd. Shanghai, 201702, China
Received Date: 07 November, 2022
Accepted Date: 21 November, 2022
Published Date: 25 November, 2022
Citation: Wang B, Wang F, Gao H (2022) Prinsepia Utilis Royle Oil Extract Improve Skin Barrier on Reconstructed Skin Model. Int J Nurs Health Care Res 5: 1363. DOI: https://doi.org/10.29011/2688-9501.101363
Abstract
Background: The skin barrier is mainly provided by the Stratum Corneum (SC). Skin barrier function mainly can be effected by tight junction and skin barrier genes/proteins, also include surfactants, UV and other environmental factor. However, Prinsepia Utilis Royle Oil (PURO) which have rarely report about improving the skin barrier. Method: We use immunofluorescence to observe protein localization on reconstructed skin models, the MTT assay to analyze tissue activity, and the ELISA assay to test the release of IL-1α/IL-8. We also use Real-time quantitative PCR analysis to detect the expression of genes and skin penetration study to detect the barrier function. We also use cell migration to detect the activity of wound healing. Results: We find that PURO can increase the cell migration in HaCAT with the higher healing rate. Application of PURO on damaged skin model increase the expression of tight junction proteins; tight junction protein 1 (TJP1) and claudin 5 (CLDN5), keratin 10 (CK10) and skin barrier proteins; loricrin (LOR), filaggrin (FLG), involucrin (IVL), Caspase-14 (CASP14), Bleomycin hydrolase (BLMH), ceramide synthase 3 (CER3), transglutaminase (TGM)-1. Application of PURO on normal skin model increase the expression of LOR, IVL and FLG. Also, the cytotoxicity of a skin irritant, was alleviated by exposure of PURO. The ET50 (Time of Half Lethal) time extended by the pretreatment of PURO. PURO can reduce the SDS (Sodium Dodecyl Sulfate)-enhanced dye permeability which further certifies its skin barrier function. We also find that PURO can inhibit the expression and release of IL-1α, IL-8 and PGE2 on SDS/UVB-induced skin model. Conclusion: Collectively, we propose that PURO may have protective effects on the skin barrier and can be used on cosmetics as an important repairing ingredient.
Keywords: PURO; Reconstructed skin model; Skin barrier
Introduction
As the first line of defence, skin forms an effective barrier between the organism and the environment fending off chemical and physical attacking and preventing pathogens invasion, also including the unregulated loss of water and other solutes [1,2]. The skin includes epidermis, dermis and subcutaneous tissue. The skin barrier function is mainly provided by the stratum corneum, with cells enriched of proteins, like cornified envelopes and cytoskeletal elements, and intercellular domains enriched of lipid.
This skin barrier is referred as a ‘brick and mortar’ structure. The structure contains the keratin microfibrils, filaggrin and cornified envelopes. All of these form the bricks. The lipids form the mortar [3]. The desmosomes form the tight, gap and adherens junctions. In simple epithelia, Tight Junctions (TJ) that contain claudins, occludin, and tight junction proteins which serve as a physical barrier to prevent solutes and water unregulated lossing through forming cell-cell junctions [4]. Proteins like loricrin (LOR), filaggrin (FLG), involucrin (IVL), Caspase-14 (CASP14), Bleomycin Hydrolase (BLMH), Ceramide Synthase 3 (CER3), Transglutaminase (TGM)-1 also contribute to the protective skin barrier [5-9].
Alterations in intercellular lipids and epidermal differentiation can lead to barrier defects, damage and, finally, skin disease. Decreasing the expression of Claudin-1 can result in significantly impaired in scratch wound healing, with delayed in cell migration and reduced in cell proliferation [10]. The TJ proteins, such as Claudin-1, Claudin -4, Occludin, and TJP1 contributed to the skin barrier to ions; Claudin -1 and TJP1 also contributed to the skin barrier to larger molecules on NHEKs models [11]. Claudin-5 (CLDN5) may which belong to claudins proteins which play an important role in the epidermal barrier with sensitive skin [12]. Compared to healthy skin models, knock downing FLG can lead to a disorganized skin lipid barrier and reduce the content of Uric Acid (UCA) and pyrrolidone carboxylic acid (PCA) [13]. The major protein of the epidermal cornified cell envelope is loricrin, approximately 70% by mass. Deficiency of loricrin protein leaded to a delay in the formation of the skin barrier in mice embryonic development [14]. Lossing of keratin 10 (CK10) expression can result in recessive epidermolytic ichthyosis, and then impaired barrier function [15,16]. Deficiency of ceramide synthase 3 (CER3) results in persistent periderm, hyperkeratosis. More seriously, losing ceramide synthase 3 can arrest keratinocyte maturation at an embryonic pre-barrier stage in mice [6]. Caspase14-deficient epidermis model can reduce skin-hydration levels and increased water loss [17]. These mean that TJ proteins, keratinized mantle protein, skin barrier protein and keratin have the important function to skin barrier.
Prinsepia utilis Royle is classified as the Rosaceae. It grows mainly in Yunnan province of China and finds in bushes at elevations of up to 1000-3000 m above sea level [18]. Prinsepia utilis has been used to treat skin diseases, rheumatism, inflammation, and leprosy in Chinese and Indian folk medicine. Prinsepia utilis contain hydrocyanic acid fatty oil, prinsepiol and triterpenoids [18]. The main fatty acids in the oil were palmitic acid (15.15 %), stearic acid (6.85 %), oleic acid (38.82 %), linoleic acid (37.15 %) and linolenic acid (1.13 %) [19]. Lipid composition in skin is one of the important structures of corneum barrier. Skin surface lipids such as free fatty acids (palmitic acid and oleic acid) maintain skin surface moisture permeability, moisture retention and barrier functions [20-22]. However, to date, there have been few studies of the function on Prinsepia Utilis Royle Oil (PURO), especially on skin barrier in reconstructed skin models or other in vitro models.
Here, we analyzed the ingredient of PURO by GC-MS. PURO mainly contain tetradecanoic acid, palmitic acid, palmitoleic acid, heptadecanoic acid, stearic acid, oleic acid, linoleic acid, linolenic acid, eicosanoic acid, eicosenoic acid. Therefore, we investigated whether the PURO have the function of skin barrier in a reconstructed human epidermis model. In our study, we find that PURO can affect the cell migration in HaCAT with the higher healing rate. The MTT and ELISA assay show that PURO have no skin irritation on reconstructed skin model. PURO can contribute to the barrier to Rhodamine B, enhance the skin barrier and reduce the skin permeability. The ET50 data suggest that PURO maintain the barrier function on skin model. Following the topical treatment of PURO, immunofluorescence staining and QRT-PCR are used to investigate differentiation of epidermal structural components of barrier function. The results show that PURO can increase the expression of FLG, LOR, TJP1, CASP14, CER3, IVL, BLMH, TGM-1 and CLDN5 on SDS damaged skin model; the expression of FLG, IVL and LOR on normal skin model. We also find that PURO can inhibit the inflammatory factor such as IL-1α, IL-8 and PGE2. We consider that PURO have the function of enhance and repair skin barrier which may have contribution to Atopic Dermatitis (AD) patients.
