Benefits of Botulinum Toxin A Treatment Combined with Controlled Dynamic Stretching Orthotics for The Treatment of Passive Range of Motion in Children with Cerebral Palsy
by Lieske van der Stam1-3, Markus Buelow1-3, Angela M. Kaindl1-4, Joanna Schneider1-4*
1Charité-Universitätsmedizin Berlin, Center for Chronically Sick Children, Berlin, Germany
2Charité-Universitätsmedizin Berlin, Department of Paediatric Neurology, Berlin, Germany
3German Center for Child and Adolescent Health (DZKJ), section CNS development and neurologic disease, partner site Berlin, Germany
4Charité-Universitätsmedizin Berlin, Institute of Cell Biology and Neurobiology, Berlin, Germany
*Corresponding author: Joanna Schneider, Pediatric Neurology, Charité – Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany
Received Date: 11 September 2025
Accepted Date: 19 September, 2025
Published Date: 22 September, 2025
Citation: van der Stam L, Buelow M, Kaindl AM, Schneider J (2025) Benefits of Botulinum Toxin A Treatment Combined with Controlled Dynamic Stretching Orthotics for The Treatment of Passive Range of Motion in Children with Cerebral Palsy. Int J Nurs Health Care Res 8:1670. DOI: https://doi.org/10.29011/2688-9501.101670
Abstract
Background: Children with spasticity often have contractures. The aim of our study was to determine to what extent the use of BTA in combination with controlled dynamic stretching (CDS) orthotics was superior to BTA alone. Methods: On 20 patients with cerebral palsy, BTA and CDS orthotic treatment (BTA+CDS, n=10) was compared with that with BTA alone (BTA, n=10). Passive range of motion (PROM) of the ankle joint was measured at baseline, 1 month (FU1), 3 months (FU2), and 6 months (FU3) post initial BTA injections. Results: The BTA+CDS group showed significant improvements in PROM at FU1 and FU2 compared to baseline. No improvements were seen in the BTA group (n=10) at these time points. Conclusions: Combined treatment of children with spasticity with BTA and CDS orthotics shows a benefit over treatment with BTA alone with respect to their pre-existing contractures, resulting in a greater improvement in PROM.
Keywords: Cerebral palsy; Botulinum toxin A; Dynamic orthotics; Contractures; Gait
Introduction
Cerebral palsy (CP) is the most common cause of motor disability in childhood with an incidence of 2 in 1000 live-born infants and is most frequently associated with spasticity [1,2]. Spasticity itself can result in secondary comorbidities such as muscle contractures and pain, causing gait and functional motor difficulties that are strongly associated with participation limitations [3]. Muscle contractures are the permanent shortening of a muscle tendon, which leads to a reduction in the passive range of motion (PROM) of joints. Treatment of contractures is difficult when spasticity persists. Starting in the 1980s, [4] botulinum toxin A (BTA) injections into target muscles have been used to treat spasticity by blocking presynaptic acetylcholine release in the neuromuscular end plate. The effect lasts for about 3 months, and repeated injections are necessary for a sustained effect [4-6]. During this period of reduced muscle tension, affected joints can be treated more effectively with physical therapy, stretching methods and orthoses.
Although spasticity can be reduced by BTA, pre-existing contractures cannot. Reduction of spasticity is a prerequisite for stretching and working on improving PROM of the affected joint [7]. Therefore, over the last years, BTA has been used in combination with other therapies such as physical therapy, night splints, and casting [8-10]. Serial casting has been shown to be the most effective approach in treating PROM after BTA injections [9,11]. This treatment consists of applying a cast to the affected joint in its most stretched position every two weeks for a period of six weeks to achieve the best PROM improvement. The six-week period was chosen because the peak performance of BTA is reached around 4 weeks post-injection [12]. Although this approach can improve the PROM, there are disadvantages. Common problems caused by serial casting include skin irritation, pain, pressure sores, and lower limb muscle atrophy [13-15]. Moreover, another important disadvantage is that the cast prevents active physical therapy including gait training [14]. The goal must be to reduce spasticity and improve PROM in order to work on gait pattern and functional motor abilities. The latter two play an important role in a child’s participation in everyday activities. In recent years, researchers have studied and classified the gait patterns caused by spasticity in the lower limbs in children with cerebral palsy [16-18]. The role of spasticity in gait was highlighted by the description of typical patterns. In addition, identifying the patient´s gait is also an indicator for the decision which muscles should be treated with BTA [19]. Not only the physical function, but also the activity and participation is treated at the level of the International Classification of Function, Disability and Health (ICF).
