Flowcytometric Based Analysis of Sox4 Gene Expression-An Early Transcription Factor Influenced by 5-Azacytidine and Compare with EpCAM in Circulating Tumor Cells Isolated from Breast Cancer Patients
by Saxena Ajit Kumar1*, Shalini1, Tiwari Meenakshi1, Singh Pritanjali2, Kumar Manoj3
1Human Cytogenetic and Molecular Genetics Laboratory, Department of Pathology/Lab Medicine, All India Institute of Medical Sciences, Patna, Bihar, India.
2Department of Radiation Oncology, All India Institute of Medical Sciences, Patna, Bihar, India.
3Department of General Surgery, All India Institute of Medical Sciences, Patna, Bihar, India.
*Corresponding author: Saxena AK, Human Cytogenetic and Molecular Genetics Laboratory, Department of Pathology/Lab Medicine, All India Institute of Medical Sciences, Patna, Bihar, India.
Received Date: 07 September, 2024
Accepted Date: 13 September, 2024
Published Date: 13 September, 2024
Citation: Saxena AK, Shalini, Tiwari M, Singh P, Kumar M (2024) Flowcytometric based Analysis of Sox4 Gene Expression-An Early Transcription Factor Influenced by 5-Azacytidine and Compare with EpCAM in Circulating Tumor Cells Isolated from Breast Cancer Patients. J Oncol Res Ther 9: 10247. https://doi.org/10.29011/2574-710X.10247.
Abstract
Circulating tumor cells (CTCs) are the frontier area of cancer medicine for early diagnosis during the management of breast cancer (BC) patients. Flowcytometry is a highly sensitive and powerful technique used for quantitative analysis of epithelial - mesenchymal transition (EMT) expression of Sox4 and EpCAM using monoclonal antibodies in CTCs population, conjugated by PE (phycoerythrin) that detects more than 98.90 % score after gating.Sox4, is an early transcription factor regulating various developmental process including EMT during tumor progression. The mean values of Sox4 showing variation between -ve mAbSox4 (7427.8) and +ve mAbSox4 (6482.3), while, highest frequency (40.00%) were observed between 51-60 years age-group and O.R (4.0) with confidence interval (C.I) at 95% varying between 1.074 -14.896 showing significant (p<0.002) differences. EpCAM mean values varying between –ve mAbEpCAM (22050.8) and +ve mAbEpCAM (21251.5) and highest frequency (40.00 %) were observed between 51-61 years age-groups. Significant (p<0.003) differences with O.R (2.0) and C.I at 95% that varying between 1.074 -14.896, suggesting confirm signaling of Sox4 act as transition inducer for CTCs in BC patients. Sensitivity was further evaluated after in-vitro exposure (1.0 ugm/ml) to peripheral blood lymphocytes (PBL) with 5-azacytidine (5AzaC) and compare with controls, that showing increased mean CTCs population in 5AzaC +ve mAbSox4 (302397) and -ve mAbSox4 (251443).Statistical analysis showing observed values of O.R (1.11) and C.I at 95% varies between 0.225 - 5.46, showing lack of significance (p<0.089) differences but data of the EpCAM showing decreasing trend of cell population with significant (p<0.001) differences, suggesting, EpCAM marker seems to be more sensitive EMT marker during tumorogenesis in BC.
Keywords: Flowcytometry; CTCs; Sox4; EMT marker; Breast Cancer.
Introduction
Circulating tumor cells (CTCs), play an important role during transition of epithelial to mesenchymal (EMT) cells and act as rich source of biomarker for early diagnosis in variety of tumors. CTCs shows genetic heterogeneous group of different subpopulation and may act as the promising tool for early monitoring of BC patients. BC, one of the most common cancer is associated with women health worldwide. Several studies have been shown in the literature that number of CTCs may be correlated with poor prognosis during progression of disease and metastasis in different cancer patients [1-7]. Liquid biopsy, is an integral part of CTCs, a minimal invasive technique is approved by FDA in cell-search system with limitations during monitoring of sub-population and their phenotypic expression using two different markers, CD45 and EpCAM [8-13]. Hence, CTCs, play a “central role” at all the stages of tumor including primary and metastatic event involving cytokeratin act as an active role to maintain tissue architecture and their plasticity [14-16].