Methods
Reconstructed Human Epidermis EpiSkin®
A commercial epidermal tissue is EpiSkin® (L’Oréal Research and Innovation Center, Shanghai, China), made from normal human keratinocytes. EpiSkin® which is an in vitro reconstructed human epidermis that culture on a collagen matrix at the air-liquid interface. EpiSkin ® are similar to those of in vivo human epidermis by histologic sections. After pre-incubation, tissues were treated with 0.3% SDS or PBS for 10 min. Then the tissues were washed by DPBS, as SDS damaged skin model or normal skin model. Then, the tissues exposure with 20 μl of various concentrations of PURO confect with Base material. After 24-48 h treatment, tissues were washed with DPBS.
HaCAT Cell Line
HaCAT cell line (National Infrastructure of Cell Line Resource) Cell scratch assay was performed by using in vitro scratch assay. HaCaT cells were cultured on 12-well plate at a density of 5×105 cells/well and incubated overnight at 37°C. The wound area was scratched with Culture-Insert 2 Well in µ-Dish 35 mm. Then, the cells were treated with 5% and 10% of PURO diluted by DMSO on 0h, 16h and 24h. The method referred to Hanittinan [23].
Materials and Reagents
Prinsepia Utilis Royle Oil (PURO) was obtained from Yunnan Botanee Bio-technology Group Co., Ltd. (Yunnan, China). TritonX-100 solution was bought from Sangon Biotech (Shanghai,
China). Sodium lauryl sulfate (SDS) was obtained from SigmaAldrich (Saint Louis, MO, USA). Calcium/magnesium-free Dulbecco’s Phosphate-Buffered Saline (DPBS) was obtained from Basal Media (Shanghai, China). MTT (Methyl Thiazolyl Tetrazolium), DAPI (4,6-amidine-2-phenylindole), Rhodamine
B and Anti-fluorescent quench sealant was obtained from BIOLIGHT BIOTECH (Shanghai, China). IL-1α, IL-8 and PGE2 ELISA kits were purchased from NEOBIOSCIENCE (Shenzhen, China). The ingredient of Base, 5% PURO and 10% PURO formula were shown on supplemental Table S1.
|
NO. |
Name |
|
1 |
H2O |
|
2 |
PEMULEN TR-1 POLYMER |
|
3 |
PMX200-350cst |
|
4 |
Moon K Glycerin |
|
5 |
GRINDSTED Xanthan 200 |
|
6 |
GRESURF GMS165 |
|
7 |
Hydrolite-5/Purolan PD-LO |
|
8 |
AMP-ULTRA PC 2000 |
Table S1: Base.
|
NO. |
Name |
|
1 |
H2O |
|
2 |
PEMULEN TR-1 POLYMER |
|
3 |
PMX200-350cst |
|
4 |
Moon K Glycerin |
|
5 |
GRINDSTED Xanthan 200 |
|
6 |
GRESURF GMS165 |
|
7 |
Hydrolite-5/Purolan PD-LO |
|
8 |
AMP-ULTRA PC 2000 |
|
9 |
PURO |
Table S1: PURO Formula.
Table S1: The ingredients of Base and PURO formula.
Skin Irritation
This method referred to Wang [24]. After overnight incubation (37 °C, 5.0% CO2), the EpiSkin® models were used in the following experiments. After 18 h of post-exposure incubation with the PURO formula at 37°C, 5.0% CO2, the surface of the EpiSkin ® was washed with DPBS. Then, using the MTT assay to detect the tissue activity and ELISA assay to detect the release of IL-1α/ IL-8 24.
Quantitative RT-PCR
Total RNA was extracted from the skin tissue using a TRIzol kit (Invitrogen) following the manual. According to the explanatory memorandum (Takara), first-strand complementary DNA (cDNA) was synthesized. Using a Roche LC96 (Roche) to perform quantitative RT-PCR with SYBR Green I master mix (Roche). The sequences of PCR primers were attached to Supplemental Table S2. The RT-qPCR results are shown as the relative expression levels normalized to the expression of β-Acting, which was used as an internal reference (β-Acting -F/R), and three biological replicates were included for every experiment.
|
CK10-F |
CAACTCACATCAGGGGGAGC |
|
CK10-R |
CAGCTCATCCAGCACCCTAC |
|
CK14-F |
AGATGATTGGCAGCGTGGA |
|
CK14-R |
ACATCTCTGGATGACTGCGA |
|
IL1a-F |
ACTCAATTGTATGTGACTGCCCAAG |
|
IL1a-R |
TGGTCTCACTACCTGTGATGGTTT |
|
CLDN5-F |
CCTTCCCTGATGTCTGATTTTTG |
|
CLDN5-R |
GCCTCTTCTCCCATTTGTTTTTC |
|
DSG1-F |
CACTCAGATTGTGCTGCAAAC |
|
DSG1-R |
CATCTGCCCCACCATCTC |
|
TJP-1-F |
GGGACAACAGCATCCTTCCA |
|
TJP-1-R |
GCAAAAGACCAACCGTCAGG |
|
AQP3-F |
CCCTGGATCAAGCTGCCCATCTA |
|
AQP3-R |
GGCGAAGTGCCAGATTGCAT |
|
IL8-F |
GCTCTCTTGGCAGCCTTCC |
|
IL8-R |
CAGAGCTCTCTTCCATCAGAAAGCT |
|
Acting-F |
GCAAAGACCTGTACGCCAAC |
|
Acting-R |
GATCTTCATTGTGCTGGGTGC |
|
FLG-F |
GAGGGCACTGAAAGGCAAAA |
|
FLG-R |
CTTCCGTGCTGAGAGTGTCT |
|
LOR-F |
CCAGACCCAGCAGAAGCAG |
|
LOR-R |
TGCCCCTGGAAAACACCTC |
|
TGM1-F |
GAGAGCACCACACAGACGAG |
|
TGM1-R |
CTCGGGGTTGTTTCCGATGA |
|
CER3-F |
ACATTCCACAAGGCAACCATTG |
|
CER3-R |
CTCTTGATTCCGCCGACTCC |
|
CASP14-F |
GACCTGGATGCTCTGGAACACA |
|
CASP14-R |
GAATCGATGGCCTGCTGGA |
|
BLMH-F |
TGTGGTTTGGCTGTGATGTT |
|
BLMH-R |
GCACCATCCTGATCATCCTT |
|
IVL-F |
CCATCAGGAGCAAATGAAACAG |
|
IVL-R |
GCTCGACAGGCACCTTCTG |
Table S2: The list of primers that used in Quantitative RT-PCR.
