Effect of Ferrum phosphoricum D12 on Mitochondrial Function and COX-2 Expression in Human Peripheral Blood Mononuclear Cells
by Maria Gevezova1,2, Tatyana Todorova1,2, Elitca Tanova1,2, Victoria Sarafian1,2*
1Department of Medical Biology, Medical University – Plovdiv, Bulgaria
2Research Institute at Medical University – Plovdiv, Bulgaria
*Corresponding author: Victoria Sarafian, Department of Medical Biology, Faculty of Medicine, Medical University-Plovdiv, 4000, 15 A Vasil Aprilov Blv, Bulgaria.
Received Date: 1 October 2024
Accepted Date: 7 October 2024
Published Date: 10 October 2024
Citation: Gevezova M, Todorova T, Tanova E, Sarafian V (2024) Effect of Ferrum phosphoricum D12 on Mitochondrial Function and COX-2 Expression in Human Peripheral Blood Mononuclear Cells. Curr Res Cmpl Alt Med 8: 256. https://doi.org/10.29011/2577-2201.100256
Abstract
Ferrum Phosphoricum D12 (FP D12) has antioxidant potential and anti-inflammatory action with a proven therapeutic effect in the early stages of fever, inflammation, muscle fatigue and anaemia. Therefore, we aimed to investigate the influence of different concentrations of FP D12 on mitochondrial function and inflammation in human peripheral blood mononuclear cells (PBMC). Both mitochondrial activity (Seahorse XFp) and gene expression (qPCR) of the pro-inflammatory markers IL-1β and COX-2, were measured in PBMCs, isolated from healthy volunteers (n=12). Our study provides additional evidence to the limited number of studies on homeopathic FP D12 as a medication in inflammatory conditions. PBMCs treated with FP D12 showed activated mitochondrial function and increased maximal respiration and respiratory reserve capacity. The tissue salt also reduced the gene levels of Cyclooxygenase - 2 (COX - 2) and Interleukin - 1 beta (IL - 1β) and demonstrating anti-inflammatory potential. We report new data on the effects of FP D12 on mitochondrial activity and gene expression of inflammatory molecules such as COX-2 and IL-1ß. FP D12 appears to be a reliable therapeutic agent in cases of acute inflammation and mitochondrial dysfunction.
Keywords: Ferrum phosphoricum D12; Mitochondrial activity; Cyclooxygenase – 2; Interleukin - 1 beta
Introduction
Ferrum Phosphoricum D12 (FP D12), known as the "salt of the immune system", has antioxidant and anti-inflammatory activity with a proven therapeutic effect in the early stages of fever, inflammation, muscle fatigue and anaemia [1]. These observations can be explained by the fact that iron is an essential element involved in the physiological functions of the body, such as oxygen and lipid metabolism, protein production, cellular respiration and DNA synthesis [2]. In addition, mitochondria play an important role in iron metabolism [3-5]. They are also involved in energy metabolism, and in ion homeostasis [3-5]. The altered iron metabolism is considered as a mechanism underlying anaemia and inflammation [5]. Its dysregulation is suggested to be a part of the defence machinery against pathogens as it is mediated by inflammatory cytokines and nitric oxide (NO) [6,7]. Mitochondria-associated iron functions as a cofactor in iron-sulphur cluster-containing proteins and hem-containing proteins (cytochrome bc1, cytochrome c, cytochrome c oxidase, and succinate dehydrogenase), which are involved in the electron transport chain (ETC) in the inner mitochondrial membrane [4].
Important iron-sulphur-containing mitochondrial proteins are electron-transfer flavoproteins, NADH: ubiquinone oxidoreductase, Rieske iron-sulphur protein and subunits of succinate dehydrogenase. They are also implicated in respiratory chain function or act like enzymes as lipoic acid synthase, biotin synthase, and radical S-adenosylmethionine (SAM) enzymes [4].
The role of FP D12 as an antioxidant could be essential in inflammatory diseases as it is known that that oxidative stress induces inflammatory processes [8,9]. Several molecules and complex interactions are involved in the induction of inflammation. Some of the basic players are COX-2 and IL-1 [10,11].
