Chronic Exposure of Ciprofloxacin Antibiotic Residue Above the MRL Level and Its Pathophysiological Effects in Mice
by Md. Shafiqul Islam1*, Bipasha Belal1, Sharmy Dash1, Md. Shakil Islam2, Md. Mahmudul Hasan3, Kazi Rafiq1, Md. Zahorul Islam1
1Department of Pharmacology, Faculty of Veterinary Science, Bangladesh Agricultural University, Mymensingh-2202, Bangladesh.
2Department of Pharmacology and Toxicology, Faculty of Animal Science & Veterinary Medicine, Sher-e-Bangla Agricultural University, Dhaka -1207, Bangladesh.
3Department of physiology & pharmacology, Faculty of Veterinary and Animal Science, Hajee Mohammad Danesh Science and Technology University, Bangladesh.
*Corresponding author: Md. Shafiqul Islam, DVM, MS, PhD, Professor, Department of Pharmacology, Faculty of Veterinary Science, Bangladesh Agricultural University, Mymensingh-2202, Bangladesh.
Received Date: 26 July 2024
Accepted Date: 6 August 2024
Published Date: 14 August 2024
Citation: Islam MS, Belal B, Dash S, Islam MS, Hasan MM, et al., (2024) Chronic Exposure of Ciprofloxacin Antibiotic Residue Above the MRL Level and Its Pathophysiological Effects in Mice. Curr Res Cmpl Alt Med 8: 249. https://doi.org/10.29011/2577-2201.100249
Abstract
The research was investigated in mice. The mice were acclimatized and randomly divided into two groups namely the control and treated groups (n=10) respectively. The treated mice were supplied ciprofloxacin antibiotic 50ppm drinking water ad libitum for a consecutive period of one year. The body weight of treated mice was found significantly higher from 3rd month onwards. The pattern of body weight was highest in the 10th month followed by decreasing in pattern but was significant until the day of sacrifice. Treated mice showed a decreased number of lymphocytes, neutrophils & monocyte. Both ALT and AST enzymes up-regulated in treated mice. Liver histopathology showed steatosis, enlarged central vein, and infiltration of inflammatory cells into the central vein. Kidney histopathology demonstrated atrophy, fragmentation of glomeruli, degeneration & necrosis. TLC analysis revealed ciprofloxacin antibiotic residue further deposited antibiotic residue in the liver, kidney, spleen, intestine, breast muscle, and thigh muscle.
Keywords: Ciprofloxacin antibiotic; Residue; Health hazards; ALT & AST
During the last decade, poultry industries become more popular agribusiness in Bangladesh. Mostly fluoroquinolones especially ciprofloxacin antibiotic is used on a large scale in poultry production to cure or prevent diseases and to stimulate growth. Indiscriminate and extensive use of ciprofloxacin in livestock industry sometimes led to the emergence of resistance microbes [1,2]. Ciprofloxacin contaminated food of poultry & livestock may significantly affect the life & health of human and may have effect on consumer confidence in animal product.
Livestock is a potential source of animal protein. The indiscriminate use of veterinary drugs in food-producing animals leads to drug residues in animal derived products (meat, milk and eggs) and poses serious public health hazard. Irrational and inappropriate use of antibiotics in commercial chicken may cause antibiotic resistance in humans [3]. In Bangladesh, the growing commercial broiler farming is playing significantly important roles in the food value chain [3]. Chronic exposure of antibiotic residues to human health produces hypersensitivity reaction, carcinogenicity, mutagenicity, teratogenicity and the development of antimicrobial drug resistance and the possible transfer of resistant pathogens from animal food products to human [1,2] resistance [4-8]. Antibiotics use more in animal than the human and the global consumption of antibiotics in animals is almost twice that of humans [9]. Research analysis estimated that globally 63.1±1.5 tons of antibiotics are annually used in livestock [10] and more than 80% is used in food producing animals [10]. If antibiotics are misused in food producing animals they ultimately goes to human body via met, milk, eggs etc. To combat the misuses of antibiotics the World Health Organization (WHO), the American Medical Association, and the American Public Health Association have urged a ban on growth-promoting antibiotics [11]. The antibiotic residues above the maximum residue limit are considered harmful and hazard to human health. The maximum residue level (MRL) of antibiotics in food has been recognized worldwide by the public and government authorities. The public and government authorities should strictly monitor the products contain above the MRL of antibiotics or drugs when they are sold for human consumption. In veterinary practices, antibiotics are widely used as therapeutic, prophylactic and growth promoting agents for better production of livestock and poultry to meet the increasing demand [12]. Furthermore, withdrawal time and physicochemical analyses are mandatory to ensure the antibiotics in food animals do not exceed the maximum residue limit (MRL) before the food is marketed and appropriate use of antibiotics in poultry and livestock industry [6].