Skin Penetration Study
Rhodamine B is a hydrophilic dye. Using Rhodamine B to evaluate the permeability of the skin [25]. EpiSkin® or SDS damaged skin model was treated with PURO formula for 24 h and washed with PBS 0.02% rhodamine B was treated for 1h. The tissues were fixed in 4% paraformaldehyde for 20 min at 4°C. Then the tissues were washed in 0.01 mol/L PBS for 5 min. After that, the tissues were dehydrated in an ethanol gradient, and then vitrified by xylene, immersed and embedded in wax overnight. Using a Leica rotary microtome (RM2235, Germany) to slice up the tissue. The sections were 5 μm thick. The slices were dewaxed by xylene, rehydrated in an ethanol gradient, washed in 1×PBS two times for 3 min respectively. After 2 times washing, 1000 times diluted DAPI in 1× PBS were treated on slides, followed by 3 washes in PBS. In the end, samples were covered by anti-fluorescent quench sealant and immunofluorescence images were captured by ZESS microscopic examination (Axio Scope, Germany).
UV Irradiation
After pre-incubation, tissues were treated with UVB=90mJ or dark. Then, the tissues exposure with 20 μl of various concentrations of PURO confect with Base material. After 42 h treatment, tissues were washed with DPBS. The medium are diluted to measure the release of PGE2 by using the ELISA kit.
MTT Assay
This method referred to Wang [24].
ELISA Assay
The test media were diluted to measure the release of IL1α/ IL-8/PGE2 by using the ELISA kit. The relative expression of IL-1α/ IL-8 release are compared to untreated EpiSkin® samples (controls).
ET50
The skin model was pretreated with samples (10%PURO formula and Base) with 0.2%SDS working solution. After 24h treatment, tissues were rinsed with DPBS. Then, the tissues were treated with 1% TritonX100 with 0min, 5min, 20min and 30min. After rinsing with DPBS, the tissues were incubated for 24 h at 37°C, 5.0% CO2. After that, the tissues were detected by MTT assay. Furthermore, calculating ET50 by SPSS software. ET50 means that the time required for 50% tissue activity.
Immunofluorescence Staining (IF)
For immunofluorescence, skin samples were cut into 5 μm section. The slices were proceeded rehydration steps with descending graded series of ethanol. The sections were incubated in boiled citrate buffer (pH 6.4) for 20 min and then cooled to room temperature. The slides were washed three times in PBS (pH
7.4) for 5 min. Then, the sections were blocked with 1.5% BSA + 0.025% TritonX-100 in PBS for 30 min to 90 min. Anti-Filaggrin (abcam ab81468 1:1000)/ Anti-Loricrin (abcam ab85679 1:200)/ Anti-Cytokeratin 10 (abcam ab111447 1:200) were diluted in blocking buffer, and the slides were then incubated at 4°C overnight or 1-2h at room temperature. Then, the slides were washed three times in PBS (pH 7.4) for 5 min and incubated with Goat AntiRabbit IgG H&L (FITC) diluted 1:2500 in PBS (pH 7.4) for 1 h at room temperature. Then, washing three times in PBS for 5 min, and DAPI were treated on slides, followed by three washes in PBS for 5min. Samples were covered by anti-fluorescent quench sealant. Using ZESS microscopic examination (Axio Scope, Germany) to capture the immunofluorescence images.
Gas Chromatography-Mass Spectrometry (GC-MS)
Qualitative and quantitative determination of Prinsepia Utilis Royle oil (PURO) by Gas Chromatography-Mass Spectrometry: The PURO was identified by gas chromatographymass spectrometry (GC-MS). The determination conditions were Agilent 7890A GC-7000MS. The chromatographic column was Agilent VF-WAX 60 m×0.25 mm×0.25 μm, and the ion source type was EI. 230°C, heating procedure: the initial temperature is 100°C, and the temperature is heated to 240°C at the rate of 10°C / min for 70 min.
Statistical Analysis
The statistical significance was determined with Two way ANOVA (p < 0.05 was considered to be statistically significant).
Results
Analysis of Main Components of PURO
Here, we use GC-MS to analyze the main components of PURO. The most abundant of PURO are palmitic acid, oleic acid and linoleic acid. The time-to-Peak force of palmitic acid is 21.675-21.853min, oleic acid is 26.385-26.743min, linoleic acid is 27.751-28.104min (Figure 1a and supplemental Figure S1a-c). The ingredient of PURO is tetradecanoic acid (0.1%), palmitic acid (17.9%), palmitoleic acid (0.4%), heptadecanoic acid (0.1%), stearic acid (9.1%), oleic acid (36.5%), linoleic acid (33.1%), linolenic acid (1.1%), eicosanoic acid (0.7%), eicosenoic acid (0.2%) (Figure 1a and b). The most abundant of these are palmitic acid, oleic acid and linoleic acid which belong to seburm free fatty acids that have the function of skin barrier and skin moisturizing [21,22,26].
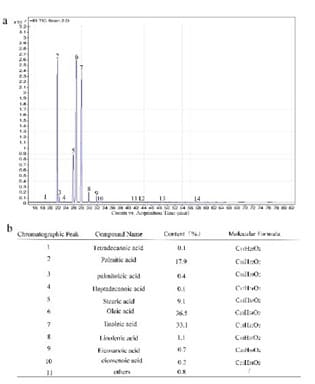
Figure1: Component analysis of PURO
Figure 1a: The Integration Peak of PURO. The abscissa axis is counts vs Acquisition time. The y-axis is TIC Scan. Figure 1b: The composition and content of PURO. The ingredient of PURO is tetradecanoic acid (0.1%), palmitic acid (17.9%), palmitoleic acid (0.4%), heptadecanoic acid (0.1%), stearic acid (9.1%), oleic acid (36.5%), linoleic acid (33.1%), linolenic acid (1.1%), eicosanoic acid (0.7%), eicosenoic acid (0.2%), others (0.8%).
Oils with a higher linoleic acid to oleic acid ratio have better barrier repair potential [27]. Palmitic acid has a direct and pronounced effect on epidermal morphogenesis via activating the proper execution of the early differentiation program and reducing the epidermal activation process which are essential for skin barrier [28]. At the same time, linolenic acid, eicosanoic acid, tetradecanoic acid, etc also can be detected in PURO. For the formation of the epidermal barrier, free fatty acids are essential for numerous processes. These compositions like seburm free fatty acids, linolenic acid, eicosanoic acid and tetradecanoic acid have good moisturizing and repairing the skin barrier function, can promote the synthesis of ceramide, moisturizing and nourishing effect, can regulate endocrine and anti-aging, between the skin and has the good affinity, good permeability, easily absorbed by skin, maintain skin nutrition and moisture and elasticity, improve skin corneum layer of degradation, repair rough skin [26,28-32]. Based on literature content, we consider that the components of PURO may be good for skin.
