Over-Expression of Epithelial–Cell Adhesive Molecule (EpCAM) - An Epithelial Mesenchymal Transition (EMT) Marker of Circulating Tumor Cells Modulated by Genetic heterogenicity of MTHFR Gene C→T Polymorphism During Metastasis in Breast Cancer Patients
by Vijay Partap Singh1, Singh C Kumar2, Saxena Ajit Kumar2*, Shambhwi Sharma1, Akash Kumar Singh1, R Kumar Chaudhary1
1R S Memorial Cancer Society and Research Centre, Kankarbagh, Patna, Bihar India.
2Savera Cancer and Multi-speciality Hospital, Kankarbagh Patna, Bihar, India.
*Corresponding author: Saxena Ajit Kumar, Savera Cancer and Multi-speciality Hospital, Kankarbagh Patna, Bihar, India.
Received Date: 21 January, 2026
Accepted Date: 27 January, 2026
Published Date: 31 January, 2026
Citation: Singh VP, Kumar SC, Kumar SA, Sharma S, Singh AK, et al. (2026) Over-Expression of Epithelial–Cell Adhesive Molecule (EpCAM) - An Epithelial Mesenchymal Transition (EMT) Marker of Circulating Tumor Cells Modulated by Genetic heterogenicity of MTHFR Gene C→T Polymorphism During Metastasis in Breast Cancer Patients. J Oncol Res Ther 11:10327. https://doi.org/10.29011/2574710X.10327
Abstract
In women, breast cancer is a leading cause of morbidity and mortality due to variety of genetic and epigenetic factors including to initiate metastatic potential either synergistic or independent manner. Present study has been designed with the aim to evaluate interaction as EpCAM (epithelial cell adhesive molecule) – a epithelial mesenchymal transition (EMT) markers of circulating tumor cells (CTC) and 3-5’methylenetetrahydrofolate reductase molecule MTHFR C677T polymorphism to evaluate “risk factors” of the disease during progression of i.e adenocarcinoma of breast cancer patients. Peripheral blood samples (5.0 ml) were collected from clinically diagnosed cases (n=25) of early age group (25-35year) and similar age matched healthy controls (n=36) having lacking history of cancer in the family. The macromolecules DNA and RNA was isolated using kits for the study of MTHFR C677T gene polymorphism using ARMS-PCR-based SNP analysis. RNA was used for EpCAM gene- expression using specific forward & reverse amplicons after cDNA synthesis. Interestingly, high expression was observed in ductal carcinoma during metastasis. Statistically, MTHFR (C677T) gene polymorphism showing significant (p<0.001) genetic heterogenicity (CT genotype), but lacking significance difference in CC (wild - type) or TT genotypes in homozygous condition with respect to controls. Hence, positive correlation was observed between EMT biomarker of CTCs (EpCAM) and genetic heterogenicity of MTHFR gene polymorphism. Further statistical analysis showing significant substitution in nucleotide frequency of C→T allele that confirm tissue specific susceptibility to increase “risk factor” during progression of disease during either pre or post metastasises in breast cancer patients.
Keywords: Breast Cancer; MTHFR Polymorphism; EpCAM Marker.
Introduction
In women, breast cancer (BC) etiopathology is highly complex result high mortality rate due complex chromosome rearrangements (CCRs) and activation of proto-oncogenes. Recent mechanism of metastasises BC cases are involvement of epithelial transition molecules (EMT) such as epithelial cell adhesive molecule (EpCAM), cytokeratin (CK19), Sox4 and vimentin consider relevant biomarkers during progression of disease. The expression of EMT markers varies due to heterogeneous morphology that develop aggressiveness of the disease with diverse clinical manifestations [1].