Good health is associated with safe food. In the recent years, farmers in every sector are trying to increase agricultural products and byproducts from paddy, vegetables, fruits, and poultry farming by using drugs, growth promoters, hormones and antibiotics legally and illegally cause antibiotics residues in the food chain and ultimately goes to the end user human body [13]. The major public health significances of antibiotics residues on humans by directly causing diseases such as hypersensitivity reaction, carcinogenicity, mutagenicity, teratogenicity, bone marrow depression, and disruption of normal intestinal flora [2,14] and indirect harm via antibiotic resistance [15]. Antibiotics are used for food production for several reasons, including their primary use as preventive or curative measures against animal infection. Others are used as growth promoters for improved feed conversion efficiency, carcass quality, and economic production [4,10].
The purpose of this study was to evaluate the food safety and health. In Bangladesh, this is the first study of long term exposure of ciprofloxacin antibiotic in residual level in mice. We found that long term exposure of antibiotic residue is hazard to animal body and produced harmful effects in vital organ in the body. Therefore ciprofloxacin antibiotic residue above the MRL level should be banned for public heath perspective in food chain.
Material and Methods
Statement of the experiment
The experiment was conducted in the Department of Pharmacology, Faculty of Veterinary Science, Bangladesh Agricultural University, Mymensingh. Swiss-Albino mice were used in this experimental.
Statement of ethical approval
The laboratory experimental mice were generated and at the end of the experiment sacrificed humanely following the ethical and welfare guidelines set by the Animal Welfare and Experimental Ethics Committee of Bangladesh Agricultural University [approval number: AWEEC/BAU/2022(15)]
Selection and preparation of the experimental shed
The animals were reared in the animal experimental room with controlled temperature (25 ± 1°C) & lighting (light/dark 12:12 h) in polypropylene cages having dimensions of 30×20×13 cm and soft wood shavings were used as bedding in every cages. They were given standard mice feed supplied by ICDDR, B (International Centre for Diarrheal Diseases and Research, Bangladesh) and water ad libitum. Animals were acclimatized to laboratory conditions at least one week prior to initiation of experiment. Mice were randomly divided into two groups namely control and ciprofloxacin antibiotic treated mice. Each group of mice consist of ten mice (n=10). Control group was kept as untreated, whereas ciprofloxacin antibiotic treated group was supplied ciprofloxacin @ 50 mg/L drinking water (50ppm) ad libitum for a period of one year. All procedures were done in accordance with the Ethical Committee of Bangladesh Agricultural University (AWEEC/ BAU/2022(15)) and in accordance with the internationally accepted principles for laboratory animal use and care.
Biosecurity measures
A strict biosecurity program was maintained inside and outside of the research laboratory as a most effective part of the disease prevention program. Entry to the experimental laboratory was highly restricted.
Collection of antibiotic
Ciprofloxacin was collected from SK+F, Dhaka, Bangladesh.
Commercial Name:Ciproflox® Vet
Generic Name: Ciprofloxacin
Formulation: Solution
Available Pack Size: 100 ml
Strength: Ciprofloxacin 10% (100 mg ciprofloxacin per ml of solution)
Trade/used dose:1ml solution per 2 Liter of drinking water (50ppm)
Weight measurement
The mice were weighed weekly and recorded in a data book. The average body weights of the mice were taken in consideration. At the end of the experiment, monthly average body weight of mice of each group was presented as mean ± SEM.
Imminent inspection of clinical signs
The mice were observed closely during the experiment and the findings were recorded accordingly. General alertness, feed intake, water intake, locomotion, and mortality were also observed regularly.
Sacrificing and sampling
At the end of the experiment, mice were sacrificed ethically for blood, serum and tissue samples for different analyses.Blood was collected into sterile heparinized and non-heparinized vials during sacrifice and were immediately stored into refrigerator separately to perform hematological tests and enzymatic analysis. Liver, kidney, spleen, intestine, breast muscle and thigh muscle samples were collected, washed individually properly in physiological saline solution and preserved at -20° C for their extraction and analysis. For histological analysis, samples were collected and preserved in neutral formalin buffer solution.
Solvents and chemicals
Analytical grade of solvents/chemicals used in the experiment.
Differential leucocytes count (DLC)
Preparation of blood smears
Two grease free, dry and clean slides with smooth and unbroken edges were taken and one of them was placed horizontally on the table. A medium sized drop of blood was taken on the left side of the slide (smearing slide). Another one (spreader slide) was placed at the middle of the smearing slide at 45° angle and was drawn backward up to touching the drop of the blood. The blood was spread up to the breath of the spreader slide. Then it was pushed forward at a continuous and medium pressure at 45° angle. A thin smear of blood was made which was dried in the air.
Staining of blood smears
Smearing slide was placed on the standing rack horizontally and added sufficient amount of Wright’s stain and allowed for 5 minutes for staining. Equal amount of distilled water was added gently blown for proper mixing and kept for 3-5 min for proper reaction. Then it was washed with running tap water and dried by air.
Identification and counting
The smear slide was placed under 10x objective of a microscope to see the staining quality. The immersion oil (a drop) was given on the focusing area of the slide and observed under 100 x objects. Randomly five fields of each slide were counted. Different DLC cells in each group were presented as mean ± SEM.