PURO Can Affect the Cell Migration in Hacat
Cell migration is an important process that effects many aspects of health, such as immune response, wound healing, tissue homeostasis and cancer [33,34]. In order to analyze whether the PURO can promote the cell migration, we use in vitro scratch assay on HaCAT cell line. We find that cell migrate faster in 5% PURO and 10% PURO treatment compared with blank treatment in 16 h and 24 h (Figure 2a-i). Then we use image J to calculate the healing area. The heal rate of 5% PURO treatment is 47.8%, 10% PURO treatment is 32.5% and the blank treatment is 16.6% in 16 h (Figure 2j). In 24h, the heal rate of 5% PURO treatment is
86.4%, 10% PURO treatment is 76.3% and the blank treatment is 63.2% (Figure 2j). These mean that the PURO can accelerate the cell migration and may have the function of wound healing and skin barrier.
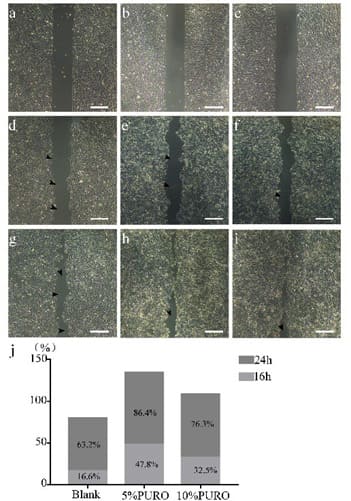
Figure 2: In vitro scratch assay on HaCAT. Figure 2a: The HaCAT cell with PBS as blank on 0h; Figure 2b: The HaCAT cell with 5% PURO diluted by DMSO on 0h; Figure2c: The HaCAT cell with 10% PURO diluted by DMSO on 0h; Figure 2d, 2e, 2f: The HaCAT cell with PBS, 5% PURO and 10% PURO on 16h; Figure 2g, 2h, 2i: The HaCAT cell with PBS, 5% PURO and 10% PURO on 24h; Bar=100 μm. Figure 2j: The healing rate of PBS (blank), 5% PURO and 10% PURO on 16h and 24h.
Treatment Of PURO Reduced The Skin Penetration
As previous results show that PURO may accelerate healing and barrier in HaCAT cell line. We still need know whether PURO may have the function of skin barrier in reconstructed human epiderm model which recapitulating natural features in human skin. Firstly, we need to detect the skin irritation of PURO. The MTT assay suggest that the tissue activity of 10% PURO, 5%PURO formula and the Base are higher than 50%, which are similar to negative control (DPBS treatment) (Figure 3a). The relative expression of IL-1α release on 10% PURO, 5%PURO formula and the Base are similar to negative control (DPBS treatment) (Figure 3b). On the contrary, the relative expression of IL-1α release on positive control (SDS treatment) is up to 15fold (Figure 3b). That means PURO has no skin irritation to skin. Furthermore, we study the skin barrier protection effect of PURO through a skin penetration. Firstly, we use 0.2%SDS and 0.3%SDS to exposure on skin model for 10min and 20min, the tissue activity of 0.2%SDS-10min treatment and 0.2%SDS-20min treatment are higher than 80%; the tissue activity of 0.3%SDS-10min treatment is 60% which can be a damaged skin tissue, also maintains tissue activity (Figure S2a). Then we use 0.3%SDS to pretreat skin model for 10 min as the SDS damaged skin model. We find that the tissue activity of SDS damaged skin which exposure on 5% PURO formula is higher than 1% PURO formula. 1% PURO formula can not improve the tissue activity of SDS damaged skin (Figure S2b). The skin penetration of rhodamine B stain show that the permeability of rhodamine B (red) was reduced by exposure with PURO in the presence of SDS pretreated. Also, reduced in the absence of SDS pretreated (Figure 3c-f). These results suggest that PURO have no skin irritation and contribute to the barrier to Rhodamine B.
Treatment Of PURO Attenuated Irritant-Induced Cytotoxicity And Increase Time Of ET50
The protection of irritant-induced tissue death can be a way to determine the protective effects of skin barrier [35]. The 10% PURO formula was applied to EpiSkin® which co-incubate with 0.2% SDS. Then, a barrier damage, 1% TritonX100 was treated at various time (5 min, 10 min, 20 min, 30 min). As a result, the ET50 of 10%PURO formula treatment is 20.734 min, Base treatment is 16.394 min (Figure 3g). The tissue activity of these PURO formula treatments showed that PURO significantly attenuated TritonX100-induced cell death than Base in EpiSkin® by MTT assay (Figure 3h). The longer the time, the better the barrier. That means PURO formula may remain the skin barrier of these tissues.
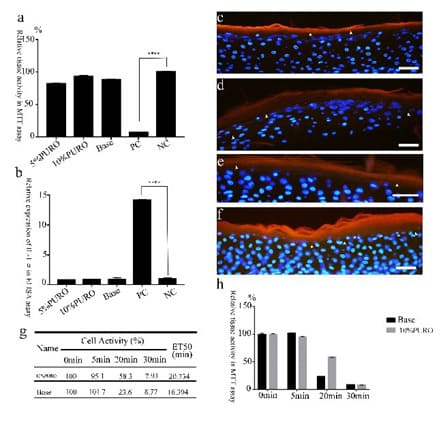
Figure 3: The skin penetration and irritant-induced cytotoxicity with PURO.
Figure 3a: The relative tissue activity of skin model with PURO formula treatment in MTT assay; Figure 3b : The relative expression of release of IL-1α with PURO formula treatment in skin model; Figure 3c and 3d: The Permeability of rhodamine B staining detected after 24h PURO formula and Base treatment of with SDS pretreatment; Figure 3e and 3f: The Permeability of rhodamine B staining evaluated after 24h PURO and Base treatment of with PBS pretreatment. The fluorescent images shown rhodamine B (red), DAPI staining (blue). The Bar= 50μm. Figure 3g: The ET50 data of 10% PURO formula and Base. Different treatment time has different tissue activity. The ET50 data calculate by SPSS. Figure 3h: The MTT assay of 10% PURO and Base with different treatment time.
