Assessing Global Lifetime Skin Carotenoids Scores (2003 to 2025) Implications to Wellness, Lifestyle Factors Potentially Enhance or Diminish Healthspan -A Narrative Review
by Helen Knaggs1, Jesse Barrett1, Nathan Fisk1, Scott Ferguson1, Melanie Riggs1, Edwin D. Lephart2,*
1NSE Products, Inc. Provo, Utah, 84601, USA
2Department of Cell Biology, Physiology and The Neuroscience Center, College of Life Sciences, Brigham Young University, Provo, Utah, 84602, USA
*Corresponding author: Edwin D. Lephart, Department of Cell Biology, Physiology and The Neuroscience Center, College of Life Sciences, Brigham Young University, Provo, Utah, 84602, USA
Received Date: 18 November 2025
Accepted Date: 26 November 2025
Published Date: 28 November 2025
Citation: Knaggs H, Barrett J, Fisk N, Ferguson S, Lephart ED, et al. (2025) Assessing Global Lifetime Skin Carotenoids Scores (2003 to 2025) Implications to Wellness, Lifestyle Factors Potentially Enhance or Diminish Healthspan -A Narrative Review. Advs Prev Med Health Care 8: 1084. https://doi.org/10.29011/2688-996X.001084
Abstract
Skin scanner spectroscopy-based technology that non-invasively quantifies carotenoid levels addresses bias/error found in dietary questionnaires and difficulties with processing invasive blood samples. The purpose of this review was to: 1) review carotenoids as to their chemical composition and distribution in foods and supplements, 2) cover, in brief, the benefits of carotenoids in health and disease, and 3) assess the spectroscopy methods for estimating skin carotenoids by non-invasive scanning technologies. However, the primary objective of this review aimed to analyse and present (via biological computation) of global lifetime skin carotenoid scores that cover more than a 20-year interval from many different countries/regions by utilizing mega-databases. This summary review enhances our awareness and understanding of carotenoid levels worldwide and underscores the translational potential to enhance health and wellness along with determining the influence of lifestyle factors. This was accomplished using the following database searches [PubMed (US National Library of Medicine at the National Institutes of Health); Google Scholar; Science Direct and Scopus by Elsevier]. In general, the findings of this review support the use of non-invasive spectroscopy-based carotenoid quantification for estimating and validating fruit and vegetable consumption (FVC) in diverse populations of adults and children. Plus, the present results confirm and extend previous findings that lifestyle factors like smoking, high body mass index (BMI), and the lack of carotenoid intake from dietary or supplementation significantly decrease skin carotenoid levels. Thus, measuring carotenoid levels in skin non-invasively is promising as a way to potentially assess health and wellness, and by increased consumption of carotenoids, an individual can presumably improve their wellbeing for the duration of their life.
Keywords: Carotenoid; Spectroscopy measurement; Healthspan; Supplementation; Lifestyle; Nutrition
Introduction
People are living longer, and in fact, increases in life expectancy worldwide are recognized as not only a societal achievement but also have social/economic challenges and health concerns. For example, by 2030, 1 in 6 people in the world will be aged 60 years or older [1]. Notably, data show that while lifespan is increasing, the number of years with quality of life or ‘healthspan’ is decreasing (healthspan represents having good quality of life free of significant illnesses or disease) [2]. This is evident where cardiovascular, cancer, neurodegenerative and metabolic disorders [like obesity and type 2 diabetes (T2D)] represent the major four cornerstones of age-related diseases [3].
Optimal health and wellness can be greatly influenced by factors such as diet, lifestyle, quantity and quality of sleep, and social connections, etc. [4,5]. These form important foundations to maintain optimal wellbeing throughout one’s entire life [4,5]. Various factors contribute to aging or ill health, but as early as the 1950s it was proposed that aging is caused by free radical reactions [6]. However, recent theories include a link between epigenetics and lifestyle [7], and the most recent characteristics of aging include at least 9 factors that cover genetic, biochemical, molecular and cellular dysfunction/communication that have been reported elsewhere [8,9]. There is universal agreement that free radicals are involved in the physical, biochemical, and pathological changes associated with aging [10-12]. Clearly, oxidative stress leads to damage of proteins, lipids, and DNA, and this damage accumulates and increases with age and is associated with agerelated diseases [10-12].
Diet is a key contributor providing many important nutrients to combat oxidative stress and promote health [11,12]. Dietary patterns have been identified, which are associated with healthy aging such as diets rich in plant-based foods and diets having moderate amounts of healthy animal-based foods [13,14]. One of the emerging dietary interventions is carotenoid intake due to their antioxidant ability to neutralize free radicals and their antiinflammatory actions [15,16]. Carotenoids are naturally occurring pigments found in plants and have been linked to various health benefits including reducing the risk of age-related diseases and promoting healthy aging [13-16]. Studies suggest that higher dietary intake of carotenoids such as β-carotene, lutein, and zeaxanthin is associated with slower aging at the biological level [17]. For example, a cross-sectional study in 27,338 adults from NHANES 1999-2018 found that increased dietary intakes of carotenoids was associated with parameters reflecting lower biological aging [18]. Additionally, the Mediterranean diet is one of the most widely studied diets and probably has the highest carotenoid content due to its high proportion of fruits and vegetables that appears to enhance health, wellbeing and potentially enhance the lifespan [16,19-21]. However, how would an individual know (determine) their carotenoid activity levels? Carotenoids derived from fruit and vegetable consumption (FVC) are metabolized then deposited into the blood, skin and tissues that enhance antioxidant activity [16,17,22]. The best method (traditionally) for monitoring carotenoid levels was to analyse blood samples using high-performance liquid chromatography (HPLC) along with mass spectrometry (MS), which made the process invasive, expensive and time-consuming [16,17,22]. The advancements in technology have provided a non-invasive method to determine carotenoid antioxidant levels by scanning the skin by via spectroscopy measurements to quantitatively estimate and validate the FVC in diverse populations of adults and children [22]. While skin carotenoid levels have been reported using non-invasive techniques, no long-term investigations have been conducted covering global lifetime skin carotenoid scores.
Objective
The objective of this narrative review aims to analyse and present (via biological computation of very large databases) global lifetime skin carotenoid scores that cover more than a 20-year period and suggest how the antioxidant and anti-inflammatory properties of carotenoids may provide predictive health and wellness outcomes that presumably lead to enhancing or diminishing the human healthspan by lifestyle choices such as smoking, obesity, lack of dietary intake of fruits and vegetables, and/or nutritional supplementation of carotenoids.
This narrative review aims to summarize recent global and lifetime data findings to enhance our awareness and understanding of how carotenoids (estimated by skin scanner technology) underscore the translational potential of enhanced health, wellness and possibly augment longevity and the human healthspan.
Carotenoids: Chemical Nature, Distribution in Foods & Supplements
Carotenoids are essential pigments in plants with multifaceted roles including absorbing light energy to supplement chlorophyll during photosynthesis and protecting the photosynthetic machinery from excessive light damage [16,22-24]. They also provide vivid colours in flowers and fruits, but, moreover, help to quench free radicals and prevent the oxidation of lipids within the chloroplast membranes, ensuring the proper functioning of the photosynthetic system [23,24] . More than 600 naturally occurring carotenoids, fat-soluble yellow, orange, and red pigments mostly synthesized in fruits and vegetables, have been identified [16,22-24]. Traditionally, carotenoids are classified into two main structural
groups: hydrocarbon carotenoids called carotenes that only have carbon and hydrogen atoms, and oxygenated carotenoids called xanthophylls that have different functional groups (i.e., the presence or absence of oxygen atoms) in addition to carbon and hydrogen atoms (Figure 1) [16,23,24].
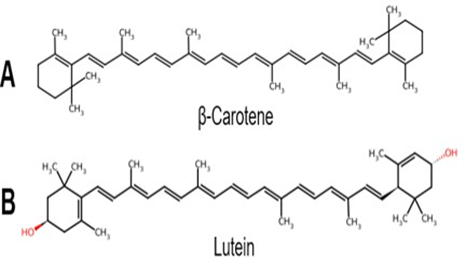
Figure 1: A. Carotene Structure and B. Xanthophyll-like Structure. This classification is based on the chemical structures containing only carbon and hydrogen atom (A) or the presence or absence of oxygen atoms (B). Chemical structures modified from ChemSpider. com (Royal Society of Chemistry, London, UK).
Humans cannot synthesize carotenoids; therefore, they must be obtained through diet from sources like fruits, vegetables, and other plants [16,22-24]. These pigments are synthesized by plants, algae, bacteria, and fungi but are absent in animals and humans, making dietary intake their sole source [16,22-24]. However, some carotenoids can also be found in foods of animal origin such as meat, egg yolks, dairy products, and fatty fish [16,22-24]. Notably, forty to sixty carotenoids have been reported in humans, but the six major carotenoids are α-carotene, β-carotene, lycopene, β-cryptoxanthin, lutein and zeaxanthin. These are metabolized and then accumulate in human plasma, skin and tissues (Figure 2) and contribute to the antioxidant activity (to reduce oxidative stress) within the body [16,20].
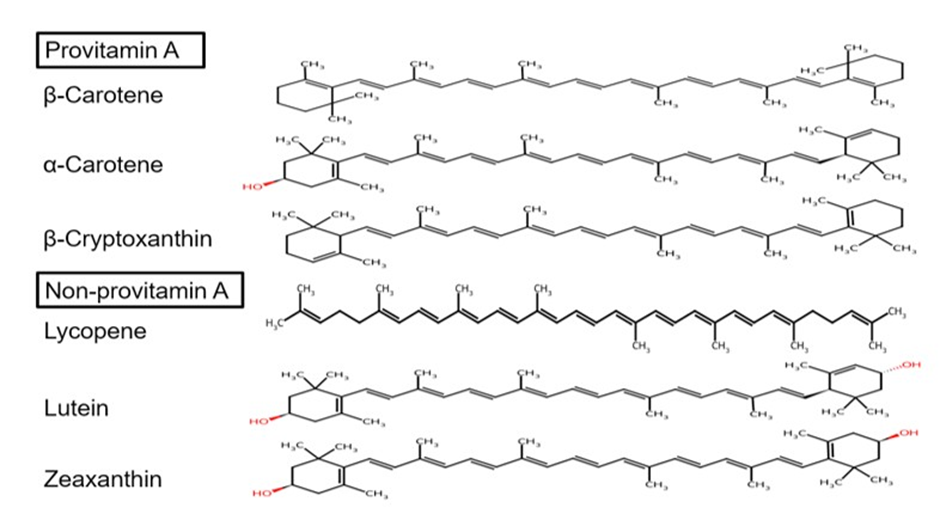
Figure 2: Structures of the Six Major Carotenoids Found in Human Blood, Skin and Tissues.
Adapted and modified from ChemSpider.com (Royal Society of Chemistry, London, UK).
In humans, vitamin A is obtained from two dietary sources: Preformed vitamin A (retinol and retinyl esters) derived from animal products such as meat, dairy, eggs and fish products [14,2224] and, Provitamin A carotenoids, which are plant pigments found typically in fruits and vegetables that include α-carotene, β-carotene, and β-cryptoxanthin. These are the carotenoids, which can be converted into retinoids for the body to use [16,22,23]. This classification is denoted by how the body converts provitamin A carotenoids into vitamin A in the intestine by the beta-carotene monooxygenase type 1 BCMO1 enzyme [22,24]. Conversely, lycopene, lutein, and zeaxanthin are classified as non-provitamin a carotenoids because they are not converted into vitamin A [22-24]. However, the non-provitamin A compounds have other important activities such as acting as potent antioxidants protecting against oxidative stress, supporting immune cell function, promoting eye/liver health and decreasing the risk of certain types of cancer [22,24,25].
The major carotenoids present in food products, β-carotene, α-carotene, β-cryptoxanthin, lycopene, lutein and zeaxanthin, contribute to the antioxidant and anti-inflammatory actions within the body to decrease oxidative stress, modulate immune defense and have protective roles in age-related diseases such as cardiovascular, metabolic, ocular, skin and bone health [21,22,24]. However, the contents of carotenoids can vary widely due to various factors such as climate, soil and cultivation [16,22,24]. The food sources that contain the six major carotenoids have been reviewed in detail elsewhere, especially on the US Department of Agriculture (USDA) weblink [26] and the dietary intake recommendations for carotenoids have also been reviewed [27]. Therefore, this information will only be summarized in brief. Examples of food sources for the carotenes include apricots, mangos, cherries, carrots and grapes where β-carotene is the most abundant, followed by α-carotene and γ-carotene, where it is present in lower concentrations [16,22,24].
For β-crytoxanthin high levels are found in Hubbard squash, peppers, tangerines and papaya. Additionally, lycopene is classified as xanthophylls (with an acyclic characteristic) (see Figure 2), that is the main pigment in tomatoes and most abundant in many reddish-pink fruits such as in watermelons and grapefruit [16,22,23]. For the other xanthophylls, lutein is the most abundant in leafy green vegetables, kale, spinach, collard greens, muster greens and Swiss chard [16,22,23]. Other sources of lutein include egg yolks, broccoli, followed by yellow and orange peppers, corn, peas, avocado, Kiwi fruit and squash [16,22,23]. Whereas, in especially green vegetables lower amounts of zeaxanthin, neoxanthin and luteoxanthin are present [16,22,24]. For example, zeaxanthin is found in egg yolks, kale, spinach, collard greens, tangerines and honeydew melons. Thus, in summary, the essential sources of carotenoids are found principally in fruits and vegetables but also in cereals and some animal food products such as meat, dairy, fish and eggs [16,22-25,27].