In eukaryotic system folate, is an essential molecule for DNA methylation followed by proliferation and differentiation of malignant cells through 3,5methylene tetrahydrofolate reductase (MTHFR) gene assigned in human chromosome 1p36.3, with 11 exons that encodes a protein comprising 656 amino contains and two promoters, producing two isoforms with molecular weights of approximately 70, & 77 KD proteins [2]. Folate metabolism is regulated by MTHFR gene polymorphism have C677T and A1298 C alleles that play significant role in variety of tumours to maintain equilibrium and also other than tumours in human disorders, including cardiovascular diseases, thrombosis, pregnancy-related complications, neural tube defects, and psychiatric disorders to increase “risk factor” during heterogenicity. Epidemiology study showing high risk with differential frequency in BC cases of Asians or Caucasian women of different ethnicities, and agegroups perhaps due to involvement of MTHR polymorphism to regulate folate metabolism [3, 4, 5]. Several genetic factors including environmental and endocrine factors also influences, the development and progression of disease like breast cancer. The variation in the frequency of genotypes (CC, CT and TT) of MTHFR C677T polymorphism either in homozygous or heterozygous states varies to different cellular stages or tissue specific genetic susceptibility during cell-to-cell interaction after signalling of p53 mediated gene mutation [6, 7, 8].
Clinicians and scientist are highly confused due to high mortality rate in cancer patients because of lack of early diagnosis and conventional techniques like histopathology or immune technology are showing lack of sensitivity but time taking too. Therefor circulating tumour cell (CTC), play an important role for early diagnosis using epithelial-mesenchymal transition (EMT) molecules markers such as epithelial cell adhesive molecules (EpCAM) , cytokeratin (CK19) both are glycoproteins , Sox4 ( early nuclear transcription factor) , and vimentin , a metastatic EMT markers to initiates after invasion to metastasises after activation of oncogene either independently or with p53 gene mediated signalling either synergistic fashion or independently to initiate transcription in BC patients [9, 10, 11]. EpCAM is a relevant transmembrane conjugated glycoprotein of 40KD act as CTCs biomarker biomarkers primarily localized in intercellular boundaries for cell-to-cell adhesion to increase genetic tissue specific genetic susceptibility. Differential expression of EpCAM were observed in variety of cancer tissues prostate, pancreas, gastric, ovary, lungs including breast cancer has been observed. The present study has been designed to evaluate the tissue specific genetic susceptibility using heterogenicity of MTHFR C677T gene polymorphism and also correlate to the EpCAM gene- expression in the circulating tumor cell CTCs- a significant biomarker during for early diagnosis in breast cancer patients.
Materials and Methods
Isolation of DNA and RNA from Breast Cancer Patients.
Genomic DNA was isolated from peripheral blood samples under sterile conditions from clinically diagnosed breast cancer patients (n= 25) and healthy controls (n=36) using kit (Life Sciences, India). Two (0.2 ml) of EDTA-anticoagulated blood sample was used, mixed with lysis buffer to disrupt cell membranes. Samples were incubated at 60°C using a dry. A single pre-wash step. The lysate was passed through a silica gel-based spin column to bind DNA and purified DNA was eluted and kept for 200C till further use.
Quantification of DNA and RNA by Nanodrop Spectrophotometer.
Macromolecules (DNA/RNA) was quantified by Nanodrop spectrophotometer by measuring absorbance at wavelengths of 260 nm and 280 nm. The concentration of the extracted DNA was approximately 50 ng/µL.
MTHFR-C677T and A1298C Gene Analysis.
MTHFR C677T Polymorphisms were carried out using TetraPrimer ARMS-PCR. Primer Sequences For MTHFR C677T, the following tetra-primers were used: forward (T):GCACTTGAAG GAGAAGGTGTGTCTGCGCGCGTFor (C allele specific, Poly G): GGCGGGCGGCCGGGAAAGCTGCGTGATGATGAAATAGGReverse (cf):TGTCATCCCTATTGGAGGTTACCCCAAA reverse primers (cr): CCATGTCGGTGCATGCCTTCACAAAG, PCR amplification was carried out in a thermal cycler under initial Denaturation: at 95°C for 5 minutes 30 seconds, Annealing: 55°C for 30 seconds, Extension: 72°C for 30 seconds. Final Extension: 72°C for 5 Minutes, Cycling (40 cycles): SYBR Green was used as the fluorescent dye to detect amplified products. Cycle threshold (Ct) values were recorded, and a melting curve (tm) analysis was performed to evaluate the specificity and presence of polymorphic alleles. The tetra-primer ARMS-PCR strategy allowed discrimination of the wild-type, heterozygous, and homozygous mutant genotypes based on differential amplification patterns [12]. The melting temperature (tm) was used to confirm the presence of the C677Tmutation in the heterozygous (CT) condition and to assess A1298C variant frequencies.
