Pien Tze Huang, a 500-Year-Old Traditional Composite Medicine in China for Liver Ailments Alleviates Some Effects of the Alcoholic Liver in a Mouse Model
by Juan Yu1, Gigi C. T. Leung2,3, Zhiliang Chen1*, Sharon L.Y. Wu2,3*, David T. Yew2,3*
1Fujian Provincial Key Laboratory of Pien Tze Huang Natural Medicine Research and Development, Zhangzhou Pien Tze Huang Pharmaceutical Co., Ltd. Fujian 363000, People's Republic of China
2Hong Kong College of Technology, Hong Kong, People's Republic of China
3School of Chinese Medicine, The Chinese University of Hong Kong, Hong Kong, People's Republic of China
*Corresponding authors: David Tai Wai Yew and Sharon L.Y. Wu, School of Chinese Medicine, The Chinese University of Hong Kong, Hong Kong, People's Republic of China.
Zhiliang Chen, Fujian Provincial Key Laboratory of Pien Tze Huang Natural Medicine Research and Development, Zhangzhou Pien Tze Huang Pharmaceutical Co., Ltd. Fujian 363000, People's Republic of China.
Received Date: 03 August 2024
Accepted Date: 09 August 2024
Published Date: 16 August 2024
Citation: Yu J, Leung GCT, Chen Z, Wu SLY, Yew DT (2024) Pien Tze Huang, a 500 Year Old Traditional Composite Medicine in China for Liver Ailments Alleviates Some Effects of the Alcoholic Liver in a Mouse Model. Curr Res Cmpl Alt Med 8: 250. https://doi.org/10.29011/2577-2201.100250
Abstract
The liver reacted to alcohol intoxication in several stages, from fatty degeneration, inflammation, cell death to finally fibrosis. Pien Tze Huang (PTH) is a composite Chinese medicine that has been used for liver ailments until this day. Despite many reported its beneficial effects on various diseases (e.g., cancers, ischemic brain and encephalitis), there are few studies on whether PTH can attenuate alcohol induced liver ailments as an alternative therapy. Therefore, this study aims to investigate if PTH possesseshepatoprotective effect against alcoholism. ICR mice were divided into four groups. Alcohol group was treated with alcohol. PTH pretreatment group was treated with PTH before alcohol treatment. Rotation treatment group received PTH and alcohol treatment alternately. Control group was treated with saline. Liver histopathology was observed via hematoxylin and eosin stain and Sirius red stain. Proliferation and necrosis in liver tissue were demonstrated by immunohistochemistry (IHC) targeting proliferating cell nuclear antigen (PCNA) and lactate dehydrogenase (LDH) respectively. Apoptosis was evaluated by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL). Tumor necrosis factor alpha (TNFα) and interleukin 4 (IL-4) in liver tissue were measured by enzyme-linked immunosorbent assay (ELISA). In histopathology, both PTH pretreatment and rotational treatment prevented fibrosis. When compared with alcohol group, both PTH treatments decreased LDH-positive cells. PTH pretreatment could further increase PCNA-positive cells and decrease positive cells in TUNEL. In ELISA, both PTH treatments decreased liver TNFα while PTH pretreatment increased liver IL-4. From our results, PTH could alleviate the alcohol effects as a complementary treatment.
Keywords: Pien Tze Huang; Chinese medicine; Alcohol; Liver; Hepatoprotective
Introduction
Alcohol intoxication can be classified into chronic or acute drinking. Chronic drinking usually refers to a continuous pattern of drinking almost every day while acute drinking refers to drinking large amounts of alcohol abruptlyon certain days. In essence, this kind of binge drinking (latter mode) is common in Asian countries where it could happen two or three times per week, usually after hard work. It is difficult to define the limit of alcohol pathology for different countries as each has its own mode of drinking. For example, in colder regions, people drink more extensively and frequently. For acute binge drinking, more than five servings with 1.5oz of hard liquor a serving or 5 oz of wine a serving were considered by many as the limit [1]. Today, there are many different animal models of research in alcohol [2]. The liver reacts to alcohol intoxication in several stages, 1) fatty degeneration of the liver, 2) inflammation of the liver cells, 3) cell death in the liver, either necrotic or apoptotic, and finally 4) fibrosis of the liver cells. There is no clear distinction between stages in some models and one stage may mingle with another [3].