AST and ALT Enzymatic Analysis
Blood was collected from wing vein, left for 30 min for coagulation, centrize @ 7000rmp and serum was collected for biochemical AST and ALT analysis.
AST
AST and ALT both were measured as described by Fatema et al., [16].
Principle
AST catalyzes the transfer of amino group between L-Aspartate and α -ketoglutarate to form oxaloacetate and glutamate. The oxaloacetate reacts with NADH in the presence of Malate Dehydrogenase to form NAD. The rate of oxidation of NADH to NAD is measured as a decrease in absorbance which is proportional to AST activity in the sample.
Kinetic determination of the aspartate aminotransferase (AST) activity:
L-Aspartate + α - Ketoglutarate ------ (AST) ------> Oxaloacetate + L-Glutamate
Oxaloacetate + NADH + H+ ----- (Malate Dehydrogenase)-->LMalate + NAD+
Preparation
Working reagent (WR): Mix: 4 vol. (R1) buffer + 1 vol. (R2) substrate.
In assay conditions the wave length (340 nm), cuvette (1 cm light path) and constant temperature (25°/30°/37°C) were strictly maintained in all measurement. The instrument was calibrated with to zero with distilled water. 1mL working solution and 100mL of sample were taken into the cuvette. Mixed, incubated for 1 minute. Reading was taken at 0 minute and then after 1 minutes intervals for three times. Absorbance differences were calculated and expressed per minute absorbance
Calculation
ΔA/min x 1750 = U/L of AST.
ALT
Principle
ALT or Glutamate pyruvate transaminase catalyzes the reversible transfer of an amino group from alanine to a-ketoglutarate resulting in the formation of pyruvate and glutamate. The pyruvate produced is reduced to Lactate by Lactate dehydrogenase (LDH) and NADH. The resulting rate of decrease in concentration of NADH, measured photometrically, is proportional to the catalytic concentration of ALT present in the sample.
Kinetic determination of the alanine aminotransferase (ALT) activity:
L-Alanine + a- Ketoglutarate ----ALT----> Pyruvate + L-Glutamate
Pyruvate + NADH + H+ ---LDH-----> L-Lactate + NAD+
Preparation
Working reagent (WR): Mix: 4 vol. (R1) buffer + 1 vol. (R2) substrate
Procedure
In assay conditions the wave length (340 nm), cuvette (1 cm light path) and constant temperature (25°/30°/37°C) were strictly maintained in all measurement. The instrument was calibrated with to zero with distilled water. 1mL working solution and 100mL of sample were taken into the cuvette. Mixed, incubated for 1 minute. Reading was taken at 0 minute and then after 1 minutes intervals for three times. Absorbance differences were calculated and expressed per minute absorbance
Calculation
ΔA/min x 1750 = U/L of ALT.
Histopathology analysis
Tissues were fixed with 10% neutral formalin, embedded in paraffin, and then manually sectioned with a microtome to obtain 4–5 mm-thick paraffin sections. De-waxed sections are then stained with hematoxylin and eosin (H&E). The mounted specimens were observed and were scored under light microscopy [17,18].
Materials used for TLC
For the detection of antibiotic by Thin Layer Chromatography, these samples were stored in deep freeze at -20ºC until further advanced procedures were performed. Samples (heart, liver, kidney, spleen, thigh muscle and breast muscle) were blended with a mortar and pestle until tissues were mashed properly. These samples were taken into properly cleaned and sterilized petri dishes with proper care as well as covering. From this 0.5g of sample was taken into beaker with the help of electric balance and spatula. Then homogenization was done with addition of 1ml phosphate buffer (pH 7.2). After proper mixing, protein contents of these samples were precipitated with the addition of 0.25ml trichloroacetic acid (TCA) (30%) maintaining sufficient care and attention. Then the mixed samples were taken into properly cleaned and sterilized centrifuge tubes for centrifugation. Then centrifugation was performed at 7000 rpm for 20 minutes with the help of automatically time regulated centrifuge machine.
The supernatant was extracted and an equal volume of diethyl ether was added and mixed properly in order to perform de-fatation. Then the mixture was kept for 10 minutes to separate layers into an upper oily layer and bottom layer. Then these mixtures were separated from each other. After discarding the upper oily layer, only bottom layer was collected. This extraction of supernatant was repeated twice with diethyl ether. The extracts were evaporated until dryness. Then, extracts were collected and investigation was done three times a sample [19].
Statistical analysis
Statistical analysis was performed by one way ANOVA using Graphpad Prism; version 6. The results were expressed as mean ± standard error mean (S.E.M).
Results
Long term exposure of ciprofloxacin antibiotic residue above the MRL and its effect on body weight in mice
Body conditions and body weight represent vital health status in the bio-system. In Physiology animal always grows up in a regular pattern; however, hazards or diseases hamper the body weight indicating the abnormalities of the body. In some cases, over growth is an indicator of the abnormalities of the body. It is to be mentioned that mice body weight was recorded weekly but presented in the figure monthly (Figure 1). In control mice, body weight up regulated steadily up to 10th month followed by decreasing pattern until the end of the experiment (Figure 1). On the other hand, residual level of ciprofloxacin antibiotic exposure in mice up regulated body weight abnormally. On 3rd month, a significant (P<0.05) body weight differences was recorded and it was continued until the end of experiment. The highest body weight (P<0.01) was recorded on 10th month of ciprofloxacin exposure (Figure 1).
