Insertion Efficacy of a Peripheral Intravenous Catheter with a Novel Glide-on-Contact Cannula Tip: A Prospective Investigation
by Michael G. Tal1, Anne Covey2, Aracelys Rijo Castro3, Yenifer Mejia Perez3, Ada Guzman3, Elizabeth Lisbeth Agustin Charles3, Juana Dilenia Castro Rincon3, Digna Scarli Nolasco Rodriguez3
1Palmetto General Hospital, Hialeah, FL 33016, USA
2Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, USA
3Hospital de Clinica Canela C2CP+7X6, Calle Benito Monción, La Romana 22000, Dominican Republic
*Corresponding author: Michael G. Tal, 4201 Collins Ave, Miami Beach, FL 33140, USA
Received Date: 06 October 2025
Accepted Date: 14 October, 2025
Published Date: 17 October, 2025
Citation: Tal MG, Covey A, Castro AR, Perez YM, Guzman A, et al. (2025) Insertion Efficacy of a Peripheral Intravenous Catheter with a Novel Glide-on-Contact Cannula Tip: A Prospective Investigation. Int J Nurs Health Care Res 8:1677. https://doi.org/10.29011/2688-9501.101677
Abstract
Background: Peripheral intravenous catheterization, one of the most common clinical procedures, poses procedural challenges with respect to the first-attempt insertion. Failure to insert a catheter on the first attempt can result in treatment delays and complications, morbidity, and catheter failure. Objective: The objective of this study was to assess, in a pragmatic setting, the first-attempt insertion success rate of a novel peripheral intravenous catheter featuring a glide-on-contact design of the plastic cannula tip. Methods: This was a prospective, single-arm, single-center trial. One hundred consecutive emergency department patients requiring peripheral venous access who provided informed consent were enrolled. Nine emergency department operators (5 physicians and 4 nurses) took part in the study. Veins were located by visualization and palpation in all cases. Results: First-attempt insertion was successful in 89%. Insertion site, laterality, demographic and medical history parameters, and the order in which participants were enrolled in the study did not significantly affect first-attempt success. A total of 116 insertion attempts were required to obtain access in all 100 participants. The mean (SD) grade of operator satisfaction with the procedure on a 5-point Likert scale (5–very satisfied) was 4.98 (0.14). Conclusions: The novel peripheral intravenous catheter with a glide-on-contact plastic cannula tip resulted in a high firstattempt success rate for accessing the target vessel.
Keywords: Catheters; Veins; Catheterization; Cannulation
Introduction
Peripheral intravenous catheterization, the introduction of a short plastic catheter into a peripheral vein, is the most common method of obtaining vascular access for venous sampling and intravenous administration of solutions, medications, and blood products. In the past few decades, peripheral intravenous catheterization has been one of the most common clinical procedures, with over a billion of short peripheral catheters (SPCs) marketed worldwide per annum and over 400 million inserted annually in hospitalized patients in the United States [1-3]. Nurses are the primary SPC inserters worldwide, responsible for roughly 70% of all procedures, though considerable regional variation exists (26% to 97%) [1].
Despite the long history of use, SPC insertion attempts may fail repeatedly [4], exposing patients to more pain and increasing the likelihood of extravasation, infiltration, and treatment delays [3,5,6]. First-attempt insertion failure is associated with phlebitis, a predictor of SPC failure [4,7] linked to catheterassociated bloodstream infection [4]. Failure to promptly insert SPC also presents a formidable financial challenge, considering the equipment costs, clinician time, and treatment of potential complications [4,8].
The gauge of the catheter and its relation to the vessel diameter and the needle tip form factor are the major catheter-related attributes predicting first-attempt success [9,10]. The importance of the angle of penetration for cannula insertion with different shaped needle tips [11,12] and the superiority of an SPC featuring a guidewire over a “regular” SPC apparent in some settings [13] suggest additional factors may affect the insertion success. While the shape of the plastic cannula tip has been associated with catheter-related complications and catheter failure [4], the interaction between the cannula tip and vessel wall during insertion has been overlooked in the literature.