We have recently reported an improvement of muscle PROM within 12 weeks through the implementation of controlled dynamic stretching (CDS) orthotics by wearing them for just 1-2 hours a day. CDS combinational orthotics have built-in clockwork springs, both working towards knee-extension and ankle dorsal extension, thereby stretching calf and hamstring muscles. Since these CDS orthotics require only a short period of daily wear at rest, they do not interfere with active physical therapy and therefore allow for gait training. Thus, we suggested CDS orthotics should be combined with BTA, active physical therapy, and stretching during the peak functional time of BTA. Here, we investigated the effect of combined BTA and CDS orthotics on PROM and functional outcome of the lower extremity and compared it to that of isolated BTA.
Material and Methods
Participants
A retrospective study was performed on a cohort of 20 patients with cerebral palsy treated at the Center for Chronically Sick Children, Charité University Medicine Berlin between 2015 and 2021. All patients were treated as outpatients. The BTA+CDS group consisted of 10 children who were treated with the combination of BTA and CDS orthotic. The BTA group consisted of 10 children treated with BTA only (Table 1), performed between 2015 and 2021. Starting in 2019, the CDS orthotics for knee extension in combination with ankle dorsal extension were introduced at our center as a combination therapy for children treated for lower limb spasticity, mostly affected by knee flexion spasticity and calf plantar flexion spasticity. From this point on, the combination of BTA and CDS orthoses was offered to patients with both spasticity and contractures, or the likelihood of developing a contracture due to the presence of spasticity.
|
# Patient |
Sex |
Age at injection |
GMFCS level |
Target muscles |
Dose |
Side |
Additional
muscles |
|
Treated with Botulinum
Toxin A and CDS othotics (treatment group) |
|||||||
|
1 |
m |
8.5 |
II |
gastroc caput medialis muscle |
10 U |
right |
|
|
soleus muscle |
10 U |
||||||
|
gastroc caput medialis muscle |
10 U |
left |
|
||||
|
soleus muscle |
20 U |
||||||
|
2 |
m |
5.8 |
II |
gastroc caput medialis muscle |
20 U |
right |
|
|
soleus muscle |
20 U |
||||||
|
gastroc caput medialis muscle |
10 U |
left |
|
||||
|
soleus muscle |
10 U |
||||||
|
3 |
m |
7.8 |
I |
gastroc caput medialis muscle |
20 U |
right |
|
|
soleus muscle |
20 U |
||||||
|
gastroc caput medialis muscle |
20 U |
left |
|
||||
|
soleus muscle |
20 U |
||||||
|
4 |
m |
5.3 |
II |
gastroc caput medialis muscle |
40 U |
right |
|
|
soleus muscle |
40 U |
||||||
|
5 |
m |
16.5 |
IV |
gastroc caput medialis muscle |
20 U |
right |
m. biceps femoris |
|
soleus muscle |
20 U |
||||||
|
gastroc caput medialis muscle |
20 U |
left |
|
||||
|
soleus muscle |
20 U |
||||||
|
6 |
f |
11 |
V |
gastroc caput medialis muscle |
20 U |
right |
m. adductor longus |
|
gastroc caput medialis muscle |
20 U |
left |
|
||||
|
7 |
m |
6.8 |
I |
gastroc caput medialis muscle |
20 U |
left |
|
|
soleus muscle |
20 U |
||||||
|
8 |
m |
6.4 |
IV |
gastroc caput medialis muscle |
30 U |
right |
m. adductor longus |
|
soleus muscle |
30 U |
||||||
|
gastroc caput medialis muscle |
30 U |
left |
m. adductor longus |
||||
|
soleus muscle |
30 U |
||||||
|
9 |
f |
8.7 |
I |
gastroc caput medialis muscle |
25 U |
left |
|
|
soleus muscle |
25 U |
||||||
|
10 |
f |
10.3 |
II |
gastroc caput medialis muscle |
50 U |
left |
|
|
soleus muscle |
50 U |
||||||
|
Treated with Botulinum Toxin A solely (control
group) |
|||||||
|
11 |
f |
5.3 |
I |
gastroc caput medialis muscle |
30 U |
right |
m. tibialis posterior |
|
12 |
m |
4.8 |
I |
gastroc caput medialis muscle |
100 U |
left |
|
|
13 |
m |
5.1 |
I |
gastroc caput medialis muscle |
40 U |
right |
|
|
gastroc caput lateralis muscle |
20 U |
||||||
|
soleus muscle |
40 U |
||||||
|
14 |
m |
8.5 |
IV |