Fatty degeneration could present itself after other kinds of insults in the liver (e.g. ketamine or carbon tetrachloride [4] and thus it was not unique for alcohol. Cohen and Inaba (2014) pointed out that alcohol intoxication facilitated the building up of fat from glucose in liver cells. In alcohol intoxication, the degradation of alcohol via alcohol dehydrogenase forms a toxic intermediate acetaldehyde and then acetic acid, while acetic acid is further degraded to water and carbon dioxide. During this alcoholic catabolism, a lot of cofactors e.g.nicotinamide adenine dinucleotide phosphate (NADPH) were produced [5,6]. On the other hand, glucose in the liver cells tended to be converted into glycogen and some to fat. The fat conversion process needed to go through the stage of acetyl coenzyme A (acetyl CoA) and while in this procedure, a large amount of NADPH facilitated acetyl CoA formation. Finally, fat got into the cell by citrate reaction. This appeared to be a likely mechanism of fat accumulation in the liver after alcohol consumption. In the liver, there should be a delicate balance between production of glycogen and production of triglyceride and cholesterol ester to ensure the absence of massive fat deposit unless there was an extraordinary amount of fat intake. At this point, one would speculate that it was alcohol or other toxins that upset the balance between glycogen versus fat deposit. For example, downregulation of the action of glycogen synthase limited the manufacturing of glycogen and thus tilted the balance to fat production. Along with the massive fatty production, the liver parenchymal cells bearing these macro and micro fat molecules became swollen and lost the diamond architecture of the lobules. Such squeeze also put much stress on the cytoskeleton of the cells and affected the health of Golgi apparatus and mitochondria. At this point of injury, the inflammatory cells came into the liver and these were leukocytes, lymphocytes and macrophages. Along with these pathological episodes, the degenerating liver cells were now ready to move towards cell death. Some considered that in cell death, the epigenetic factors also played a role [7]. But what happened before the alcohol entered the liver? The current hypothesis was that the ingested alcohol broke down the barrier of the basement membrane of the intestine and the existing bacterial or viral toxin, in addition to the fact that alcohol and its toxic metabolites travelled in the portal system reaching the liver. The vascular barrier was then disrupted and the toxins gained entry into the liver. Vascular involvement of blood vessels in alcoholics had also been suggested earlier [8,9]. Subsequent cell deaths in the liver included those of necrosis and apoptosis [10]. With cell death, fibrosis became apparent and led to cirrhosis. No widely accepted treatment was advocated for alcoholic fibrosis or cirrhosis. At times corticosteroid had been used to slow down the disease progress [11].
Pien Tze Huang (PTH) is an herbal mixture well known in China for over 500 years, initially formulated in the Ming Dynasty when a monk who had been a court physician of the late Ming Emperor, toured southern China and found people dying of hepatitis and related liver diseases. He then decided on a mixture of several herbal and animal components which together formed a formula with the purpose of hepatoprotection and promoting liver health. Such mixture is known as Pien Tze Huang and has been used in liver ailments until this day. The mixture has four principal components, namely cow bile stone, snake gall, musk and Panax notoginseng (Burkill) F.H.Chen [Araliaceae]. Experimentally there has been no toxicity found with this formula [4]. Many papers appeared in recent years suggesting beneficial effects of PTH on hepatoma [4], colorectal cancer [12-14], and ovarian cancer [15], as well as on the ischemic brain [16] and blood vessel formation [10,13,17]. A recent paper by Qiu et al on PTH stated PTH would affect immune mechanism in a model of experimental encephalitis [18]. The pattern of PTH in high-performance liquid chromatography (HPLC) had been worked out and there were prominent peaks of 2.043, 2.995, 3.717, 6.325, 15.709 at 204nm [4]. However, there had been little study on whether PTH could affect the alcohol induced liver ailments as an alternative therapy. The alcoholic constituent in drinks is ethanol with concentration varies from 5% in beer to more than 40% in liquor and this study focused on this class of alcohol. Amouse model combining chronic and acute ethanol feeding was documented to demonstrate histological and molecular features in liver which mimics acute-on-chronic alcoholic liver injury in patients [19-21]. In this study, the chronic and binge ethanol feeding model was employed to investigate if the formulae of PTH could indeed exert hepatoprotective effect on alcoholism
Materials and Method
Experimental animals
All animal experiments were approved by the Research Ethics Review Panel for Animal Experiments of the institute. Twelve-week-old male ICR mice weighing 35-40g were kept in cages with water and liquid diet providedad libitum, in a room kept at 22 +/- 2 °C with 12:12 light dark cycles.