5-Azacytidine (5AzaC), a DNA methylation cytostatic drug used by the clinicians for the management of acute leukemia and chronic myelogeneous leukemia belongs to pediatric agegroups [17- 19]. Besides this, it is also being used for solid tumors including adenocarcinoma during metastasis by interfering denovo pyrimidine synthesis. 5-AzaC, a DNA methyltransferase inhibitor that induces double strand breaks (DSBs) including chromosome aberrations (chromatid breaks) and segregation of chromosomes due to pronounced stickiness in proliferating cells in G2 and S phase of cell-cycle [20-22]. These neoplastic agents showing epigenetic modification of progenitor (stem) cells and makes the study more complex due to predisposition of tumor gene involving several signaling pathways like p53 (tumor suppressor gene) and transforming growth factors (TGF) during tumor progression [23]. Sox4 gene is Sry-box transcription factor belongs to the high mobility group (HMG) region and their over-expression associated with cellular-proliferation in pre/post metastasis [24]. Epithelial cell adhesive molecule (EpCAM), a transmembrane conjugated glycoprotein that showed overexpression in variety of cancer including ovarian tumors [25]. Mechanism of transformation from epithelial to mesenchymal cells in CTCs has not been defined clearly in BC patients. However, the present study has been designed with the aims to evaluate the comparative sensitivity of Sox4 and EpCAM in CTCs population using monoclonal antibodies in flowcytometery. To validate of the above findings, the sensitivity was again evaluated using invitro system, where the peripheral blood lymphocytes (PBL) were exposed with 5-AzaC and compared with controls to evaluate genetic susceptibility towards drug sensitivity during therapeutics in breast cancer patients.
Materials and Methods
Peripheral blood lymphocytes (3.0 ml) samples were collected in EDTA sterile vials and stored at 40C, till further study, after clinical diagnosis of breast cancer patients (n=47) from the OPD of All India Institute of Medical Sciences, Patna. Present study is dually approved by the IRC (Institute Research Committee) and Institute Ethical Committee (IEC).The samples were collected after informed consent from the patient or attendant.
Isolation of Circulating Tumour Cells using Ficoll’s Gradient Method
Short-term lymphocyte culture (n=47) were setup using RPMI1640 media, supplemented with 10% foetal bovine serum and phytohemagglutinin (PHA), and antibiotic. After harvesting, the cultured cells were used for isolation of CTCs using Ficoll’s density gradient centrifugation from blood mononuclear cells (PBMC) ring at 400g for 30 min. Cells were washed twice with 1.0 ml PBS and again centrifuge at 1200rpm for 10 min at 40C and stored at -200C, till further characterization of CTCs using the standard protocol. The morphological characteristic of individual cells were also visualized under phase contrast microscope after staining with 1.0 % Giemsa to check the viability cells before flowcytometry.
CTCs staining for Sox4 and EpCAM markers
Sox4 (B-7) PE Lot # B2420, Catalog No sc-518016 monoclonal conjugated antibodies is used to detect intra cellular antigen (Santa Cruz Biotechnology USA). EpCAM PE (REF 12-579182, Lot 2384305) monoclonal antiMo-CD326 was purchased from Thermo fisher (USA) for extra cellular detection of antigen. Cell were fixed and permeabilization buffer is used according to the protocol of the kit (PerFix-nc, Beckman Coulter, France). Staining procedures were strictly followed by the manufacture’s protocol and 50 ul of CTCs were stained with 200 µg/ml for Sox4 PE and 100 µg for EpCAM PE monoclonal antibodies and incubated for 60 minutes. Cell were fixed for 10 minute in 5 µl of Per Fix-nc buffer 1 followed by PerFix-nc buffer 2 and then add antibodies , incubated for 30 minutes wash two times with 3.0 ml of phosphate buffer and centrifuge at 1600 rpm for 5 minutes to remove excess amount of residues. Now cell were allowed to keep in permeabilization buffer 3 (1:10 dilution) and centrifuge at 1600 rpm , finally resuspend pellet in 500 ul of PerFix-nc buffer 3 and cells are ready for acquisition on flowcytometry.