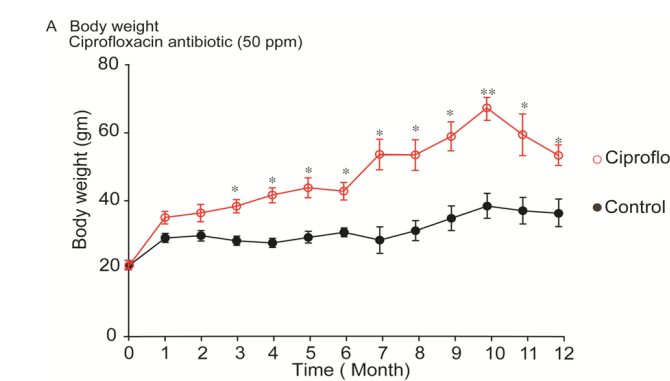
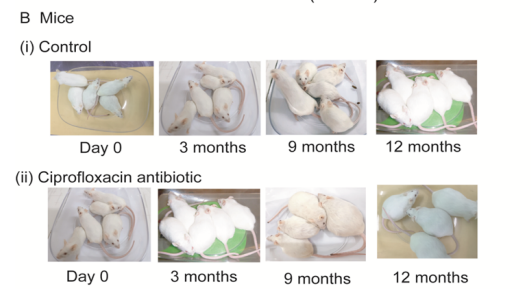
Figure 1: Mice body weight. (A), Body weight of control and ciprofloxacin residue exposure mice. Control mice were supplied drinking water; whereas, ciprofloxacin exposed mice were supplied 50ppm medicated drinking water ad libitum respectively. Mice pellet was supplied as feed for both groups of mice. (B), Representative photographs of control and ciprofloxacin residue exposed mice. *, P<0.05 and **, P<0.01; comparisons between control and ciprofloxacin groups. n=10.
Long term exposure of ciprofloxacin antibiotic residue above the MRL and its effect on immune system in mice
White blood cells are immune cells and are like warriors in the body. They come from bone marrow and are part of the lymphatic system. They fight against infection and defend the body against other foreign materials. Various types of immune cells viz. neutrophil, lymphocyte, monocyte, eosinophil, and basophil perform different functions. Antibiotic exposure mice showed lower neutrophil, lymphocyte & monocyte and higher in eosinophil & basophil than that of the control mice (Figure 2).
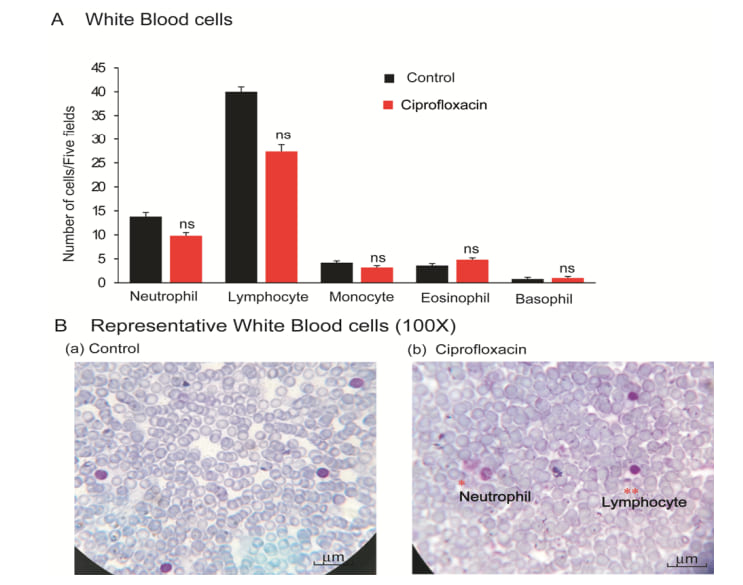
Figure 2. White blood cells. (A), Graphical presentation of neutrophil, lymphocyte, monocyte, eosinophil and basophils of control and ciprofloxacin residue exposed mice. (B), Representative photographs of WBCs of control and ciprofloxacin residue exposed mice.
Image=100x. n=10.
Long term exposure of ciprofloxacin antibiotic residue above the MRL and its effect on enzymatic analysis AST and ALT
Aspartate transaminase (AST) and alanine aminotransferase (ALT) are important enzymes in liver. These enzymes are produced by liver and indicator of body function. Under normal circumstances, these enzymes reside within liver cells to perform normal cellular functions and integrity. Liver damage causes sweep the enzymes into bloodstream.
The results of AST and ALT were represented in figure 3. Long-term exposure of ciprofloxacin antibiotic increased the both AST and ALT than the control mice; however, the increased level was found statistically insignificant (Figure 3). Therefore, AST and ALT indicated a clue that long term exposure of ciprofloxacin residue could induce liver damage.