The authors hypothesized that an SPC cannula with a glide-oncontact tip design could minimize traumatic collision with the vessel wall and, therefore, facilitate insertion success rates. The purpose of this study is to evaluate the first-attempt and overall success rate of catheter insertion as well as the safety of a gliding peripheral intravenous catheter (GPIV). The uniquely-shaped tip of the GPIV catheter plastic cannula features asymmetry, with the negative, convex slope in the plane perpendicular to the longitudinal axis of the tip resulting in bottom part of the cannula being shorter and establishing a surface that is hypothesized to glide on contact with the vessel wall, thereby reducing the likelihood of tissue trauma (Figure 1).
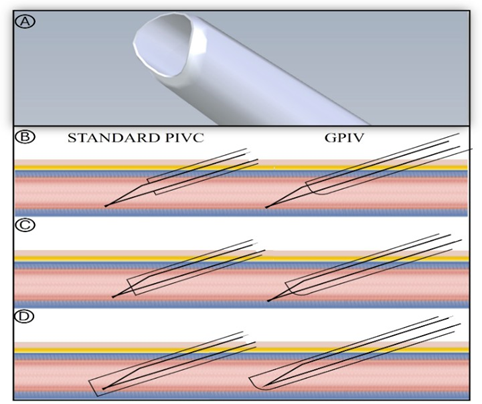
Figure 1: GPIV vs “regular” SPC: (A) GPIV cannula tip; (B) cannula and needle are advanced into the vein; (C) cannula is advanced over the needle and encounters the vessel wall opposite the site of penetration; (D) the potentially traumatic contact of the advancing plastic cannula tip of a “regular” SPC with vessel wall opposite the site of penetration is envisioned to be avoided with the glide-on-contact design of the tip of GPIV.
The design of this trial, conducted in a low-resource hospital, was pragmatic regarding the setting, users, selection of participants, intervention, outcomes, and relevance to practice, with the standard institutional clinical practice preserved, to maximize external validity of the results [14,15].
Methods
This was a prospective, single-arm study. SPCs were inserted by 9 emergency department (ED) clinicians, five of whom were physicians (2 with 25 and one each with 5, 10, and 20 years of experience) and 4 nurses (1, 5, 7, and 20 years of experience) at a single site in the Dominican Republic (Clinica Canela, La Romana, Dominican Republic).
Participants
Consecutive patients, 18 years of age or older, requiring SPC insertion were recruited to the study. All participants had to be able to understand and sign the consent form. Exclusion criteria were pregnancy or lactation, need for emergency venous access, and history of venous grafts or previous surgery at the target vessel access site.
Investigational Device
GPIV (ViTal, Embrace Medical Ltd., Tel Aviv, Israel) is an 18- or 20-G, 1.25- or 1.75- inch–long (respectively) SPC with a radiopaque polyurethane cannula and a stainless-steel needle with a passive safety shield, attached to an acrylonitrile butadiene styrene flash chamber. No training was provided on the use of the device.
Investigational Procedure
After consent was obtained, participants underwent SPC insertion following the standard institutional procedures. There were no protocol limitations or guidance on insertion site selection. The protocol did not require or preclude use of difficult intravenous access (DIVA) assessment tools and/or assistive technologies. Up to 4 attempts were allowed before GPIV insertion would be deemed a failure, in which case the institutional standard SPC would be used. All successive attempts were made at a site different from the one(s) at which access had already failed.
Outcomes
The primary endpoint was the first attempt success rate. Successful insertion was defined as withdrawal of non-pulsatile blood or infusion of saline without evidence of extravasation. GPIV insertion would be regarded as having failed if there was extravasation with initial infusion, inability to draw blood, or inability to obtain access.
The secondary endpoint was the incidence of the device- and procedure-related adverse events.
Exploratory analyses included the number of attempts required to obtain successful access; user (nurse or physician) satisfaction at the time of insertion, measured on 5-point Likert scale (5 - very satisfied, 4 - somewhat satisfied, 3 - neutral, 2 - somewhat unsatisfied, 1 - very unsatisfied); participant-graded pain associated with insertion, measured on 5-point Likert scale (5 being the maximum and 1 being the minimum); and the incidence of device deficiencies and of adverse events, both expected and unexpected, related or unrelated to GPIV or the procedure.