COX-2 (Cyclooxygenase-2) catalyses the conversion of free arachidonic acid to prostaglandins, and is triggered by bacterial endotoxins and cytokines during the acute inflammatory response [10]. The proinflammatory cytokine IL-1 is found to induce COX-2 in the central nervous system (CNS), contributing to inflammatory pain hypersensitivity [12]. On the other hand, iron is a regulatory component for the synthesis of human IL-1β (Interleukin 1 beta) [13], which may explain the important role of FP D12 in metabolic and inflammatory processes [13]. In addition, upon bacterial infection, iron leads to the production of signals for its own exporting from phagosomes thereby causing “starvation” of intracellular pathogens [14,15]. Figure 1 summarizes the basic pathways in which iron is involved in inflammation and oxidative stress.
There is a very limited information published on the homeopathic tissue salt FP D12. Only one study examines the molecular mechanisms of action of FP D12 on cell proliferation and mRNA expression of genes related in iron metabolism, antioxidant defence and inflammation but in the mouse J774A.1 macrophages cell line. There is no available investigation on the effect of FP D12 on human cells.
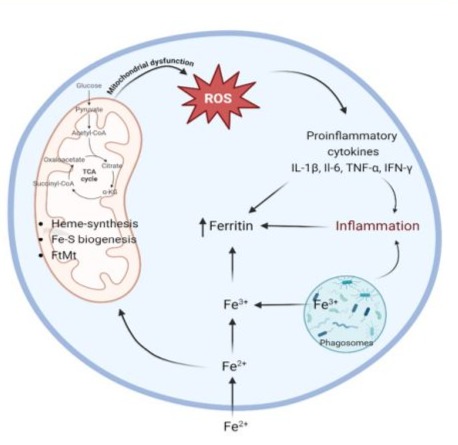
Figure 1: Cellular iron homeostasis in inflammation and oxidative stress (BioRender).
Therefore, we aimed to examine the influence of different concentrations of FP D12 on mitochondrial function and inflammation in human peripheral blood mononuclear cells (PBMCs). Our research provides additional information to the restricted number of studies available on homeopathic FP D12. We report novel data on the effects of FP D12 on mitochondrial activity and gene expression of inflammatory molecules like COX-2 and IL-1ß.
Materials and Methods
Peripheral blood from volunteers:
Twelve healthy volunteers (n=12,mean age 35±16.01) were included in the study according to the following criteria: absence of acute infections, anti-inflammatory treatment or systemic comorbidities. All participants filled in an informed consent (with inclusion and exclusion criteria noted). Venous blood was taken by venipuncture in EDTA-Vacutainer monovettes (S -Monovette 2.6 ml, Z - Sarstedt) following all safety requirements. Routine haematological tests (blood cell count, erythrocyte sedimentation rate, C-reactive protein) were performed to further assess the overall health status prior to inclusion in the study. All examined parameters were within the normal range.
Isolation of peripheral blood mononuclear cells (PBMCs):
PBMCs were isolated via gradient centrifugation using Pancoll (Pan Biotech Cat № P04-60500) following the manufacturer's protocol.
Laboratory mixture of Mineral Salts:
Тablets from FP D12 (DHU-Arzneimittel GmbH & Co. KG, Germany, provided by Alpen Pharma AG Ltd., Bulgaria) were triturated of powder and weighed. Stock solutions (10 mg/mL) were prepared as follows: 250 mg of the tablets was dissolved in 25 mL cell culture medium RPMI-1640 medium (Pan Biotech Cat № P04-22100)
The powder is placed in a 50 ml tube and dissolved in medium RPMI-1640 and vortexed (Corning® LSE™ vortex mixers, Cat # CLS6776, Merck, Darmstadt, Germany) intensively.