Alcohol treatments
For alcohol intoxication treatment, the chronic feeding of Lieber-DeCarliliquid diet (F1258SP, BioServ, Flemington, USA) with 5% (w/v) ethanol and the multiple ethanol binges (5g/kg) were employed, and they werereported to demonstrate histological and molecular features of alcoholic diseases [20-22]. During the non-alcohol treatment period, animals were fed on Lieber-DeCarli liquid diet (F1259SP, BioServ, Flemington, USA)without alcohol.
Twenty-fivemice were divided into five groups: NORM group (n=5), PCTRL group (n=5), PRE group (n=5), RCTRL group (n=5), and ROTgroup (n=5). PRE group received PTH pretreatment before alcohol treatment. PCTRL group was the control of PRE group and received control saline pretreatment before alcohol treatment. ROT group received PTH and alcohol rotation treatment.RCTRL group was the control of ROT group and received control saline and alcohol rotation treatment. NORM group was thenormal non-alcohol treated group. PCTRL group was pre-treated with normal saline orally for 24 days and followed by 60 days of alcohol intoxication during which the animals were voluntarily fed on ethanol liquid diet as the only energy source [23]. On average, each mouse consumed about 21.25g ethanol/kg/day ethanol through the liquid diet from our previous study [24]. PRE group was pre-treated with Pien Tze Huang (Zhangzhou Pien Tze Huang, Zhangzhou, China) orally (0.37g/kg, equivalent to the dose recommended for human adult) for 24 days and then intoxicated by alcohol with the same dosages and period as in PCTRL group. Pien Tze Huang was manufactured and provided by Zhangzhou Pien Tze Huang Pharmaceutical Co.,Ltd. Treatment schedule for ROT group was that in every week there were five days of alcohol treatment followed by two days of PTH on a rotational basis, for a total of 12 week. Therefore the total days of alcohol treatment in ROT group was also 60 days and PTH treatment was also 24 days. RCTRL received same rotation treatment of ROT with PTH replaced by saline.NORM group was fed with liquid diet without alcohol. All PCTRL, PRE, RCTRL and ROT group received an additional ethanol binge orally (5g/kg) after every five days of alcohol treatment. NORM group received a normal saline treatment orally only. After 84-day treatment, all animals were sacrificed and livers were excised. Part of the liver tissues were fixed in 10% phosphate buffered formalin for histological studies and part of the tissues were snap frozen in liquid nitrogen and then stored at -80°C for enzyme-linked immunosorbent assay (ELISA).
Histological studies
The formalin-fixed tissues were processed, embedded and sectioned for hematoxylin and eosin (H&E) stain, sirius red stain, terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay, and immunohistochemistry (IHC) targeting on proliferating cell nuclear antigen (PCNA) and lactate dehydrogenase (LDH). The methods were describedin the previous publication [24]. In IHC, rabbit anti-PCNA antibody (PLA0079, Merck, Darmstadt, Germany) or rabbit anti-LDH antibody (ab52488, Abcam, Cambridge, UK) was used as the primary antibody depending on the target, and the secondary antibody was biotinylated goat anti-rabbit antibody (ab6720, Abcam, Cambridge, UK). To quantify the results of IHC and TUNEL, positive cells were counted manually under bright-field microscope at a magnification of 400X. Three optical fields from each of the three animals from each group and nine optical fields in total were counted.
Enzyme-linked immunosorbent assay (ELISA)
The tissue homogenate for ELISA were prepared as previously described [24], Tumor necrosis factor alpha (TNFα) and interleukin-4 (IL-4) content in liver were measured by TNFα (500850, Cayman Chemical, Ann Arbor, USA) and IL-4 (ab100710, Abcam, Cambridge, UK) ELISA kits respectively.
Statistical analyses
Statistical analyses were performed with the GraphPad Prism7.0 (GraphPad Software Inc., USA). The differences between groups werecompared by one-way analysis of the variance (ANOVA), and followed by a post hoc Tukey test.The differences between groups with a p value less than 0.05 (p < 0.05) were considered to be statistically significant. The results were presented as means and standard derivations.