Safety Analysis
All adverse events that occurred, whether considered related to the study device and/or procedure or not, were reported, coded with the Medical Dictionary for Regulatory Activities (version 24.1), and mapped to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) v. 5.0. The International Medical Device Regulators Forum (IMDRF) technical document IMDRF terminologies for categorized Adverse Event Reporting (AER): terms, terminology structure and codes Annex E (Health Effects-Clinical Signs, Symptoms and Conditions Terms and Codes) was consulted at the time of event coding. Safety analyses were conducted by reviewing safety listings and narratives.
Statistical Analysis
Safety analysis was conducted on the intention-to-treat (ITT) dataset comprising all consented participants. Efficacy analyses were conducted on the modified ITT (mITT) dataset comprising all participants from the ITT dataset in whom GPIV insertion was attempted. Continuous variables were summarized by a mean, median, standard deviation, minimum, and maximum, and categorical variables by a count and percentage. Fisher’s exact test calculating two-sided P value was used for analysis of potential risk factors for failure. Cochran-Mantel-Haenszel test was used to assess the relationship between the demographic and medical history parameters of the study participants and their order of enrolment in the study, in terms of success in the primary outcome. Statistical analysis was performed with IBM SPSS Statistics version 29.0.0 (IBM, Armonk, NY, USA).
Results
One hundred consecutive ED patients were screened for participation in the study between November 2021 and March 2022. There were no screen failures, and catheterization with GPIV was attempted in all consented participants, who then formed the analysis set for safety and efficacy analyses. No participants withdrew or were withdrawn from the study or excluded from analysis. Demographic variables and vital signs of the study participants are summarized in Table 1. Thirty-two percent of the study participants were dehydrated. Fifteen percent suffered from heart disease.
|
Sex |
||
|
Female |
% (n/N) |
56% (56/100) |
|
Male |
% (n/N) |
44% (44/100) |
|
Ethnicity |
||
|
Hispanic, Latino/a, or of Spanish origin |
% (n/N) |
91% (9/100) |
|
Not of Hispanic, Latino/a, or Spanish origin |
% (n/N) |
9% (9/100) |
|
Race |
||
|
Black or African American |
% (n/N) |
89% (89/100) |
|
White |
% (n/N) |
11% (11/100) |
|
Age, years |
N |
99 |
|
Mean (SD) |
37 (13.67) |
|
|
Median [Range] |
33 [19 – 81] |
|
|
Height, ft |
N |
100 |
|
Mean (SD) |
5.5 (0.31) |
|
|
Median [Range] |
5.4 [4.9 – 6.9] |
|
|
Weight, lb |
N |
100 |
|
Mean (SD) |
161 (35.27) |
|
|
Median [Range] |
156 [100 – 313] |
|
|
Body mass index, kg/m2 |
N |
100 |
|
Mean (SD) |
26.9 (5.66) |
|
|
Median [Range] |
25.9 [17.7 – 50.5] |
|
|
Systolic blood pressure, mmHg |
N |
99 |
|
Mean (SD) |
112 (12.86) |
|
|
Median [Range] |
110 [69 – 160] |
|
|
Diastolic blood pressure, mmHg |
N |
99 |
|
Mean (SD) |
70 (6.87) |
|
|
Median [Range] |
70 [51 – 90] |
|
|
Heart rate, beats per minute |
N |
100 |
|
Mean (SD) |
86 (10.65) |
|
|
Median [Range] |
86 [63 – 113] |
|
|
Body temperature, ºC |
N |
99 |
|
Mean (SD) |
37 (0.71) |
|
|
Median [Range] |
37 [36 – 39.5] |
Table 1: Demographics and Vital Signs.