EpCAM Gene Expression in Breast Cancer Patients.
Total RNA from cells or tissues using an RNA isolation kit was used for cDNA synthesis: The cDNA) with a reverse transcriptase enzyme and appropriate primers help to prepare PCR master mix containing Syber Green. EpCAM and GAPDH act as genomic controls. Add cDNA template in the PCR mixture. Primer Design use BLAST (NCI) to find specific forward F-CCATCTTCCAGGAGCGAGATC Gand Reverse R-ATGGTGGTGAAGACGCCAGTG for GAPDH and forward F-CGCAGCTCAGGAAGAATGTG. Reverse R-TGAAGTACACTGGCATTGACG RT-PCR Amplification: To perform RT-PCR, amplify the EpCAM gene expression, with respect to controls
Histopathology
Breast tissue (biopsy sample) were fixed in 10% neutral buffered formalin for 24 hours followed by dehydration using a graded series of ethanol. Tissues were finally cleared in xylene, embedded in paraffin wax. After preparation of blocks 5 µm thick sections were used for observation (cellular architectures) using haematoxylin and eosin staining.
Statistical Analysis
Statistical analysis were carried using software (Graph Pad Prism) to evaluate significance differences i.e. p < 0.05 values and Chisquare test for EpCAM gene expression. in breast cancer patients.
The provided the statistical methods used.
Results
RT-PCR based analysis showing differential expression of EpCAM gene after compare with gnomic controls (GAPDH) as shown in figure-1. Expression of EpCAM were observed between clinically diagnosed cases of adenocarcinoma of breast tissues like acute chronic metastatic, metastatic ductal carcinoma and infiltrating ductal Carcinoma. The highest four fold expression in metastatic ductal carcinoma were observed as details are documented in table- 1.
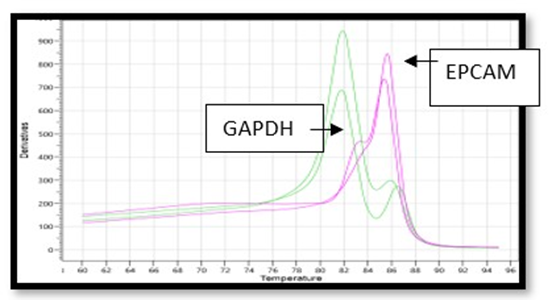
Figure 1: RT-PCR analysis showing differential expression of EpCAM in variety (types) of biopsy in BC Patients.
|
S.No. |
Types in Pathology |
No. Cases |
Frequency (%) |
ΔΔ CT |
Fold changes |
|
|
1. |
Infiltrating ductal Carcinoma |
10 |
40.00% |
0.66 |
2.07 |
|
|
2. |
Metastatic ductal Carcinoma |
8 |
32.00% |
1.42 |
4.71 |
|
|
3. |
Acute chronic Metastatic |
7 |
28.00% |
0.95 |
2.95 |
Table 1: Frequency (%) of EpCAM gene in variety of breast cancer tissues.
There is interesting findings in two clinically diagnosed case of BC i.e acute chronic metastatic and infiltrating ductal carcinoma showing almost the expression of EpCAM i.e.2.95 & 2.07, respectively but the maximum expression (4.0 %) were observed in metastatic ductal Bar graph shows the distribution of high and low EpCAM expression in breast cancer patients. The findings were also supported by bar diagram (figure-1).