Results
Histology confirmed the appearance of some macrophages and a few leukocytes a month after alcohol ingestion, along with fatty degeneration of hepatocytes (Fig. 1A, B and C) in the model. Macrophages were seen in the liver parenchyma and even invaded from the omentum (Fig 1C). Starting from two months of alcoholic ingestion, fibrosis began to be apparent (Fig. 2A, 3) and PTH treatment of either pretreatment (PRE group) or rotational treatment (ROT group) prevented fibrosis (Fig. 2B, 3). Representation images of different groups from different histological techniques were shown in figure 3.
PCNA is a marker of cell proliferation. Treatment with alcohol (PCTRL and RCTRL group) increased the number of PCNA-positive cells in IHC and thus the proliferation of the liver while pretreatment of PTH before alcohol (PRE group) further promoted proliferation significantly, while proliferation after PTH and alcohol rotation treatment (ROT group) was slightly higher than the corresponding control (RCTRL) (Fig. 3, 4A).
TUNEL positive cell density counts revealed that these apoptotic cells increased after sole alcohol intoxication in the liver (PCTRL and RCTRL group) versus the control, but went down significantly after pretreatment of PTH (PRE group) and slightly in the PTH and alcohol rotation treatment group(ROT group) (Fig. 3, 4B).
For necrosis, sole alcoholic treatment increased necrotic cells (PCTRL and RCTRL), while both PTH pretreatment (PRE group) and rotational PTH treatment (ROT group) decreased the number of necrotic cells (Fig. 3, 4C).
TNFα ELISA (Fig. 4D) demonstrated higher values in all alcohol treated groups than the normal mice (NORM group). Particularly, the PCTRL group with 24 days saline plus 60 days alcohol treatment had the highest amount of TNFα. Both PRE group with 24 day PTH pre-treatment plus 60 day alcohol treatment and the ROT group with PTH and alcohol rotation treatment had lower quantities of TNFα.
IL-4 ELISA (Fig. 4E) showed that the PRE group and ROT group had higher levels of IL-4 than their corresponding control groups (PCTRL and RCTRL group).
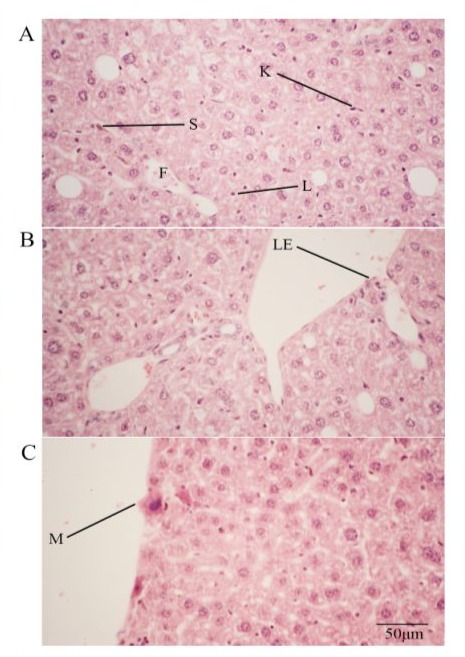
Figure 1: (A-C) H&E stained PCTRL liver sections showing infiltration of lymphocyte (L), macrophage (M) and leukocyte (LE) into alcoholic liver. Note also fatty degeneration (F). S denotes stellate cell while K denotes Kupffer cell. 400X.
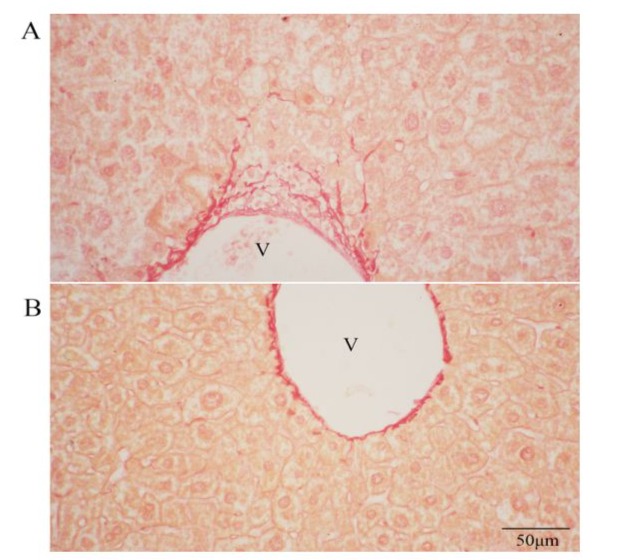
Figure 2: (A) Sirius red stained PCTRL liver sections. (B) Sirius red stained PRE liver sections. Note many fibers at the vicinity of the portal vein (V). 400X.