Samples Acquisition and Analysis of the Data analysis
Beckman Coulter Life Science DxFLEX software (Serial No BG10007 Model No DxFLEX) equipment is used for detection of CTCs with blue argon laser at 488 nm. FlowClean Cleaning Agent (100 ul) from Beckman Coulter is used for daily clean of instrument and QC/Standardization is passed using DxFLEX Daily QC Fluorospheres (REF C39283, LOT 15AQHF Beckman Coulter) before performing experiments. FSC and SSC data were analysed using CytExpert version 2.2 for DxFLEX software. Gating strategy were selected the Sox4 and EpCAM positive circulating tumor cells, exclude lymphocytes and erythrocytes. More explicitly on a CD326/SSC dot plot a gate was drawn to select only CD326 population.Sox4/SSC dot plot a gate was drawn to select Sox4 population. In another set of experiment, where, the cells were exposed with 5-AzaC for detection of Sox4 and EpCAM positive population of CTCs and compare with controls.
Statistical Analysis
Significance difference (p values) for odd ratio and confidence interval (C.I) at 95% was calculated between cases and controls using SPSS 27.0 version software (USA). X2- test (two tailed) was also used to observe the p values between experiment and controls.
Results
Table-1 showing the details statistical analysis data of two EMT markers Sox4 & EpCAM in CTCs of BC patients.
Sox4, a pluripotent stem cell marker and compare to EpCAM (intracytoplasmic) EMT markers were assessed by flowcytometer using two different specific monoclonal antibodies for CTCs population that varying significantly findings between cases and controls. Forward cell scattering (FCS) showing cell-size in mix population including CTCs, while pure population after gating is more than 98.27 % cells were score for EpCAM with (+) or without (-) antibodies labeled cells as shown in figure-1 A,B,C&D . The calculated values of odd ratio and C.I at 95% for EpCAM showing highly significant (p<0.001) difference with respect to controls. The sensitivity of these two markers Sox4 and EpCAM were again evaluated by in - vitro exposure with 5AzaC (24 hour) exposure in CTCs. EpCAM cell number (mean values) decrease more than two times i.e. +ve5AzaC 45540 to -ve5AzaC 17357 (controls). Graphically and histogram represents total cell population +veEpCAM and –ve EpCAM that showing the frequency of CTCs decrease significantly (p<0.05) in + EpCAM (98.27%) and - EpCAM (89%) as depicted in figure 2 A,B,C &
2D due to selective specificity towards antigen. The sensitivity of EpCAM was further evaluated using 5AzaC (1.0 µg/ml) in -vitro PBL exposed in CTCs of BC patients and compare with controls (unexposed), cell population again showing decrease (p<0.05) significantly. Interestingly, Sox4, cells number increases in treated (exposed) group 5+ ve AzaC 251443, as compare with controls (unexposed cells) 5AzaC -ve 12882.66 (fig.3 A,B,C&D). Age wise distribution of CTCs showing that highest frequency (40%) of EpCAM in CTCs were observed between 51-60 & 61-70 year of age groups, but frequency of Sox4 decreases (30%) in 61-70 age groups in breast cancer patients (fig 4 A&B).
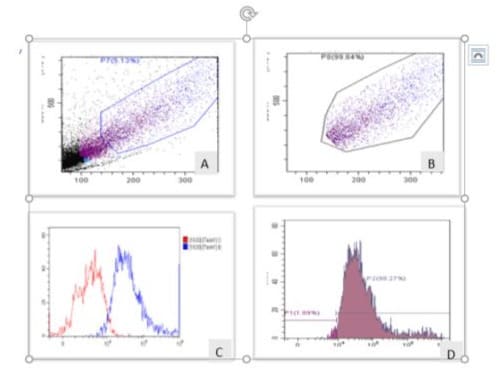
Figure-1A,B,C&D: Flowcytometric analysis for the detection of EpCAM in CTCs population from breast cancer patients. Forward cell scattering (FCS) showing cell-size in mix CTC population (fig.1A) and pure CTC population after gating (fig.1B). Graphically represents total cell population +ve EpCAM increase (blue) and –ve EpCAM (red) as shown in fig.1C. Histogram showing frequency of cell population is 98.27 (%) in + EpCAM and - EpCAM (89%) as depicted in fig.1D.