gastroc caput medialis muscle |
20 U |
left |
|
|
soleus muscle |
20 U |
||||||
|
15 |
m |
3 |
IV |
gastroc caput medialis muscle |
50 U |
right |
|
|
soleus muscle |
25 U |
||||||
|
gastroc caput medialis muscle |
50 U |
left |
|
||||
|
soleus muscle |
25 U |
||||||
|
16 |
m |
2.11 |
III |
gastroc caput medialis muscle |
10 U |
right |
|
|
soleus muscle |
10 U |
||||||
|
gastroc caput medialis muscle |
10 U |
left |
|
||||
|
soleus muscle |
10 U |
||||||
|
17 |
m |
5.7 |
II |
gastroc caput medialis muscle |
10 U |
left |
|
|
soleus muscle |
10 U |
||||||
|
18 |
f |
8.2 |
II |
gastroc caput medialis muscle |
150 U |
left |
|
|
19 |
m |
3.3 |
I |
gastroc caput medialis muscle |
15 U |
right |
|
|
soleus muscle |
15 U |
||||||
|
20 |
f |
3.4 |
II |
gastroc caput medialis muscle |
20 U |
left |
|
|
soleus muscle |
20 U |
||||||
Table 1: Characteristic of the BTA+CDS and BTA group. Gastroc=gastrocnemicus muscle
Measures
Inclusion criteria for a BTA+CDS treatment were (i) the presence of spastic cerebral palsy, (ii) treatment of the lower extremity, i.e. medial head of gastrocnemius and/or soleus muscles, possibly in combination with other muscle groups such as semitendinosus and semimembranosus and/or adductor longus muscles, (iii) the presence or risk of developing talocrural joint contracture due to high spasticity, risk assessment included measurements of spasticity using the modified Ashworth scale (MAS) and modified Tardieu scale (MTS) [20]. A high score in either MAS (>2) or MTS (>2) indicated risk for developing a muscle contracture, (iv) reduced PROM due to presence of spasticity, not at fault due to orthopedic deformities(v)data available for at least 3 months post-injection, and (vi) treatment with an CDS combination orthotics for knee and ankle joint. In the BTA group, the same inclusion criteria applied, except that no CDS orthotics had been used.
Exclusion criteria were (i) treatment of other muscle groups without injecting the target muscle group, such as upper extremity only, and (ii) the lack of clinical data on pre- and post-treatment periods including PROM. For BTA treatment, after informed consent target muscles with pathological activity were identified by gait analysis and spasticity quantified by standardized measurements. Dosage and number of injection sites were selected according to consensus guidelines (Dressler et al. 2021, Fehlings et al. 2000, www.wemove.org, see Table 2). BTA was dissolved with physiologic saline to a concentration of 50 U/ml. After local anesthesia with lidocaine patches or ice spray, the injection site was disinfected. The target muscles were localized by ultrasound and injected out-of-plane under visual control.
|
Muscle |
Dose range (U/kg) |
Injection sites |
|
M. adductor longus/magnus/brevis |
3- |
1-3 |
|
medial ischiocrural muscles (M. semitendinosus, M. Semimembranosus) |
3-8 |
3-4 |
|
M. gastrocneunius medialis |
3-6 |
2-4 |
|
M. soleus |
2-3 |
1-2 |
|
M. tibialis posterior |
1-2 |
1 |
Table 2: Dosage and number of injection sites with botulinum toxin A.
Procedure
Patients receiving CDS orthotics were measured at initial appointment. CDS orthotics were prescribed by treating doctor and fitted by an orthopedic technician, specialized in orthotic treatment for children with neurological disorders. At the fitting appointment, a physical therapist was present, in order to support the families with the use of the orthotic. First when the CDS orthotic was available to the family, the BTA injections would start. It was recommended to use the CDS orthotic daily: rather often with low spring intensity than seldom with a high spring intensity. The intensity of the spring force was adjusted to the patient’s pain threshold, allowing to feel the stretch but to endure at least half an hour to an hour with it. At every follow-up appointment the families would bring in the orthotic to asses it uses and adjustments were made when necessary.