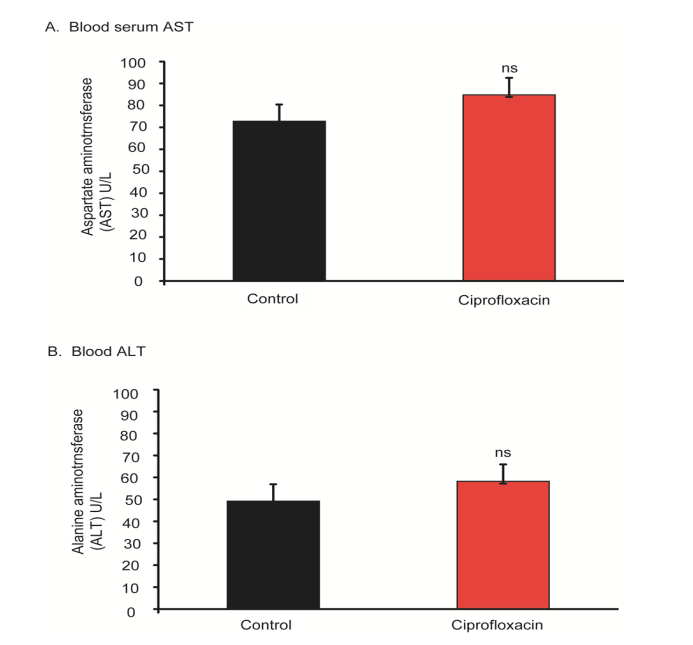
Figure 3: AST and ALT enzyme level in blood serum. (A), AST in blood serum in control and ciprofloxacin exposed mice. (B), ALT in blood serum in control and ciprofloxacin exposed mice. ns, no significant. n=10.
Long term exposure of ciprofloxacin antibiotic residue above the MRL and its effect on liver histology
In control mice, histology of liver showed ideal hepatic cells, sinusoids, central vein and kupffer’s cells. On the other hand, ciprofloxacin residue induced mice demonstrated marked steatosis, enlarged central vein, infiltration of inflammatory cells into the central vein, decreased size & shape of cells as well as decreased number of hepatic cells (Figure 4). Like AST & ALT liver histopathology also indicated that long-term exposure of ciprofloxacin residue could change the cellular functions and structural aberration in liver texture.
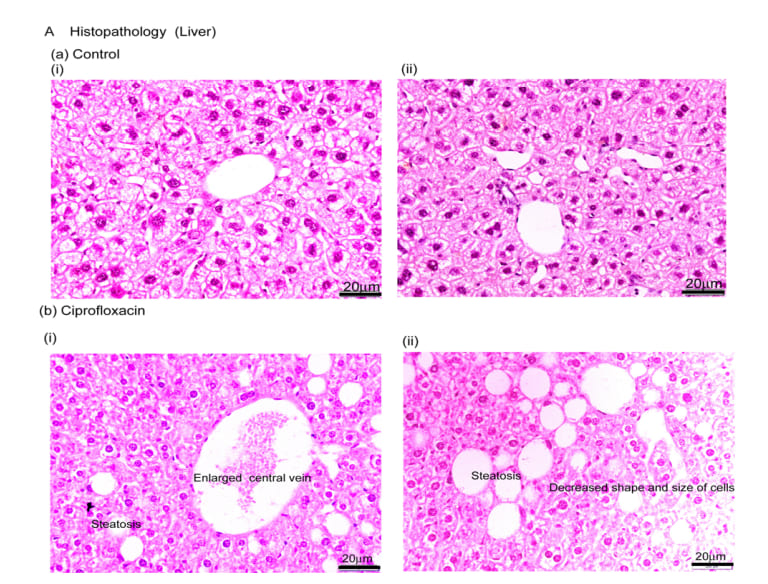
Figure 4: Liver histology. HE staining showing normal liver architecture with orderly cords of hepatocytes and sinusoidal structure in control mice(A, a-i-ii); (A, b-i-ii) ciprofloxacin residue exposed mice displayed the liver architecture with steatosis, central vein enlargement, scattered aggregates of infiltrated inflammatory cells and proliferating LPCs. Scale bar = 20µm. n=10.
Long term exposure of ciprofloxacin antibiotic above the MRL level and its effect on kidney histology
In control mice, histology of kidney demonstrated normal architecture of renal corpuscle with their glomeruli and renal tubules (Figure 5). Histology of ciprofloxacin exposure mice displayed glomerular atrophy, fragmentation of glomeruli with many renal tubule showing degeneration and necrosis as well as wide capsular space, degenerated proximal tubule with exfoliating cells, accumulation of hyaline cast within its wide lumina, wide distal tubules, and interstitial inflammatory cell infiltration are seen in ciprofloxacin residue exposure mice (Figure 5).