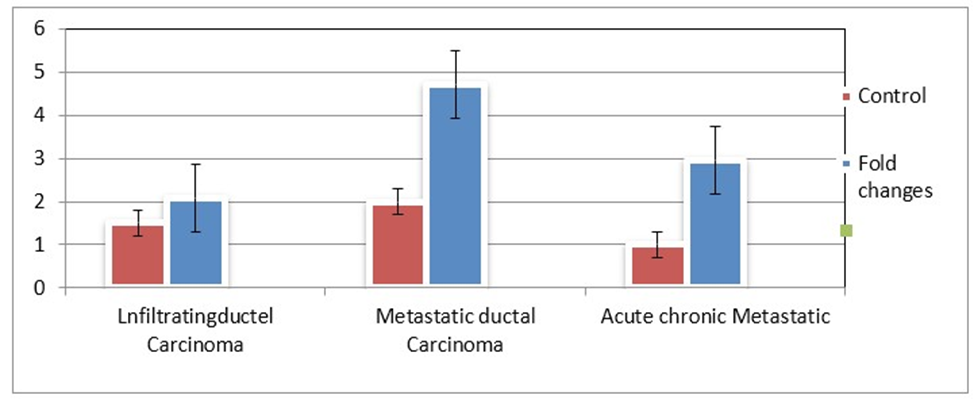
Figure 2: Bar diagram showing the folding low (pink) and high (blue) expression of EpCAM gene in three types of adenocarcinomas of breast cancer patients.
The finding of MTHFR C677T gene polymorphism showing variation in the frequency of the genotypes-CC (wild type), TT (rare types) in homozygous conditions and CT (risk factor) in heterozygous condition in breast cancer patients. This study was carried out by using ARMS PCR, a highly sensitive techniques and Tm values were compared with GAPDH as genomic controls. Table-2 showing the significant difference (p < 0.001) in CT genotype in heterozygous condition, while genotypes CC and TT in homozygous condition showing lack of significant differences.
|
S.No. |
Type of MTHFR C677T Genotypes |
Frequency (%) |
Odd Ratio |
C.I at 95% |
p-values |
|
|
1. |
CC |
12(48.0%) |
0.65 |
0.29 - 1.48 |
0.314 |
|
|
2. |
CT |
08(32.0%) |
0.61 |
0.24 - 1.55 |
0.001* |
|
|
3. |
TT |
05(28.0%) |
9.6 |
3.27 -28.12 |
0.305 |
Table 2: MTHFR C677T gene polymorphism showing variation in frequency of genotypes and level of significance in Breast Cancer Patients.
Figure 3 bar diagram showing significant correlation (frequency) between types of MTHFR C677T genotypes i.e CC, CT & TT and their pathological grading or tissue specificity in breast cancer patients. The metastatic ductal carcinoma showing significant correlation in CT genotypes in heterozygous condition. Interestingly, decreasing trend in clinically diagnosed, (pathologically) tissue types metastatic ductal carcinoma and acute chronic metastatic were observed with the frequency of MTHFR genotypes.
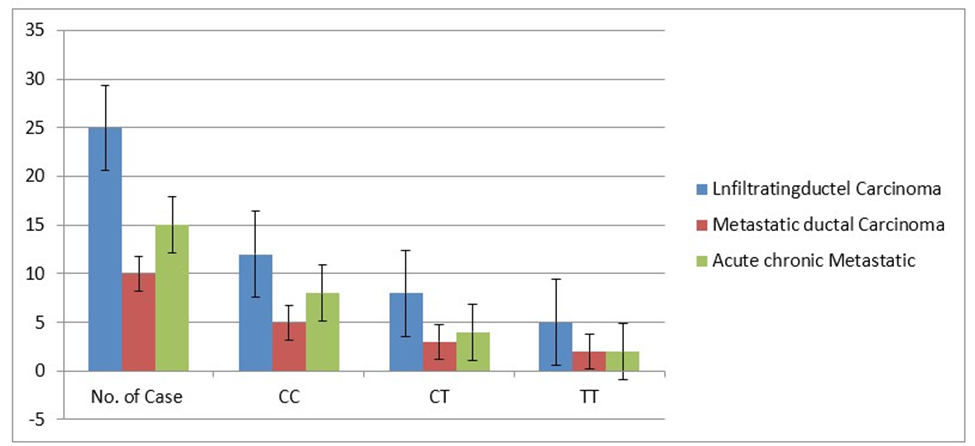
Figure 3: Bar diagram showing the decreasing trend of frequency of MTHFR genotypes and different types of breast cancer patients.