The first-attempt success rate was 89% (two-sided exact 95% CI, 81.17%-93.75%). The majority of first attempts to insert GPIV involved dorsal (n=50) and antecubital (n=41) sites (Table 2). In 61 of the 100 participants, right-sided sites were chosen for the first attempt. Insertion site selection nor laterality significantly affected first-attempt success rate (Fisher’s exact test, P=.47 and P=.49, respectively).
|
Attempts |
||||||||||||||||||||||||
|
1 |
2 |
3 |
4 |
|||||||||||||||||||||
|
Accumulating success rate |
Accumulating success rate |
Accumulating success rate |
Accumulating success rate – 100% |
|||||||||||||||||||||
|
– 89% |
– 96% |
– 99% |
||||||||||||||||||||||
|
N sites |
Success |
N sites |
Success |
N sites |
Success |
N sites |
Success |
|||||||||||||||||
|
All |
R |
All |
R |
L |
All |
R |
All |
R |
L |
All |
R |
All |
R |
L |
All |
R |
All |
R |
L |
|||||
|
Sites |
N |
N |
N |
% |
% |
% |
N |
N |
N |
% |
% |
% |
N |
N |
N |
% |
% |
% |
N |
N |
N |
% |
% |
% |
|
all |
100 |
61 |
89 |
89 |
90.2 |
87.2 |
11 |
6 |
7 |
63.6 |
83.3 |
40 |
4 |
2 |
3 |
75 |
50 |
100 |
1 |
1 |
1 |
100 |
100 |
-- |
|
AC |
41 |
21 |
37 |
90.2 |
95.2 |
85 |
3 |
2 |
3 |
100 |
100 |
100 |
2 |
1 |
2 |
100 |
100 |
100 |
1 |
1 |
1 |
100 |
100 |
-- |
|
dorsal |
50 |
33 |
45 |
90 |
90.9 |
88.2 |
4 |
1 |
2 |
50 |
100 |
33.3 |
2 |
1 |
1 |
50 |
0 |
100 |
0 |
0 |
0 |
-- |
-- |
-- |
|
forearm |
9 |
7 |
7 |
77.8 |
71.4 |
100 |
4 |
3 |
2 |
50 |
66.7 |
0 |
0 |
0 |
0 |
-- |
-- |
-- |
0 |
0 |
0 |
-- |
-- |
-- |
|
R – right; L – left; AC – antecubital |
||||||||||||||||||||||||
Table 2: Insertion Attempts and Success.
There was no association between first-attempt success and the demographic variables or the medical history- including gender (Fisher’s exact test, P=.86), ethnicity (Fisher’s exact test, P=1), race (Fisher’s exact test, P=.42), dehydration (P=.43), and history of heart disease (Fisher’s exact test, P=.46) of the participants. There was no difference in first attempt success between the first 30 participants and the latter 70.
Vascular access with GPIV was obtained in all participants. A second attempt was required in 11 participants, a third-in 4, and a fourth-in 1 participant. A total of 116 insertion attempts were made to obtain successful access in 100 participants, bringing the mean (SD) number of attempts to 1.16 (0.51).
The mean (SD) grade of pain at the time of insertion, as reported by the participants, was 1.8 (0.92). Users were very satisfied (Likert scale grade 5) with the insertion procedure in 98% of the participants. In the two remaining cases, users graded their satisfaction as 4. Mean (SD) satisfaction was 4.98 (0.14).
Adverse events included CTCAE Grade-1 extravasation (n=4) and hematoma (n=1), all of which resolved spontaneously without sequelae.
Discussion
The SPC insertion success research focuses mainly on the use of assistive technologies and the size, shape, and design features of the SPC needle [9-12,16-19]. To the best of the authors’ knowledge, this is the first report of a first-attempt insertion success rate assessment in which the tip of the plastic cannula is the element of interest. In this study, the first-attempt insertion success rate with a catheter featuring a glide-on-contact design of the plastic cannula tip was 89%. The lower bound of the first-attempt SPC insertion success rate with and without assistive devices varies greatly in literature [9]. The performance goal of 90%, advocated by some authors as “clinically relevant and acceptable” [20], has rarely been sustained in the data published in the last two decades [9,2128], including recent publications of the threshold proponents [17]. The reported inpatient and ED first-attempt success rates in adults infrequently exceed 80% [5,21,22,28].