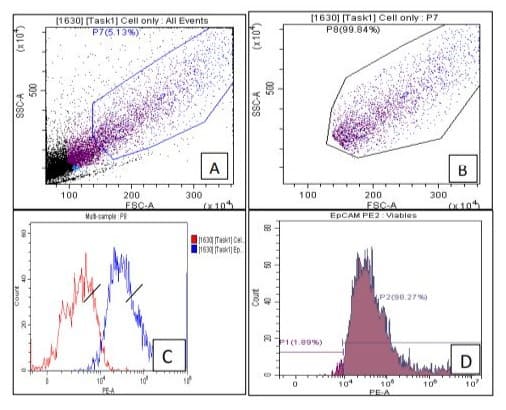
Figure-2A,B,C & D: In-vitro 5-Azacytidine showing mix and pure CTCs population of EpCAM after gating (fig. 2A& B). Graphically represents total cell population +ve EpCAM (blue) and –ve EpCAM (red) as shown in fig. 2C. Histogram showing frequency of + EpCAM (98.27 %) cell population (fig.2D) with decrease trend significantly, when compare with controls due to cytotoxic nature of the drug.
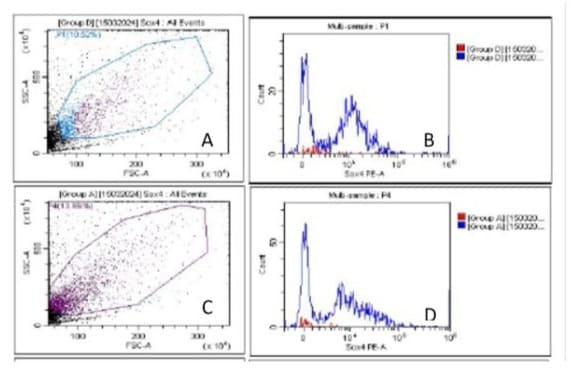
Figure 3A,B,C &D: Sox4 showing after gating pure CTCs population from breast cancer patients (fig3A) visualize by two different colors, red –ve mAbSox4 and +vemAbSox4 (blue) as shown in fig.3B. In-vitro detection of CTCs population after exposure with 5-AzaC showing cell population after gating (fig.3C) and graphically represents total cell population significantly decrease with +vemAbSox4 (blue) and –ve mAbSox4 (red) as shown in fig.3D.
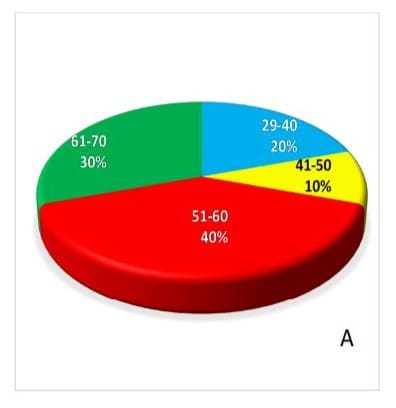
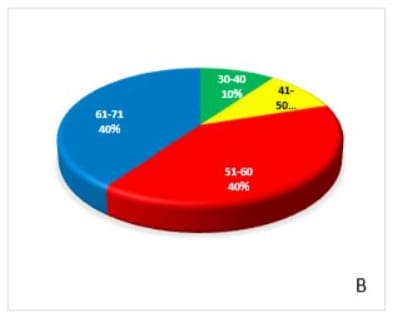
Figure-4A &B: Age-wise distribution and their percentage (%) frequency of transcription factor and epithelial-mesenchymal transition marker-Sox4 and EpCAM, respectively in circulating tumor cells isolated from breast cancer patients.
Table-1: Statistical analysis showing mean and their (%) frequency between two different markers Sox4 & EpCAM in circulating tumor cells population of breast cancer patients.