Treatment Of PURO Increased The Expression Of Skin Barrier Genes On SDS Damaged Skin Model
Filaggrin and loricrin as the keratinized mantle protein have the function on skin barrier [3]. To further analyze the effects of PURO on epidermal differentiation, we detected the immunofluorescence with antibodies against filaggrin (FLG) and loricrin (LOR) on the treated tissues. We use 0.3%SDS to pretreat skin model for 10min as the SDS damaged skin model. Then the 10% PURO, 5% PURO formula and Base are treated on SDS damaged skin model. In the case of FLG, after 24h, there is intensity increase on 10% PURO, 5% PURO formula treatments, but no significant difference in the localization, (Figure 4a-c). In the case of LOR, immunofluorescence intensity increased on 10% PURO, 5% PURO formula treatments compared with Base treatment (Figure 4d-f). To confirm IF data (Figure 4) and investigate whether PURO can promote epidermal barrier repairmen indeed, mRNA levels of FLG and LOR were evaluated using QRT-PCR assay. PURO is topically applied to SDS damaged skin model for 24h and 48h. As a result, 10% PURO, 5% PURO formula treatments significantly increase the expression of FLG and LOR in 24h and 48h (Figure 5a and b). With the extension of incubate time, the expression of FLG and LOR that treated with PURO increase significantly (Figure 5a and b). As we all know, LOR, TJP1, CK10, DSG-1, involucrin (IVL) and CLDN5 are also involved in skin barrier [4,5,12]. The IF data show that the immunofluorescence intensity of CK10 significantly up-regulate after treated with PURO formula (Figure 6a-c). Furthermore, the QRT-PCR analyze shows that CK10, TJP1 and CLDN5 significantly up-regulate after treated with PURO formula (Figure 6d). Caspase-14, calpain 1, and bleomycin hydrolases can degrade filaggrin (FLG) protein into amino acids and amino acid metabolites in the stratum corneum. Those pivotal natural moisturizing factors are amino acids and amino acid metabolites, like rans-urocanic acid and Pyrrolidone Carboxylic Acid [8]. Bleomycin Hydrolase (BLMH) is a well-conserved cysteine protease that are extensively expressed in several mammalian tissues, especially on skin which contains high levels. BLMH take part in the degradation of citrullinated filaggrin monomers into free amino acids [7]. This is important to skin hydration. In atopic dermatitis (AD) patients, the expression and activity of BLMH is reduced [7]. Ceramides (CERs) are important for skin barrier function which belong to epidermal lipids. Deficiency of ceramide synthase 3 (CER3) results in lacking of continuous extracellular lipid lamellae and a non-functional cornified lipid envelope in mice [6]. Transglutaminases (TGMs) are membrane associated enzymes. TGMs have the function of imparting integrity to the stratum corneum by catalyzing e-(g-glutamyl) lysine crosslinking reactions [9]. The QRT-PCR show that involucrin (IVL), caspase-14 (CASP14), Bleomycin hydrolase (BLMH), ceramide synthase 3 (CER3) and Transglutaminases-1 (TGM-1) notably up-regulate in SDS damaged skin models, especially with 10% PURO formula (Figure 6e-g). We consider that high concentration of PURO could promote epidermal differentiation and skin barrier repairmen.
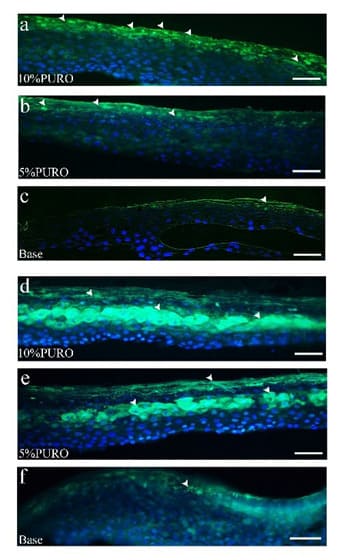
Figure 4: The immunofluorescence staining of FLG and LOR in SDS damaged skin model on 24h.
Figure 4a : The immunofluorescence staining of FLG protein on 10% PURO formula treatment with location and intensity; Figure 4b: The immunofluorescence staining of FLG protein on 5% PURO treatment with location and intensity; Figure 4c: The immunofluorescence staining of FLG protein on Base treatment with location and intensity; Figure 4d, 4e, 4f: The immunofluorescence staining of LOR protein on 10% PURO, 5% PURO formula and Base treatment with location and intensity. The Bar= 50μm.
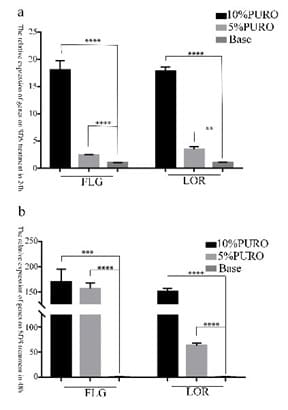
Figure 5: The relative expression of FLG and LOR gene on damaged SDS skin model.
Figure 5a and 5b: Quantitative RT-PCR analysis of FLG and LOR expression on damaged SDS skin model treated with 10% PURO, 5% PURO formula and Base formula on 24h and 48h. The levels of FLG and LOR were normalized to those of β-Acting and compared with those of Base. Error bars indicate SD and were calculated from three biological replicates.
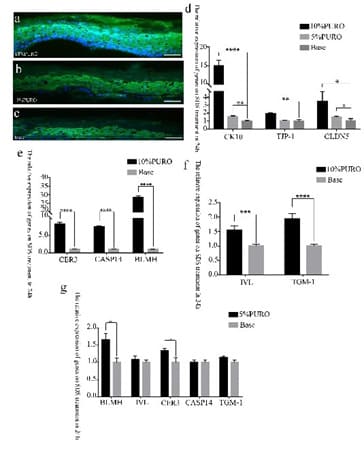
Figure 6: The Immunofluorescence and Quantitative RT-PCR analysis of CK10, TJP1 and CLDN5 on damaged skin model.
Figure 6a, 6b, 6c: The immunofluorescence staining of CK10 protein on 10% PURO, 5% PURO and Base treatment with location and intensity. The Bar= 50μm. Figure 6d: Quantitative RT-PCR analysis of CK10, TJP1 and CLDN5 in SDS damaged skin model with 10% PURO, 5% PURO formula and Base treatment on 24h. Figure 6e: Quantitative RT-PCR analysis of CER3, CASP14 and BLMH in SDS damaged skin model with 10% PURO formula and Base treatment on 24h. Figure 6f: Quantitative RT-PCR analysis of IVL and TGM-1 in SDS damaged skin model with 10% PURO formula and Base treatment on 24h. Figure 6g: Quantitative RTPCR analysis of CER3, CASP14, IVL, TGM-1 and BLMH in SDS damaged skin model with 5% PURO formula and Base treatment on 24h. The levels of CK10, TJP1, CER3, CASP14, IVL, TGM1, BLMH and CLDN5 were normalized to those of β-Acting and compared with those of Base.
PURO Increases The Expression Of Normal Skin Barrier Genes
As previous results, we suggest that PURO may promote the skin barrier on damaged skin. We still need know that whether PURO may promote the skin barrier on normal skin model. The IF data also show that intensity of LOR protein significance increase after treated with 10% PURO formula on normal skin model at 24h (Figure 7b and c). The QRT-PCR analyze show that intensity of IVL protein significance increase after treated with 5% PURO formula on normal skin model at 24h (Figure 7e). The 10% PURO can significantly increase the expression of FLG, IVL, CER3 and LOR (Figure 7d-e). That means PURO can enhance the expression of keratinized mantle protein and may increase skin barrier.