Dietary supplements are also a source of carotenoids, and these increase antioxidant activity for body defences (to decrease oxidative stress and provide anti-inflammatory protection) [16,22,27]. Vitamin A is available as a stand-alone supplement and found in most multivitamins (in the form of retinyl acetate, retinyl palmitate, provitamin A beta-carotene, or some combination thereof) [27,28]. A dose of 3,000 micrograms of retinol activity equivalents (RAE) of vitamin A in supplements represents the tolerable upper intake level (UL) and suggests this is the maximum amount that adults may consume daily without experiencing adverse health effects, such as liver damage or birth defects [28]. While some stand-alone vitamin A supplements typically contain 3,000 RAE, most often multivitamins contain lower doses (e.g. 750 to 1,050 mcg RAE) [28]. This limit applies to preformed vitamin A (from animal sources and supplements containing retinyl palmitate). The UL does not apply to provitamin A carotenoids (like beta-carotene) found in fruits and vegetables, as the liver regulates their conversion into active vitamin A [28]. Additionally, lutein, zeaxanthin, and lycopene are often combined in supplements particularly for eye health [15-17,22,25,27]. These carotenoids act as antioxidants protecting cells from damage and have been linked to reduced risk of eye diseases like age-related macular degeneration (AMD) [15-17]. Lutein and zeaxanthin are particularly concentrated in the macula of the eye, where they help filter blue light and protect against oxidative stress [17,29]. Lycopene, while not as directly concentrated in the eye as lutein and zeaxanthin, also has antioxidant properties and can contribute to overall eye health [15-17].
Benefits of Carotenoids in Health and Disease
Dietary carotenoids are essential compounds for the survival of photosynthetic plants, and many investigations suggest that blood carotenoid levels are associated with a lower risk of chronic and age-related diseases due to their potent antioxidant and antiinflammatory properties [15-19,21,22,24]. Additionally, some experimental investigations suggest that carotenoids may extend the lifespan (using a model organism, C. elegans), while in clinical studies carotenoid levels were associated with a reduced risk of Alzheimer’s disease [20,22,24].
Carotenoids ↑ Antioxidant Capacity and ↓ Reactive Oxygen Species (ROS)
Carotenoids act as powerful antioxidants and can extinguish singlet oxygen and neutralize free radicals preventing damage to living cells [15-19, 22, 23]. In humans, they play an indispensable role in the overall antioxidant defense system by upregulating antioxidant enzymes via the stimulating of Nrf2, which is the master gene for further antioxidant production, detoxification enzymes and mitochondrial function [15-17,20,30-38]. Additionally, carotenoids influence other cellular signaling pathways such as inhibiting the pro-inflammatory factor NFkB [17,18,20,36-38].
In this regard, several studies suggest that carotenoids can positively impact aging and healthspan [17,18,20,24,30-38]. Increased dietary intake or nutraceutical supplementation of carotenoids has been linked to lower biological aging parameters by reducing oxidative stress [20,37,39,40]. Oxidative stress (OS) is caused when the production of reactive oxygen species (ROS) exceeds the body’s antioxidant defenses and leads to cellular damage, lipid peroxidation, protein oxidation and DNA fragmentation, which can lead to multiple cells signaling dysfunctions [20,37,40-42]. In 2023, Fekete et al. showed that nutritional supplementation might be an appropriate treatment to consider for COPD patients, especially the supplementation of carotenoids with their high antioxidant activity [39]. Finally, in 2024, Chen et al. showed from 19,280 participants in the NHANES survey conducted from 2009 to 2018 that higher carotenoid intake was linked with a reduction in the hastening of biological aging highlighting their protective anti-aging actions [40].
↓ Risk of Cardiovascular Disease with Carotenoids
Several clinical investigations suggest that carotenoids may help reduce the risk of cardiovascular disease (CVD), which have been reviewed elsewhere [15,18,22,24,36,37,41,43-49]. In brief, carotenoids, found in fruits and vegetables, act as strong antioxidants and apparently prevent cholesterol oxidation in arteries (i.e., atherosclerosis), a key process in the development of CVD [17,43-47]. By preventing cholesterol oxidation in arterial walls, carotenoids slow down the buildup of plaque, enhance endothelial cell wall function, reduce blood pressure and prevent clots [44,45]. Various studies have shown that higher consumption of carotenoidcontaining foods or higher blood levels of carotenoids are linked to a lower incidence of CVD and cardiovascular mortality [45-47]. For example, Sumalla-Cano et al. (2024) performed a systematic review of 31 interventional clinical studies (randomized and non-randomized) from 2011 to 2024 [45]. They concluded from this analysis that increased blood carotenoid concentrations were associated with a reduction in CVDs (for cardiovascular risk factors based upon inflammatory biomarkers) [45]. Further analysis was conducted by Yao et al. in 2021, which reported a comprehensive review of 13 observational studies with over 100,000 subjects along with the analysis of 22 randomized clinical trials with over 1,000 individuals [48]. This analysis supported the positive effects of carotenoids against CVDs by attenuating OS and decreasing the inflammatory response [48]. Lastly, two reports from 2023 examined the association of carotenoids (total levels and the six most common carotenoids found in plasma) [44,49]. In the first study carotenoids were associated with a decrease in CVDs along with congestive heart failure (CHF), angina, heart attack and stroke [44] (which was adjusted for age, race, gender, poverty, educational level, smoking and alcohol history, BMI, physical activity and lipid panel levels), while the results from the second study reported a positive association of serum carotenoids with decreasing the risk of all-cause mortality in hypertensive adults [49].
Some of the reasons carotenoids reduce CVDs, as well as other benefits of carotenoids on health and wellbeing remain controversial, which may be due, in part, to the well-known variability in clinical trials, the dosages and types of carotenoids tested (whether known or unknown), and the limited brief time interval(s) of the interventions [45].
In the study by Obana et al. skin carotenoid levels were estimated via refraction spectroscopy, which the authors suggested might be a good indicator for recommending carotenoids to prevent CVD [46]. Finally, in 2015, Gammone et al. examined how carotenoid compounds might help lower blood pressure, reduce inflammation, and improve insulin sensitivity in muscle, liver and adipose tissues, which are factors that can contribute to cardiovascular health [47]. While the current evidence suggests that carotenoids reduce the risk of CVD, more research is warranted to understand the mechanisms by which they exert their protective effects (collectively, individually and/or synergistically).
Carotenoids ↑ Skin Health & Acts as a UV Protectant
Much research attention has been focused on carotenoid’s positive impact on skin health and as a protectant against ultraviolet (UV) rays, which have been reviewed elsewhere [51-59]. In summary, carotenoids, especially beta-carotene and lycopene, offer several skin health benefits including photo-protection against sunburn, reducing skin aging, improving skin elasticity, hydration, and increased collagen and elastin production to reduce the appearance of wrinkles and improve skin firmness [16,17,22,50,51]. Additionally, carotenoids may help reduce inflammation and improve skin texture and tone [52,53,56,57]. Recent studies suggest the role of ingestible carotenoids in reference to skin protection, which decreases OS by inhibiting the molecular biomarker intercellular adhesion molecule 1, heme oxygenase-1, and matrix metalloproteinases 1 and 9 [58,59]. However, while carotenoids offer some photo protection, they should not replace the use of broad-spectrum sunscreen products. Reviews of clinical studies have shown that lycopene to be a promising topical and nutraceutical product for cosmetic applications to decrease skin photo damage and skin photo-aging and for the treatment of melasma [56-58].
In 2021 Baswan et al. reviewed 25 intervention studies from 1970 until 2020, where the total number of subjects was 966 [56]. Only four of the randomized placebo-controlled studies will be summarized here and represent 262 subjects tested from 2018 to 2020. Ito et al. in 2018 reported a 10-week study of 23 healthy Japanese subjects (21 female & 2 male) administered 4 mg of astaxanthin per day, which showed a significant improvement in protection against UV-induced erythema along with significant improvement in skin texture [60]. In 2019, Groten et al. studied 149 healthy volunteers (34 men and 115 women) for 12 weeks administered 15 mg lycopene along with 5.8 mg of phytoene and phytofluene and 0.8 mg of β-carotene daily, which resulted in protection against UVB-induced erythema and decreased levels of proinflammatory cytokines (IL-6 and TNF-α) compared to placebo values [61]. Notably, phytoene and phytofluene are known as colorless carotenoids, which are present in apricots, tomatoes, red grapefruit, watermelons and carrots that provide good photoskin protection as well as known for lowering the risk of CVDs and certain cancers [62,63]. In 2020, Baswan et al. reported a 12week study of 60 healthy females administered a multi-carotenoid softgel containing 12.8 mg β-carotene, 3.3 mg α-carotene, 3.4 mg lutein and 0.2 mg of zeaxanthin daily that significantly reduced UV-induced pigmentation and erythema, while significantly increasing blood carotenoid levels [64]. Also in 2020, Zmitek et al. reported a 12-week study in 30 healthy Caucasian females administered 20 mg of lutein daily, which resulted in significant improvement in photo protection against UV-induced erythema compared to placebo values [65]. Finally, Varghee et al. presented a recent report, in 2025, on the mechanisms of how carotenoids enhance skin health [59].
↑ Immune Health via Carotenoids
Great research attention has been directed into the potential role of carotenoids to enhance the immune response. Vitamin A deficiency in children suffer from compromised immunity and have xerophthalmia (night blindness) and from mortality due to infections like measles or diarrhea [66]. In general, carotenoids, β-carotene and lutein specifically, are known for their role as antioxidants, and can benefit the immune system by supporting cell-mediated (increased lymphocyte production) and humoral immune (increased production of antibodies by B cells) responses, boost immune cells to stimulate phagocytic and bacteria-killing blood neutrophils and peritoneal macrophages and enhance natural killer (NK) cell cytotoxicity, which helps the immune system combat abnormal cells [67-70]. The presence of carotenoids in immune cells protects them against OS and cellular damage thus helping to ensure optimal functions that include apoptosis (programmed cell death), cell signaling, and gene regulation [67,68,70,71]. For example, carotenoids and their metabolites may interact with nuclear receptors involved in immune system regulation and influence downstream target genes and proteins involved in OS, inflammation and cellular differentiation [70]. Such immune system differentiation can act through vitamin-Aactive retinoids receptors RAR/RXR [37,70]. Earlier studies on carotenoids, in the late 1990s, showed that β-carotene enhanced NK cells especially in elderly men [72,73], and a diet low in carotenoids had a suppressive effect on the mitogenic proliferation of blood lymphocytes, which was corrected with when the subjects increased their dietary intake of vegetables rich in carotenoids [74].
Only three clinical trials will be summarized here reported from 2003 to 2023. In 2003, Watzl et al. reported a blinded, cross-over study of male subjects on a low-carotenoid diet that consumed 330 ml/day of tomato juice (37 mg/day lycopene) or carrot juice (27 mg/ day of β-carotene and 13 mg of α-carotene) for 2 weeks [75]. The results showed activation of NK cells and lymphocyte proliferation during the depletion period. The authors concluded that increased plasma carotenoids levels after vegetable juice consumption modulated immune function in a time-delayed manner in healthy men [75]. In 2011, Kim et al. reported the protective effects of carotenoids on the oxidative stress status in healthy smokers [76]. In this clinical study 39 heavy smokers (> 20 cigarettes per day) and 39 non-smokers were tested and randomly divided into 3 treatment groups that received 5, 20, or 40 mg of astaxanthin (n =13 each) once daily for 3 weeks. Oxidative stress biomarkers were quantified at baseline, after 1, 2 and 3 weeks. The results suggest that the carotenoid treatments decreased the levels of the OS biomarkers in a dose-dependent manner in heavy smokers [76]. The last clinical study examined the role of carotenoids in a common autoimmune disease such as systemic lupus erythematosus (SLE) in a crosssectional investigation. Pocovi-Gerardino et al. in 2021 studied the beneficial effect of a Mediterranean diet on disease activity and cardiovascular risk in 280 systemic lupus erythematosus patients (average age 47 years) [77]. The authors concluded that adherence to the Mediterranean diet exerted a beneficial effect on disease activity and cardiovascular risk in SLE patients.
Finally, Median-Garcia et al. examined the influence of carotenoids on the immune system by in vitro, in vivo, observational and clinical studies (of more than 40 reports) published in 2025 [78]. Based upon their review of the literature these authors concluded that: “it can be conclusively stated that carotenoids play a fundamental role in regulating and strengthening the immune system….by modulation of cytokine expression, regulation of cell differentiation and proliferation, and reduction in oxidative stress…and attenuating the progression of autoimmune diseases by modulating an overactive immune response and suppressing autoantibody production.”[78].
↑ Bone Health (↓ in Osteoporosis) with Carotenoids
Studies have shown that higher consumption of carotenoids is associated with a lower risk of osteoporosis and a higher bone mineral density (BMD), which have been reviewed elsewhere [16,22,33,79]. For example, Crupi et al. in 2023 reviewed the beneficial effects of carotenoids in health and wellbeing that highlighted carotenoids as natural antioxidants by neutralizing ROS to protect against OS and bone loss [16]. In 2009, Sahni et al. in a cross-sectional and longitudinal analyses of 334 men and 540 women (mean age 75 years) from the Framingham Osteoporosis Study found protective effects of carotenoids in bone mineral density in men and the lumbar spine in women [80].
In 2016, Xu et al. reported in a meta-analysis of five prospective and 2 case-control studies with 140,265 participants and 4,324 cases that circulating carotenoid levels of β-carotene may lower the risk of hip fracture [81]. Hayhoe et al in 2017, reported via a cross-sectional investigation (n = 14,803) for bone density status and in a longitudinal analysis (n = 25,439) in middle-aged men and women that higher plasma α-carotene and β-carotene levels were associated with higher bone density and reduced bone fracture [82].