The 89% (two-sided exact 95% CI, 81.17%-93.75%) first attempt success rate in the current pragmatic study with a heterogenous group of inserters, exceeds other studies with the exception of a trial of an over-the-wire SPC reported by Idemoto et al more than a decade ago [13]. Unlike the device reported here, use of the overthe-wire SPC required extensive preclinical and clinical training, prior to enrolling patients [13]. First-attempt insertion of the overthe-wire SPC comparator in that study (Insyte Autoguard, Becton, Dickinson and Company, Sandy, UT, USA) was successful in only 47% of the cases, underscoring the extent to which the first-attempt success rate can vary with different SPCs in adult populations. The results of Idemoto et al were not reproduced in a later study with registered nurses inserters [29] and a comparable study population. The apparent difficulty that a study of army combat medics had with the over-the-wire SPC [30], even after receiving training analogous to that reported by Idemoto et al, suggests that SPCs requiring adaptation of the insertion technique may not be appropriate for all users. Further, a recent study also suggests that clinicians think that over-the-wire SPC may not be suitable for all patients [31].
As expected in a pragmatic setting, the insertion preferences of operators in this study deviated from the recommendations of the Infusion Nurses Society (INS) with respect to insertion site selection [32]. Globally most SPCs are placed in the hand, antecubital veins, and the wrist, and only the minority of SPCs, as low as 21% in some territories, are placed in an area of non-flexion (forearm) [1]. In Latin America, doctors are much more likely to insert SPCs into the hand, wrist, or antecubital fossa than they are into forearm, while nursing technicians, nursing assistants, registered nurses, and IV teams are equally likely to choose recommended and non-recommended sites [33]. The root of the practice of antecubital fossa insertions by ED operators is the belief that patients may require “fluid resuscitation and administration of highly concentrated drugs during clinical deterioration scenarios” [34]. The departure from the guideline recommendations is not expected to affect interpretation of the effectiveness results of this study [9].
This study was conducted in a low-resource center and neither required nor precluded use of assistive technologies. None of the inserters used such technologies in any of the study participants. This deviates from the INS recommendations but aligns with the known issue of inserters’ preference and limited availability of devices such as ultrasound, especially in “small, underfunded healthcare services” where “ultrasound ranks low on priority list”, despite the evidence-based recommendations [35,36]. With regard to the primary effectiveness outcome assessment in this study, not using assistive technologies could be speculated to represent the worst-case scenario.
Finally, in this pragmatic study, the inserters were not required to use DIVA assessment tools, and none of them utilized such tools voluntarily.
This is in line with the known worldwide difficulties in implementing evidence-based practice recommendations due to insufficient resources, especially in regional, rural, and remote settings [33,35]. In addition, the 2024 update of the INS guidance asserts that DIVA assessment tools must be population-specific and acknowledges that generalizability of such tools is uncertain [32]. With the ever-growing number of instruments using diverse terminology to assess DIVA [20,37-40], such non-harmonized approach could be one of the reasons DIVA reporting in clinical trials is often subpar [27]. When reported, the prevalence of DIVA in clinical trials ranges between 6% and 87.7%, depending on the definition applied [37]. Some researchers also argue that adoption of DIVA assessment tools in the context of clinical practice is impeded because grading on the existing scales takes up a lot of valuable time and the tools are perceived as complex and inconvenient [41]. Alternatives to DIVA assessment have recently been proposed [41]. Overall, the impact of the lack of DIVA assessment in this study on interpretation of the study results may not be different than the impact of use of different assessment tools in the absence of harmonization of DIVA assessment.
Limitations of the Study
This was a single-center study. Single-center studies may have the proclivity for overestimation of the effect size. However, this bias has been, at least partly, offset by not recruiting roll-in participants and not providing any training or guidance on the use of the investigational device to the multiple inserters who were unfamiliar with the device prior to the study.
The single-arm study design lacks innate mechanisms for reduction of the selection bias. In this study, the selection bias has been significantly reduced through consecutive sampling and performance of calculations on the dataset comprising data from all consented participants. Also, as discussed above, DIVA assessment was not conducted in this study, which could mean that the first-attempt and overall success rate could be lower in certain populations which may have been underrepresented in the study.
Conclusions
Efficiency and speed are hallmarks of medicine. SPC insertions are often made with limited information on the patient [42], limited access to assistive technologies and limited, if any, experience with their use [35,43]. Considering the expected reduction in complications associated with repeated insertion attempts, the 60% worldwide prevalence of SPCs in inpatient wards alone [44], and the cost savings anticipated under the conditions of reduction in the number of insertions and personnel time [45], an SPC with a high first-attempt success rate is where the interests of all stakeholders converge. This is especially true in light of the questionable value of infrared devices [28], the non-negligible procurement and maintenance costs of ultrasound being a major obstacle to its use [46-48], and the substantially longer time it may take to achieve first‐attempt insertion when assistive devices are used, time inserter may not have in emergency [49].
