Evaluation of Jugular-Applied Coherent Low-Level Laser Therapy for Glucose Modulation
by Travis Sammons1*, Iliana Sosa Teste2, David Huff3
1Clinical Affairs Manager, Erchonia Corporation, Fountain Inn, SC, USA
2Researcher and Full Professor, National Center for Laboratory Animal Production (CENPALAB), USA
3Practicing Veterinarian, Plantation Park Animal Hospital, USA
*Corresponding author: Travis M. Sammons, Clinical Affairs Manager, Erchonia Corporation, 112 Southchase Blvd, Fountain Inn, SC 29644, USA.
Received Date: 15 August 2025
Accepted Date: 20 August 2025
Published Date: 25 August 2025
Citation: Sammons T, Teste IS, Huff D (2025) Evaluation of Jugular-Applied Coherent Low-Level Laser Therapy for Glucose Modulation. Curr Res Cmpl Alt Med 9: 273. https://doi.org/10.29011/2577-2201.100273
Abstract
This experimental study evaluated whether a single session of jugular-applied coherent low-level laser therapy (LLLT) could acutely reduce blood glucose levels in diabetic dogs through systemic mitochondrial activation. Chronic hyperglycemia in diabetic animals leads to widespread metabolic dysfunction, reduced quality of life, and shortened lifespan. This study explored a novel systemic approach by targeting the jugular vein to stimulate circulating free-floating mitochondria. Ten adult Beagle dogs with confirmed diabetes mellitus were randomized to receive either a 20-minute session of 640 nm coherent LLLT applied over the jugular vein (n = 5) or placebo (sham device; n = 5). Venous glucose was measured before and one hour after treatment under an analyst-blinded protocol.
The LLLT group demonstrated a significant reduction in blood glucose (−33.4 mg/dL; −19.9%, p = 0.0001) compared to the placebo group (−14.0 mg/dL; −8.2%, p = 0.0006), with a statistically significant between-group difference (p = 0.0002), suggesting a rapid and biologically meaningful metabolic effect. In conclusion, jugular-applied LLLT produced an acute and substantial glucose-lowering effect in diabetic dogs, likely mediated by enhanced mitochondrial activity.
Introduction
Chronic hyperglycemia is a hallmark of diabetes mellitus and is associated with a wide array of systemic complications, including endothelial dysfunction, impaired tissue repair, peripheral neuropathy, and progressive organ damage [1]. These pathologies collectively diminish quality of life and reduce lifespan in affected animals. Early and effective modulation of metabolic dysregulation is essential for minimizing long-term morbidity and mortality. Mitochondria play a central role in glucose homeostasis by generating ATP and regulating critical cellular signaling pathways involved in insulin sensitivity, redox status, and inflammatory control. In diabetic states, mitochondrial dysfunction is both a consequence and contributor to hyperglycemia, characterized by impaired glucose oxidation, reduced ATP synthesis, and increased oxidative stress [2]. Therapeutic strategies aimed at restoring mitochondrial function represent a promising adjunct to conventional pharmacologic approaches for glycemic control.
Low-level laser therapy (LLLT) is a non-invasive technique that has been demonstrated to enhance mitochondrial respiration, increase ATP production, and attenuate oxidative stress. While most LLLT applications have focused on localized tissue effects, recent investigations have explored systemically directed delivery methods. This study introduces a novel approach by applying coherent LLLT over the jugular vein—a high-flow vascular structure—to stimulate both intracellular mitochondria and circulating free-floating mitochondria. These extracellular mitochondria, now recognized as viable and metabolically functional, circulate systemically and may act as mobile bioenergetic regulators [3]. Activation of this mitochondrial population through jugular-applied LLLT may facilitate a rapid, body-wide improvement in metabolic function, including enhanced glucose utilization.
Methods
Ten adult Beagle dogs (5 males, 5 females; ages 5–9 years) with confirmed diabetes mellitus were randomized into LLLT or placebo groups. The LLLT group received a single 20-minute session of coherent 640 nm red laser (Erchonia Corp) applied over the jugular vein, whereas the placebo group underwent the same procedure using a device that emitted incoherent light. Venous blood samples were collected immediately before treatment and one hour post-treatment to assess changes in glucose concentration (mg/dL) using standard enzymatic assays.
All biochemical analyses were performed at the National Center for Laboratory Animal Production (CENPALAB). The study was conducted under a double-blind protocol: veterinarians responsible for applying treatments and collecting samples were not involved in biochemical analyses, and analysts performing laboratory measurements were blinded to group assignments. Statistical analysis was performed using paired t-tests within groups and Welch’s t-test to compare changes between groups, with significance set at α = 0.05.