The association of dietary carotenoid intake and bone mineral density in Korean adults age 30-75 years (that included 8,026 subjects; 3,767 males and 4,259 females) was studied from 2008 to 2011 by Regu et al. in 2017 [83].This report along with others suggested that higher β-carotene and β-cryptoxanthin along with lutein/zeaxanthin intake was linked to positive effects on bone health, particularly in postmenopausal women [80,83,84]. In addition, lycopene may offer some protection against bone loss, especially in the lumbar spine in women and pelvis (hip) region in men [80].
In 2020, Charkos et al found an inverse association between dietary carotenoids (especially β-carotene) and the risk of high fractures in a meta-analysis of nine observational studies with 190,545 participants [85]. The Charkos study is supported by broader scientific research on the role of carotenoids in bone health [16,33,80,83,84]. For example, Kan et al. in 2021 [86] reported on the link between dietary carotenoid intake and osteoporosis from the National Health and Nutrition Examination Study (NHNES) from 2005 to 2018 with more than 10,000 participants and Zheng et al in 2025 from 2023-2014 with 2,053 participants [87]. The average age in these two studies was 50 and 62 years, respectively. In the first study using the NHNES database Kan et al. showed that higher β-carotene and β-cryptoxanthin levels were linked to a decreased risk of osteoporosis [86]. While the second NHNES investigation found that dietary intake of carotenoids (especially β-carotene) was associated with decreased vertebral fractures in women [87]. Thus, carotenoids may promote bone health through their antioxidant activity particularly in reducing OS and inhibiting bone resorption while stimulating bone formation [16,22,33,80,83-87]. While promising, more research is needed, especially randomized controlled trials to fully understand the mechanisms, and to what extent carotenoids benefit bone health.
↓ Neurodegenerative Disease (Alzheimer’s) with Carotenoids
Due to the very high interest in neurodegenerative diseases (Alzheimer’s, Huntington’s, Parkinson’s and amyotrophic lateral sclerosis), there is an abundance of research suggesting that carotenoids may offer neuroprotective benefits and play a role in supporting brain health [15,17,20,33,37,88-97]. Neurodegenerative diseases are characterized by the gradual loss of neuronal structure/function and other protective mechanisms that lead to the loss of cognitive and motor function and intellectual impairment [97]. Currently, there are no cures or effective treatments for neurodegenerative diseases. However, carotenoids acting as: 1) powerful antioxidants, protect brain cells from damage by free radicals [88-96], 2) anti-inflammatory compounds to reduce inflammation, a key factor in neurodegeneration [88,96,98-101], 3) inhibitors of or reduction in microglial function [98-100] and 4) anti-plaque agents that reduce or prevent the buildup beta-amyloid (Aβ) plaques and neurofibril formation that are the hallmarks of neurodegeneration [90-92,98,99].
It is beyond the scope of this sub-subsection to present even a fraction of the available research that shows carotenoids help prevent age-related neurodegeneration. However, there appears to be a significant correlation (from several studies) between higher carotenoid levels with better cognitive function in humans [8996,98], decreased incidence of Alzheimer’s disease and disease remediation [89-91,93-96,98-101], and even protection of the physical brain structures with aging [102].
For example, in 2021 three studies examined dietary carotenoids and cognitive function. In the first study, Davinelli et al. reported on the meta-analysis of nine studies with a total of 4,402 participants (aged 45 to 75 years) found that dietary carotenoids significantly reduced the risk of cognitive impairment and dementia [89]. The second study by Liu et al. found that high blood α-carotene levels were linked with better cognitive function(s) using the Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) trial, which was a randomized controlled intervention of 295 participants over a 3-year period where plasma nutrients were quantified in subjects (65-84 years of age) [90]. The third study, Yuan et al., reported on the Rush Memory and Aging Project with 927 participants (a community-based cohort of older adults; mean age 80 years), where a clear link between dietary carotenoid intake protecting against Alzheimer’s disease incidence and neuropathology was found [91]. The Yuan et al. study confirmed and extended a previous report with 960 participants followed for over 4 years from the Memory and Aging Project (MAP), where it was found that dietary carotenoids slowed cognitive decline and neurodegeneration [92]. Finally, in 2023 Wang et al. reported via a systematic review and meta-analysis (from twenty-three studies, n = 6,610 with 1,422 patients with dementia, 435 patients with mild cognitive decline, and 4,753 controls that blood carotenoid levels were significantly lower in subjects with dementia compared to controls [93].
Additionally in this milestone cross-sectional study of 2,050 people (median age, 61 years, 61 % female) underwent magnetic resonance imaging (MRI) scans to determine whether intake of carotenoids protected against neurodegenerative parameters (e.g., choroid plexus volume, lateral ventricle volume and perivascular spaces in the brain) [92]. The investigators showed that β-carotene concentrations in serum were associated with better neurological volumes and parameters, most likely due to the antioxidant activity maintaining the glymphatic system function of the brain [102]. Specifically, the glymphatic system is a unique clearance pathway in the brain that relies on cerebrospinal fluid (CSF) and glial cells to remove metabolic waste and other harmful substances and is an emerging therapeutic approach for neurological disorders [103].
While many studies have examined carotenoids in general, other investigations have focused on lutein and lycopene that have promising in vitro and in vivo results supporting the amelioration of neurodegenerative parameters [104-109]. For instance, in 2018 Power et al. showed that supplementation with “retinal” carotenoids (lutein at 10 mg, zeaxanthin at 10 mg and meso-zeaxanthin at 2 mg) for 12-months enhanced memory in 91 healthy participants with low levels of macular pigmentation in a randomized, doubleblind, placebo-controlled clinical trial reflecting enhancement of cognitive function given the antioxidant, anti-inflammatory and neuroprotective properties of these phytochemicals [109].
In summary, carotenoids are thought to act through several mechanisms in the fight against neurodegenerative diseases by- enhanced antioxidant defenses via the Keap1/Nrf2 pathway to decrease ROS and neuroinflammation by suppressing cytokines and reducing apoptotic factors, while promoting clearance of Aβ plaques [15,17,88,102,110]. Thus, carotenoids may help to reduce the risk of neurodegenerative disorders that are associated with aging along with depression [111-121], a topic that will not be covered herein due to limiting the scope of this review.
↓ Metabolic Syndrome, Type 2 Diabetes (T2D) & Weight Loss/ Control with Carotenoids
Several studies suggest that carotenoids are associated with reduced risk of metabolic syndrome [15-17,123-128], type 2 diabetes (T2D) [15-17,37,68,88,129-131], and weight gain or obesity [132-135]. For metabolic syndrome, there is an inverse association between higher intake of total carotenoids and the presence of metabolic syndrome [123-128]. For example, two studies in 2021 and four studies in 2024 highlighted the therapeutic potential of carotenoids in preventing and managing metabolic disorders, supporting their antioxidant and anti-inflammatory roles in mitigating metabolic syndrome (MetS) [123-128]. In 2021 Takayanagi et al. examined the relationship between skin carotenoid scores (SCS) quantified by reflection spectroscopy in 1,812 Japanese participants (mean age 58 years; 859 males and 953 females) total [those with MetS (n = 151) and those without (n=1,661)] underwent physical examinations, provided lifestyle factors (smoking, medications/ prescriptions, etc.) and had their blood chemistries quantified [124]. The authors concluded that lower SCS were observed in patients with MetS compared to those without MetS, and smoking and poor lipid profiles were significantly associated with lower SCS in both groups [124]. Additionally, the report by Yen et al. in 2021 also found that obese patients displayed a higher prevalence of MetS and lower β-carotene levels compared to non-obese subjects [123].
The review by Ortega-Regules et al. [127] along with other reports in 2024 [125-128], (particularly the observational and Mendelian randomization study by Sun et al. [126]) showed that high carotenoid levels mitigated and potentially prevented MetS, which was attributed to their regulating lipid metabolism, decrease blood glucose levels, and enhancing the activity of antioxidant enzymes (via Nrf2) to prevent and protect against cellular damage. Moreover, some studies have shown that specific carotenoids like α-carotene, β-carotene, lutein and zeaxanthin have been linked to reduced risk of metabolic syndrome particularly in women [124].
In examining T2D, evidence suggests that dietary intake of carotenoids (especially β-carotene) is linked with a reduced risk of T2D [16,17,33,69,129-131]. Also, in 2021, Jiang et al showed that higher levels of carotenoids (especially β-carotene) were associated with a lower risk of T2D in a dose-dependent response in a meta-analysis [130]. Lastly, lutein and zeaxanthin supplementation has been shown to have a positive impact on regulating blood sugar levels (HbA1c) [132,133]. Weight loss has also been shown to be linked to higher intake of carotenoids, where lower levels of carotenoids increase adiposity, including increased waist circumference, and visceral and subcutaneous fat mass [130,133-135]. From several studies evidence suggests that carotenoids promote fatty acid oxidation, which may account, in part, for lower weight gain [133-135]. For instance, Yao et al. in 2021 in a systematic and meta-analysis review showed a clear association in the prevalence in overweight or obese subjects that displayed low carotenoid levels [135]. Moreover, carotenoids have been shown to improve obesity and fatty liver disease via the gut microbiome [132,136,137]. While these studies show associations, they may not prove causation, more randomized controlled trials are needed to confirm the causal link between carotenoids and reduced risk of metabolic syndrome, T2D, and weight gain.
Summary Linking Carotenoid Intake to Enhancing the Body’s Antioxidant Capacity and Potentially the Human Healthspan
In summary carotenoids act through a variety of mechanisms to enhance healthspan. For example, they influence gene expression by regulating transcription factors and signal transduction pathways involved in aging [15-17,33,37]. Carotenoids may enhance SIRT1 activity, a critical enzyme linked to cellular longevity plus stress resistance while at the same time can reduce chronic inflammation contributing to age-related disease by inhibiting NFkB signaling [33,37,88]. Carotenoids exert additional antioxidant activity by neutralizing ROS, inhibiting additional inflammatory compounds, and modulating cytokine production like interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), which are involved in systemic inflammation [15-17,37,88]. Carotenoids also improve mitochondrial function through their antioxidant activity by reducing oxidative damage and enhancing cellular energy metabolism [22,24,36,37,68]. Also, more direct effects on immune modulation include the ability of β-carotene to increase immunoglobulin synthesis, supporting adaptive immune defenses by stimulating macrophage function and T-cell proliferation, to strengthen innate immunity [36,37,67,68]. Carotenoid supplementation has been linked to protection from infections by fortifying both the epithelial barrier and immune surveillance mechanisms [59,68,138,139]. In this regard, Zhuang et al, in 2022, examined the effects of oral carotenoids on oxidative stress and other parameters in a 20-year meta-analysis showing that intake duration of 8 weeks should be sufficient to reach effective systemic concentrations for improved physiological function [140]. Finally, Shanaida et al. in 2025, reported on the antiaging benefits of nutraceutical, pharmaceutical and cosmetic applications [141].
Spectroscopy Methods for Quantification of Skin Carotenoids (introduction and peer-reviewed reports)
Since humans cannot synthesize carotenoids, the concentrations of these pigments traditionally were determined in blood samples, which provided the best markers of FVC through foods or supplements [143-146]. However, due to the invasive nature of blood collections and the associated high costs of conducting large-scale studies alternative methods have emerged, where noninvasive techniques for estimating carotenoid levels that reflect FVC were developed and have been in use from 10 to over 20 years that use spectroscopy-based technologies [143-146]. The two major methods employed to determine skin carotenoid levels are presented, in brief, below.
RS Spectroscopy-based Skin Carotenoid Measurements
Pressure-Mediated Reflection Spectroscopy (RS) uses a broad-band light source (460-500 nm) to measure the levels of skin carotenoids [143,144,146]. Pressure is applied during the measurement to temporarily limit blood flow minimizing interference from hemoglobin [143,146]. This RS method is considered to be more cost-effective and portable compared to Resonance Raman Spectroscopy (RRS), and the Veggie Meter® is an example of an RS-based device, which was invented in 2015 [144]. RS-based measurements have shown good correlations in infants, children, adolescents and adults using both RRS and plasma carotenoid levels as reported in many journal articles and in several reviews over the past 10 years [143-146].
For example, Radtke et al. in 2020 published a systematic review, which covered over 20 studies on the validity of spectroscopybased skin carotenoid measurements as an estimate for fruit and vegetable intake [145]. In 2025, Wu et al. reported that RS technology quantification of skin carotenoid scores (SCS) highly correlated with plasma concentrations (determined by HPLC) for all the major dietary carotenoids compounds except for lycopene [147]. Additionally, Hwang et al. in 2023 and Ahn et al. in 2024 evaluated the Veggie Meter in quantifying skin carotenoid levels in randomized control trials [148,149]. The first study showed that the RS method could be an alternative to the RRS technology in testing 80 participants in a randomized control trial [148], and the second study, also with 80 subjects where skin and blood carotenoid levels were quantified weekly, demonstrated that after 4 weeks the RRS method corresponded to serum carotenoid concentrations (determine by HPLC), which supported using SCS to assess carotenoid FVI [149].
Furthermore, the use of the Veggie Meter as a tool to objectively estimate FVI has been studied in: a) pre-school and school-based nutritional meals [151,152], b) adolescents in Southern Italy that reflected carotenoid consumption on a Mediterranean diet [153], c) in positive pregnancy outcomes [154], d) older urban adults [155], and e) among American indigenous families of the Osage Nation (in children, adolescents and adults) [156]. In addition, some studies noted that obesity and smoking significantly decreased SCS [152,154-156].
In another clinical study, the RS method quantified SCS in a 6-week randomized control feeding trial in 162 subjects of racially and ethnically diverse origins (25 % Black, 25 % Asian, 27 % non-Hispanic White and 23 % Hispanic) showed that Veggie Meter SCS reflected daily changes in FVI, but this study was not statistically powered to determine differences between the racial/ ethnical groups [157].