|
S. No. |
Age |
Sox4 |
Age |
EpCAM |
||
|
-ve (%) +ve (%) |
-ve (%) +ve (%) |
|||||
|
1 |
58 |
4384 |
3845 |
69 |
10000 |
10200 |
|
16.17 |
20.86 |
19.84 |
28.58 |
|||
|
2 |
67 |
6614 |
6377 |
71 |
10010 |
10090 |
|
31.22 |
23.77 |
22.97 |
14.22 |
|||
|
3 |
29 |
2221 |
11672 |
30 |
47569 |
43400 |
|
24.27 |
41.9 |
12.05 |
10.28 |
|||
|
4 |
39 |
4895 |
6205 |
43 |
42326 |
42437 |
|
4.1 |
2.72 |
3.58 |
4.53 |
|||
|
5 |
55 |
4054 |
3256 |
68 |
42071 |
42146 |
|
7.97 |
6.11 |
12.54 |
12.27 |
|||
|
6 |
54 |
3803 |
5025 |
55 |
52223 |
41178 |
|
7.07 |
9.03 |
10.87 |
7.64 |
|||
|
7 |
70 |
6914 |
5123 |
62 |
5963 |
6280 |
|
12.79 |
13.94 |
11.29 |
18.79 |
|||
|
8 |
48 |
3050 |
3640 |
52 |
3823 |
9481 |
|
20.32 |
18.96 |
12.76 |
27.95 |
|||
|
9 |
63 |
32866 |
4524 |
51 |
3199 |
4789 |
|
22.02 |
8.13 |
11.32 |
26.06 |
|||
|
10 |
58 |
5477 |
15156 |
53 |
3334 |
2804 |
|
14.72 |
21.61 |
12.63 |
10.07 |
|||
|
Mean |
54.1 |
7427.8 |
6482.3 |
55.4 |
22050.8 |
21251.5 |
|
s.d |
11.93 |
8592.5 |
3681.07 |
12.1 |
19901.3 |
17321.18 |
|
C.I @95% Max-Min |
54.1-7.39 |
7427.8-5325.6 |
6482.3-2281.5 |
54.4-7.5 |
22050.8-12335.01 |
21251.5-10735.78 |
|
*Significant differences (p<0.05) with respect to controls. |
||||||
Discussion
Flowcytometry is highly sensitive and reliable technique for the detection of epithelial mesenchymal transition (EMT) biomarkers. Cancer death has been multiply due to poor prognosis by the clinicians because classical invasive (biopsy) histopathology or cytochemistry techniques are much time consuming and less sensitive. Present advancement of technology, liquid biopsy is the emerging field for early diagnosis after using EMT markers either with RT-PCR or specific antibodies of Sox4, EpCAM, CK19 and Vimentin in different types of tumors. CTCs population shows genetic heterogeneity during epithelial-mesenchymal transition (EMT) and are non-invasive prognostic biomarker in breast cancer [3-5]. CTCs are the ‘key player’ during tumor progression and difficult to identify either due to aggressiveness or small in number (1-2%), but can be detected from the peripheral blood samples at all the stages including early and metastatic [6, 8, 9]. In the present study we used magnetic beads during flowcytometry analysis of Sox4 and EpCAM quantitative expression in CTCs population in both controls and experimental group (cells were exposed with 5AzaC). Unequivocally, findings showing lack of consistency in individual frequency (%) in CTC population between (-/+) mAb probably either due to different specificity or sensitivity towards using two different antigens based on their functional point of views at different stages of tumor (M0/M1) in different age-groups. Earlier study showing elderly group (>50 year) have significant +ve CTCs number as compared to the women belongs to early age group (less than 50 years) of female patients. Our study also found the similar findings that CTCs sensitivity of EpCAM observed in elderly age groups (51-61years) is more either due to stability of antigen in cytoplasm or abundance in quantity during transition of epithelial-mesenchymal transition from pre / post metastatic during angiogenesis. Interestingly, EpCAM, an intracytoplasmic marker showing more sensitivity (98.98%) for CTCs using mAbEpCAM and even after 5AzaC exposure again validates the significant role during progression of the disease as relevant biomarker in BC patients. Data of the sensitivity for CTCs between two populations was further analyzed and compare with 5AzaC in-vitro PBL experiments showing decreasing trend (down-regulation) with significant differences in EpCAM, suggesting extracellular cytoplasmic marker is more prone for cytotoxicity due to antineoplastic nature of the drug. However, the biological activity of 5AzaC is controversial and affects DNA, RNA and protein synthesis, due to increasing enzymatic activity in-vitro experiments [19]. Similarly, our study also confirm that the significant reduction of cell number with +ve5AzaC after using EpCAM as EMT biomarker for CTCs in BC. EpCAM is an epithelial cell surface antigen of 40kD and over expressed (97%) in colon cancer, but in primary tumor of breast patients shows only 41.7%, this variation may be either due to tissue specificity or different metastatic stages (M0/M1) of during progression of the disease. [7,9]. Sox4 showing increased frequency of CTCs population that confirming early initiation of tumor progression as transcription factor. Interestingly, the variation in mean values of Sox4 cell-population increases with respect to controls may be due to selective sensitivity towards cell-kinetics or over-expression act as growth promoter during metastasis in BC. Side scattering is the relevant features for the characterization of three major subset population including polymorphic cells, monocytes and lymphocytes. After gating, it’s become necessary to know cellproliferation rate (cell-kinetics) in tumor cells during progression of disease, which might helpful for the clinicians during management of BC. Most of the studies shows variation in antigen expression using two different approaches either MCF-7 cells or immunochemistry after collection of 10-15 ml of samples for CTCs isolation and confirm genetic heterogeneity. Although, our approach have an advantage to reduce sample volume (quantity) with heterogeneity in CTCs with increase and maintain sensitivity of EMT markers in BC patients [22.23]. Interestingly, the data of the present study is dually evaluated which confirm the sensitivity using two different antibodies to detect two different antigens (+mAbEpCAM or +mAbSox4) and 5AzaC exposure to PBL showing statistical significant difference with respect to controls that confirm the CTCs role in metastatic activity during tumor progression. Earlier study also shows that Sox4 gene promotes metastasis through over-expression and responsible for phenotypic aggressiveness in cancer patients [24]. Our study also emphasizes that the behavior changes occurs due to Sox4 (intranuclear) signaling to promote EpCAM (intracytoplasmic) regulation of during epithelial to mesenchymal transition in synchronize or paracrine fashion to promotes tumor progression in BC.