We compared data from both treatment options, retrospectively. We assessed as the primary endpoint, the PROM of the talocrural joint at baseline (T0), 1 month (FU1), 3 months (FU2), and 6 months (FU3) post-injection. PROM was measured independently by the same two clinicians, a medical doctor and a physical therapist, using the zero-neutral method [21,22]. Patients who were treated bilaterally were given one value corresponding to the average of the measurements of both sides. Gross motor function data were evaluated using the Gross Motor Function Measure (GMFM-66) and were extracted from the paper and electronical patient records. Video data were recorded on an internally protected iPad and were always collected by the same two clinicians, who by default filmed all their patients pre- and post-injecting the lower extremity. Videos were filmed in frontal (ventral and dorsal) and sagittal (bilateral) planes and evaluated by the physical therapist using the Edinburgh visual gait scale (EVGS). All data were pseudonymized and stored in an electronic database. The Ethics Committee of the Charité – Universitätsmedizin Berlin approved this study (EA2/202/19).
Data Analysis
Statistical analysis was performed using IBM SPSS Statistics 26.0 (IBM Corp., NY, USA). Graphs were created using GraphPad Prism 6 (GraphPad Software, La Jolla, USA). The comparison of PROM between T0 and FU1, FU2, and FU3 were analyzed using paired t-test. The differences between BTA+CDS and BTA group were tested using t-test. Test results with p ≤ 0.05 were considered statistically significant.
Results
The cohort comprised 20 children with spastic CP affecting the lower limb, at a mean age of 6.8 years (range 2.11 – 16.5) at the time of the study. Ten children with a mean age of 8.7 years (range 5.3-16.5), a total of 16 lower limbs were treated with combined BTA and CDS orthotic (BTA+CDS group). In ten children with a mean age of 4.9 years (range 2.11-8.5), a total of 12 lower limbs were treated with BTA only (BTA group). The differences of age between both groups were significant (p=0.005, U-test).
The demographics, treated muscles and gross motor function classification system (GMFCS) levels are summarized in Table 1. In the BTA+CDS group, four patients were treated for unilateral spasticity and six for bilateral spasticity. They all received injections to treat spasticity of the medial head of the gastrocnemius and soleus muscles (n=16 injected limbs). One patient received an additional injection into the biceps femoris muscle and two into the adductor longus muscle. Most patients in this group had GMFCS motor function level II (n=4), followed by GMFCS level I (n= 3), IV (n=2), and V (n=1). In the BTA group, only two patients were treated for bilateral and eight for unilaterally spasticity. All patients in this group received injections into the medial head of gastrocnemius and soleus muscle. Most patients were categorized in GMFCS level I (n=4), followed by level II (n=3), IV (n= 2) and III (n=1). No patient was classified to have a GMFCS level V.
In the BTA+CDS group, six out of ten patients were treated bilaterally and only two out of ten in the BTA group. In addition to the lower extremity, a total of five patients from both groups were treated on the upper extremity in this study.
PROM 1-month post-injection
To identify the effect of the two treatment regimens, we analyzed the change of PROM of treated joints between baseline and one month after BTA injection (FU1), the time of maximum BTA effect. PROM measurements were available for all patients at baseline and for 16 patients at FU1 (n=9 BTA+CDS group, n=7 BTA group).
The combined treatment (BTA+ CDS) resulted in a significant improvement of the ankle joint PROM at baseline: median -1,5° (min. -12,5°, max. 10°), to the median 10° at FU1 (min. -5°, max.
17,5°, p=0.001). The BTA group showed no change in ankle joint
PROM from baseline: median 0° (min. -5°, max. 10°) and at FU1 median 0°, (min. -5°, max. 20°) (p=0.175) (Figure 1). The BTA+CDS and BTA groups differ significantly from each other (p=0.02) (Figure 2).
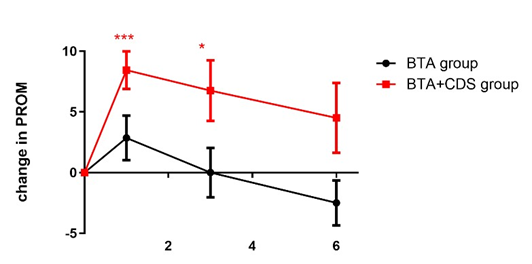
Figure 1: Change in PROM over the course of time.
Changes in PROM in the BTA+CDS group (red line) one and three months after Botulinum toxin A (BTA) injection in the affected muscles showed a significant improvement compared to baseline. There were no significant changes between baseline and follow-up time 1, 3 and 6 months after the BTA injection in the BTA group (black line). BTA+CDS group received combined therapy with BTA and CDS orthosis. The BTA group received BTA only. PROM at baseline time was set to 0 for each patient. PROM=passive range of motion. BTA= Botulinum toxin A injection. CDS= controlled dynamic stretching orthotic. *** p<0.001, * p<0.05, paired t-test. Data are shown as mean +/- SEM.