Johnston et al. in 2025, reported the correlation between Veggie Meter SCS to self-reported carotenoid FVI among older adults in a diabetic prevention program in a geriatric population (n = 79, age > 65 years) [158]. The author’s findings supported the Veggie Meter SCS that reflected carotenoid FVI records and suggested the potential utility of Veggie Meter to assess carotenoid status in older adults with prediabetes [158]. However, this positive correlation was not significant in smokers or individuals with high BMIs that displayed lower SCS [158].
In 2023, Obana et al. demonstrated good inter-device correspondence of the Veggie Meter’s reliability and accuracy to estimate SCS to FVI [159]. Finally, Hasin et al. in 2023 and Obana in 2025 reported systematic reviews of RS-based technology to quantify SCS and their association in health and disease [160,161] and more than 50 peer-reviewed articles have been published since its invention.
For the Veggie Meter, scores range from 0 to 800 with higher scores indicating a greater presence of carotenoids suggesting more FVC. Each 100 units on the Veggie Meter is thought to generally correspond to about one cup of FVC daily [144]. Notably, the manufacturer of the Veggie Meter states it is not designed to give specific dietary advice [162]. However, a skin carotenoid score at 350 or below suggests an inadequate FVC, while a score above 400 indicates good FVC [144,162]. An example of the Veggie Meter display is shown in Figure 3. While figure 3 is just an example, many reports describe skin carotenoid scan levels to range between 250 to 350 [144,148,160].
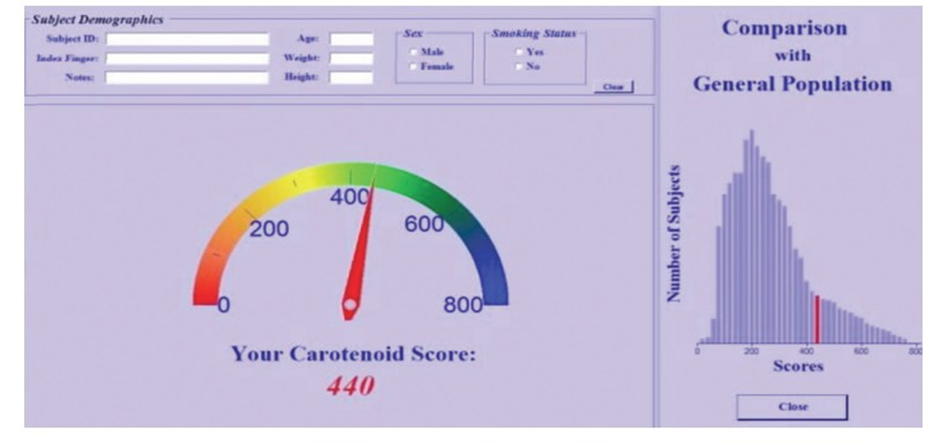
Figure 3: Veggie Meter example display, showing the overall skin carotenoid score (red pointer-indicator) and in the right-hand panel a comparison (red bar) to a reference population histogram indicating the general population. Adapted and modified from Radtke et al. [144], license under CC BY 4.0.
RRS Spectroscopy-based Skin Carotenoid Measurements
Resonance Raman Spectroscopy (RRS) uses a laser, typically around 488 to 514 nm, to excite the carotenoids in the skin. This excitation generates an inelastic scatter response including characteristic Stoke or spectral lines (around 530 nm), whose magnitude is used to determine the carotenoid levels in the skin. The RRS is a validated method via numerous published studies, which correlate with blood carotenoid levels and corresponding dietary intake journal surveys [142,143,145,161,163]. In general, it is highly specific to carotenoids in the skin due to the strong resonance enhancement of their Raman signal(s), when excited with their absorption bands [142,143,145,161,163]. For example, devices like the NuSkin BioPhotonic S1 Scanner utilize RRS technology, which was invented in 2003, and subsequent next generation versions were introduced in 2006 (S2) and 2013 (S3 BioPhotonic Scanner) [164].
In this regard, the review by Obana in 2025 entitled the measurement of skin carotenoids and their associations with diseases described:
a) the factors associated with skin carotenoid levels, b) how skin carotenoids are measured by RS and RRS methods (with figure illustrations), c) how skin carotenoids serve as biomarkers for disease prevention and covered the following diseases/disorders such as CVD, MetS, asthma, age-related macular degeneration (AMD), and cognitive function(s) [161]. In this review, Obana concluded that “extensive research has been conducted on the relationship between carotenoid levels and disease, with carotenoid indices indicating susceptibility to certain diseases …and disease prevention”[161]. In 2021, Pitts et al. performed a meta-analysis of 15 studies that included 1,155 individuals (963 adults and 192 children), where the overall results demonstrated a positive, significant correlation between RRS quantified skin and blood carotenoid levels [165].
Finally, in 2020, Radtke et al. performed a systematic review of spectroscopy-based skin carotenoid measurements with age and/ or disease status, which included 29 research articles, where most of the studies used RRS technology [145]. For utilization of RRS technology in this review, seven investigations used the BioPhotonic scanner. One study examined asthma and FVI [166]. Others studied children and adults [167-170] that showed a positive association with FVC [142,171]. In another five-year study that quantified SCS in breast cancer operated patients provided evidence for a positive impact of FVI on decreasing oxidative stress in the body and favourable BC prognostic indications [172]. Particularly, in this five-year study, women, who had significant reductions in their waist circumference and, BMI, displayed higher SCS [172].
Since the SCS data presented in the review used the Nu Skin BioPhotonic Scanner the results range from 10,000 to over 89,000 Raman Intensity Units (RIU), with 32,000 RIU being an approximate average score (see below). This range indicates a moderate consumption of fruits and vegetables. Scores below 37,000 RIU may suggest a need for increased FVC or antioxidant supplementation, while scores above 46,000 RIU indicate a high level of carotenoids likely from a diet rich in carotenoids and/or supplementation. As shown in Figure 4, the color-coded skin carotenoid scan scores are displayed.
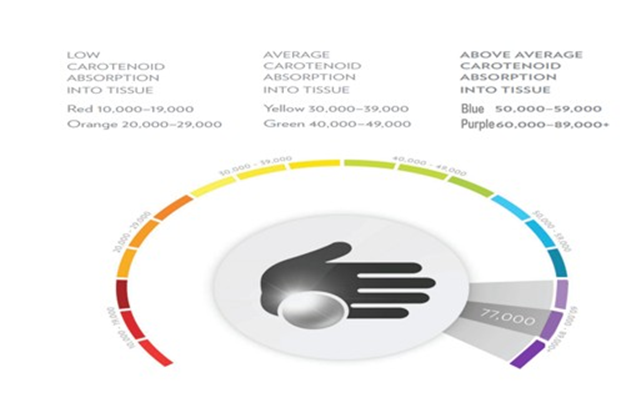
Figure 4: Example of skin carotenoid (color-coded) ranges with a NuSkin BioPhotonic S3 Scanner used with permission.
Finally, more than 75 publications over 20 years have utilized NuSkin’s BioPhotonic Scanner [142,143,145,163,164].
Advantages and Limitations of RS and RRS Spectroscopy-based Skin Carotenoid Measurements
The advantages of both RS and RRS technologies, as stated previously, are non-invasive, rapid (taken within 30 to 60 seconds), do not require specialized training or data processing (compared to blood sample analysis) and have been shown to correlate with plasma carotenoid levels in several research investigations [142,145,161,173].
The limitations of both RS and RRS technologies can be identified by knowing the scans do not quantify all carotenoids (minor compounds, isomers, etc.) and this is especially the case for the colorless carotenoids (like phytoene and phytofluene) that are not taken into account at all during skin scans [37,161].
There are various factors associated with skin carotenoid levels that have been reviewed in detail elsewhere, but in brief include: smoking, high BMI, gender, alcohol intake, medication use, genetics, health status, dietary fat and fiber intake, the food matrix, plant stanol/sterol intake and skin melanin and hemogloblin levels in the skin’s blood vessels affecting underlying redness, of different skin tones [37,55,143,145,161,173,174].
Other Methods of Estimating Skin Carotenoid Concentrations
Some reports suggest that near-infrared, mid-infrared and molecular fluorescence can detect carotenoids in food samples [175]. Therefore, the two main methods of estimating carotenoids levels in a non-invasive manner are RRS and RS using skin scanners.
Non-invasive RRS Spectroscopy-based Skin Carotenoid Levels, Implications to Healthspan
Can Daily Habits Influence Healthspan?
Since recent data shows that our diets are lacking in adequate micronutrients [176], and it is challenging to get people to change their diet to increase carotenoids by eating more fruits and vegetables, supplementation offers an attractive alternate approach. With the growing increase in digital technologies, there are now a number of non-invasive measurement tools and trackers, which can be used to monitor various aspects of health like apps for glucose monitoring, sleep quality, number of steps on a daily basis, monitoring food intake etc. One approach that does seem to be proving effective in getting people to change their habits is leveraging these non-invasive tools to help individuals understand their current practices, monitor their health situation, and more importantly, provide feedback on how they can positively change their habits [3-5].
For the first time, individuals are able to measure things regularly and provides positive reinforcement to influence habits in a healthier direction. This concept has been emphasized in the highly cited publication in the British Journal of General Practice, in 2012, by Gardner et al. that encouraged patients to make health habitual by making healthy habits for simple and sustainable changes in behaviour leading to healthier lifestyles [177]. More recently Ballard et al. in 2024 reported how “habits are powerful determinants of daily decisions that contribute to goal-directed behaviours for a lasting impact on life”, and how past histories influence future selections toward favourable outcomes [178].
For example, blood glucose levels are influenced by various factors such as nutrient composition, meal timing, physical activity, circadian rhythm and stress (cortisol) levels [179]. Continuous glucose monitoring (CGM) is now a behaviour modification tool [180] for not only diabetes but also non-diabetic individuals wearing CGM can avoid pre-diabetes or diabetic conditions, improve mental and physical performance, and modify food intake pattern for a healthier life [179-181]. Similar benefits have been reported in tracking daily step counts that decrease all-cause mortality, cardiovascular disease and metabolic disorders [182184]. In addition, tracking sleep patterns [185] enhance wellbeing, and using internet-base smartphone apps improve healthy eating behaviours [186], which potentially may enhance an individual’s healthspan. As a result of the growth in these non-invasive tracking devices, there has been a rapid increase in peoples’ understanding of one’s own wellbeing with a goal to be healthier on an individual level for a more personalised approach. Not only do they allow a person to keep track over time, but also they can provide actionable insights on ways an individual can improve their health. Thus, putting all these pieces together, we propose that measuring carotenoids noninvasively can actually provide a guide to an individual’s overall healthspan with carotenoids playing a key role in many aspects that lead to positive wellness outcomes. Moreover, these measurements can be effective in helping to encourage behaviours and interventions to improve health.
Methods for Lifetime and Worldwide Skin Carotenoid Scanning RRS Data
Skin carotenoids scores (SCS) were obtained from worldwide scans at corporate events, academic institutions and schools, where individuals filled out: a) consent form to process an individual’s data relating to their health and ethnicity for research and statistical purposes (signed by a parent or guardian if a minor) and b) answered an electronic survey that contained demographic information such as birthdate, sex, height/weight, ethnicity, daily sun exposure (time in hours), tobacco use (per day), fruit and vegetable (daily) intake, and use of other supplements, including marine omega, LifePak or an antioxidant juice blend (G3). There were no exclusions of individuals who gave their consent and desired a scan to obtain a skin carotenoid score. Individuals ranged in age from 1 to 90 years of age.
The skin carotenoid scans were performed in a uniform manner using the palm of the hand below the little finger, but above the wrist, where there is low skin pigmentation [164]. These data were collected worldwide over more than a 20-year period using three generations of Nu Skin’s BioPhotonic scanners. Due to the volume of data collected from 2002 to 2025 using the BioPhotonic Scanners the skin carotenoid scanner scores represent thousands to millions of scans.
Results of Global, Lifetime Skin Carotenoid Scores by RRS Technology by Country or World Regions
Some of the data presented herein has been provided in the public domain in past presentations at scientific and life science society meetings as posters or as preprints in non-peer-reviewed reports [187-190]. Only with the advent of better computational entry and management of large data sets has it been possible to create this magnitude of database, which can be queried (mined) in a consistent and accurate manner.
While skin carotenoid scanning data has advanced through non-invasive measurements that can estimate FVI and/or carotenoid supplementation, all previous published studies reported on defined and/or presented limited subject populations (i.e., with no more than 2,000 subjects) in various areas and regions throughout the world. This is the first time this magnitude of data analysis by biological computation has been presented on skin carotenoid scores via RRS technology and represents a milestone in scientific reporting for antioxidant research. Therefore, this analysis and present results have now made it possible to demonstrate global-lifetime SCS [187190] that may have implications to wellness as demonstrated by the large volume of research reported on carotenoids health benefits (see section 3).
For example, lifetime SCS globally averaged 32.74K RIU. Korea displayed the highest SCS of 46.28K RIU of all countries studied (that had at least 1,000 scans) and displayed significantly higher SCS compared to the global average of 32.74K RIU (see Figure 5 for global data and Figure 6 for Korean data).