Treatment with FP D12:
The isolated PBMCs were divided into 3 groups - an untreated control group and cells treated with two different concentrations of FP D12 (0.0125 mg/ml and 0.0250 mg/ml), respectively. PBMCs from all 3 groups were cultured for 24 h in RPMI-1640 medium (Pan Biotech Cat № P04-22100) supplemented with 10% FBS, 1% penicillin/streptomycin in a cell culture incubator at 37 °C, 5% CO2, and high humidity in 24-well plates. The workflow of the study is shown in Fig. 2.
Before measuring mitochondrial function, cell viability and number were determined using a "LUNA" automated counter (Logos Biosystems, Anyang, South Korea). In addition, cells were aliquoted and sorted for bioenergetic profile and gene expression studies.
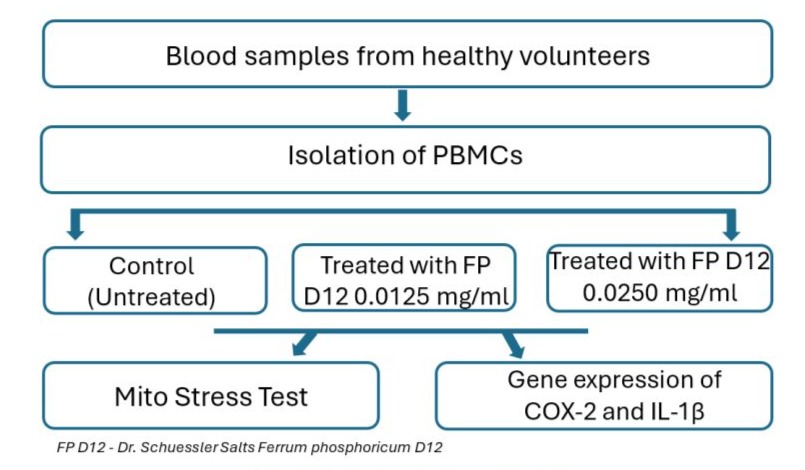
Figure 2: Design of the experimental workflow.
Real-time analysis of mitochondrial function in living cells:
To assess mitochondrial function in PBMCs, energy metabolism was explored in real time on metabolic analyzer (Seahorse XFp) that measures the oxygen consumption rate (OCR). Baseline OCR was determined first, followed by successive OCR measurements of the electron transport chain (ETC) after the injection of the following inhibitors: oligomycin (1.5 µM), carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (1 µM) (FCCP) and rotenone (0.5 µM) which were added according to the manufacturer's instructions. This allowed the determination of key parameters of mitochondrial function such as:
- Basal respiration - The initial respiratory rate in intact cells that reflects the resting adenosine triphosphate (ATP) demand.
- ATP Production - Production of ATP as a result of biological oxidation.
- Maximal Respiration - The maximum rate of oxygen consumption during oxidation of substrates (sugars, fats and amino acids).
- Spare Respiratory Capacity - The amount of extra ATP that can be produced by oxidative phosphorylation in case of a sudden increase in energy demands.
- Non-mitochondrial respiration - The consumption of oxygen in the form of oxidases and other cellular enzymes. Residual oxygen consumption may increase in the presence of a stress response indicating activated inflammation.
- Proton leak depicts the protons that migrate into mitochondrial matrix without producing ATP.
To measure Мito Stress, inhibitors (Oligomycin, FCCP and Rotenon) were added to alternate OCR. On Figure 3 the principle of mitochondrial function detection is presented.
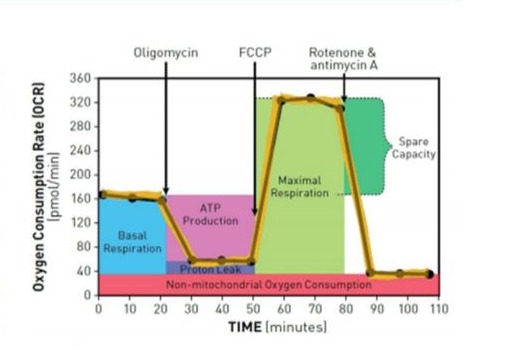
Figure 3: Schematic representation of the detection of mitochondrial function using Agilent Seahorse XF analyzer (www.agilent.com/en/products/cell-analysis-(seahorse)/mitochondrial-respiration-xf-cell-mito-stress- test).