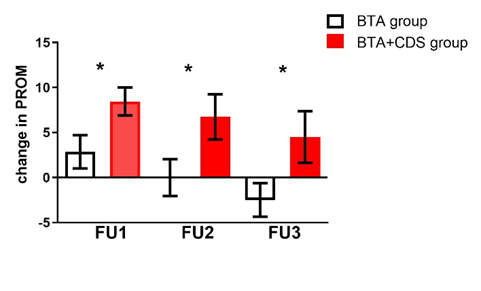
Figure 2: Difference in PROM in patients’ groups.
This figure shows the differences between BTA+CDS group (red) and BTA group (black). Both groups differ significantly from each other in terms of PROM at FU1, FU2 and FU3. PROM=passive range of motion. BTA= Botulinum toxin A injection. CDS= controlled dynamic stretching orthotic. FU1 = follow-up 1 month, FU2 = 3 months and FU3 = 6 months after Botulinum toxin A injection. * p<0.05, t-test.
PROM 3 months post-injection
To test the treatment effect after the BTA effect had tapered off, PROM was evaluated about 3 months (range 12-14 weeks) after the first BTA injection (FU2). At FU2, the joints of all ten patients within the BTA+CDS group still showed significant improvement at FU2 compared to baseline: median: 5° (min. -10°, max. 25°, p=0.02). The median of PROM at FU2 did not change significantly compared to FU1 (p=0.48). At this timepoint, when spasticity had returned (MAS ≥ 2 / MTS ≥ 2), eight out of ten patients were reinjected. Only one patient discontinued wearing the CDS orthotics due to lack of compliance.
Nine patients of the BTA group showed a median 5° (min. -10°, max. 10°, p=1.0) in terms of absolute ankle joint PROM at FU2. The median of FU2 did not change compared to FU1 (p= 0.44). Four out of ten patients had recurrent spasticity (MAS ≥ 2 / MTS ≥ 2) and received BTA reinjections. In three patients there was no information on whether they received reinjection or not. BTA+CDS and BTA groups are still significantly different (p=0.04).
PROM 6 months post-injection
Six months after initial injection (FU3), the PROM was compared to the median PROM at baseline and FU2 to follow-up changes between possible injections. In the BTA+CDS treatment it showed a median of 2.5° (min. -10°, max. 15°) at FU3. Compared to baseline (p=0.18) and FU2 (p=0.44), there were no changes. In the BTA group, the ankle PROM had worsened since FU2 to a median of -2.5° (min. -10°, max. 10°, p=0.42). Similarly, there was no change compared to the baseline (p=0.21). Finally, the difference between the two groups at FU3 stayed significant (p=0.03). The individual time course of PROM in all patients is shown in Supplement Figure 1 A-F.
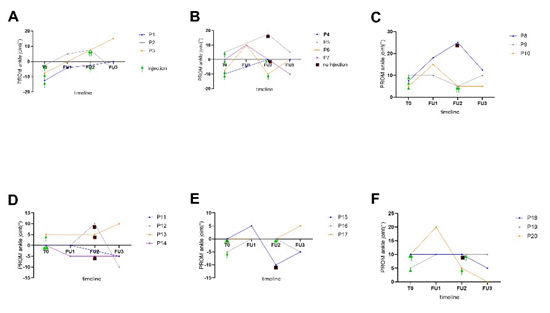
Supplement Figure 1: Individual course of PROM development within 6 months after BTA injections for BTA + CDS group (P1P10) and BTA group (P11-P20). PROM = passive range of motion. BTA= Botulinum toxin A injection. FU1 = follow-up 1 month, FU2 = follow-up 3 months and FU3 = follow-up 6 months.
GMFM at baseline and 6 months post-injections
The gross motor function measurement was evaluated only in the BTA+CDS group at baseline and 6 months post-injection. An increasing score is considered an improvement in gross motor function. At baseline, the median score was 66.35 (range 43.0 - 96.0) and changed to 69.90 (range 42.8 - 96.0, p=0.16, n=9) after 6 months post-injections.
Gait at baseline, 3- and 6-months post-injections
The gait patterns were filmed in six of the ten patients in the BTA+CDS group. Video recordings were made at baseline, 3 months and 6 months post-injections. A reduction in scores is considered as an improvement of the gait pattern. At baseline, median of gait score was 16.75 (range 7.0-26.0, n=7), which improved to a median of 12,50 (range 6.0-24.0, p=0.3, n=8). At six months, the median significantly improved to 10,50 (range 4.015.0, p=0.009, n=8) (Figure 3).