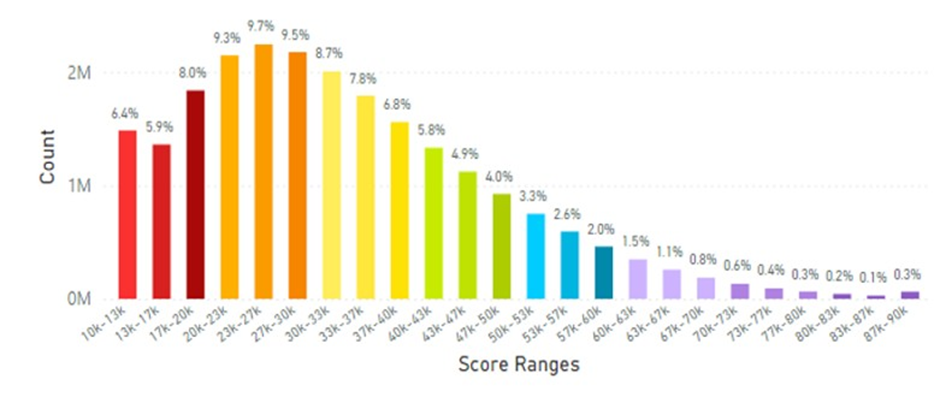
Figure 5: Total Global Data Collected from 2002 to 2025 Representing 21.27 Million Scans.
The average skin carotenoid score was 32.74K RIU globally. The count on the y-axix represents the percentage of scans within a given bar of the histogram profile that is color-coded. Low Carotenoid Concentration in skin: Red-10,000-19,000 RIU, Orange 20,00029,000 RIU; Average Carotenoid Concentration in skin: Yellow- 30,000-39,000 RIU, Green 40,000 -49,000 RIU and High Carotenoid Concentration in skin: Blue 50,000-59,000 RIU, Purple 60,000-90,000 RIU.
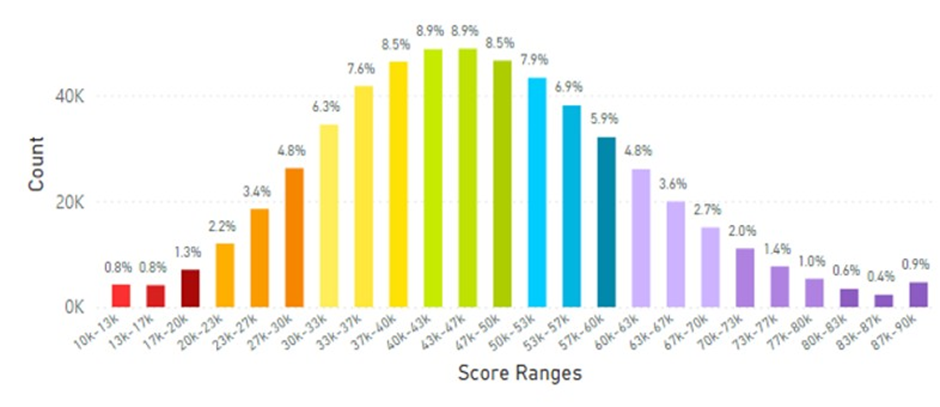
Figure 6: Korean Average Skin Carotenoid Score = 46.28K RIU from 547,780 scans. (See Figure 5 legend for additional information about this histogram.)
Japan displayed an average skin carotenoid score of 36.18K RIU from a total of 2.29 million scans (Figure 7), which was significantly below that of the Korea average of 46.28K RIU.
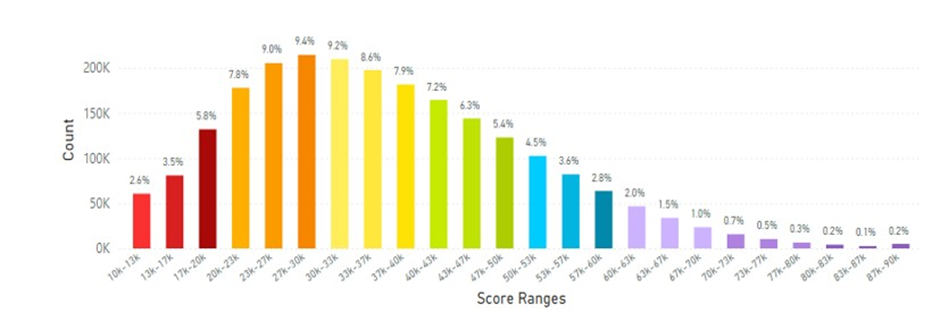
Figure 7: Japanese Average Skin Carotenoid Score = 36.18 K RIU from 2.29 million scans. (See Figure 5 for more information about this histogram information.)
Skin carotenoids scores from mainland China are shown in Figure 8 and the average skin carotenoid score = 32.23K RIU (from 3.99 million scans), which was lower than the Korean or Japanese average scores.
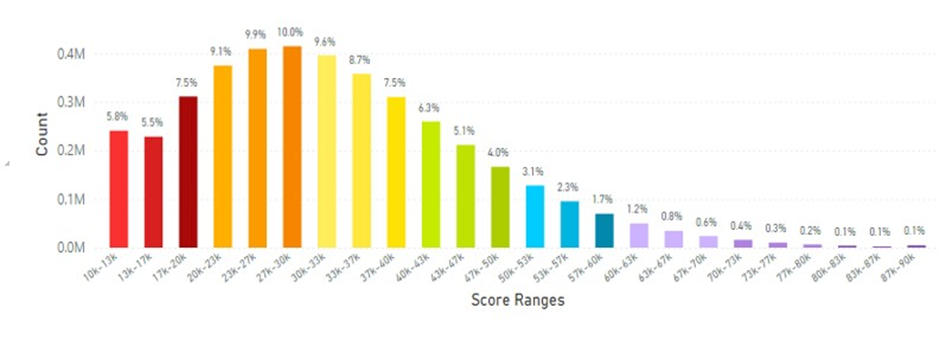
Figure 8: Mainland China Average Skin Carotenoid Score = 32.23K RIU from 3.99 million scans. (See Figure 5 legend for additional information about this histogram.)
Skin carotenoid scanner information from Taiwan is displayed in Figure 9 for the average skin carotenoid score.
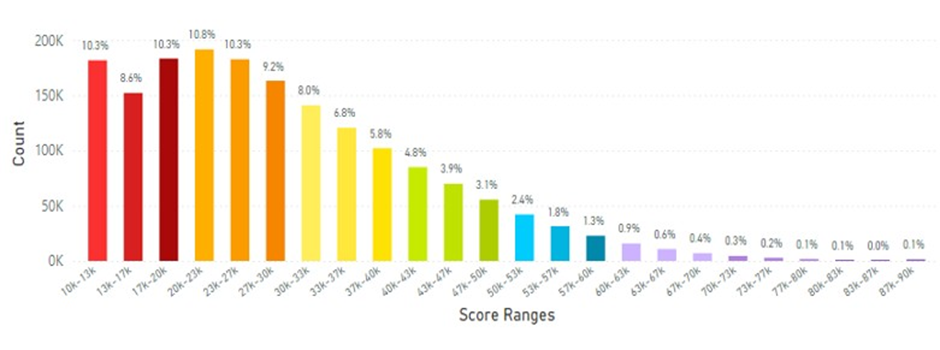
Figure 9: The Average Skin Carotenoid Score in Taiwan was 28.85K RIU from 1.73 million scans. (See Figure 5 legend for additional information about this histogram.)
When other Southeastern Asia countries (Malaysia, Indonesia, Philippines, Singapore, Thailand & Vietnam) were examined the average skin carotenoid score = 27.20K RIU that was lower than the global average of 32.73K RIU (see Figure 10).
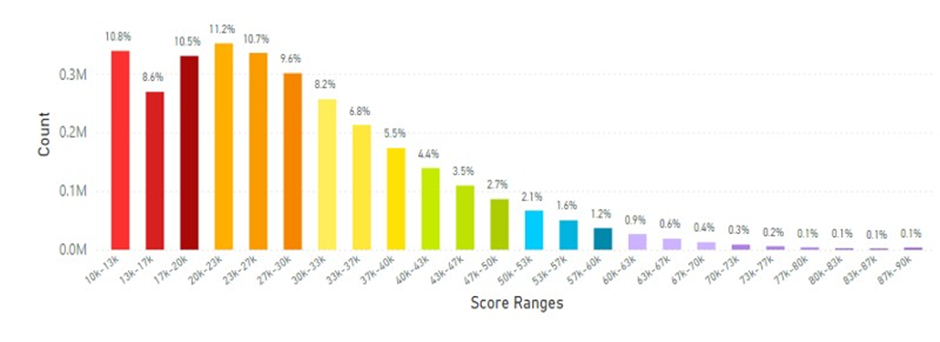
Figure 10: The Average Skin Carotenoid Score in Southeastern Asia was 27.70K RIU from 2.92 million scans. (See Figure 5 legend for additional information about this histogram.)
In particular the Philippines and Indonesia displayed the lowest average skin carotenoid scores compared to the global data at 20.65K RIU and 25.29K RIU, respectively. (see Figures 11 and 12). These populations might be at risk for carotenoid deficiency and may benefit from supplemental dietary carotenoids.
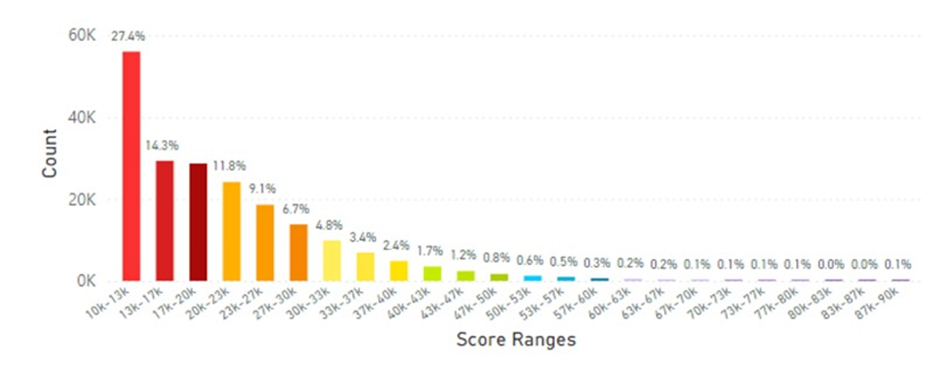
Figure 11: The Average Skin Carotenoid Score in the Philippines was 20.65K from 204,500 scans. See Figure 5 legend for additional information about this histogram).
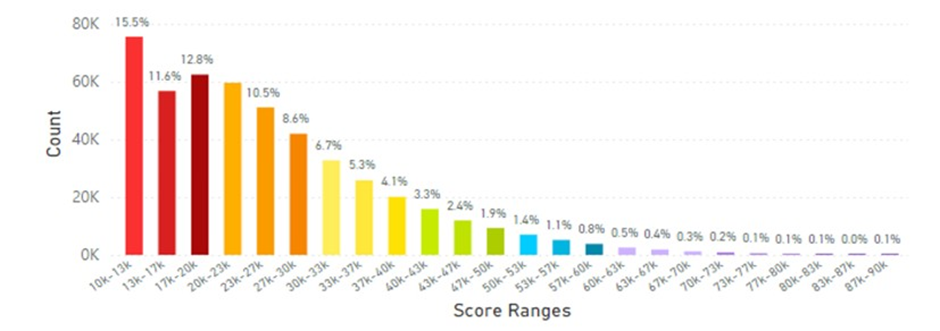
Figure 12: The Average Skin Carotenoid Score in Indonesia was 25.29K from 204,500 scans. See Figure 5 legend for additional information about this histogram).
In analyzing the data from Thailand the average skin carotenoid score = 29.16K RIU from 705,790 scans shown in Figure 13.
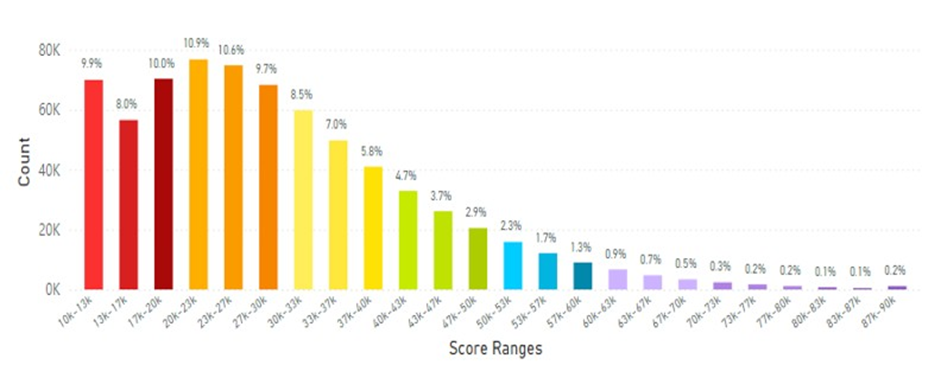
Figure 13: Thailand Average Skin Carotenoid Score = 29.16K RIU from 705,790 scans. (See Figure 5 legend for additional information about this histogram.)
The data from South East Asia and Pacific region that included Australia and New Zealand are shown in Figure 14 for the score range profiles.
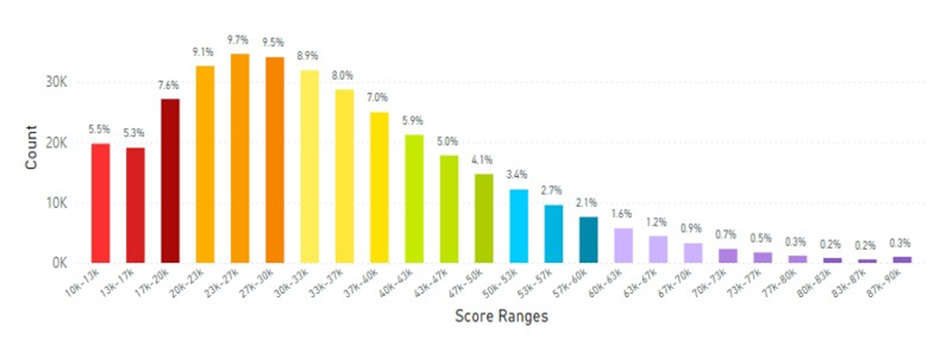
Figure 14: Australia and New Zealand Average Skin Carotenoid Score = 28.90K RIU from 3.48 million scans. (See Figure 5 for additional information about this histogram.)