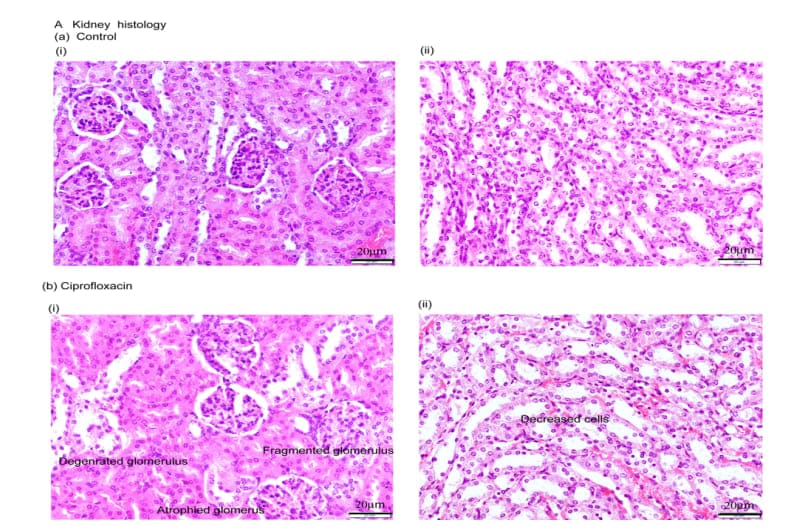
Figure 5: Kidney histology. HE staining showing normal structure of renal corpuscles with their glomeruli and renal tubules in control mice (A, a-i-ii); (A, b-i-ii) ciprofloxacin residue exposed mice showed glomerular atrophy, fragmentation of glomeruli with many renal tubules degeneration as well as wide capsular space, degenerated proximal tubule with exfoliating cells, accumulation of hyaline cast within its wide lumina and wide distal tubules. Scale bar = 20µm. n=10.
Long term exposure of ciprofloxacin antibiotic above the permissible level and its effect on colon histology
Histology of control mice showed normal colon architecture with epithelial cells, goblet cells, crypt, lumen, stromal cells (Figure 6). Like control colon structure, we found ciprofloxacin exposure did not affect the colon histology visibly (Figure 6). This is also a great information in medical science that gastrointestinal tract is not affected by antibiotic residue exposure.
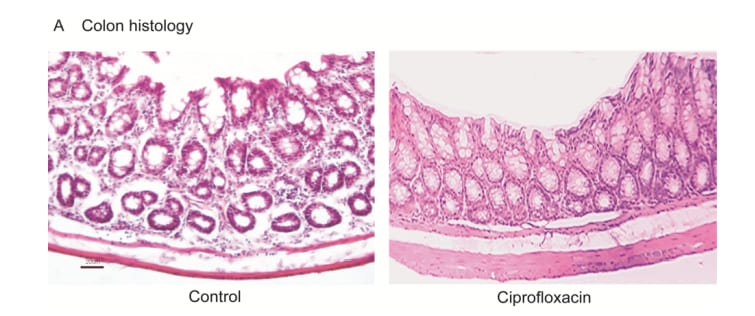
Figure 6: Colon histology. (A), HE staining showing normal colon structure in control mice and ciprofloxacin residue exposed mice.
No observable architectural aberration was observed in any section of the colon tissues in control and treated mice. Scale=20m. n=10.
Long term exposure of ciprofloxacin antibiotic residue above the MRL level and its deposition in the soft tissues in the body
Appearances of antibiotics residue in edible animal tissues remain a global issue. Therefore, antibiotics residues in food chain to safe levels are very important concern for public health importance. Human consumption of toxic levels of antibiotic residues in food origins caused several pathological defects. We analyzed ciprofloxacin residue in liver, kidney, spleen, intestine, breast muscle and thigh muscle. We found that long term exposure of ciprofloxacin residue further deposited in the soft tissues in mice (Figure 7). The level of deposition was more in liver, kidney & spleen followed by intestine, thigh muscle and breast muscle (Figure 7). This investigation gave a clue that there is no alternative ways rather to discriminate use and proper monitoring of antibiotic regimen in human and animal.
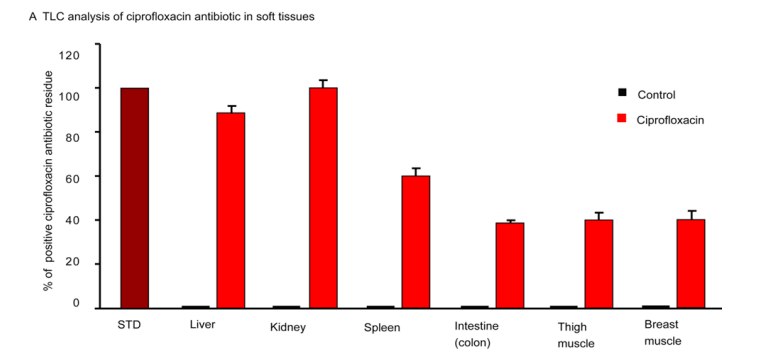
Figure 7: Thin layer chromatographic analysis. Control group of mice showing no residue, whereas, ciprofloxacin residue induced mice showing different level of residues in the soft tissues. n=10.