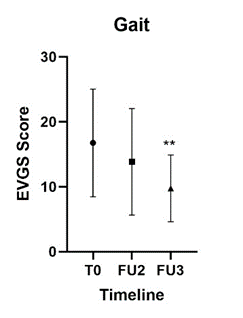
Figure 3: Changes in Gait.
This figure shows the improvements of the BTA+CDS group in gait measures by EVGS at baseline, FU2 and FU3. EVGS = Edinburgh visual gait score. T0 = baseline, FU2 = 3 months and FU3 = 6 months after Botulinum toxin A injection. ** p<0.01, t-test.
Discussion
In this study, we compared the effect of combined BTA and CDS orthotics on PROM, gait and general gross motor function of the lower extremity with that of BTA treatment alone in a pediatric cohort with spasticity. Our results support a benefit of a combinational treatment of BTA and CDS orthotic on the mean PROM as well as on gait.
With BTA alone, no significant changes in PROM were found, in contrast to BTA+CDS group, where PROM improved significantly already at 1-month post-injection, and stayed significantly improved at 3 months post-injection. The development of PROM during the 6-month period was significantly different between the BTA+CDS and BTA groups.
Several studies have highlighted the effectiveness of BTA in treating spasticity of the lower limb in children with CP.[23] Although BTA relaxes the muscles and thus provides the possibility to work on improving PROM, pre-existing contractures are not treated with BTA.[24] Our results show similar effect in the BTA group, namely non-significant improvements in PROM at the peak of the BTA effect, with a return to baseline within 3 months after a single BTA injection has lost its effect. At 6 months postinjections, there was even a deterioration in PROM, although at least 40% of the patients were re-injected with BTA after 3 months. In the BTA+CDS group, 82% of the patients were re-injected at 3 months, which could have been a major factor in the differences in outcome at 6 months post-injection. However, comparing the changes in PROM between baseline and FU2 with the changes between FU2 and FU3, the first injection appears to have had a greater impact on PROM than the second injection. A normal PROM in the ankle joint is only 20° towards dorsal extension. For contractures starting between -20° and 0°, a certain plateau can be expected, as normal PROM of the ankle joint is achieved.
Given this limitation of an effect of BTA alone, BTA is often used in combination with serial casting on the lower limb. This additional treatment has shown significantly improved contractures in the past [25,26]. Besides the benefits, the disadvantages have had a great impact on the daily lives of the children and their families. After the peak period of BTA, casting is removed without followup treatment of the contracture. As the spasticity slowly returns, there is no treatment for the contractures, which could lead to a worsening of the previously achieved PROM [27]. As shown in a study by Newman et al., contracture management with delayed serial casting has a longer effect on PROM improvement than serial casting applied directly after BTA injection [28]. Keeping the cast on for longer than six-weeks is not considered an option, as it drastically disrupts a child’s daily life. The advantage of the CDS orthotics is that it can be used after the peak time of BTA, as it does not need to be worn all day. By using the CDS orthotics for only 1-2 hours per day, not only is an improvement in PROM seen, but the treatment does not limit the patient´s daily life activities. Children can wear functional orthotics during their active time to improve their gait and only wear the CDS orthotics during their resting time. By staying active, the child treats themselves and will only see further improvements [29]. By 6 months post initial injection, patients in the BTA+CDS group of this study showed a significant improvement in gait pattern. This could be due to the ability to walk and stay active during BTA working period by wearing the CDS orthotic instead of serial casting. As serial casting can cause pain and skin ulcers, this treatment can be potentially traumatic for children and may not be repeated [15]. In our studies in 2020, the CDS orthotic was a highly accepted and well tolerated treatment option. Because of the way it is fabricated, it can be adjusted and fitted as the child grows. This could be a relief for families who already have many appointments.
The main limitation of this study is its retrospective study design, resulting in the heterogeneity between both groups. It is striking that more than half of the patients (6/10) in the BTA+CDS group were treated bilaterally, whereas only 2 out of 10 in the BTA group were treated bilaterally. Patients treated unilaterally do not necessarily use the affected leg more after BTA treatment, as the healthy side is their dominant side in everyday life. Therefore, they might not show such great improvements compared to patients treated bilaterally. Patients treated bilaterally have to focus on both affected legs, which seems like a disadvantage but allows both treated legs to be mobilized. The BTA+CDS group had the disadvantage of a significant higher age, and higher GMFCS levels, with this in mind, the BTA only group would have had better prerequisites for improvements in PROM.