When Europe was examined the skin carotenoid scores are shown in Figure 15 and the average skin carotenoid score = 34.50K RIU from 3.18 million scans.
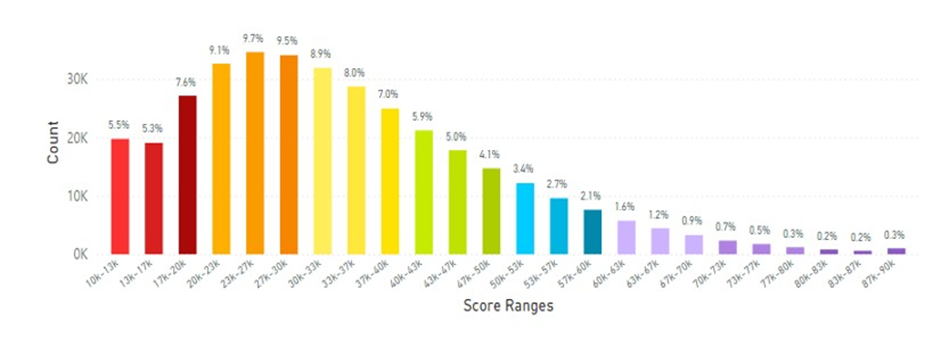
Figure 15: The Europe Average Skin Carotenoid Score = 34.50K RIU from 3.18 million scans. (See Figure 5 legend for additional information about this histogram.)
In examining certain European regions, the skin carotenoid data from the United Kingdom is displayed in Figure 16, while data from France and Germany that were combined due to the similarities of the data results, which are displayed in Figure 17.
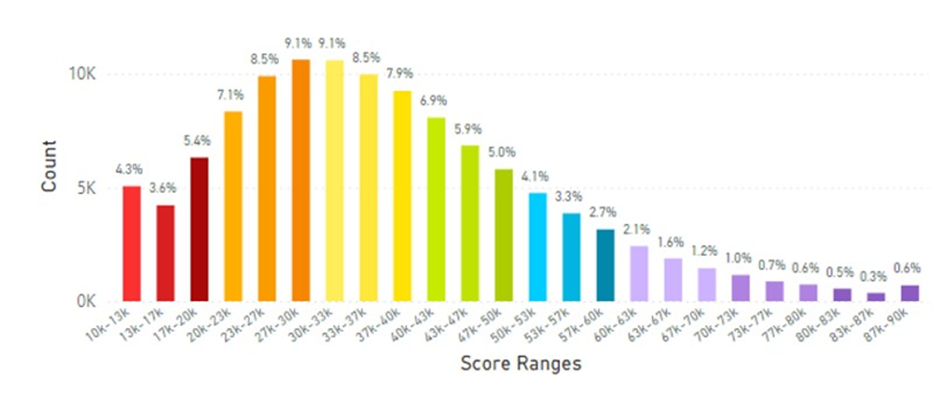
Figure 16: United Kingdom Average Skin Carotenoid Score = 36.68K RIU from 104,990 scans. (See Figure 5 legend for additional information about this histogram.)
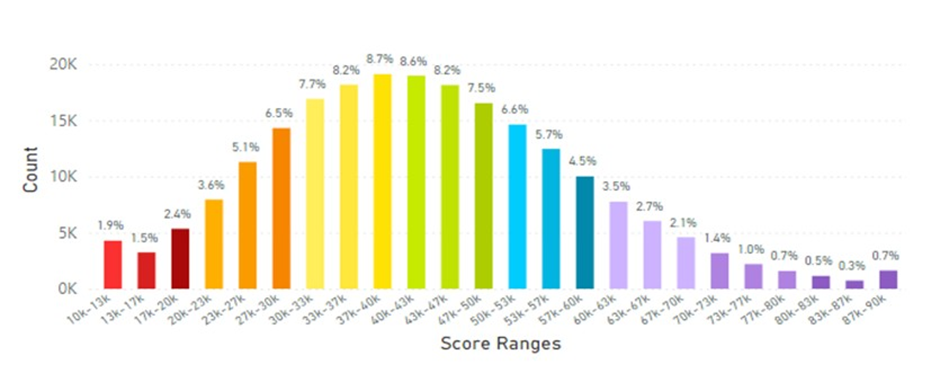
Figure 17: France and Germany Average Skin Carotenoid Score = 42.68K RIU from 155,700 scans. (See Figure 5 legend for additional information about this histogram.)
When the European data was analysed during the period of the pandemic (April 2020 to January 2025) interesting results were discovered where skin carotenoid scores increased, possibly reflecting a greater interest in a healthier lifestyle and dietary FVC. This information is displayed in Figure 18.
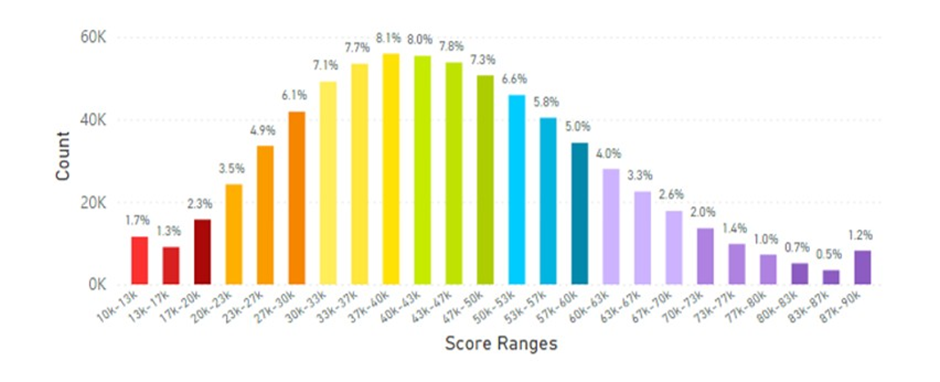
Figure 18: European Average Skin Carotenoid Scores = 44.34K RIU from 392,600 scans during the Pandemic. (See Figure 5 legend for additional information about this histogram.)
When North America (Canada and the United States of America) data was analysed the results are displayed in Figure 19.
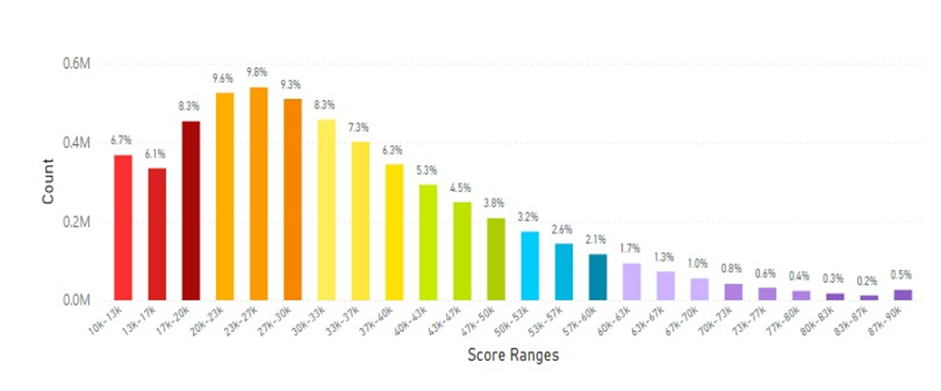
Figure 19: North America (Canada and The United States of America) Average Skin Carotenoid Score = 33.00K RIU from 4.89 million scans. (See Figure 5 legend for additional information about this histogram.)
In analyzing results from Latin America for skin carotenoid scores, this data is displayed in Figure 20.
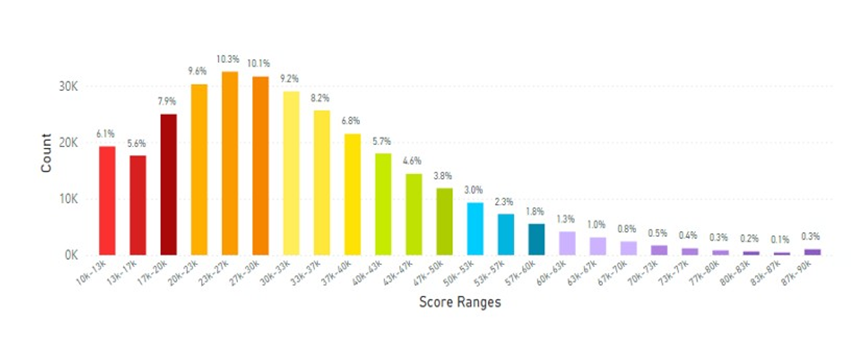
Figure 20: Latin America Average Skin Carotenoid Score = 32.39K RIU from 306,490 scans. (See Figure 5 legend for additional information about this histogram.)
Skin carotenoid score data from Iceland, Sweden, Norway and Russia were also analysed as displayed in Table 1.
|
Country |
Average Skin Carotenoid Score (RIU) |
From Number of Scans |
|
|
Iceland |
40.00K |
1086 |
|
|
Sweden |
42.99k |
34110 |
|
|
Sweden (Pandemic) |
46.83K |
10280 |
|
|
Norway |
42.15K |
70560 |
|
|
Russia |
27.89K |
125970 |
Table 1: Skin Carotenoid Scores from the Nordic Countries and Russia.
During the pandemic (February 21, 2020 to January 31, 2025) the average skin carotenoid scores increase by almost eleven percent in Sweden, suggesting that like the data from the United Kingdom individuals become more interested in a healthier lifestyle and dietary intake of fruits and vegetables, even though there was a hesitancy to vaccinate, especially children, in Sweden [191]. RIU=Raman
Intensity Units
Additionally, the global average skin carotenoid scores were analysed by ethnicity which is shown in Table 2 for White Caucasians, for African Americans, for Hispanics and for Asians.
|
Low Concentration |
Average Concentration |
High Concentration |
Average Score |
Number of Scans |
||||
|
Red |
Orange |
Yellow |
Green |
Blue |
Purple |
30.30K |
732,730 |
|
|
10-19K |
20-29K |
30-39K |
40-49K |
50-59K |
60-89K |
|||
|
White Caucasian |
22.9% |
31.4% |
24.2% |
12.8% |
5.6% |
3.1% |
||
|
African American |
36.1% |
31.9% |
18.5% |
8.2% |
3.3% |
2.0% |
25.99K |
23,870 |
|
Hispanic |
23.3% |
32.1% |
23.5% |
12.3% |
5.5% |
3.3% |
30.00K |
97,000 |
|
Asian |
20.0% |
28.3% |
25.3% |
15.6% |
7.3% |
3.5% |
32.00K |
1,650,000 |
Table 2: Global Average Skin Carotenoid Scores (RIU) by Ethnicity.
Low Concentration=Low Carotenoid Concentration in Skin; Average Concentration=Average Carotenoid Concentration in Skin and High Concentration=High Carotenoid Concentration in Skin. The percentages displayed above fall within a specific range under the color-coded concentration labels. RIU=Raman Intensity Units.
Previous reported studies on SCS are mixed, with some studies finding significant differences by race/ethnicity [37,55,145,192], while other find no significant differences [55]. From an extensive review by Madore et al. in 2023, a majority of the published research has not identified significant differences in skin carotenoids across ethnic groups [55]. However, in a few reports Asian adults had significantly higher skin carotenoid compared to other ethnic groups [55]. In the present analysis by ethnicity Asians displayed the highest average skin carotenoid score at 32.00K RIU, while African Americans displayed the lowest score at 25.99K RIU. Both White Caucasians and Hispanics scores were similar at 30.30K RIU and 30.00K RIU, respectively. Notably, previous investigations on this topic typically studied relatively small numbers of subjects (from less than 100 to 500) of different ethnicities [55]. In the present analysis, African Americans had the lowest number of scans at 23,870 while Asians had the highest number of scans at 1.65 million. The results in Table 3 imply the differences observed among the test groups suggest that geographical location and national dietary patterns play a greater role on skin carotenoid levels compared to an individual’s ethnic origin.
Finally, the ranking of the global lifetime average skin carotenoid scores are displayed in Table 3 for 20 different countries/regions (including specific time periods), see next page.
|
Rank |
Average Skin Carotenoid Score (RIU) |
Number of Scans |
|
Sweden (Pandemic) |
46.80K |
10,280 |
|
Korea |
46.38K |
547,780 |
|
European (Pandemic) |
44.34K |
392,600 |
|
Sweden |
42.99K |
34,110 |
|
France & Germany |
42.68K |
155,700 |
|
Norway |
42.15K |
70,560 |
|
Iceland |
40.00K |
1,086 |
|
United Kingdom |
36.68K |
104.990 |
|
Japan |
36.18K |
2,290,000 |
|
Europe |
34.50K |
3,180,000 |
|
North America (Canada & USA) |
33.00K |
4,890,000 |
|
Latin America |
32.39K |
306,490 |
|
China (Mainland) |
32.23K |
3,990,000 |
|
Thailand |
29.16K |
705,790 |
|
Australia & New Zealand |
28.90K |
3,480,00 |
|
Taiwan |
28.85K |
1,730,000 |
|
South-eastern Asia |
27.79K |
2,920,000 |
|
Russia |
27.89K |
125,970 |
|
Indonesia* |
25.29K |
487,430 |
|
Philippines* |
20.65K |
204,500 |
Table 3: Global Ranking of the Average Skin Carotenoid Scores in Different Countries/Regions and during the Pandemic.
USA=United States of America. The average skin carotenoid scores correspond to the range of values for carotenoid pigment concentration in skin. For example, 10-19K RIU and 20-29K RIU indicate low concentration of carotenoids in skin; 30-39K RIU and 40-49K RIU indicated average concentration of carotenoids in skin; and 50-59K RIU and 60-90K RIU indicate high concentration of carotenoids in skin. * indicates for Indonesia and the Philippines displayed particularly low skin carotenoid levels and the risk of carotenoid deficiency may be high. RIU = Raman Intensity Units.