Conclusions
EpCAM seems to be more sensitive and reliable biomarkers for early diagnosis using in CTCs for BC patients using flowcytometric analysis.. However, our focus on functional aspects to open new avenues of research in tissue engineering techniques and stem cell therapeutics still continue to reverse the transition i.e from mesenchymal to epithelial by developing new inhibitors or antineoplastic agents to reduce the cellular toxicity and enhance the survival of the tumor bearing patients.
Author Contributions: AKS executed experiment designed, implementation, S help for validation of findings, MT help for proof reading, during illustration of the manuscript, while PS and MK helped for clinical diagnosis of the breast cancer patients.
Funding: This study was funded by Department of Science and Technology, New Delhi, India (Grant number: DST/TDP/ BTDT16/2021).
Informed Consent Statement: Informed consent was obtained from all subjects involved in the study.
Availability of data and materials: The data presented in this study are available on request from the corresponding author. Ethics approval and consent to participate
Study was approved by Institute ethical committee of AIIMS Patna (AIIMS/Pat/IRC/2020/610), and informed consent form was dually signed by patients.
Competing interests
There are no competing interests among the authors.
Acknowledgments: AKS is thankful to the Department of Science and Technology (DST), New Delhi for providing financial assistance. Moreover, we are also thankfully acknowledge to the patients participated in this study.
References
- Krebs MG, Sloane R, Priest L, Lancashire L, Hou JM, et al. (2011) Evaluation and Prognostic Significance of Circulating Tumor Cells in Patients with Non-Small-Cell Lung Cancer. J Clin Oncol 29:1556-63.
- Cohen SJ, Punt CJA, Iannotti N, Saidman BH, Sabbath KD, et al. (2008) Relationship of Circulating Tumor Cells to Tumor Re sponse , Progression-Free Survival, and Overall Survival in Patients with Metastatic Colorectal Cancer. J Clin Oncol 26:3213-21.
- Bono JSD, Scher HI, Montgomery RB, Parker C, Miller MC, et al. (2008) Circulating Tumor Cells Predict Survival Benefit from Treatment in Metastatic Castration-Resistant Prostate Cancer. Clin Cancer Res 14:6302-9.
- Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, et al. (2004) Circulating Tumor Cells, Disease Progression, and Survival in Metastatic Breast Cancer. N Engl J Med 351:781-91.
- Saxena AK (2022) Discordance between Stem Cell Oct4, Sox2, Sox4 and Epithelial Mesenchymal Transition CK 19, EpCAM Markers in Circulating Tumor Cells - MTHFR C677T Gene Variant Increase Risk Factor in Pancreatic Tumor. International Journal Cancer Medicine. 6: 1-9.
- Poruk KE, Valero V, Saunders T, Blackford AL, Griffin JF, et al. (2016) Circulating Tumor Cell Phenotype Predicts Recurrence and Survival in Pancreatic Adenocarcinoma. Ann Surg 264:1073-1081.
- Saxena AK (2022) Non-Synonymous Variants of Methylenetetrahydrofolate Reductase C677T Gene Polymorphism showing Discordance with KRAS Mutation in Circulating Tumor Cells of Hepatocellular Carcinoma - A Rare Case Report. Clinical Oncology 6:1-5.