Mito Stress Test
Before starting the experiment, the Seahorse XF cartridge was hydrated by adding 200 µL of calibrator to each working well and left overnight in a CO2-free incubator at 37 °C. On the day of the assay, the cultured PBMCs from each experimental group were centrifuged at 500 relative centrifugal force (RCF) for 10 min. After that the supernatant was removed and the pellet was resuspended in Seahorse RPMI (Seahorse XF RPMI medium, pH 7.4, 500 mL Cat. #103576- 100). PBMCs were seeded at a density of 2.105 with 180 µL of Seahorse RPMI (Seahorse XF RPMI medium, pH 7.4, 500 mL Cat. No. 103576-100) in poly-D-lysine-pretreated wells of a Seahorse XFp cartridge.
To ensure that PBMCs were evenly distributed in the wells, they were visualized by microscopy before analysis (Nikon eclipse TS 100, NIKON, Amstelveen, Netherlands). Each sample was tested in duplicate, and the results were averaged. The data were analyzed using the Seahorse Report Generator, which automatically averages the values from all duplicates per sample.
Raw OCR values were recorded without an assay inhibitor challenge (baseline readings), and in the presence of an assay inhibitor - oligomycin, carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP) and rotenone/antimycin A. The inhibitors were added consecutively to the cartridge in volumes, 20 µL, 22 µL, and 25 µL, respectively at the time points recommended by the Seahorse software. After analysis, the number of cells in each well was normalized with a Corning Cell Counter (Cytosmart, Axion BioSystems, Atlanta). Normalization is necessary because there may be differences in cell survival, division, and/or mitochondrial content during the assay, even though the number of cells is the same in each well.
Gene expression of COX-2 and IL-1β:
Isolation of total RNA
RNA was isolated from PBMCs using Trizol (Thermo Fisher Scientific, Waltham, MA, USA, Lot. No. 1559602), following the manufacturer's instructions. Samples were treated with the TURBO DNA-free kit (Thermo Fisher Scientific, Waltham, MA, USA, Lot. No. AM1907) after RNA extraction, to remove residual DNA. Extracted RNAs were quantified at 260/280 nm absorbance by NanoDrop Nucleic Acid Quantification (Thermo Fisher Scientific, Walthan, MA, United States).
Reverse transcription and qPCR
For the reverse transcription by RevertAid H Minus First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Waltham, MA, USA, Cat. No. 00648151) 2 μg of total RNA was as applied. The resulting cDNA was used to quantify COX-2 and IL-1β expression. It served as a template for amplification in a quantitative PCR reaction by Genaxon GreenMasterMix (2x) (Genaxxon bioscience GmbH, Ulm, Germany, Cat. No. M3023.0500) following the manufacturer's recommendations. qPCR was performed with the primer combinations indicated in the Table 1. For quantification of gene expression, we selected three housekeeping genes - GAPDH, ACTINβ, hUBC. The expression level of the housekeeping genes did not vary under the experimental conditions and combining them allowed reliable quantitative studies to be performed.
|
Gene |
Sequence of Forward primer |
Sequence of Reversed primer |
|
COX-2 |
5' TCCTAGTCCTCATCGCCCTC-3 |
5'-AGATTAGTCCGCCGTAGTCG -3' |
|
IL-1β |
5'-AGTGTCTGAAGCAGCCATGG-3' |
5'-AGTCATCCTCATTGCCACTGT-3' |
|
GAPDH |
5'-AGG TCCACCACTGACACGTTG-3' |
5'-AGCTGAACGGGATGCTCACT-3' |
|
ACTINβ |
5'-AGTGTGACG TGGACATCCGGA-3' |
5'-GCCAGGGCAGTGATCTCCTCCT-3' |
|
hUBC |
5'-TCCTCAGGCAGAGGTTGATCTT-3' |
5'-GGACCAAGTGCAGAGTGGACTCTT-3' |
Table 1: Nucleotide sequence of the primers used (Integrated DNA Technologies, Leuven, Belgium).
qPCR reactions were performed on a Rotor-Gene Q 600 (Qiagen, Germany). Relative mRNA levels were calculated using the 2-ΔΔCT method. All samples were analyzed in duplicate.