Results
|
Group |
n |
Pre (mg/dL) |
SD |
Post (mg/dL) |
SD |
Δ (mg/dL) |
SD |
% Change ±SD |
|
LLLT |
5 |
168.2 |
14.79 |
134.8 |
13.57 |
-33.4 |
5.18 |
-19.9 ± 2.88 |
|
Placebo |
5 |
170.4 |
7.23 |
156.4 |
8.56 |
-14.0 |
3.16 |
-8.24 ± 2.03 |
Table 1: Effects of Jugular-Applied Coherent LLLT on Venous Glucose Levels in Diabetic Dogs.
Laser-treated dogs exhibited a significant reduction in venous glucose levels (−33.4 mg/dL; −19.9%, p = 0.0001), compared to a smaller reduction in the placebo group (−14.0 mg/dL; −8.2%, p = 0.0006). The between-group difference in glucose reduction was statistically significant (p = 0.0002), indicating a robust glucose-lowering effect associated with jugular-applied LLLT.
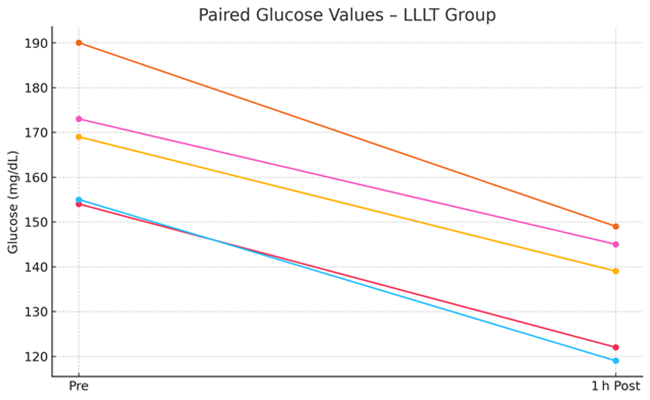
Figure 1: Pre- and Post-Treatment Glucose Values in the LLLT Group.
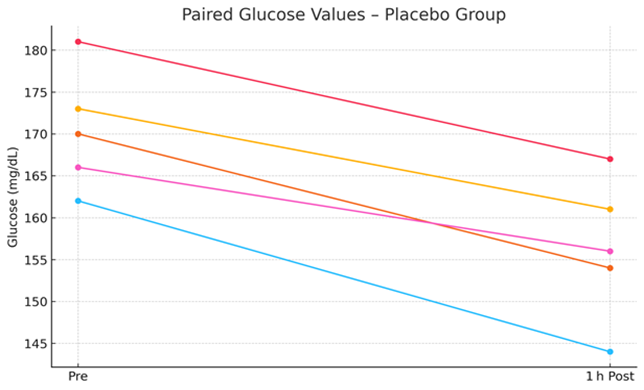
Figure 2: Pre- and Post-Treatment Glucose Values in the Placebo Group.
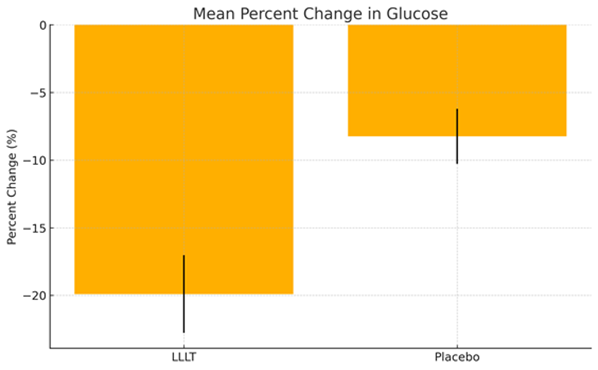
Figure 3: Mean Percent Change in Glucose – LLLT vs Placebo.