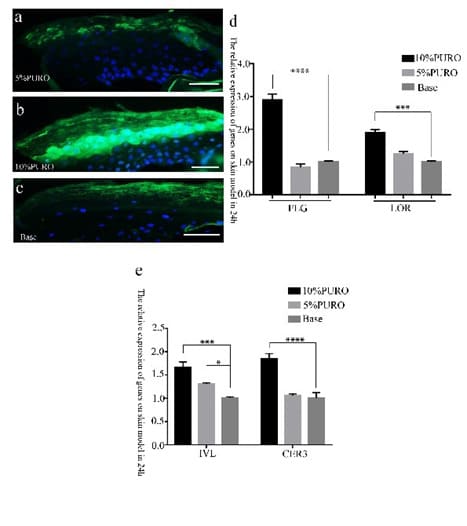
Figure 7: The Immunofluorescence and Quantitative RT-PCR analysis of LOR on normal skin model.
Figure 7a: The Immunofluorescence staining of LOR on normal skin model with 5% PURO formula treatment on 24h; Figure 7b: The Immunofluorescence staining of LOR on normal skin model with 10% PURO formula treatment on 24h; Figure 7c: The Immunofluorescence staining of LOR on normal skin model with Base treatment on 24h; The Bar= 50 μm. Figure 7d: Quantitative RT-PCR analysis of LOR and FLG in normal skin model with 10%PURO, 5% PURO formula and Base treatment on 24h. Figure 7e: Quantitative RT-PCR analysis of IVL and CER3 in normal skin model with 10%PURO, 5% PURO formula and Base treatment on 24h. The levels of FLG, LOR, IVL and CER3 was normalized to those of β-Acting and compared with those of Base.
Treatment Of PURO Reduced The Expression Of AntiInflammatory Genes On Skin Models
As the previous results, we can confirm that PURO have the function on repairing the damaged skin and increasing skin barrier. As we all know, the dysfunctional skin barrier, typically Atopic Dermatitis (AD) also causes an inflammatory response in the skin [36]. Furthermore, we want to explore whether PURO have function on anti-inflammatory. When the keratinocyte (KC) is damaged, it will release IL-1, which essentially is a primary event in skin defense. IL-1 also stimulates the production and release of IL-6, IL-8 and GM-CSF from the neighboring KC [37]. UV irradiation is a potent inducer of cyclooxygenase (COX)2 enzyme and produces high levels of PGE2 in the skin [38]. Here, we use QRT assay to detect the expression of IL-1α and IL-8. We find that PURO have no effect to IL-1α and IL-8 on normal skin model (Figure S2d). This suggest that PURO cannot cause inflammation on skin model which may imply its safety. The QRTPCR analyze show that the relative expression of IL-1α and IL-8 are significant down-regulate after treated with high concentration of PURO on SDS-damaged skin model (Figure 8a). Then, we use UVB to induce and damage the skin model and detect the PGE2 release. The result shows that both 5% PURO and 10% PURO significantly inhibited the release of PGE2 on UVB-induced skin model (Figure 8b). That means PURO may have the function on anti-inflammatory in skin model.
Discussion
An important function of the epidermis is the barrier function. The stratum corneum protects the viable cell layers beneath and the whole body by avoiding being harmed by virus, microorganism and environment [2,39,40]. Loricrin, filaggrin and involucrin are major component of the cornified envelope and have effect on the formation of the cornified envelope. These play a key role in the skin barrier function [41]. In the stratum granulosum, typical TJ (Tight Junction) structures are major processes on the preparation of the stratum corneum. Here, we analyze the composition and content of PURO (Figure 1 and S1). The palmitic acid, oleic acid and linoleic acid and free fatty acids are important for lipid formation and skin barrier [27,28,42]. oleic acid may have irritation for skin [27]. However, in our study, the PURO are good for skin barrier. It may be due to the synergistic effect of different proportion of fatty acids and free fatty acids in the PURO.
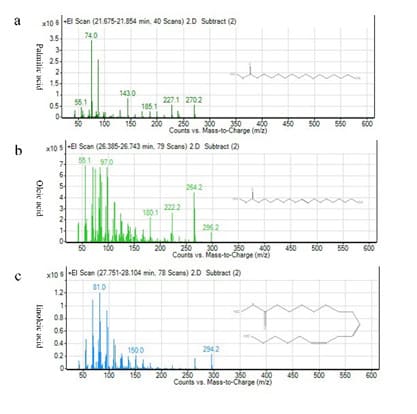
Figure S1: The molecular formula and mass-to-charge of palmitic acid, oleic acid and linoleic acid.
Figure S1a : The molecular formula and mass-to-charge of palmitic acid; Figure S1b: The molecular formula and mass-to-charge of oleic acid; Figure S1c: The molecular formula and mass-to-charge of linoleic acid.
We demonstrate that PURO can improve the cell healing rate in scratch-wound which means PURO may promote the cell migration (Figure 2). We also find that at 16 hours, the 5% PURO-treated group showed higher cell migration capacity than the 10% PURO-treated group (Figure 2j). The composition of PURO is fatty acid. We consider that the concentration of PURO may be too high, which had a certain effect on cell growth. PURO can enhance the expression of FLG, CK10, TJP1, CLDN5, IVL, BLMH, CASP14, TGM-1, CER3 and LOR on damaged skin model (Figure 4-6) which may demonstrate that PURO have the function of repairing skin barrier. Furthermore, PURO can also accelerate the skin barrier function by reducing skin permeability and increasing the expression of LOR, IVL and FLG (Figure 3 and Figure 7). Meanwhile, the expression of AQP3 and DSG1 have no significant difference on damaged and normal skin model after treated with PURO formula (Figure S2 c). We also find that 5% PURO does not up-regulate a number of skin barrier genes (Figure 6 and 7). We think that may low concentration of PURO not as effective as high concentrations for repairing the skin barrier. We consider that PURO may improve the skin barrier by enhance the expression of keratinized mantle protein and tight function protein family. This may be important to explore the function of PURO.
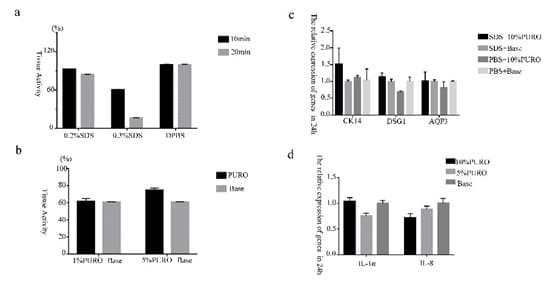
Figure S2: The relative expression of genes on damaged and normal skin model.
Figure S2a : The tissue activity of skin model that treated with 0.2% SDS 10min and 20min,0.3%SDS 10min and 20min. Figure S2b : The tissue activity of skin model that pretreated with 0.3%SDS 10min, the exposure with 1%PURO formula and 5% PURO formula.Figure S2c: Quantitative RT-PCR analysis of CK14, DSG1 and AQP3 in damaged and normal skin model treated with PURO formula on 24h; Figure S2d: Quantitative RTPCR analysis of IL-1α and IL-8 in normal skin model. The levels of CK14, DSG1, AQP3, IL-1α and IL-8 were normalized to those of β-Acting and compared with those of Base.