The current report of a promising nominal success of GPIV in establishing peripheral venous access on the first attempt suggests that the shape of the plastic cannula tip may play a role in the success of peripheral intravenous catheterization. Comparative, multicenter studies, potentially involving use of vascular visualization technologies, may help further understand the contribution of the plastic cannula tip form factor to the efficacy and safety of SPCs.
Funding
This study was sponsored by Embrace Medical Ltd.
Conflicts of Interest
Michael G. Tal has equity in Embrace Medical Ltd., Tel Aviv, Israel, which manufactures the ViTal peripheral intravenous catheter The other authors have nothing to disclose.
Trial Registration
ClinicalTrials.gov NCT07016607; https://clinicaltrials.gov/study/ NCT07016607
References
- Alexandrou E, Ray-Barruel G, Carr PJ, Frost SA, Inwood S, et al. (2018) Use of Short Peripheral Intravenous Catheters: Characteristics, Management, and Outcomes Worldwide. J Hosp Med 5.
- Liu J (2022) Vascular Access Devices | Market Insights | US | Medtech Insights.
- Bahl A, Haddad L, Hoerauf K, Mares A, Alsbrooks K (2023) The Clinical and Economic Burdens of Infiltration and Extravasation with Peripheral Intravenous Catheters: A Contemporary Narrative Review. International Journal of Nursing Studies.
- Helm RE, Klausner JD, Klemperer JD, Flint LM, Huang E (2019) Accepted but Unacceptable: Peripheral IV Catheter Failure. J Infus Nurs 3: 151-164.
- Parker SI, Benzies KM, Hayden KA, Lang ES (2017) Effectiveness of interventions for adult peripheral intravenous catheterization: A systematic review and meta-analysis of randomized controlled trials. Int Emerg Nurs Mar 31:15-21.
- Parker SIA, Benzies KM, Hayden KA (2017) A systematic review: effectiveness of pediatric peripheral intravenous catheterization strategies. J Adv Nurs. 7:1570-1582.
- Ray-Barruel G, Polit DF, Murfield JE, Rickard CM (2014) Infusion phlebitis assessment measures: a systematic review. J Eval Clin Pract 2: 191-202.
- Gala S, Alsbrooks K, Bahl A, Wimmer M (2024) The economic burden of difficult intravenous access in the emergency department from a United States’ provider perspective. J Res Nurs 1: 6-18.
- Carr PJ, Rippey JCR, Cooke ML, Trevenen ML, Higgins NS, et al. (2019) Factors associated with peripheral intravenous cannulation first-time insertion success in the emergency department. A multicentre prospective cohort analysis of patient, clinician and product characteristics. BMJ Open. 4: e022278.
- Moureau NL (2019) The VHP Model. In: Moureau NL, ed. Vessel Health and Preservation: The Right Approach for Vascular Access. Springer International Publishing. 3-8.
- Suzuki T, Tanaka A, Fukuyama H, Nishiyama J, Kanazawa M et al. (2004) Differences in penetration force of intravenous catheters: effect of grinding methods on inner needles of intravenous catheters. Tokai J Exp Clin Med 4: 175-81.
- Tanabe H, Oosawa K, Miura M, Mizuno S, Yokota T et al. (2022) Effect of a thin-tipped short bevel needle for peripheral intravenous access on the compressive deformation and displacement of the vein: A preclinical study. J Vasc Access 1: 265-273.
- Idemoto BK, Rowbottom J, Reynolds JD, Hickman RL (2014) The AccuCath Intravenous Catheter System with Retractable Coiled Tip Guidewire and Conventional Peripheral Intravenous Catheters: A Prospective, Randomized, Controlled Comparison. Journal of the Association for Vascular Access. 2: 94-102.
- Zwarenstein M, Treweek S, Gagnier JJ, Altman DG, Tunis S et al. (2008) Improving the reporting of pragmatic trials: an extension of the CONSORT statement. BMJ
- Ford I, Norrie J (2016) Pragmatic Trials. N Engl J Med 5: 454-63.