Discussion
The medical science is on a big question for antibiotic resistance and a steady increase in the number of pathogens that show multidrug resistance [20,21]. Antibiotic residue is a global problem; however, developing countries are more vulnerable than the developed countries. Antibiotic residues are associated with antibiotic resistance, immunosuppression, allergy, hepatotoxicity, bone marrow toxicity, nephrotoxicity, mutagenicity and even carcinogenicity [4]. In Bangladesh, this is the first study of chronic exposure of ciprofloxacin antibiotic residue (50ppm) above the MRL level in laboratory animal. We have investigated body weight, blood & enzyme analysis, histopathology etc. to understand the effects of long-term exposure of ciprofloxacin antibiotic residue in mice. Antimicrobial agents for growth promotion in farm animal were practiced since the past. Sometimes, it was practiced as sub-therapeutic doses, sometimes as prophylactic measures, sometimes directly for growth promotion [13,22,23]. We found that ciprofloxacin exposure in sub-therapeutic/or residual level to mice started significant body weight gain from 3 months onwards. The peak body weight gain was observed at 10th months of exposure followed by decreasing in pattern. As a matter of fact, the mechanisms by which antibiotics promote growth are not yet defined; however, studies have indicated that antibiotics may stimulate growth by their antimicrobial activities against pathogens and harmful bacteria.
Body weight gain/or loss is associated with the integrated functions of all major organs in the body. Liver function is determined by its internal milieu of AST & ALT level. Any kind of hepatotoxicity, infections, stresses, and diseases in the liver causes AST & ALT leaked out to blood.
Antibiotics do not directly disturb the immune system in the body. Long-term exposure and unnecessary antibiotic burden in the body reduce the ability of macrophage cells to engulf the bacteria and foreign particles [24]. Health status is truly an index of immune status of the body, although in many circumstances, the immune system alone is not enough [25]. Antibiotic residue above the MRL associated with drug toxicity, allergic reactions, dermatitis, alteration of intestinal micro flora, antibiotic resistance [5], bone marrow & nephrotoxicity [4] and carcinogenicity [4], gallstones and quinolone cholestasis [26]. In our study we found that longterm exposure of ciprofloxacin antibiotic residue altered immune cells in mice. Neutrophil, lymphocyte and monocyte were found decreased in number, while eosinophil was higher and basophil was found unchanged in antibiotic treated group. Some antibiotics not only clear the bacterial infections but also affect the immune system in the host [25].
The mode of administration might cause the accumulation of drugs in the adipose tissue, liver, kidney, muscle and other vital organs and gradually execrated from the body through biotransformation. Limiting the metabolism and the elimination of drugs can result drug residues [27]. However, if the deposition remains for a long time it starts to affect the major functions of the body. We have detected ciprofloxacin antibiotic residues in liver, kidney, spleen, breast muscle and thigh muscle by TLC analysis in mice. Other scientists also reported antibiotics residues in liver and kidney in piglets above the MRL level [28]. The great problem of antibiotic residue is to alter the organ’s function and cellular alteration. We found ciprofloxacin antibiotic residue induced glomerular atrophy, fragmentation of glomeruli, renal tubules degeneration, necrosis, accumulation of hyaline cast. In case of liver, antibiotic residue induced architectural abnormalities including fatty changes, enlarged central vein, infiltration of inflammatory cells [29]. In our study, we could find any major architectural aberration in colon tissues. This research suggested stopping the exposure of antibiotic residues through food chain to the human health.
Conclusion
Long-term exposure of ciprofloxacin residue above the MRL to mice caused liver & kidney damage and lowered immune cells in blood. Therefore, ciprofloxacin antibiotic residue above the MRL in food chain should be banned for human consumption (Figure 8).
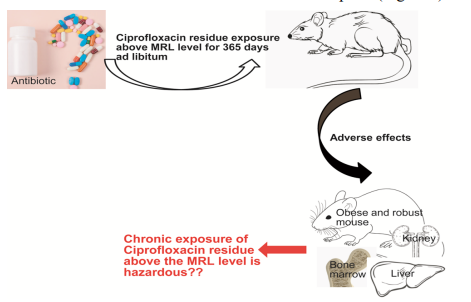
Figure 8: Schematic of the hazards of chronic exposure of ciprofloxacin antibiotic residue above the MRL level was shown.
Declaration of interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding: This work was supported in part by a Grant-in-Aid for scientific research from the Ministry of Education, Government of the People’s Republic of Bangladesh by a grant in research. (Project No. 37.20.0000.004.033.020.2016.1053; LS2019925).
References
- Arsene MM, Davares AK, Viktorovna PI, Andreevna SL, Sarra S, et al., (2022) The public health issue of antibiotic residues in food and feed: Causes, consequences, and potential solutions. Vet World 15:662671.
- Okocha RC, Olatoye IO, Adedeji OB (2018) Food safety impacts of antimicrobial use and their residues in aquaculture. Public Health Rev 39:21.