Discussion
Chronic hyperglycemia, the hallmark of diabetes mellitus, imposes progressive and multifaceted damage across virtually all major organ systems. Sustained elevations in blood glucose contribute to the formation of advanced glycation end products (AGEs), heightened oxidative stress, and persistent low-grade inflammation, factors that collectively drive the pathogenesis of diabetic complications [4]. These include microvascular and macrovascular disorders such as nephropathy, retinopathy, neuropathy, and cardiovascular disease. In veterinary populations, particularly among aging dogs, cats, and horses, poorly controlled diabetes mellitus is associated with reduced quality of life, impaired wound healing, decreased mobility, susceptibility to infection, and ultimately shortened lifespan. Conventional therapeutic strategies for diabetes management in animals primarily focus on exogenous insulin administration, dietary modification, and, in some cases, adjunctive pharmacologic agents such as oral hypoglycemic [5]. While these interventions are effective in controlling glucose levels, they require frequent monitoring, strict adherence, and do not directly target the underlying cellular and metabolic dysfunctions—particularly those related to mitochondrial impairment—that contribute to disease progression.
Mitochondria are central to glucose metabolism and cellular redox regulation. In diabetic states, mitochondrial dysfunction is characterized by impaired oxidative phosphorylation, decreased ATP synthesis, and excessive generation of reactive oxygen species (ROS), all of which can exacerbate insulin resistance and accelerate tissue damage [6]. Targeting mitochondrial bioenergetics has therefore emerged as a promising therapeutic avenue for metabolic disorders. Low-level laser therapy (LLLT), a non-invasive modality, has been shown to enhance mitochondrial function, increasing ATP production, and modulating oxidative stress. Although most LLLT applications to date have involved localized treatment of affected tissues, the current study introduces a novel systemic approach: delivering coherent LLLT over the jugular vein to activate circulating free-floating mitochondria. These mitochondria, recently recognized as viable and functionally active in the bloodstream, may act as mobile bioenergetic units capable of modulating systemic metabolic activity. The findings of this study are notable. A single 20-minute session of jugular-applied LLLT resulted in a statistically significant 19.9% reduction in venous glucose levels within one hour of treatment. This magnitude of glucose reduction, achieved through a non-pharmacologic and non-invasive intervention, is clinically meaningful. For comparison, similar reductions in glycemia typically require several days to weeks of consistent pharmacologic treatment with insulin or oral hypoglycemic agents, which may be associated with adverse effects such as hypoglycemia, gastrointestinal distress, or injection-related complications. Furthermore, the between-group difference in glucose reduction was highly significant (p = 0.0002), reinforcing the metabolic efficacy of LLLT beyond placebo or stress-related responses. The observed effects may be mediated by insulin-independent pathways, including increased glucose transporter (GLUT4) translocation, improved endothelial function, and enhanced microvascular perfusion [7]. All of which are consistent with mitochondrial activation and improved cellular energetics.
Conclusion
This experimental study demonstrates that a single 20-minute session of jugular-applied coherent LLLT can produce a rapid and clinically meaningful reduction in blood glucose levels in diabetic dogs. By targeting circulating free-floating mitochondria, this novel approach may offer a safe, non-invasive adjunct to conventional diabetes therapies. These findings support further investigation into systemic LLLT as a promising tool for metabolic regulation in veterinary care. Long-term studies are warranted to determine whether these improvements are sustained over time and to assess the potential cumulative benefits.
References
- Giri B, Dey S, Das T, Sarkar M, Banerjee J, et al. (2018) Chronic hyperglycemia mediated physiological alteration and metabolic distortion leads to organ dysfunction, infection, cancer progression and other pathophysiological consequences: An update on glucose toxicity. Biomed Pharmacother 107:306-328.
- Iheagwam FN, Joseph AJ, Adedoyin ED, Iheagwam OT, Ejoh SA (2025) Mitochondrial Dysfunction in Diabetes: Shedding Light on a Widespread Oversight. Pathophysiology 32:9.
- Al Amir Dache Z, Otandault A, Tanos R, Pastor B, Meddeb R, et al. (2020) Blood contains circulating cell-free respiratory competent mitochondria. FASEB J 34:3616-3630.
- Nowotny K, Jung T, Höhn A, Weber D, Grune T (2015) Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules 5:194-222.
- Behrend E, Holford A, Lathan P, Rucinsky R, Schulman R (2018) 2018 AAHA Diabetes Management Guidelines for Dogs and Cats. J Am Anim Hosp Assoc 54:1-21.
- Abdul-Ghani MA, DeFronzo RA (2008) Mitochondrial dysfunction, insulin resistance, and type 2 diabetes mellitus. Curr Diab Rep 8:173-178.
- Gong L, Zou Z, Liu L, Guo S, Xing D (2021) Photobiomodulation therapy ameliorates hyperglycemia and insulin resistance by activating cytochrome c oxidase-mediated protein kinase B in muscle. Aging (Albany NY) 13:10015-10033.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.