The molecular findings were further confirmed by histopathological study, after simple staining with H & E staining as shown in figure 4A (normal) that showing proliferating and differentiating ductal parenchyma cells. These cellular changes initiate shrinkage followed by reduction in cell-size perhaps due to apoptosis. These changes are responsible morphology of the ductal carcinoma during metastasis as shown in figure 4B. Simillarly, the frequency of EpCAM and MTHFR 677T, both the gene showing highest frequency in of expression in ductal carcinoma during metastasis in breast cancer patients with respect to controls.
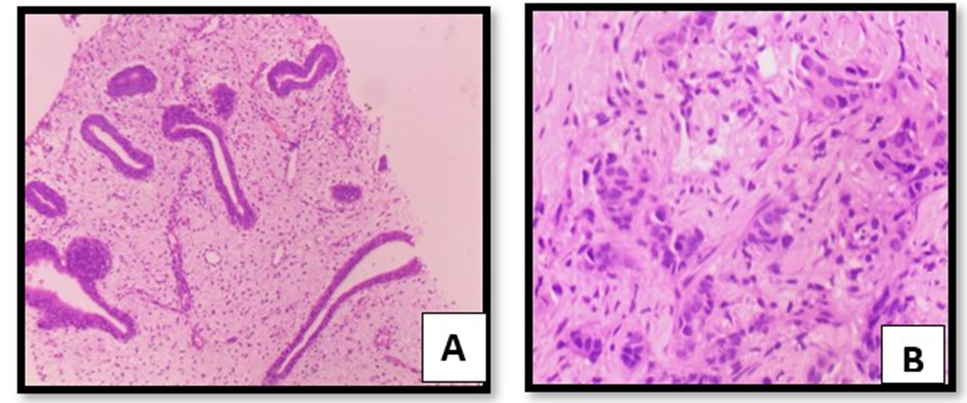
Figure 4AB: Showing breast parenchyma with cystic dilatation and showing normal proliferation of dusts in control tissue (fig4A) and invasive ductal carcinoma with Grade III (3+3+1).
Statistical Analysis
The data was analysed to evaluate the significance differences (p<0.001) between normal tissue and malignant tissue using two tailed Chi square test. The frequency of MTHFR (C677T) genotypes CC (wild type), CT (heterozygous) and TT (rare type) were calculate to access genetic heterogenicity. The correlation between odd ratio (O.R) and confidence interval (C.I) at 95% were also calculated to observe in between different stages of metastasises in breast cancer.
Discussion
Folic acid is an essential component for DNA methylation required for cellular proliferation followed by tissue differentiation during progression of disease like breast cancer. To maintain the folate equilibrium inside cells, the study of 5-10 methylenetetrahydrofolate reductase (MTHFR) gene becomes essential to explore the study of metabolism and their locus assigned on chromosome 1p 36.3 and MTHFRC677T polymorphism becomes essential to assess the genetic heterogenicity and “risk factor” to induce disease. Present study showing the positive correlation between the EpCAM, a circulating tumor cells EMT biomarker due to the highest frequency (40%) in Infiltrating ductal carcinoma, due to genetic heterogenicity of MTHFR C677T gene polymorphism, where the substitution of nucleotide cysteine change in to thymidine (C→ T) followed by changes in amino acids i.e. alanine to valine due to single gene (frame shift) mutation [13]. ductal carcinoma with Grade III (3+3+1).