- Jansson S, Bendahl PO, Larsson AM, Aaltonen KE, Rydén L (2016) Prognostic Impact of Circulating Tumor Cell Apoptosis and Clusters in Serial Blood Samples from Patients with Metastatic Breast Cancer in a Prospective Observational Cohort. BMC Cancer 16:433.
- Chen LM, Lazcano O, Katzmann JA, Kimlinger TK, Li CY (1998) The Role of Conventional Cytology, Immunocytochemistry, and Flow Cytometric DNA Ploidy in Evaluation of Body Cavity Fluids: A Prospective Study of 52 Patients. Am J Clin Pathol 109:712-21.
- Schneller J, Eppich E, Greenebaum E, Elequin F, Sherman A,et al. (1987) Flow Cytometry and Feulgen Cytophotometry in Evaluation of Effusions. Cancer 59:1307-13.
- Unger KM, Raber M, Bedrossian CW, Stein DA, Barlogie B (1983) Analysis of Pleural Effusions Using Automated Flow Cytometry. Cancer 52:873-7.
- Krishan A, Ganjei-Azar P, Jorda M, Hamelik RM, Reis IM, et al. (2006) Detection of Tumor Cells in Body Cavity Fluids by Flow Cytometric and Immuncytochemical Analysis. Diagnostic Cytopathology 34:528-541.
- Pollard TD, Cooper JA (2009) Actin, a Central Player in Cell Shape and Movement. Science 326:1208-12.
- Herrmann H, Strelkov SV, Burkhard P, Aebi U (2009) Intermediate Filaments: Primary Determinants of Cell Architecture and Plasticity. J Clin Invest 119:1772-83.
- Anderson JM, Heindl LM, Bauman PA, Ludi CW, Dalton WS, et al. (1996) Cytokeratin Expression Results in a Drug-Resistant Phenotype to Six Different Chemotherapeutic Agents. Clinical Cancer Research, 2:97-105.
- Ao Z, Shah SH, Machlin LM, Parajuli R, Miller PC, et al. (2015) Identification of cancer associated fibroblast in circulating blood from patients with metastasis breast cancer. Cancer Res 75:4681-7.
- Hrodek O, Vesely J (1971) 5- Azacytidine in childhood leukemia. Neoplasma 18:493-503.
- McCredie K B, Bodey GP, Brugess M A, Rodriguez V, Sullivan M P, et al. (1972). The treatment of acute leukemia with 5-Azacytidine. Blood 40: 975-981.
- Davidson S, Crowther P, Radley J, Woodcock D (1992) Cytotoxicity of 5- aza 2’- deoxycytidine in a mammalian cell system. Eur J Cancer 28:362-368.
- Fucik V, Michaelis A, Rieger R (1970) On the induction segment extension and chromatid structural changes in Vicia faba chromosome after treatment with 5-azacytidine and 5’-aza-2-deoxycytidine. Mutat Res 9:599-606.
- Saxena AK, Srivastava AK, Singh G (2007) Unexpected segregation of chromosome and common fragile site expression induced by 5 Azacytidine exposure in human lymphocytes of Down syndrome patients. Biomed Research 18:31-34.
- Kiziltepe T, Hideshima T, Catley L ,Raje Noopur , Yasul Hiroshi, et al. (2007) 5-Azacytidine, a DNA methyltransferase inhibitor induces ATH mediated DNA double strand breaks responses, apoptosis and synergistic cytotoxicity with doxorubicin and bortezomib against multiple myeloma cells. Mol Cancer Ther 6:1718-27.
- Saxena Ajit Kumar, Tiwari Meenakshi,,Singh Pritanjali ,, Singh Veena (2024). Comprehensive Mutational Spectra of Epithelial Mesenchymal Transition Markers-SOX4, EpCAM, CK19 and their Impact of Methylene Tetrahydrofolate Reductase C677T Gene Polymorphism Associated Risk Factor Modified by BRCA and TGFβR1 Genes in Pre/ Post-Menopausal Cases of Breast. International Journal of Cancer Medicine 7:158-182.
- Song GD, Sun Y, Shen H, Li W (2015) Sox4 over - expression is a novel biomarker of malignant status and poor diagnosis in breast cancer patients .Tumor Biol 36:4167-73.
- Konigsberg R, Obermayr E, Bises G, Pfeiler G, Gneist M et al. (2010) Detection of EpCAM positive and negative circulating tumor cells in metastatic breast cancer patients. Acta Oncologia 20:700-710.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.