It should be noted that skin carotenoid profiles may be associated with gut bacteria. In this regard, there is growing evidence suggesting that the absorption and metabolism of carotenoids are influenced by the bacteria in the intestines (i.e., gut microbiome) [136]. The gut microbiome is constantly changing throughout life, and adolescence is a period of significant development and change, where a greater abundance and diversity of certain bacteria has been reported compared to adults [193-195]. However, this association warrants further investigations to determine whether this factor is involved in age-related profiles of carotenoid scores.
Can Nutraceutical Supplementation Increase Skin Carotenoids Levels?
Early studies published in the 1990s clearly showed that administering an oral nutraceutical supplement containing carotenoids increased serum and skin carotenoid levels to enhance skin attributes in women [196]. However, in a recent published review in 2023, some authors suggested that “is it not currently known whether the magnitude of these increases (with carotenoid oral supplementation) is greater than that produced by a similar intake of carotenoids from food sources” [55]. While this may or may not be the case depending on the nutraceutical supplement given, there are several current reviews published in 2025 that suggest nutraceutical interventions to be effective for a variety of human health applications [197-199].
For example, a recent randomized, doubled-blind, placebocontrolled study compared two non-invasive methods (RS and RRS) to detect changes in skin carotenoid levels with the 12-week administration of a multivitamin supplement to men and women ages 20 to 65 years old (n = 46) that subsequently quantified six quality of life parameters at the end of the treatment [200]. The effectiveness of the supplementation treatment was validated by significant increases in vitamin C (by 44 %) and selenium (by 25 %) in plasma compared to placebo values, where the treatment supplement contained vitamins, minerals, carotenoids and phytonutrients, while the placebo supplement contained maltodextrin. The comparison of the RRS vs RS skin carotenoid levels are shown in Figure 21.
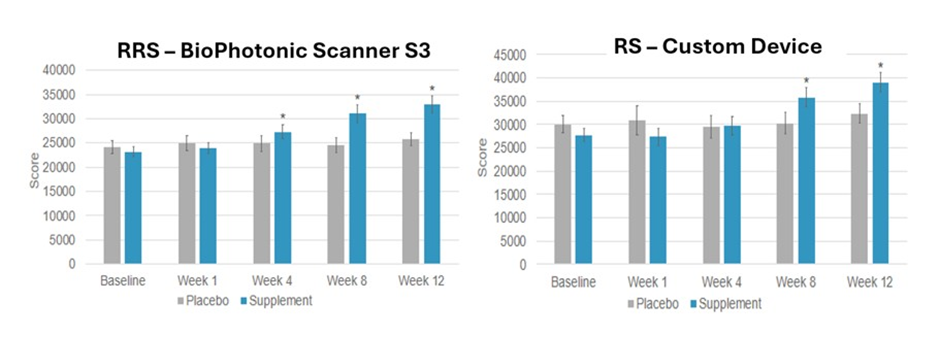
Figure 21: Left-panel RRS Skin Carotenoid Levels. Right-panel RS Skin Carotenoid Levels.
* = significantly increased skin carotenoid levels compared to placebo-control values.
This comparison suggests that oral nutraceutical supplementation can increase skin carotenoid levels, but it appears that the RRS method was more sensitive compared to the RS method in detecting changes in skin carotenoid levels. However, both the RRS and RS methods were able to detect the significant changes in skin carotenoid levels from taking a nutraceutical supplement for 12 weeks [200]. Finally, all six of the quality of life parameters (energy, health, immune, recovery from illness, improved life quality and enhancement in longterm health) were significantly improved that ranged from 44% to 58 % over control values.
Again, through data biological computational analysis, it is now possible to determine how oral nutraceutical supplementation of carotenoids compare with FVC recorded over a very large data set of 21.27 million scans performed with RRS technology along with correlating information for demographic/dietary intake questionnaires [187-190]. This information is shown in Figure 22, which shows that individuals consuming less than 2 servings of FV daily displayed a skin carotenoid score mean at 27.90K RIU, those consuming 2-3 servings a day displayed a mean SCS of 31.10K RIU. Those taking supplementation irregularly had a mean SCS of 31.00K RIU, while individuals taking Life Pak twice daily improved skin carotenoid levels to 37.77K RIU, which was greater than multiple servings of RVC daily (Figure 22).
The data presented in Figure 22 suggests that oral nutraceutical supplementation can provide a similar or greater magnitude for the increase in skin carotenoid levels compared to high or multiple daily servings from food sources [187-190], which has not been previously reported. Additionally, the data showed 8.6 million people, who never took LifePak supplementation, and 4.2 million respondents, who consumed less than 2 servings of FV daily, compared to a mere 327,000 respondents, who indicated they consumed more than 6 servings daily of FVC. Clearly, there are a lot more people with poor eating and supplementation habits in the world than there are people with good habits implying a need for better lifestyle choices as a broad population issue. This data is consistent with the recent Lancet paper identifying global micronutrient deficiencies [176].
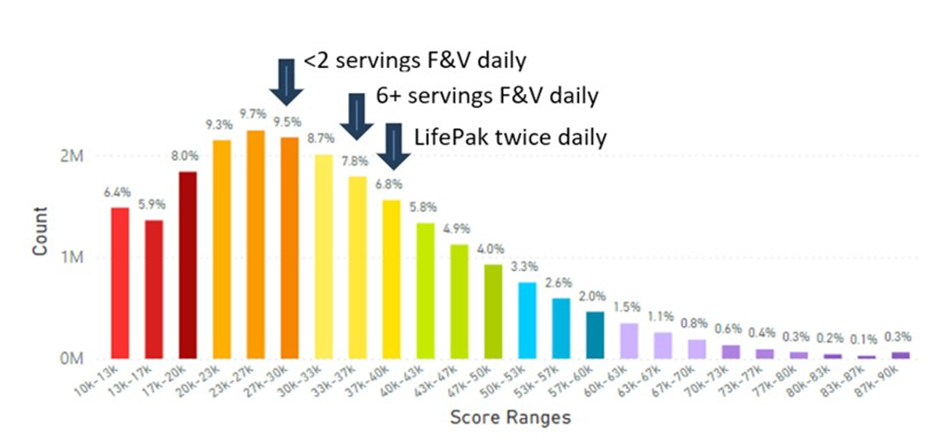
Figure 22: Global Skin Carotenoid Scores Correlated with Fruit and Vegetable (F&V) Daily Intake or with Individuals Taking the Nutraceutical Supplement (Life Pak) twice daily. Life Pak contains vitamins, minerals, carotenoids and phytonutrients. (See Figure 5 legend for additional information about this histogram.)
Of course, while carotenoids exhibit protective effects at low concentrations, high doses (20-30 mg/day) may lead to pro-oxidative effects and toxic metabolic interactions. For example, in smokers, β-carotene can degrade into reactive aldehydes and epoxides, which can lead to tissue damage, and high doses of β-carotene can disrupt retinoid receptor balance and potentially increase cancer risk [201]. A proposal of scanning your skin to know your carotenoid levels in order to go forward and enhance one’s healthspan by lifestyle factors is shown in Figure 23.
Figure 23: The Importance of Carotenoids in Well-being to Enhance Human
Healthspan by Knowing Skin Carotenoid Levels.
Lifestyle factors such as: a) smoking*, b) high body mass index (BMI of 30 or greater), c) no or low FVC and d) no nutraceutical supplementation provide a different histogram curve skewed to the left (Figure 24). Here 60% of individuals are scoring low for skin carotenoids. This is a major concern for this demographic. Not only are they not getting an adequate level of carotenoids in their diet, but they are also being exposed to oxidative stress with a less-than-ideal body volume to distribute their antioxidants [202,203].
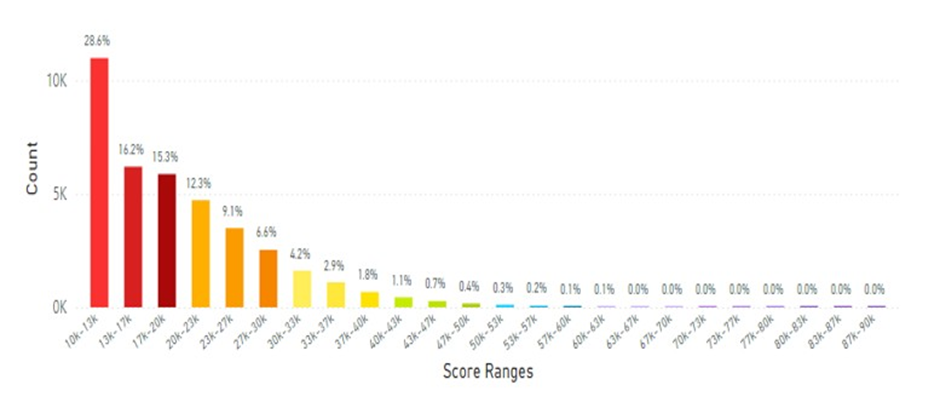
Figure 24: This figure displays data from 38K scans of individuals pursuing ‘unhealthy’ lifestyle factors, such as smoking with a high BMI, no or low FVC and no nutraceutical supplementation. The average scan score was 19.16K RIU. A smoker * is an adult who smokes any amount of tobacco (cigarettes etc.) regularly every day. (See Figure 5 legend for additional information about this histogram.)
For consideration, we propose that SCAN-KNOW-GO educates people on lifestyle factors supporting healthspan and provides information around key lifestyle modifications that can enhance carotenoid levels and provide healthspan benefits. For example by making one lifestyle modification such as choosing not to smoke (but having a high BMI and no nutraceutical supplementation), the skin carotenoid scores improved as shown in Figure 25. Also, if two lifestyle changes are made, such as not smoking and having a normal BMI, but having less than 2 servings of FVC daily, the skin carotenoid scores were further improved (Figure 26).
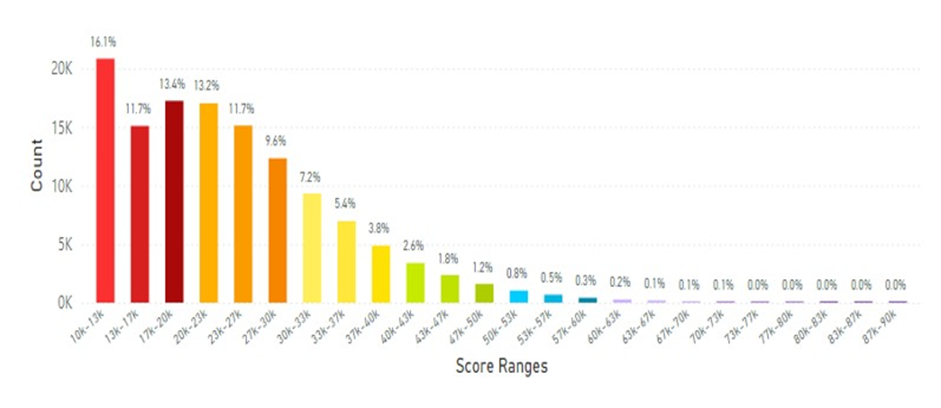
Figure 25: This figure displays data collected from 128K scans of individuals with high BMI parameters, less than 2 servings FVC daily and did not smoke. The average scan score improved to 23.65K RIU. (See Figure 5 legend for additional information about this histogram).
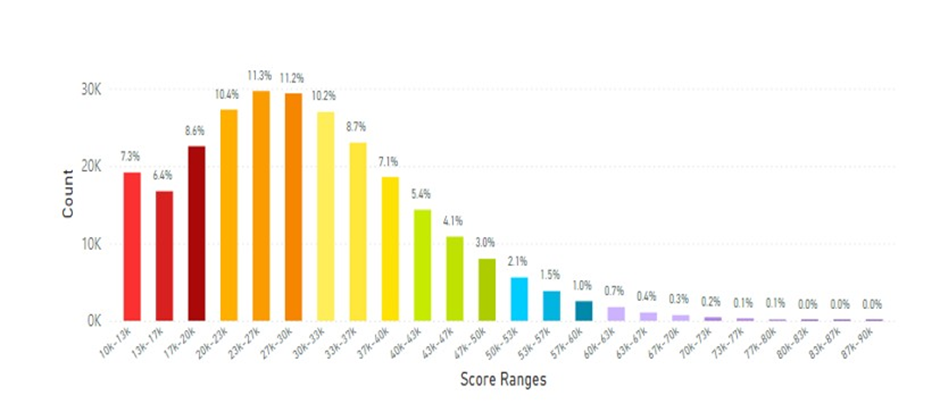
Figure 26: This figure displays data collected from 263.3K scans of individuals that had a normal
BMI, were not smokers, had less than 2 servings of FVC daily and did not consume any nutraceutical supplementation. The average scan score improved to 29.74K RIU. (See Figure 5 legend for additional information about this histogram.)
Comparing the impact of lifestyle factors in tabular form of the data shown above in figures 24 through 26 is shown in Table 4, below. Thus, the ranking of how lifestyle factors affects skin carotenoids levels is apparent from this data.
|
Smoker |
High BMI |
No OR Low FVC |
No Nutrition Suppl. |
Skin Carotenoid Score |
Scans |
|
YES |
YES |
YES |
YES |
19.16K RIU |
30,000 |
|
NO |
YES |
YES |
YES |
23.65K RIU |
128,000 |
|
NO |
NO |
YES |
YES |
29.74K RIU |
263,300 |
|
Compared to global data (without knowledge of lifestyles factors ) |
32.74K RIU |
21,270,000 |
Table 4: Lifestyle changes impact global skin carotenoid scores and influences healthspan.