Statistical analysis:
The differences between the studied parameters were evaluated by ANOVA test (Tukey). Significant differences are indicated in the graphs as * = P<0.05. Statistical analysis and graphical representations were performed using GraphPad Prism V8 (San Diego, CA).
Schematic draw:
The illustration on Figure 1 was created in BioRender: Scientific Image and Illustration Software, BioRender.
Results
Effect of FP D12 on mitochondrial function:
A change in the bioenergetic parameters maximal respiration (MR) and spare respiratory capacity (SRC) was recorded after the application of FP D12 compared to control untreated cells. Baseline OCR (basal respiration) was first measured to determine differences in initial metabolic rates between treated and untreated PBMCs. No statistically significant difference in basal respiration was detected, which is evidences the homogenous start of the study (Fig. 4A).
A significant increase in MR (pmol/min) compared to the control group was reported in PBMCs treated with FP D12 0.0250 mg/ml (p=0.036).
PBMCs treated with FP D12 0.0250 mg/ml reached higher levels of oxidative respiration after FCCP injection (16.94±7.15 pmol/min), compared to those of the control group w- 5.96±2.25 pmol/min (Fig. 4B). In addition, they had significantly higher SRC than controls (Fig. 4C). SRC for FP D12 0.0250 was 110.5±41.14, while for the control group SRC was 76.66±12.87. The differences between the studied groups were statistically significant (p= 0.0152) (Table 2).
No statistically significant difference was found in the rest of the examined mitochondrial parameters (Proton leakage, ATP production and Non-mitochondrial respiration).
|
Mitochondrial parameters |
Control cells (Untreated) |
Cells treated with FP D12 0.0125 mg/ml |
Cells treated with FP D12 0.0250 mg/ml |
|
Maximal respiration (pmol/min) |
5.96±2.25 |
8.64±3.69 |
16.94±7.15 |
|
Spare respiratory capacity (pmol/min) |
76.66±12.87 |
81.63±18.6 |
110.5±41.1 |
Table 2: Bioenergetic parameters of PBMCs measured with Seahorse Bioscience Analysis.
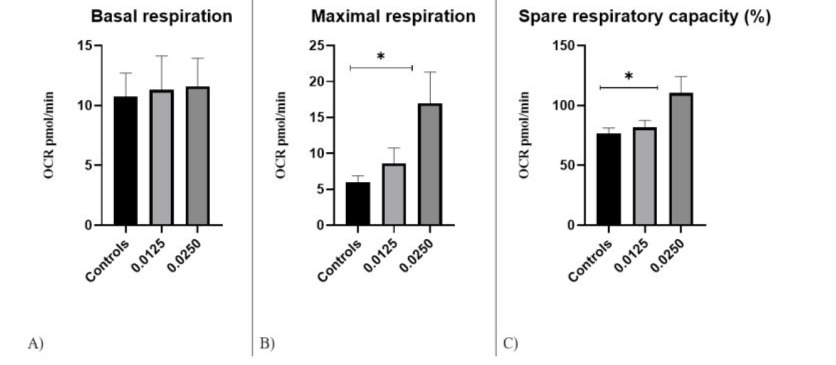
Figure 4: Respiratory metrics of PBMCs by Seahorse Bioscience Analysis: A) Basal respiration; B) Maximum respiration; C) Spare respiratory capacity in the studied groups, * P<0.05.
Gene expression of COX-2 and IL-1β:
The qPCR results showed that COX-2 expression levels were significantly decreased after FP D12 treatment, compared to controls (1.09±0.7). COX-2 mRNA expression indicated a statistically significant difference between FP D12 0.0125 mg/ml (p=0.01), and FP D12 0.0250 mg/ml (p=0.03) compared to control cells. The mean mRNA expression levels in FP D12 0.0125 mg/ml treated PBMCs was 0.26±0.1, and for FP D12 0.0250 mg/ml treated cells it was 0.29±0.1 (Fig. 5A). The data indicated that IL-1 expression levels did not differ significantly between the treated and the control groups, but there was a tendency for decreased mRNA expression after the application of FP D12 0.0125 mg/ml (1.49±0.95) and FP D12 0.0250 mg/ml (2.05±0.91) in comparison with controls (2.35±1.54)) (Fig.5B).