Here, we find that 10% PURO formula can improve the expression of LOR (Figure 7a and b). 5% PURO formula can improve the expression of IVL on normal skin model; the 10% PURO formula can rapidly improve the expression of LOR, IVL, FLG and CER3 on normal skin model (Figure 7). That means high concentration of PURO possibly raise the barrier, low concentration of PURO not as effective as high concentrations for repairing the skin barrier. For SDS-damaged skin model, both 5% PURO formula and 10% PURO formula can repair the skin barrier by improving the expression of FLG and LOR (Figure 4 and Figure 5), 10% PURO formula reducing skin permeability and lengthening the ET50 time (Figure 3). That means PURO can be used on people with sensitive skin to repair the skin barrier.
Here, we demonstrate that PURO have the function in skin barrier. We still find that PURO may have the function on antiinflammatory effect. When the keratinocyte (KC) is damaged, it will release IL-1, which essentially is a primary event in skin defense. Furthermore, the response amply via IL-1 stimulating the production and release of IL-1 from neighboring KC [37]. IL-8 is strong neutrophil chemokines. When the skin is affected by allergen and stimulator, it can be produced by KC. High expression can be found in psoriasis, pemphigus, herpetiformis pemphigus, large autoimmune diseases such as pemphigoid and squamous cells tumor cells such as carcinoma [43]. UVBinduced inflammation is characterized by dramatic increases in prostaglandin E2 (PGE2) synthesis due to enhanced arachidonate deacylation from the membrane on skin [44]. The QRT-PCR analyze and ELISA assay show that the relative expression of IL1α and IL-8 are significant down-regulate after treated with high concentration of PURO on damaged skin model, as well as PGE2 (Figure 8). These may suggest that high concentration of PURO may have the anti-inflammatory function. All results point to the important function of PURO on epiderm, including skin barrier and anti-inflammatory.
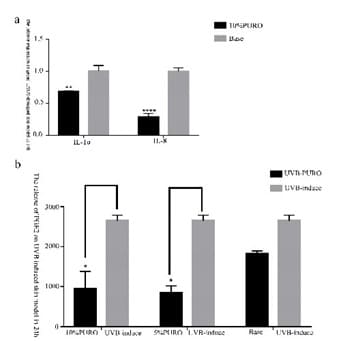
Figure 8: The expression of inflammatory cytokines. Figure
8a : The quantitative RT-PCR analysis of IL-1α and IL-8 in SDS-damaged skin model. The levels of IL-1α and IL-8 were normalized to those of β-Acting and compared with those of Base. Figure 8b: The release of PGE2 with PURO formula treatment on UVB-induced skin model.
Because of its important role in strengthening and repairing the skin barrier, PURO can be applied to cosmetic and adjunctive therapy in dermatology in the future. We still need to explore the function of PURO on skin barrier thoroughly. Using emollients with Prinsepia utilis Royle during the maintenance period of infantile AD can significantly reduce the risk of AD flares via clinical test [45]. Furthermore, we can study the effect of PURO on other skin barrier genes, the interactions of genes and proteins, finding the target gene of the barrier function pathway affected by PURO. However, high concentration of PURO plays a certain role in inhibiting inflammatory factors, which provides an idea for further research on other functions of PURO in skin diseases. Meanwhile, Prinsepia utilis Royle also have the function on antioxidant activity which content monounsaturated fatty acid and polyunsaturated fatty acid [46]. All of these demonstrate that PURO play an important role on skin healthy, especially in skin barrier and AD patients. PURO can be useful for nutraceuticals and cosmeceuticals field.
Author Contribution
All authors agree with the manuscript content. Bo Wang and Feifei Wang led study design. Bo Wang and Han Gao performed experiments and data analysis. Bo Wang prepared the manuscript and data analysis. Feifei Wang provided suggestions and modified the manuscript.
Funding Information
This study was supported by the Kunming Major Science and Technology Project (2019-1-N-25318000003083).
Data Availability
The data used to support the findings of this study are included within the article.
Ethics Approval
We sincerely thank the L’Oréal Research and Innovation Center (Shanghai, China) for their relevant technical assistance on the EpiSkin® kits and National Infrastructure of Cell Line Resource for HaCAT cell line.
Reference
- Chau DYS, Johnson C, MacNeil S, Haycock JW, Ghaemmaghami AM (2013) The development of a 3D immunocompetent model of human Biofabrication. 5: 035011.
- Proksch E, Brandner JM, Jensen JM (2008) The skin: an indispensable Exp Dermatol 17: 1063-1072.
- Kalinin A, Marekov LN, Steinert PM (2001) Assembly of the epidermal cornified cell envelope. J Cell Sci 114: 3069-3070.
- Kim S, Kim GH (2017) Roles of claudin-2, ZO-1 and occludin in leaky HK-2 cells. PLoS One 12: e0189221.
- Draelos ZD (2012) New treatments for restoring impaired epidermal barrier permeability: skin barrier repair creams. Clin Dermatol 30: 345
- Jennemann R, Rabionet M, Gorgas K, Epstein S, Dalpke A, et (2012) Loss of ceramide synthase 3 causes lethal skin barrier disruption. Hum Mol Genet 21: 586-608.
- Riise R, Odqvist L, Mattsson J , Monkley S, Abdillahi SM, et al. (2019) Bleomycin hydrolase regulates the release of chemokines important for inflammation and wound healing by keratinocytes. Sci Rep 9:
- Kim Y, Lim KM (2021) Skin barrier dysfunction and filaggrin. Arch Pharm Res 44: 36-48.
- Lee JO, Hwang SH, Shen T, Kim JH, You L, et al. (2021) Enhancement of skin barrier and hydration-related molecules by protopanaxatriol in human keratinocytes. J Ginseng Res 45: 354-360.
- Volksdorf T, Heilmann J, Eming SA, Schawjinski K, Zorn-Kruppa M, et al. (2017) Tight Junction Proteins Claudin-1 and Occludin Are Important for Cutaneous Wound Healing. Am J Pathol 187: 1301-1312.
- Basler K, Brandner JM (2017) Tight junctions in skin inflammation. Pflugers Arch 469: 3-14.
- Yang L, Lyu L, Wu W, Lei D, Tu Y, et al. (2017) Genome-wide identification of long non-coding RNA and mRNA profiling using RNA sequencing in subjects with sensitive skin. Oncotarget 8: 114894
- Vavrova K, Henkes D, Struver K, Sochorova M, Skolova B, et al. (2014) Filaggrin deficiency leads to impaired lipid profile and altered acidification pathways in a 3D skin construct. J Invest Dermatol 134: 746-753.
- Koch PJ, de Viragh PA, Scharer E, Bundman D, Longley MA, et al. (2000) Lessons from loricrin-deficient mice: compensatory mechanisms maintaining skin barrier function in the absence of a major cornified envelope protein. J Cell Biol 151: 389-400.