- Hill S (2019) Insertion. In: Moureau NL, ed. Vessel Health and Preservation: The Right Approach for Vascular Access. Springer International Publishing. 69-80.
- van Loon FH, Timmerman R, den Brok GP, Korsten EH, Dierick-van Daele AT, et al. (2022) The impact of a notched peripheral intravenous catheter on the first attempt success rate in hospitalized adults: Blockrandomized trial. J Vasc Access 2: 295-303.
- Prunet B, Meaudre E, Montcriol A, Asencio Y, Bordes J et al. (2008) A prospective randomized trial of two safety peripheral intravenous catheters. Anesth Analg 1:155-8.
- Matthews R, Gavin NC, Marsh N, Marquart-Wilson L, Keogh S (2023) Peripheral intravenous catheter material and design to reduce device failure: A systematic review and meta-analysis. Infect Dis Health 4: 298-307.
- van Loon FHJ, van Hooff LWE, de Boer HD, Koopman SSHA, Buise MP, et al. (2019) The Modified A-DIVA Scale as a Predictive Tool for Prospective Identification of Adult Patients at Risk of a Difficult Intravenous Access: A Multicenter Validation Study. J Clin Med 2: 144.
- Tran QK, Flanagan K, Fairchild M, Yardi I, Pourmand A (2022) Nurses and Efficacy of Ultrasound-Guided Versus Traditional Venous Access: A Systemic Review and Meta-Analysis. J Emerg Nurs 2: 145-158.
- Tran QK, Fairchild M, Yardi I, Mirda D, Markin K, Pourmand A (2021) Efficacy of Ultrasound-Guided Peripheral Intravenous Cannulation versus Standard of Care: A Systematic Review and Meta-analysis. Ultrasound Med Biol 11: 3068-3078.
- Oakley E, Wong AM (2010) Ultrasound-assisted peripheral vascular access in a paediatric ED. Emerg Med Australas 2: 166-70.
- Perry AM, Caviness AC, Hsu DC (2011) Efficacy of a near-infrared light device in pediatric intravenous cannulation: a randomized controlled trial. Pediatr Emerg Care 1: 5-10.
- Peterson KA, Phillips AL, Truemper E, Agrawal S (2012) Does the use of an assistive device by nurses impact peripheral intravenous catheter insertion success in children? J Pediatr Nurs 2:134-43.
- Heinrichs J, Fritze Z, Vandermeer B, Klassen T, Curtis S (2013) Ultrasonographically guided peripheral intravenous cannulation of children and adults: a systematic review and meta-analysis. Ann Emerg Med 61: 444-454 e1.
- Berlanga-Macias C, Diez-Fernandez A, Martinez-Hortelano JA, SequíDomínguez I, Saz-Lara A, et al. (2022) Ultrasound-guided versus traditional method for peripheral venous access: an umbrella review. BMC Nurs 21: 307.
- Drugeon B, Schults JA, Ray-Barruel G, Hui Xu G, Ball D, et al. (2025) Infrared Devices Versus Traditional Palpation Approach for Peripheral Intravenous Catheter Insertion in Adults: A Systematic Review and Meta-Analysis. J Adv Nurs 29: 17007.
- Chick JFB, Reddy SN, Chen JX, Seamark T, Chittams J, et al. (2017) A randomized comparison between an intravenous catheter system with a retractable guidewire and conventional intravenous catheters. J Vasc Access 18: 530-534.
- Jin LM, Medeck S, Ruley J, Riddle M, Aden J (2018) “Guidewire Intravenous Catheter Systems Do Not Improve First-Pass Success Rates for Peripheral Access When Placed By Army Combat Medics (68W) in a Pre-hospital Setting.” A Prospective, Randomized Controlled Trial with Crossover Study Design. Mil Med 183: e730-e734.
- Xu HG, Hyun A, Kang E, Marsh N, Corley A (2024) Exploring clinicians’ insertion experience with a new peripheral intravenous catheter in the emergency department. Australas Emerg Care 27:192-197.