- Chowdhury S, Ghosh S, Aleem MA, Parveen S, Islam MA, et al., (2021) Antibiotic Usage and Resistance in Food Animal Production: What Have We Learned from Bangladesh? Antibiotics 10:1032.
- Bacanli M, Basaran N (2019) Importance of antibiotic residues in animal food. Food Chem Toxicol 125:462-466.
- Cunha BA (2001) Antibiotic side effects. Med Clin North Am 85:149185.
- Fei Z, Song S, Yang X, Jiang D, Gao J, et al., (2022) Occurrence and Risk Assessment of Fluoroquinolone Residues in Chicken and Pork in China. Antibiotics 11:1292.
- Muaz K, Riaz M, Akhtar S, Park S, Ismail A (2018) Antibiotic Residues in Chicken Meat: Global Prevalence, Threats, and Decontamination Strategies: A Review. J Food Prot 81:619-627.
- Pereira AM, Silva LJ, Rodrigues J, Lino C, Pena A (2018) Risk assessment of fluoroquinolones from poultry muscle consumption: Comparing healthy adult and pre-school populations. Food Chem Toxicol 118:340-347.
- Aarestrup F (2012) Sustainable farming: Get pigs off antibiotics. Nature 486:465-466.
- Van Boeckel TP, Brower C, Gilbert M, Grenfell BT, Levin SA, et al., (2015) Global trends in antimicrobial use in food animals. Proc Natl Acad Sci U S A 112:5649-5654.
- Graham JP, Boland JJ, Silbergeld E (2007) Growth promoting antibiotics in food animal production: an economic analysis. Public Health Rep. 122:79-87.
- Donoghue DJ (2003) Antibiotic residues in poultry tissues and eggs: human health concerns? Poult Sci 82:618-621.
- Hao H, Cheng G, Iqbal Z, Ai X, Hussain HI, et al., (2014) Benefits and risks of antimicrobial use in food-producing animals. Front Microbiol 5:288.
- Rather IA, Koh WY, Paek WK, Lim J (2017) The Sources of Chemical Contaminants in Food and Their Health Implications. Front Pharmacol 8:830.
- Chen J, Ying GG, Deng WJ (2019) Antibiotic Residues in Food: Extraction, Analysis, and Human Health Concerns. J Agric Food Chem 67:7569-7586.
- Fatema K, Auditi TI, Biswas S, Ayesha SB, Helal Uddin M, et al., (2023) Investigations of hemato-biochemical and histopathological parameters, and growth performance of walking catfish (Clarias batrachus) exposed to PET and LDPE microplastics. Environ Toxicol Pharmacol 102:104250.
- Islam MS, Kusakabe M, Horiguchi K, Iino S, Nakamura T, et al., (2014) PDGF and TGF-beta promote tenascin-C expression in subepithelial myofibroblasts and contribute to intestinal mucosal protection in mice. Br J Pharmacol 171:375-388.
- Islam MS, Murata T, Fujisawa M, Nagasaka R, Ushio H, et al., (2008) Anti-inflammatory effects of phytosteryl ferulates in colitis induced by dextran sulphate sodium in mice. Br J Pharmacol 154:812-824.
- Kenyon AS, Flinn PE, Layloff TP (1995) Rapid screening of pharmaceuticals by thin-layer chromatography: analysis of essential drugs by visual methods. J AOAC Int 78:41-49.
- Lundborg CS, Tamhankar AJ (2017) Antibiotic residues in the environment of South East Asia. BMJ 358:j2440.
- Reis AC, Kolvenbach BA, Nunes OC, Corvini PF (2020) Biodegradation of antibiotics: The new resistance determinants - part I. N Biotechnol 54:34-51.
- Brussow H (2015) Growth promotion and gut microbiota: insights from antibiotic use. Environ Microbiol. 17:2216-2227.
- Ryan AM (2013) Will value-based purchasing increase disparities in care? N Engl J Med 369:2472-2474.
- Yang JH, Bhargava P, McCloskey D, Mao N, Palsson BO, et al., (2017) Antibiotic-Induced Changes to the Host Metabolic Environment Inhibit Drug Efficacy and Alter Immune Function. Cell Host & Microbe. 22:757-765.e3.
- Anuforom O, Wallace GR, Piddock LV (2015) The immune response and antibacterial therapy. Med Microbiol Immunol 204:151-159.
- Hautekeete ML (1995) Hepatotoxicity of antibiotics. Acta Gastroenterol Belg 58:290-296.
- Manyi-Loh C, Mamphweli S, Meyer E, Okoh A (2018) Antibiotic Use in Agriculture and Its Consequential Resistance in Environmental Sources: Potential Public Health Implications. Molecules 23:795.
- Gamboa-Cruz C, Barros S, Vila Pouca AS, Barbosa J, Freitas A, et al., (2021) Assessing antibiotic residues in piglet liver and kidney samples: How to manage the results obtained. Food Control 122:107819.
- Kleiner DE (2018) Histopathological challenges in suspected druginduced liver injury. Liver Int 38:198-209.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.