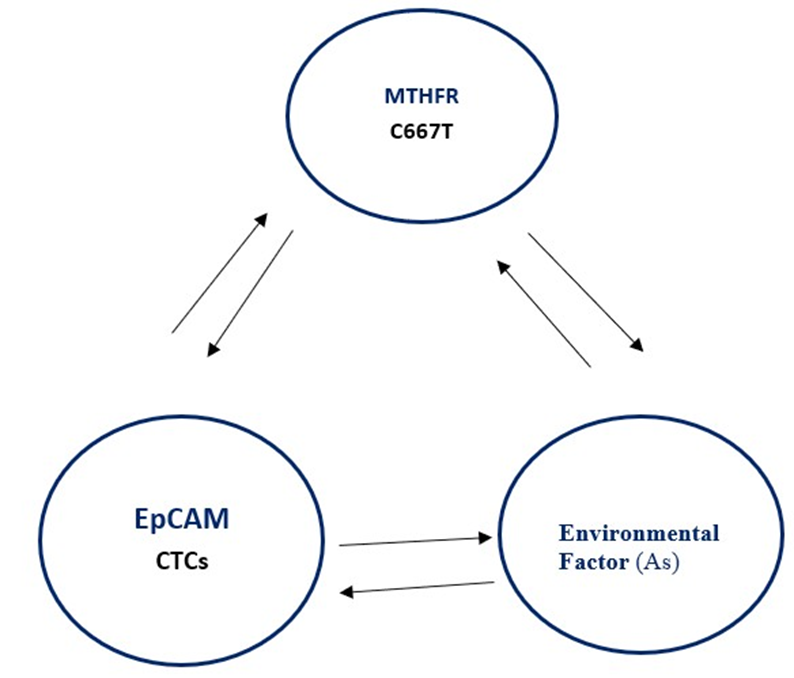
Figure 5: Proposed schematic overview showing interaction between over-expression of EpCAM – a marker of CTCs influence by genetic heterogenicity of 2, 3 methylenetetrahydrofolate reductase (MTHFR C66&T) gene polymorphism in metastatic in breast cancer patients. Epigenetics factors such as arsenic (As) may also influence the activity of MTHFR and CTCs including DNA copy number variations in breast cancer tissue.
Etiopathology of breast cancer is highly complex due to involvement of chromosome complex rearrangements (CCRs) including homologous chromosome aneuploidy that increase genomic instability in breast cancer patients. Epigenetics (environmental) factors that commonly involved arsenic molecule is mutagenic in nature, influence malignancy if exposed for longer period of time increased risk for development of breast cancer patients [14], In the present study authors try to correlate that heterogenicity of MTHFR modulate the expression of EpCAM in ductal carcinoma i.e tissue specific susceptibility were observed in adenocarcinoma [15]. Recent, flocytometrically study showing decreasing trend of EpCAM in BC patients with increase plasticity of malignant tissue during metastasise [16] Earlier study showing that environmental factor, arsenic, a mutagen exposure for longer period of timeintervals to the differentiation cells might have enter through food- chain and increased risk factor of the disease such as cancer.
Present study showing mechanistic approach and relevance for the clinicians and the scientists, that leaving a question why the CTCs marker EpCAM and MTHFR CT genotype showing positive correlation during pre to metastatic or post metastatic either synergistic manner of unknown environmental factor as shown in figure-5. Transformation from single normal cell to malignant cell is still not clear, no doubt activation of oncogenes and tissue specific genetic susceptibility makes the study more interesting but complex. The changes in frequency of the C/T allele’s variation increase “risk factor” of the disease and still required clarification allele frequency (frame shift gene mutation) increase either from maternal or paternal manner to develop genetic heterogenicity.
Conclusion: EpCAM over gene-expression varying in different types of adenocarcinoma of breast may be either due to different age-groups or pathologically stages, but clear that genetic heterogenicity of MTHFR C677T gene polymorphism is one of the relevant “risk factor” to the disease through CTCs EMT marker of EpCAM may consider as tissue specific biomarker for ductal carcinoma during progression of metastases in breast cancer. The study is small, but statistically interesting to make the study interesting, reporting first time in women of Bihar population (India).
Funding Agency: There is no funding agency at present
Contribution in the authors: V P S clinically diagnose the breast cancer patients, AKS plan and designed the study and C K S execute the research work
Ethically Approved: The present study is ethically approved by hospital clinical committee
Conflict of Interest: There is no conflict between the authors References
- Cecilio AP, Takakura ET, Jumes JJ, Dos Santos JW, Herrera AC, et al. (2015) Breast cancer in Brazil: Epidemiology and treatment challenges. Breast Cancer (Dove Med Press) 7: 43-49.