- Muller FB, Huber M, Kinaciyan T, Hausser I, Schaffrath C, et al. (2006) A human keratin 10 knockout causes recessive epidermolytic Hum Mol Genet 15: 1133-1141.
- Vodo D, Sarig O, Peled A, Samuelov L, Malchin N, et al. (2018) Recessive epidermolytic ichthyosis results from loss of keratin 10 expression, regardless of the mutation location. Clin Exp Dermatol 43: 187-190.
- Denecker G, Hoste E, Gilbert B, Hochepied T, Ovaere P, et al. (2007) Caspase-14 protects against epidermal UVB photodamage and water Nat Cell Biol 9: 666-674.
- Guan B, Peng CC, Zeng Q, Cheng XR, Yan SK, et al. (2013) Cytotoxic pentacyclic triterpenoids from Prinsepia utilis. Planta Med 79: 365-368.
- Chen G, Fuying L, Ying G, Xuheng N (2020) Study on the physicochemical properties of Prinsepia utilis Royle and its oil, and the fatty acid composition of its oil. Journal of Cereals & Oils. 33: 69-71.
- Drake DR, Brogden KA, Dawson DV, Wertz PW (2008) Thematic review series: skin lipids. Antimicrobial lipids at the skin surface. J Lipid Res 49: 4-11.
- Nakatsuji T, Kao MC, Zhang L, Zouboulis CC, Gallo RL, et al. (2010) Sebum free fatty acids enhance the innate immune defense of human sebocytes by upregulating beta-defensin-2 expression. J Invest Dermatol 130: 985-994.
- Jensen JM, Proksch E (2009) The skin’s barrier. G Ital Dermatol Venereol 144: 689-700.
- Hanittinan O, Oo Y, Chaotham C, Rattanapisit K, Shanmugaraj B, et al. (2020) Expression optimization, purification and in vitro characterization of human epidermal growth factor produced in Nicotiana benthamiana. Biotechnol Rep (Amst) 28: e00524.
- Ma X, Wang F, Wang B (2021) Application of an in vitro reconstructed human skin on cosmetics in skin irritation tests. J Cosmet Dermatol 20: 1933-1941.
- Nounou MI, Zaghloul TI, Ahmed NA, Eid AA, El-Khordagui LK (2017) Skin permeability enhancement by Bacillus subtilis alkaline protease: Application to transdermal drug delivery. Int J Pharm 529: 423-432.
- Ni Raghallaigh S, Bender K, Lacey N, Brennan L, Powell FC (2012) The fatty acid profile of the skin surface lipid layer in papulopustular Br J Dermatol 166: 279-287.
- Vaughn AR, Clark AK, Sivamani RK, Shi VY (2018) Natural Oils for Skin-Barrier Repair: Ancient Compounds Now Backed by Modern Am J Clin Dermatol 19: 103-117.
- Mieremet A, Helder R, Nadaban A, Gooris G, Boiten W, et al. (2019) Contribution of Palmitic Acid to Epidermal Morphogenesis and Lipid Barrier Formation in Human Skin Equivalents. Int J Mol Sci 20.
- Kawamura A, Ooyama K, Kojima K et al. (2011) Dietary supplementation of gamma-linolenic acid improves skin parameters in subjects with dry skin and mild atopic dermatitis. J Oleo Sci 60: 597-607.
- Simard M, Julien P, Fradette J, Pouliot R (2019) Modulation of the Lipid Profile of Reconstructed Skin Substitutes after Essential Fatty Acid Supplementation Affects Testosterone Permeability. Cells. 8:
- Simard M, Tremblay A, Morin S, Martin C, Julien P, et al. (2022) alphaLinolenic acid and linoleic acid modulate the lipidome and the skin barrier of a tissue-engineered skin model. Acta Biomater 140: 261
- Vinogradov AI, Balabolkin I I, Vinogradova NA (1991) [Eicosanoic acid levels in children with atopic dermatitis]. Pediatriia. 10: 29-32.
- Vang Mouritzen M, Jenssen H (2018) Optimized Scratch Assay for In Vitro Testing of Cell Migration with an Automated Optical Camera. J Vis Exp 138: 57691.
- Trepat X, Chen Z, Jacobson K (2012) Cell migration. Compr Physiol 2: 2369-2392.
- zur Muhlen A, Klotz A, Weimans S, Veeger M, Thorner B, et al. (2004) Using skin models to assess the effects of a protection cream on skin barrier function. Skin Pharmacol Physiol 17: 167-175.
- Mottin VHM, Suyenaga ES (2018) An approach on the potential use of probiotics in the treatment of skin conditions: acne and atopic Int J Dermatol 57: 1425-1432.
- McKenzie RC, Sauder DN (1990) The role of keratinocyte cytokines in inflammation and immunity. J Invest Dermatol 95: 105S-107S.
- Rundhaug JE, Fischer SM (2008) Cyclo-oxygenase-2 plays a critical role in UV-induced skin carcinogenesis. Photochem Photobiol 84: 322
- Johnson AM (1990) The speed of mental rotation as a function of problem-solving strategies. Percept Mot Skills 71: 803-806.
- Brandner JM, Schulzke JD (2015) Hereditary barrier-related diseases involving the tight junction: lessons from skin and intestine. Cell Tissue Res 360: 723-748.
- Jung YO, Jeong H, Cho Y et al. (2019) Lysates of a Probiotic, Lactobacillus rhamnosus, Can Improve Skin Barrier Function in a Reconstructed Human Epidermis Model. Int J Mol Sci 20.
- Ohnari H, Sekiya M, Naru E, Ogura T, Sakata O, et al. (2021) Amino Acids and Their N-Acetylated Derivatives Maintain the Skin’s Barrier Chem Pharm Bull (Tokyo) 69: 652-660.
- Schmidt E, Reimer S, Kruse N, Jainta S, Brocker EB, et al. (2000) Autoantibodies to BP180 associated with bullous pemphigoid release interleukin-6 and interleukin-8 from cultured human keratinocytes. J Invest Dermatol 115: 842-848.
- Gresham A, Masferrer J, Chen X, Leal-Khouri S, Pentland AP (1996) Increased synthesis of high-molecular-weight cPLA2 mediates early UV-induced PGE2 in human skin. Am J Physiol 270: C1037-1050.
- S Wang, L Wang, P Li, Shu H, Shen C, et al. (2020) The improvement of infantile atopic dermatitis during the maintenance period: A multicenter, randomized, parallel controlled clinical study of emollients in Prinsepia utilis Royle. Dermatol Ther 33: e13153.
- P Kewlani, DC Tewari, L Singh, VS Negi, ID Bhatt, et al. (2022) Saturated and Polyunsaturated Fatty Acids Rich Populations of Prinsepia utilis Royle in Western Himalaya. J Oleo Sci 71: 481-491.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.