In this study, we also did not compare the treatment of BTA in combination with CDS orthotics with BTA in combination with serial casting. It would be of great interest to compare both combinational treatments to improve the current standard of care. Glanzman et al. 2004 showed PROM improvements of 17° achieving a median PROM in ankle joint of 10° after combining BTA with serial casting [30]. Similarly, Hayek et al. 2010 showed improvements of the median PROM of the ankle joint of 12.2° after the first injection with BTA in combination with serial casting. After the second injection, the median was 8.3° [11]. Our study shows comparable results. If the treatment with CDS orthotics is as successful as serial casting after injecting BTA, it should be discussed whether the current treatment strategies should be changed, considering the convenience of daily life [31]. Therefore, a larger cohort should be included in a prospective, randomizedcontrolled follow-up study.
Conclusions
In conclusion, we highlight significant improvements of PROM and gait in children treated with BTA in combination with CDS orthotics when compared to those treated with BTA only. Keeping the limitations of the study in mind, a combinational therapy (BTA+CDS) appears to be a beneficial and warrants further investigations. This study data was collected retrospectively and the groups were small cohorts. A prospective, larger cohort study would be desirable to further proof this research.
Conflicts of Interest
The authors certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
Funding
This study was financially supported by albrecht GmbH. The company was not involved in the design, implementation, production or interpretation of the results.
The authors report that the sponsor had no role in the research that could have affected the outcome of this work.
AMK received research funding by the Einstein Stiftung Fellowship through the Günter Endres Fond, the Sonnenfeld-Stiftung, and the Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung, BMBF) as part of the German Center for Child and Adolescent Health (DZKJ).
Authors’ contributions
LS, JS, AMK conceptualized and designed the study. MB performed the BTA injections on the patients. LS carried out of physiotherapeutic tests (e.g. PROM etc.), collected patient data and carried out statistical analyses. LS and JS interpreted the data and drafted the initial manuscript. All authors read and approved the final version of the manuscript.
Acknowledgements
We thank our patients and their families for participation in the study. Our study was supported by the albrecht GmbH and Charité University Medicine Berlin.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Oskoui M, Coutinho F, Dykeman J, Jetté N, Pringsheim T (2013) An update on the prevalence of cerebral palsy: a systematic review and meta-analysis. Dev Med Child Neurol 6: 509-519.
- Odding E, Roebroeck ME, Stam HJ (2006) The epidemiology of cerebral palsy: incidence, impairments and risk factors. Disabil Rehabil. 4: 183-191.
- Klochkova OA, Kurenkov AL, Kenis VM (2018) Development of contractures in spastic forms of cerebral palsy: Pathogenesis and prevention. PTORS, 1: 58-66.
- Huang W, Foster JA, Rogachefsky AS (2000) Pharmacology of botulinum toxin. Journal of the American Academy of Dermatology. (2 Part 1):249-259.
- Dressler D, Saberi AF (2005) Botulinum toxin: mechanisms of action. Eur Neurol 1: 3-9.
- Barnes M (2003) Botulinum toxin--mechanisms of action and clinical use in spasticity. J Rehabil Med (41 Suppl): 56-59.
- Gracies JM (2004) Physiological effects of botulinum toxin in spasticity. Mov Disord 8: S120-128.
- Yana M, Tutuola F, Westwater-Wood S, Kavlak E (2019) The efficacy of botulinum toxin A lower limb injections in addition to physiotherapy approaches in children with cerebral palsy: A systematic review. NeuroRehabilitation. 2: 175-189.
- Kelly B, MacKay-Lyons M, Berryman S, Hyndman J, Wood E (2019) Casting Protocols Following BoNT-A Injections to Treat Spastic Hypertonia of the Triceps Surae in Children with Cerebral Palsy and Equinus Gait: A Randomized Controlled Trial. Phys Occup Ther Pediatr 1: 77-93.
- Kinnear BZ, Lannin NA, Cusick A, Harvey LA, Rawicki B (2014) Rehabilitation therapies after botulinum toxin-A injection to manage limb spasticity: a systematic review. Phys Ther 11: 1569-1581.
- Hayek S, Gershon A, Wientroub S, Yizhar Z (2010) The effect of injections of botulinum toxin type A combined with casting on the equinus gait of children with cerebral palsy. J Bone Joint Surg Br 8: 1152-1159.