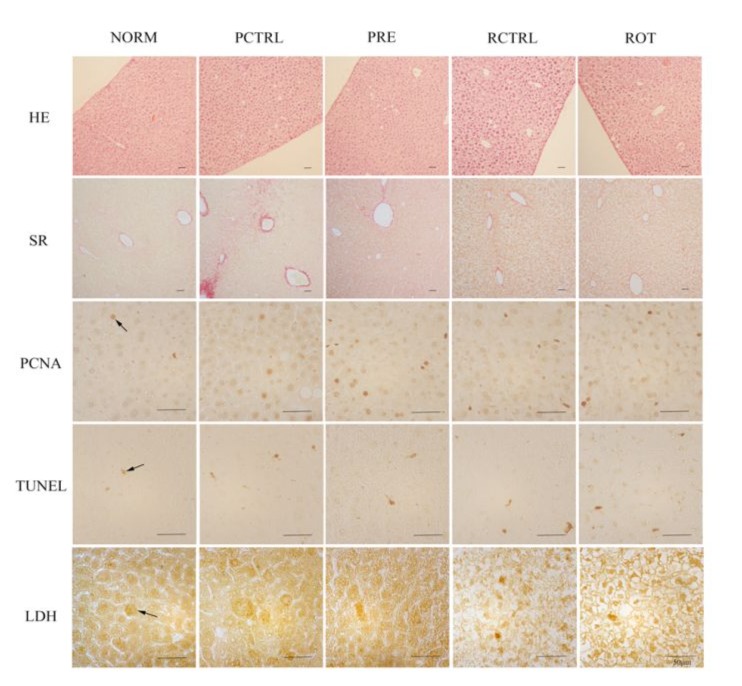
Figure 3: Representative images of different groups from H&E stain, Sirius red stain, PCNA IHC, TUNEL and LDH IHC. Arrowed are some immunoreactive cells.
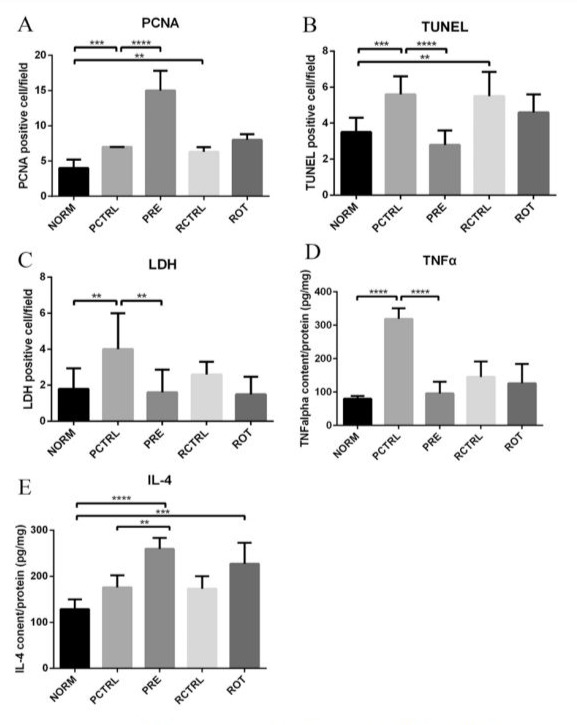
Figure 4: Graphs show quantification results of (A) PCNA IHC, (B) TUNEL, (C) LDH IHC, (D) TNFα ELISA and (E) IL-4 ELISA. Data are represented as mean ± SD, **P ≤ 0.01, ***P ≤ 0.001, ****p < 0.0001.
Discussion
In our alcohol mouse model, the polymorphonuclear leukocytes found were few while macrophages and lymphocytes were abundant. Further, macrophages were found not only coming in from the portal system, but could migrate from the omentum into the liver via penetrating the Glisson’s capsule of the liver. All these external inflammatory cells and Kupffer and Stellate cells secreted many cytokines while Kupffer and Stellate cells were TNFα producing [25].