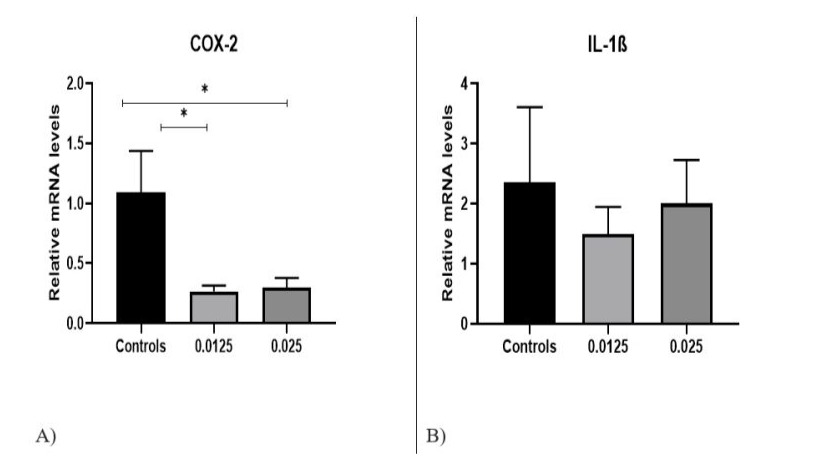
Figure 5: Gene expression of (A) COX-2 (B) IL-1β in control untreated cells and in PBMCs treated with FP D12, *p<0.05.
Discussion
Novelty:
This is the first study aimed to examine the influence of FP D12 on mitochondrial activity of human PBMCs. In addition, we investigated mRNA expression levels of the proinflammatory molecules COX-2 and IL-1β. These data demonstrate the important effect of FP D12 on mitochondrial function and its anti-inflammatory properties based on the expression of COX-2 and IL-1β.
PBMC as a model:
Human PBMCs are a suitable model for monitoring the influence of FP D12 on bioenergetic changes and inflammatory processes, because Mineral Salt № 3 stimulates metabolism, increases the activity of immune cells, has an anti-toxic effect and accelerates phagocytic activity in various inflammations and injuries (https://schuesslertissuesalts.com.au/product/ferr-phos). PBMCs are a reliable target to follow changes in the immune system [16] as they participate in the immune response, undergoing activation, proliferation and differentiation. To perform all these processes, PBMCs initiate metabolic reprogramming. It results in the activation of specific genes and proteins. In addition, PBMCs provide a more comprehensive overview of the immune system status than circulating serum or plasma markers [16].
Mitochondrial function:
The Seahorse analyzer provides a unique opportunity to measure metabolic activity in living cells. In this study, for the first time, we determined OCR in human cells treated with FP D12.
We found that the treatment with FP D12 did not affect OCR of basal respiration, which proves the homogenous onset of the experiment in all studied groups. The bioenergetic profile of the cells showed increased MR and SRC after treatment with FP D12 (0.0250 mg/ml) compared to controls.
The studied mitochondrial parameters (MR and SRC) are indicative of the viability and functional bioenergetic capacity of the organelles, as well as the overall health of the cell [17]. SRC can be considered as a predictive marker for the cell's response to endogenous/exogenous stressors [18]. Accordingly, the rate of mitochondrial respiration increases to stimulate the synthesis of more ATP, suggesting a crosstalk mechanism linking the cellular demand for ATP and the regulation of oxidative phosphorylation [19,20].
Besides ATP production, these organelles are involved in calcium regulation and in the generation of reactive oxygen species (ROS). They play an important role in cytochrome C-mediated apoptosis, having a pleiotropic effect on the cell. Our overall data show the ability of FP D12 to induce an increase in MR and SRC. It might be hypothesized that mitochondria could be stimulated for better cell adaptation.