- Paiva AD, Meunier FA, Molgó J, Aoki KR, Dolly JO (1999) Functional repair of motor endplates after botulinum neurotoxin type A poisoning: biphasic switch of synaptic activity between nerve sprouts and their parent terminals. Proc Natl Acad Sci U S A 6: 3200-3205.
- Milne N, Miao M, Beattie E (2020) The effects of serial casting on lower limb function for children with Cerebral Palsy: a systematic review with meta-analysis. BMC Pediatr. 1: 324.
- Moseley AM, Hassett LM, Leung J, Clare JS, Herbert RD, Harvey LA (2008) Serial casting versus positioning for the treatment of elbow contractures in adults with traumatic brain injury: a randomized controlled trial. Clin Rehabil 5: 406-417.
- Lee SJ, Sung IY, Jang DH, Yi JH, Lee JH, Ryu JS (2011) The effect and complication of botulinum toxin type A injection with serial casting for the treatment of spastic equinus foot. Ann Rehabil Med 3: 344-353.
- Winters TF, Gage JR, Hicks R (1987) Gait patterns in spastic hemiplegia in children and young adults. J Bone Joint Surg Am 3: 437441.
- Sutherland DH, Davids JR (1993) Common gait abnormalities of the knee in cerebral palsy. Clin Orthop Relat Res 288: 139-147.
- Sutherland DH, Santi M, Abel MF (1990) Treatment of stiff-knee gait in cerebral palsy: a comparison by gait analysis of distal rectus femoris transfer versus proximal rectus release. J Pediatr Orthop 4: 433-441.
- Rodda J, Graham HK (2001) Classification of gait patterns in spastic hemiplegia and spastic diplegia: a basis for a management algorithm. Eur J Neurol 5: 98-108.
- Yoo M, Ahn JH, Rha DW, Park ES (2022) Reliability of the Modified Ashworth and Modified Tardieu Scales with Standardized Movement Speeds in Children with Spastic Cerebral Palsy. Children (Basel). 6: 826.
- Gerhardt JJ, Rondinelli RD (2001) Goniometric techniques for rangeof-motion assessment. Phys Med Rehabil Clin N Am 3: 507-527.
- Ryf C, Weymann A (1995) The neutral zero method -A principle of measuring joint function. Injury. 26:1-11.
- Lukban MB, Rosales RL, Dressler D (2009) Effectiveness of botulinum toxin A for upper and lower limb spasticity in children with cerebral palsy: a summary of evidence. J Neural Transm (Vienna) 116: 319331.
- Kim H, Kolaski K (2021) Is botulinum toxin type A more effective and safer than other treatments for the management of lower limb spasticity in children with cerebral palsy? A Cochrane Review summary with commentary. NeuroRehabilitation. 49: 161-164.
- Park ES, Rha DW, Yoo JK, Kim SM, Chang WH, et al. (2010) Shortterm effects of combined serial casting and botulinum toxin injection for spastic equinus in ambulatory children with cerebral palsy. Yonsei Med J 51: 579-584.
- Booth MY, Yates CC, Edgar TS, Bandy WD (2003) Serial casting vs combined intervention with botulinum toxin A and serial casting in the treatment of spastic equinus in children. Pediatr Phys Ther 15: 216220.
- Kay RM, Rethlefsen SA, Fern-Buneo A, Wren TA, Skaggs DL (2004) Botulinum toxin as an adjunct to serial casting treatment in children with cerebral palsy. J Bone Joint Surg Am 86: 2377-2384.
- Newman CJ, Kennedy A, Walsh M, O’Brien T, Lynch B, et al. (2007) A pilot study of delayed versus immediate serial casting after botulinum toxin injection for partially reducible spastic equinus. J Pediatr Orthop 27: 882-885.
- Löwing K, Thews K, Haglund-Åkerlind Y, Gutierrez-Farewik EM (2017) Effects of Botulinum Toxin-A and Goal-Directed Physiotherapy in Children with Cerebral Palsy GMFCS Levels I & II. Phys Occup Ther Pediatr 37: 268-282.
- Glanzman AM, Kim H, Swaminathan K, Beck T (2004) Efficacy of botulinum toxin A, serial casting, and combined treatment for spastic equinus: a retrospective analysis. Dev Med Child Neurol 46: 807-811.
- Flett PJ, Stern LM, Waddy H, Connell TM, Seeger JD, et al. (1999) Botulinum toxin A versus fixed cast stretching for dynamic calf tightness in cerebral palsy. J Paediatr Child Health 35: 71-77.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.