Our results pointed out most importantly that PTH when taken as a remedy in alcoholic models could alleviate some of the alcohol effects. Firstly, it prevented the upregulation of TNFα , a proinflammatory molecule [25]. This decrease in TNFα could result in the possible downregulation of fibrosis. IL-4 was on the other hand, upregulated in both pre-treatment of PTH followed by alcohol group (PRE group) and the rotational PTH/alcohol group (ROT group). IL-4 is an interesting cytokine with a debatable origin, though many believed that basophils activated naïve helper T cells to form T helper 2 cells that would secrete IL-4 [26]. Original thoughts suggested this cytokine was related to allergic or autoimmune reactions. Recently, IL-4 was believed to have a regulatory role to suppress unwanted immune reactions via supporting regulatory T cells [27]. The presence of an appreciable amount of IL-4 after rotational PTH or PTH pretreatment in the alcoholic liver may be a trend for further modulation of inflammation induced by alcohol.
Our results further disclosed that in general PTH treatment before alcohol or PTH treatment in rotation with alcohol promoted proliferation of liver cells. The promotion in the pretreatment group was the best and both groups decreased apoptosis and necrosis in general. For necrosis, both PTH treatment before alcohol and rotation treatment of PTH alcohol decreased necrosis. On the other hand, protection of programmed cell death required long pretreatment of PTH rather than rotational treatment of PTH. On that account, we should take note that different regimes of treatment might have different effects in different cases. Post-treatment with alcohol first and PTH later also had been explored in our study yet did not produce much effect owing to the fact that much pathology had already been developed and thus changes would be difficult.
In this mouse model, apoptosis and necrosis were features of liver damage, along with infiltration of macrophage and lymphocytes leading to fibrosis. This aligns very well with the human drug induced liver lesion described by Krishna [28]. The only difference was that in this model, apoptosis and necrosis were mostly spotty and periportal, and no large lobular lesions were seen. In this work, apoptosis and necrosis were reduced in the liver of the PTH treated mice compared with the solely alcohol treated mice. Taken together, PTH appeared to indicate a cellular protective effect on the alcohol treated liver. On the other hand, proliferation of liver cells (number of the PCNA-expressing cells) was higher in the PTH treatment groups, particularly in the pretreatment groups (PRE group), when compared with the control (PCTRL) and the normal group (NORM), indicating upregulation of proliferation of liver cells after PTH treatment, which was conducive to regeneration.
It is also obvious some of the potential therapeutic effects worked well in the rotation PTH/alcohol model better than pretreatment PTH model while the reverse was true in other cases. This would very much depend on the half-life of the suppression molecules generated by the medicine and whether the molecules would have a longer-term accumulative effect or acted only short-term. On the whole, it appeared that the PTH treatment was useful in some ways as an alternative treatment for alcoholic liver.
Due to our limitations, the key components in this traditional formula with numerous ingredients and the exact mechanisms for the protective effectscould not beidentified in this study.There are some possible answers.Two of the components of PTH, cow bile stone and snake gall, had cholic acid which possesses bacteriostatic and viral inactivation effects as well as hepato-protective, anti-inflammatory, anti-histaminergic and anti-pyrexic properties in Chinese medicine [29,30]. Other liver injuries could also lead to necrosis and apoptosis which could be related to superoxide and acetaldehyde formation [31]. PTH appeared to protect against these cell deaths as seen in a previous study [10]. Another component, P.notoginseng upregulated the Protein kinase B (AKT)/BCL2 associated agonist of cell death (BAD) cell survival pathway while exerting cell protection [32]. Musk is a glandular secretion from the musk deers bred in farms and contains predominantly muscone. Muscone could induce autophagy in hepatoma via the adenosine monophosphate (AMP) kinase/mammalian target of rapamycin (mTOR) complex [33] and assist in the amelioration of inflammatory response [34]. This is a preliminary study to investigate whetherPTH could exert hepatoprotective effects on alcoholism.From our results, it seems that PTH would indeed alleviate some detrimental effects of hepatic alcohol intoxication in a complementary way.However, further studies are needed to delineate the mechanism and to find out the key component in PTH exerting the effects.