Gene expression of COX-2 and IL-1β:
Ferrum Phosphoricum D12 has proven anti-inflammatory properties [21]. The decreased levels of COX-2 after treatment with both concentrations of FP D12 confirm these observations.
Figure 5A shows that both concentrations of FP D12 reduced the gene expression of the cytokine IL-1β, but the difference was not statistically significant. IL-1β is an important proinflammatory mediator, participating in proliferation, differentiation and apoptosis [22]. Its expression can be increased in mitochondrial dysfunction through activation of the inflammasome, which also proves the immune-mitochondrial axis [23]. In addition, IL-1β induces COX-2 expression and has a pleiotropic effect as a participant in inflammation on the one hand and as a remodeling agent on the other [14,24].
Perspectives:
Since iron is an important component of antioxidant defence [13,37], it may be assumed that it would lead to a higher resistance of PBMCs, and possibly of other cells as well, to oxidative stress. Free radical-mediated stress has been shown to be involved in fatigue syndrome and tissue damage [25]. Dysfunctional mitochondria also lead to the generation of ROS, which further aggravates cell function. In addition, the Fe ion also plays a key role in inflammatory processes by blocking tissue iron release, reduces serum iron and total iron-binding capacity, and increases serum ferritin which is considered as a part of the acute phase response [6].
Limitations of the study:
This is the first study to show the effect of FP D12 on mitochondrial activity and markers related to inflammation in human PBMCs. In the recent years, there has been a gradual increase in research related to Shussler salt №3 and its connection with genes involved in homeostasis. The limitation of the present research is the relatively small number of samples examined and the heterogeneity of PBMCs (monocyte or lymphocyte population) between healthy volunteers. It should be also considered that the pool of PBMCs comprises of several types of immune cells like T cells, B cells, monocytes, dendritic cells, and natural killer (NK) cells (~ 10%) [26,27].
Further studies are needed to reveal the effect of FP D12 on the mRNA expression of other inflammation – related genes, and on cell metabolism. In such a way the limited information in the literature on the mechanism of action of FP D12 could be enriched.
Conclusion
Novel data on the ability of FP D12 to activate mitochondrial function in PBMCs are presented. The tissue salt leads to increase in maximal respiration (MR) and spare respiratory capacity (SRC) of cells. It also decreases gene levels of COX-2 and IL-1β which indicates its anti-inflammatory potential. FP D12 seems to be a reliable therapeutic agent in cases of acute inflammation and mitochondrial dysfunction.
Ethical Guidelines: The study was approved by the Ethics committee of Medical University of Plovdiv (Protocol No C-05-2/10.04.2020)
Conflicts of Interest: The authors declare no conflict of interest.
References
- Tasinov O, Kiselova-Kaneva Y, Ivanova D, Pasheva M, Vankova D, et al. (2022) Ferrum phosphoricum D12 Treatment Affects J774A.1 Cell Proliferation, Transcription Levels of Iron Metabolism, Antioxidant Defense, and Inflammation-related Genes.Homeopathy 111:113-120.
- Abbaspour N, Hurrell R, Kelishadi R (2014) Review on iron and its importance for human health. J Res Med Sci 19:164-174.
- Lill R., Hoffmann B, Molik S, Pierik AJ, Rietzschel N, et al., (2012) The role of mitochondria in cellular iron-sulfur protein biogenesis and iron metabolism. Biochim Biophys Acta 1823:1491-1508.
- Paul BT, Manz DH, Torti FM, Torti SV (2017) Mitochondria and Iron: current questions. Expert Rev Hematol 10:65-79.
- Horowitz MP, Greenamyre JT (2010) Mitochondrial iron metabolism and its role in neurodegeneration. J Alzheimers Dis Suppl 2: S551-568.
- Konijn AM (1994) Iron metabolism in inflammation. Baillieres Clin Haematol 7:829-849.
- Gao J, Zhou Q, Wu D, Chen L (2021) Mitochondrial iron metabolism and its role in diseases. Clin Chim Acta 513:6-12.