- Nickel B, Gorski L, Celadon T, Kyes A, DeVries M, et al (2024) Infusion Therapy Standards of Practice, 9th Edition. J Infus Nurs 47: S1-S285.
- Walker RM, Pires MPO, Ray-Barruel G, Cooke M, Mihala G, et al. (2022) Peripheral vascular catheter use in Latin America (the vascular study): A multinational cross-sectional study. Front Med (Lausanne) 9:1039232.
- Xu HG, Ullman AJ, Rickard CM, Johnston A (2023) Factors impacting emergency department clinicians’ peripheral intravenous catheter practice: A qualitative analysis. Int Emerg Nurs 71:101366.
- Schults JA, Calleja P, Slaughter E, Paterson R, Rickard CM, et al. (2022) Peripheral intravenous catheter insertion and use of ultrasound in patients with difficult intravenous access: Australian patient and practitioner perspectives to inform future implementation strategies. PLoS One. 17: e0269788.
- Engstrom A, Forsberg A (2019) Peripheral intravenous catheter difficulty - A clinical survey of registered nurse and critical care nurse performance. Journal of clinical nursing. 28: 686-694.
- Bahl A, Johnson S, Alsbrooks K, Mares A, Gala S, et al. (2021) Defining difficult intravenous access (DIVA): A systematic review. J Vasc Access 17: 11297298211059648.
- Ng M, Mark LKF, Fatimah L (2022) Management of difficult intravenous access: a qualitative review. World J Emerg Med 13: 467-478.
- Paterson RS, Schults JA, Slaughter E, Cooke M, Ullman A, et al. (2022) Review article: Peripheral intravenous catheter insertion in adult patients with difficult intravenous access: A systematic review of assessment instruments, clinical practice guidelines and escalation pathways. Emerg Med Australas 34: 862-870.
- Bell J, Moureau N, Campos C (2023) Validation and Reliability of the Comprehensive Difficult IV Access Scoring Tool. International Journal of Nursing and Health Care Research. 6: 1414.
- Bahl A, Alsbrooks K, Zazyczny KA, Johnson S, Hoerauf K (2024) An Improved Definition and SAFE Rule for Predicting Difficult Intravascular Access (DIVA) in Hospitalized Adults. J Infus Nurs 47: 96-107.
- Evison H, Carrington M, Keijzers G, Marsh NM, Sweeny AL, et al. (2022) Peripheral intravenous cannulation decision-making in emergency settings: a qualitative descriptive study. BMJ Open. 12: e054927.
- Archer-Jones A, Sweeny A, Schults JA, Rickard CM, Johnson L, et al. (2020) Evaluating an ultrasound-guided peripheral intravenous cannulation training program for emergency clinicians: An Australian perspective. Australas Emerg Care 23: 151-156.
- Alexandrou E, Ray-Barruel G, Carr PJ, Frost S, Inwood S, et al. (2015) International prevalence of the use of peripheral intravenous catheters. J Hosp Med 10: 530-3.
- Kara R, Hollmann S, Ferko N, Delatore P (2016) PMD114 - A Modeled Health Economic Analysis of the Accucath® Intravenous Catheter System with Retractable Coiled Tip Guidewire Compared with Conventional Peripheral Intravenous Catheters. Value in Health. 19: A705.
- Stone R, Walker RM, Marsh N, Ullman AJ (2023) Educational programs for implementing ultrasound guided peripheral intravenous catheter insertion in emergency departments: A systematic integrative literature review. Australas Emerg Care 26: 352-359.
- Ray-Barruel G, Pather P, Schults JA, Rickard CM (2024) Handheld Ultrasound Devices for Peripheral Intravenous Cannulation: A Scoping Review. J Infus Nurs 47: 75-95.
- Cooke M, Ullman AJ, Ray-Barruel G, Wallis M, Corley A, et al. (2018) Not “just” an intravenous line: Consumer perspectives on peripheral intravenous cannulation (PIVC). An international cross-sectional survey of 25 countries. PLoS One.13: e0193436.
- Tada M, Yamada N, Matsumoto T, Takeda C, Furukawa TA, et al. (2022) Ultrasound guidance versus landmark method for peripheral venous cannulation in adults. Cochrane Database Syst Rev 12: CD013434.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.