Conclusion
As for now, there was no reliable treatment in western medicine for alcoholic toxicity and subsequent fibrosis. Herbal components like PTH may show some potential for future research in the field of medicinal chemistry for these diseases. Traditional medicine is a useful addition to complement treatment regimens of western drugs. This is an example of how a composite formula of traditional medicine could help as an integration of treatment in which the beneficial effectscould be verified. Granting all this, there are still many issues to be evaluated including the final treatment regimen, the clinical trials, etc. At least, however, traditional medicine properly assessed does have its value in the future.
Conflict of Interest: Authors Yuan Yu and Zhiliang Chen are working in the research laboratory of Zhangzhou Pien Tze Huang Pharmaceutical Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgements: This work was supported by the Natural Science Foundation of Fujian Province [grant number 2022J01530, China].
Ethical Approval : This animal study was approved by Research Ethics Review Panel for Animal Experiments of Hong Kong College of Technology (reference no.: RERP(A)/1/2018-19/1(M)).
Data Availability Statement: The data used to support the findings of this study are included within the article.
References
- Massey VL, Arteel GE (2012) Acute alcohol-induced liver injury. Front Physiol 3:193.
- Warner DR, Liu H, Ghosh Dastidar S, Warner JB, Prodhan MAI, et al., (2018) Ethanol and unsaturated dietary fat induce unique patterns of hepatic ω-6 and ω-3 PUFA oxylipins in a mouse model of alcoholic liver disease. PloS One 13:e0204119.
- Tsukamoto H, Gaal K, French SW (1990) Insights into the pathogenesis of alcoholic liver necrosis and fibrosis: status report. Hepatology 12:599-608.
- Lee KK, Kwong WH, Chau Ft, Yew DT, Chan WY (2002) Pien Tze Huang Protects the Liver against Carbon Tetrachloride‐Induced Damage. Pharmacol Toxicol 91:185-192.
- Holmes RS, Duley JA, Algar EM, Mather PB, Rout UK (1986) Biochemical and genetic studies on enzymes of alcohol metabolism: the mouse as a model organism for human studies. Alcohol Alcohol 21:41-56.
- Inaba DS, Cohen WE (2014) Uppers, Downers, All Arounders: Physical and Mental Effects of Psychoactive Drugs, 7th: Medford, OR: CNS Productions, Inc; 2014.
- Aroor AR, James TT, Jackson DE, Shukla SD (2010) Differential changes in MAP kinases, histone modifications, and liver injury in rats acutely treated with ethanol. Alcohol Clin Exp Res 34:1543-1551.
- Szabo G, Bala S, Petrasek J, Gattu A (2010) Gut-liver axis and sensing microbes. Dig Dis 28:737-744.
- Szabo G (2015) Gut–liver axis in alcoholic liver disease. Gastroenterology 148:30-36.
- Hong F, Chen Z, Tang HC, Wu SL, Yang J, et al., (2017) Herbal medicine for liver protection in experimental animals-a histochemical , pathological study.
- Choi G, Runyon BA (2012) Alcoholic hepatitis: a clinician’s guide. Clin Liver Dis 16:371-385.
- Zhuang Q, Hong F, Shen A, Zheng L, Zeng J, et al., (2012) Pien Tze Huang inhibits tumor cell proliferation and promotes apoptosis via suppressing the STAT3 pathway in a colorectal cancer mouse model. Int J Oncol 40:1569-1574.
- Shen A, Chen Y, Hong F, Lin J, Wei L, et al., (2012) Pien Tze Huang suppresses IL-6-inducible STAT3 activation in human colon carcinoma cells through induction of SOCS3. Oncol Rep 28:2125-2130.
- Wan Y, Shen A, Qi F, Chu J, Cai Q, et al., (2017) Pien Tze Huang inhibits the proliferation of colorectal cancer cells by increasing the expression of miR‑34c‑5p. Exp Ther Med 14:3901-3907.
- He F, Wu HN, Cai MY, Li CP, Zhang X, et al., (2014) Inhibition of ovarian cancer cell proliferation by Pien Tze Huang via the AKT‑mTOR pathway. Oncol Lett 7:2047-2052.
- Zhang L, Lam WP, Lü L, Wang C, Wong YW, et al., (2010) Protective effects and potential mechanisms of Pien Tze Huang on cerebral chronic ischemia and hypertensive stroke. Chin Med 5:35.