- Hussain T, Tan B, Yin Y, Blachier F, Tossou MC, et al., (2016) Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid Med Cell Longev 2016:7432797.
- Gambini J, Stromsnes K (2022) Oxidative Stress and Inflammation: From Mechanisms to Therapeutic Approaches. Biomedicines 10:753.
- Smyth EM, Grosser T, Wang M, Yu Y, FitzGerald GA (2009) Prostanoids in health and disease. J Lipid Res 50 Suppl: S423-428.
- Cui J, Jia J (2021) Natural COX-2 Inhibitors as Promising Anti-inflammatory Agents: An Update. Curr Med Chem 28:3622-3646.
- Samad TA, Moore KA, Sapirstein A, Billet S, Allchorne A, et al., (2001) Interleukin-1beta-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature 410:471-475.
- O'Brien-Ladner AR, Nelson SR, Murphy WJ, Blumer BM, Wesselius LJ (2000) Iron is a regulatory component of human IL-1beta production. Support for regional variability in the lung. Am J Respir Cell Mol Biol 23:112-119.
- Ullah I, Lang M (2023) Key players in the regulation of iron homeostasis at the host-pathogen interface. Front Immunol 14:1279826.
- Cronin SJF, Woolf CJ, Weiss G, Penninger JM (2019) The Role of Iron Regulation in Immunometabolism and Immune-Related Disease. Front Mol Biosci 6:116.
- Sen P, Kemppainen E, Oresic M (2017) Perspectives on Systems Modeling of Human Peripheral Blood Mononuclear Cells. Front Mol Biosci 4:96.
- Memis I, Mittal R, Furar E, White I, Eshraghi RS, et al., (2022) Altered Blood Brain Barrier Permeability and Oxidative Stress in Cntnap2 Knockout Rat Model. J Clin Med 11:2725.
- Marchetti P, Fovez Q, Germain N, Khamari R, Kluza J (2020) Mitochondrial spare respiratory capacity: Mechanisms, regulation, and significance in non-transformed and cancer cells. FASEB J 34:13106-13124.
- Casanova A, Wevers A, Navarro-Ledesma S, Pruimboom L (2023) Mitochondria: It is all about energy. Front Physiol 14:1114231.
- Bennett CF, Latorre-Muro P, Puigserver P (2022) Mechanisms of mitochondrial respiratory adaptation. Nat Rev Mol Cell Biol 23:817-835.
- Tasinov O, Kiselova-Kaneva Y, Ivanova D, Pasheva M, Vankova D, et al., (2022) Ferrum phosphoricum D12 Treatment Affects J774A.1 Cell Proliferation, Transcription Levels of Iron Metabolism, Antioxidant Defense, and Inflammation-related Genes. Homeopathy 111:113-120.
- Chaudhry H, Zhou J, Zhong Y, Ali MM, McGuire F, et al., (2013) Role of cytokines as a double-edged sword in sepsis. In Vivo 27:669-684.
- Chan AH, Schroder K (2020) Inflammasome signaling and regulation of interleukin-1 family cytokines. J Exp Med 217: e20190314.
- Qu QC, Shen HH, Wang CJ, Zhang XY, Wu JN, et al., (2021) A positive COX-2/IL-1beta loop promotes decidualization by upregulating CD82. Reproduction 162:227-236.
- Kennedy G, Spence VA, McLaren M, Hill A, Underwood C, et al., (2005) Oxidative stress levels are raised in chronic fatigue syndrome and are associated with clinical symptoms. Free Radic Biol Med 39:584-589.
- Autissier P, Soulas C, Burdo TH, Williams KC (2010) Evaluation of a 12-color flow cytometry panel to study lymphocyte, monocyte, and dendritic cell subsets in humans. Cytometry A 77:410-419.
- Verhoeckx K, Cotter P, López-Expósito I, Kleiveland C, Lea T, et al., (2015) The Impact of Food Bioactives on Health: in vitro and ex vivo models. Cham (CH): Springer; 2015.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.