- Levin BL, Varga E (2016) MTHFR: Addressing Genetic Counseling Dilemmas Using Evidence-Based Literature. J. Genet. Coins 25: 901911.
- He L, Shen Y (2017) MTHFR C677T polymorphism and breast, ovarian cancer risk: A meta-analysis of 19,260 patients and 26,364 controls. Onco. Targets Ther.10: 227-238.
- Floris M, Sanna D, Castiglia P, Putzu C, Sanna V, et al (2020) MTHFR, XRCC1 and OGG1 genetic polymorphisms in breast cancer: a casecontrol study in a population from North Sardinia. BMC Cancer 20: 1-15.
- Petrone I, Bernardo PS, Dos Santos EC, Abdelhay E (2021) MTHFR C677T and A1298C Polymorphisms in Breast Cancer, Gliomas and Gastric Cancer: A Review. Genes 12: 587.
- Castiglia P, Sanna V, Azara A, De Miglio MR, Murgia L, et al. (2019) Methylenetetrahydrofolate reductase (MTHFR) C677T and A1298C polymorphisms in breast cancer a Sardinian preliminary case-control study. Int J Med Sci 16: 1089-1095.
- Gaughan DJ, Barbaux S, Kluijitmans LA, Whitehead AS (2000) The human and mouse methylenetetrahydrofolate reductase (MTHFR) genes: genomic organization, mRNA structure and linkage to the CLCN6 gene. Gene 257: 279-289.
- Jansson S, Bendah PO, Larsson AM, Aaltonen KE, Ryden L (2016) Prognostic impact of circulating tumor cells Apoptosis and clusters in Serial Blood Samples from patients with Metastatic Brest Cancer in a Prospective Oservational Cohort.BMC Cancer 16: 433
- Saxena AK, Shalini, Vaibhava V, Kumar M (2024) Discordance in the Frequency of Epithelial - Mesenchymal Transition Markers Sox4, EpCAM and CK19 Gene Expression in Circulating Tumor Cells in Variety of Cancer Patients-Significant Correlation with p53 and MTHFR C677T Gene Polymorphism”. Acta Scientific Cancer Biology 11: 11-17.
- Bono JSD, Scher H I, Montgomery RB, Parker C, Miller MC, et al. (2008). Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate. Clinical Cancer Res 14: 6302-6309.
- Konigsberg R, Obrmayr E, Bisse G, Pfeiler G, Gneist M, et al. (2011) Detective of EpCAM positive and negative circulating tumor cells in metastatic breast cancer patients 50: 700 -710.
- Saxena AK, Gupta RK, Kumar A, Kumar M (2016) ARMS-PCR based SNP analysis of MTHFR C677T allele using syber green in pancreatic tumor British Jr. Med. Medical Research 11: 1-6.
- Rahimi Z, Bozorgi M, Rahimi Z, Shakiba E, Yari K, et al. (2019) MTHFR C677T polymorphism is associated with the risk of breast cancer among Kurdish population from Western Iran. International Journal of Cancer Management 12.
- Saxena AK, Tiwari SM, Singh P (2024) Penetrance of arsenic modulates gene expression of epithelial mesenchymal transition marker Sox4, EpCAM and CK19 genes through MTHFR C677T polymorphism regulation in circulating tumour cells isolated from breast cancer patients. Clinics in Oncology 7: 1-4.
- Partap SV, Saxena AK, Kumar SC, Sharma S, Singh AK, et al. (2025) Significant Association of Methylenetetrahydrofolate Reductase (MTHFR) C677T Polymorphism Increasing Risk Factor in Adenocarcinoma of Ovary in Indian Population of Bihar. Acta Scientific Cancer Biology 3: 2-25.
- Saxena AK, Shalini, Tiwari M, Singh P, Manoj K (2024) Flowcytometric based Analysis of Sox4 Gene Expression-An Early Transcription Factor Influenced by 5-Azacytidine and Compare with EpCAM in Circulating Tumor Cells Isolated from Breast Cancer Patients. Journal of Oncology Research and Therapy 9: 1-7.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.