- Hong F, Liu L-y, Lin J-m, Zhuang Q-c, Hong Z-f, et al., (2012) Effects of Pien Tze Huang (片仔癀) on angiogenesis in vivo and in vitro. Chin J Integr Med 18:431-436.
- Qiu X, Luo H, Liu X, Guo Q, Zheng K, et al., (2018) Therapeutic potential of Pien Tze Huang on experimental autoimmune encephalomyelitis rat. J Immunol Res 2018: 2952471.
- Bertola A, Mathews S, Ki SH, Wang H, Gao B (2013) Mouse model of chronic and binge ethanol feeding (the NIAAA model). Nat Protoc 8:627-637.
- Ramirez T, Li Y-M, Yin S, Xu M-J, Feng D, et al., (2017) Aging aggravates alcoholic liver injury and fibrosis in mice by downregulating sirtuin 1 expression. J Hepatol 66:601-609.
- Xu M-J, Cai Y, Wang H, Altamirano J, Chang B, et al., (2015) Fat-specific protein 27/CIDEC promotes development of alcoholic steatohepatitis in mice and humans. Gastroenterology 149:1030-41. e6.
- Gao B, Xu M-J, Bertola A, Wang H, Zhou Z, et al., (2017) Animal models of alcoholic liver disease: pathogenesis and clinical relevance. Gene Expr 17:173-186.
- Guo F, Zheng K, Benedé‐Ubieto R, Cubero FJ, Nevzorova YA (2018) The Lieber‐DeCarli Diet—A Flagship Model for Experimental Alcoholic Liver Disease. Alcohol Clin Exp Res 42:1828-1840.
- Chen Z, Chow TC, Wang S, Leung GC, Wu SL, et al., (2021) Reaction of the Liver upon Long-Term Treatment of Fluoxetine and Atorvastatin Compared with Alcohol in a Mouse Model. J Toxicol 2021:9974969.
- Grund EM, Kagan D, Tran CA, Zeitvogel A, Starzinski-Powitz A, et al., (2008) TNF-α Regulates Inflammatory And Mesenchymal Responses Via MEK, p38, NF-κB In Human Endometriotic Epithelial Cells. Mol Pharmacol 73:1394-1404.
- Bao K, Reinhardt RL (2015) The differential expression of IL-4 and IL-13 and its impact on type-2 immunity. Cytokine 75:25-37.
- Yang W-C, Hwang Y-S, Chen Y-Y, Liu C-L, Shen C-N, et al., (2017) Interleukin-4 supports the suppressive immune responses elicited by regulatory T cells. Front Immunol 8:1508.
- Krishna M (2017) Patterns of necrosis in liver disease. Clin Liver Dis 10:53-56.
- Lowe PP, Gyongyosi B, Satishchandran A, Iracheta-Vellve A, Cho Y, et al., (2018) Reduced gut microbiome protects from alcohol-induced neuroinflammation and alters intestinal and brain inflammasome expression. J Neuroinflammation 15:298.
- Huang M, Zhao W, Li J, Wang Z, Zhang J (2018) Research advance of chemical constituents, analytical methods and pharmacological effects of cow-bezoar and its substitutes. Chinese Journal of Pharmaceutical Analysis 38:1116-1123.
- Brandon-Warner E, Schrum LW, Schmidt CM, McKillop IH (2012) Rodent models of alcoholic liver disease: of mice and men. Alcohol 46:715-725.
- Zhong H, Wu H, Bai H, Wang M, Wen J, et al., (2019) Panax notoginseng saponins promote liver regeneration through activation of the PI3K/AKT/mTOR cell proliferation pathway and upregulation of the AKT/Bad cell survival pathway in mice. BMC Complement Altern Med 19:122.
- Qi W, Li Z, Yang C, Jiangshan Dai J, Zhang Q, et al., (2020) Inhibitory mechanism of muscone in liver cancer involves the induction of apoptosis and autophagy. Oncol Rep 43:839-850.
- Zhou Ly, Yao M, Tian Zr, Liu Sf, Song Yj, et al., (2020) Muscone suppresses inflammatory responses and neuronal damage in a rat model of cervical spondylotic myelopathy by regulating Drp1‐dependent mitochondrial fission. J Neurochem 155:154-176.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.