Supplementation = Suppl.; the skin carotenoid levels in RIU represent the average scan score. Raman Intensity Units = RIU
Taking this analysis into account, the low levels of carotenoids observed in the Philippines (see Figure 11), may be due to diet, which is a significant problem that affects a large population of this country, where there is a “double burden of nutrition” that experiences high rates of undernutrition (including micronutrients) alongside a rise in individuals that are overweight and obese [204]. Conversely, Indonesia has one of the highest smoking rates in the world, where 39 % of the population (males at 75 %, while female rates are low at 4 %) smoke. This may account for the low carotenoid levels recorded in this country [205] (see Figure 12). The smoking rate in the Philippines is 19 %, which is moderate, but the malnutrition factors appear to be the leading cause of the low carotenoid levels [205].
In analyzing this database of skin carotenoid scores (Figures 24 through 26) if individuals at an ‘unhealthy’ status move toward a more healthy lifestyle will improve their SCS, therefore, higher levels of carotenoids are then available to defend the body against daily stressors, that may provide overall wellness benefits [16,17,22,24,161].
Increasing carotenoid consumption through diet or supplementation offers a route to enhance ones overall health, as measured and indicated through skin carotenoid levels. Thus, SCAN-KNOWGO is a simple, non-invasive way to monitor important health biomarkers to potentially improve the human healthspan. Measuring carotenoid levels in skin non-invasively might be a promising way to assess healthspan and wellness for a lifetime.
With regard to potential limitations regarding the computational analysis of this megadata, the carotenoid skin scans were performed in a uniform manner over a 22-year period along with individual questionnaires (where the palm of the hand was scanned, which has little melanin). However, the BioPhotonic scanners increased in accuracy with each new generation of equipment used (see section 4, S2, S3 generation of scanners), which may have influenced the datasets. Conversely, the very large number of scans undoubtedly increased the precision of the carotenoid values recorded and the factors associated with skin carotenoid levels (melanin/skin tone, age, gender, geographical region) have been reviewed in detail elsewhere [55]. Future data analysis by age, gender, and examining different time intervals (covering different years) may reveal changes in dietary habits and lifestyles. The analysis of these parameters are currently being performed that will be presented in upcoming scientific meetings, journal reports and reviews.
Conclusion
To our knowledge, this is the first review to report global, lifetime skin carotenoid levels from 20 different countries/regions over a 22-year interval using non-invasive RS-spectroscopy-based technology that represents thousands to millions of scans. For example, Korea had the highest SCS at 46.38K RIU from 547,780 scans, while the Philippines had the lowest SCS at 20.65K RIU from 204,500 scans. In comparison, to date, the largest study reported 2,078 participants in Southern Italy and the Dominican Republic among university students for skin carotenoid levels that showed higher SCS of students on a Mediterranean diet [206]. Thus, present study’s primary strength lies in its focus on analyzing very large numbers of SCSs by country over a lifetime (from individuals aged 1 to 90 years). Another distinguishing strength of the present analysis is the display of skin carotenoid levels, which were color-coded ranging from low to above average carotenoid values along with the percentage of each level over the 24 histogram bars that show the overall carotenoid profile for each country.
The present results confirm and extend previous reports, but with much larger data sets, that smoking and high BMI significantly decrease SCS [55,68,152,154-156,161,172]. The impact of these lifestyle factors is thought to have a detrimental impact on skin carotenoid levels by reducing the antioxidant capacity of the body to combat oxidative stress [55,68,88,161,207]. In this regard, previous reports suggest that carotenoid intake via diet or nutraceutical supplementation may benefit smokers, but the positive impact is greatly reduced in this lifestyle factor [201,202]. In addition, several published reports suggest high skin carotenoid scores are associated with a lower likelihood of having MetS, which demonstrates the positive actions of carotenoids (see section 3.7). Finally, some previous reported studies suggest that Asian individuals display higher skin carotenoid levels, while other investigations found no significant ethnic differences [55,145,192].
However, in the present analysis by ethnicity, Asians displayed the highest average skin carotenoid score at 32.00K RIU, while African Americans displayed the lowest score at 25.99K RIU. Both White Caucasians and Hispanics scores were similar at 30.30K RIU and 30.00K RIU, respectively. Notably, previous investigations on this topic typically studied relatively small numbers of subjects (from less than 100 to up to 400 individuals) of different ethnicities [55]. In the present analysis, African Americans had the lowest number of scans at 23,870, while Asian data had the highest number of scans at 1.65 million. Thus, the results in Table 2 imply that geographical location and national dietary patterns play a greater role on skin carotenoid levels compared to an individual’s ethnic origin.
Also, this review presented evidence that nutraceutical supplementation with carotenoids may be as good or better than multiple servings of fruits and vegetables per day, which represents a more convenient and less costly way to increase and sustain an individual’s carotenoid levels to potentially enhance health and wellbeing, which has been reported by other investigators [208210].
Since it is well established that non-invasive spectroscopy-based technologies can estimate carotenoid levels via skin carotenoid scans, this may be a method to help individuals determine their carotenoid status to improve their health and quality of life, especially when personal portable carotenoid skin scanners become available in the future. Abbreviations
α: alpha; β: beta; AMD: age-related macular degeneration; BMD: bone mineral density; BMI: body mass index; CARS: coherent anti-strokes Raman spectroscopy; CGM: continuous glucose monitoring; CHF: congestive heart failure; COPD: chronic obstructive pulmonary disease; CSF: cerebral spinal fluid; CVD: cardiovascular disease; FV: fruit and vegetable; FVC: fruit and vegetable consumption; FVI: fruit and vegetable intake; HbA1c: hemoglobin A1c (glycosylated hemoglobin); HPLC: high performance liquid chromatography; mg : milligram; MS: mass spectrometry; NFkB: nuclear factor kappa B; Nrf2: nuclear factor erythroid 2-related factor 2; OS: oxidative stress; RAE: retinol activity equivalent; ROS: reactive oxygen species; RS: reflective spectroscopy; RRS: resonance Raman spectroscopy; SCS: skin carotenoid score; SORS: spatially offset Raman spectroscopy; SR-B1: scavenger receptor class B member 1; SRS: stimulated Raman scattering spectroscopy; T2D: type 2 diabetes; TNF: tumor necrosis factor; USD: United States dollar; USDA: United States Department of Agriculture; UV: ultraviolet
Author Contributions
HK was involved in conceptualization, formal analysis, investigation, data curation, writing-original draft preparation, further writing, review and editing, providing supervision, project administration and funding acquisition of this work. JB, NF, SF, MR formal analysis, data curation, computational biology assessment, conducting investigational elements. EDL was involved in data analysis, data organization, writing-original draft preparation, further writing, review and editing and funding acquisition for this work. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by NSE Products Inc. and, in part, by the College of Life Sciences at Brigham Young University (grant number 19-2215).
Ethical Guidelines
Ethics approval and consent to participate: Informed written consent was obtained from all participants (if a minor then adults/ guardians granted permission) to use their data in scientific reports following institutional review board protocols and the ethical principles of the Helsinki Declaration.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design, presentation, collection of research information and scientific references, or in the analyses, interpretation of the presented data/information, in the writing of the manuscript; or in the decision to publish the contents of this narrative review.
References
- United Nations Department of Economic and Social Affairs. World population prospect 2022: release note about major differences in total population estimates for mid-2021 between 2019 and 2022 revisions. New York: Population Division. 2022.
- Garmany A, Terzic A (2024) Global healthspan-lifespan gaps among 183 world health organization member states. JAMA Network Open 7: e2450241.
- Attia P (2023) The Long Game. In. Outlive, the science & art of longevity. Harmony: New York.10.
- Rippe JM (2018) Lifestyle Medicine: The health promoting power of daily habits and practices. Am J Lifestyle Med 12: 499-512.
- Lephart ED, Knaggs H (2023) Enhancing skin anti-aging through healthy lifestyle factors. Cosmetics. 10: 142.
- Harman D (1992) Free radical theory of aging. Mutat Res 275: 257266.
- Alegria-Torres JA, Baccarelli A, Bollati V (2011) Epigenetics and lifestyle. Epigenetics. 3: 267-277.
- Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A (2018) Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nature Rev Endocrinol 14: 576-590.
- Li Z, Zhang Z, Ren Y, Wang Y, Fang J, et al. (2021) Aging and age-related diseases: from mechanisms to therapeutic strategies. Biogerontology. 22: 165-187.
- Rahaman K (2007) Studies on free radicals, antioxidants, and cofactors. Clin Interventions Aging 2: 219-236.
- Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D, et al. (2018) Oxidative stress, aging and disease. Clin Interventions Aging. 13: 757-772.
- Chandimali N, Bak SG, Park EH, Lim H-J, Won Y-S, Kim E-K, et al. (2025) Free radicals and their impact on health and antioxidant defenses: a review. Cell Death Discovery. 11: 19
- Kassis A, Fochot M-C, Horcajada M-N, Horstman AMH, Duncan P, Bergonzelli G, et al. (2023) Nutritional and lifestyle management of the aging journey: A narrative review. Front Nutr 9: 1087505.
- Tessier A-J, Wang F, Korat AA, Eliassen AH, Chavarro J, Grodstein F, et al. (2025) Optimal dietary patterns for healthy aging. Nat Med 31: 1644-1652.
- Roa AV, Rao LG (2007) Carotenoids and human health. Pharmacol Res 55: 207-216.
- Crupi P, Faienza MF, Naeem MY, Corbo F, Clodoveo ML, Muraglia M (2023) Overview of the potential beneficial effects of carotenoids on consumer health and well-being. Antioxidants. 12:1069.
- Bakac ER, Percin E, Gunes-Bayir A, Dadak A (2023) A narrative review: The effects and importance of carotenoids on aging and agingrelated diseases. Int J Mol Sci 24: 15199.
- Qi X, Wang X, Cheng L, Li Y, Dang K, Yang S, et al. (2025) Dietary carotenoids intakes and biological aging among US adults, NHANES 1999-2018. Nutr J 24: 9.
- Marhuenda-Munoz M, Hurtado-Barroso S, Tresserra-Rimbau A, Lamuela-Raventos RM (2019) review of factors that affect carotenoid concentrations in human plasma: differences between Mediterranean and Northern diets. European J Clin Nutr 72: 18-25
- Moron-Ortiz A, Karamelegkos AA, Mapelli-Braham P, Ezcurra M, Melendez-Martinez AJ (2024) Phytoene and Phytotone-rich microalgae extracts extend lifespan and C. elegans and protect against amyloid-β toxicity in an Alzheimer‘s disease model. Antioxidants. 13: 931.
- Tan BL, Norhaizan ME (2019) Carotenoids: How effective are they to prevent age-related diseases? Molecules. 24: 1801.
- Terao J (2023) Revisiting carotenoids as dietary antioxidants for human health and disease prevention. Food Function. 14: 7799-7824.
- Maoka T (2020) Carotenoids as natural functional pigments. J Nat Med 74: 1-16
- Gomez-Sagasti MT, Lopez-Pozo M, Artetex U, Becerrill JM, Hernandez A, Garcia-Plazaola JI, et al. (2023) Carotenoids and their derivatives: A “Swiss Army knife-like” multifunctional tool for fine-tuning plantenvironment interactions. Environmental Exp Botany 207: 105229.
- Toti E, Chen C-Y O, Palmery M, Valencia DV, Peluso I (2018) Nonprovitamin A and provitamin A carotenoids as immunomodulators: Recommended dietary allowance, therapeutic index, or personalized nutrition? Oxidative Med Cell Longevity. 4637861.
- USDA National Agriculture Library. Food Composition, nal.usda.gov/ human-nutrition-and-food-safety/food-composition Accessed 3 JUN 2025.
- Bohm V, Lietz G, Olmedilla-Alonso B, Phelan D, Reboul E, Banati D (2021) From carotenoid intake to carotenoid blood and tissue concentrations-implications for dietary intake recommendations. Nutr. Rev 79: 544-573.
- National Institutes of Health (USA) Office of Dietary Supplements, Vitamin A and Carotenoids.
- Mrowicka M, Mrowicki J, Kucharska E, Majsterek I (2022) Lutein and zeaxanthin and their roles in age-related macular degeneration- neurodegenerative disease. Nutrients, 14: 827.
- Rodriguez-Amaya DB (2015) Carotenes and xanthophylls as antioxidants. In Handbook of Antioxidants for Food Preservation, Woodhead Publishing.17-50.
- Rivera-Madrid R, Caballo-Uicab VM, Cardenas-Conejo Y, AguliarEspinosa M, Siva R (2025) Chapter 1 Overview of carotenoids and beneficial effects on human health. In Carotenoids: Properties, Processing and Applications. Elsevier. 1-40.
- Hammad M, Raftari M, Cesario R, Salma R, Godoy P, et al. (2023) Roles of oxidative stress and Nrf2 signaling in pathogenic and nonpathogenic cells: A Possible General Mechanism of Resistance to Therapy Antioxidants. 12: 1371.
- Bufka J, Vankova L, Sykora J, Krizkova V (2024) Exploring carotenoids: Metabolism, antioxidants, and impacts on human health. J Funct Foods 118:106284.
- Arslansoy N, Fidan O (2024) Carotenoids and their antioxidant power. In The Power of Antioxidants-Unleashing Nature’s Defense Against Oxidative Stress, Barros AN, Abraao AC Eds.; Intech
- Aune D, Keum N, Giovannucci E, Fadnes LT, Boffetta P et al. (2018) Dietary intake and blood concentrations of antioxidants and the risk of cardiovascular disease, total cancer, and all-cause mortality: a systematic review and dose-response meta-analysis of prospective studies. Am J Clin Nutr 108: 1069-1091.
- Bohn T (2019) Carotenoids and markers of oxidative stress in human observational studies and intervention trials: implications for chronic diseases. Antioxidants. 8: 179.
- Bohn T, Bonet ML, Borel P, Keijer J, Landrier JF, et al. (2021) Mechanistic aspects of carotenoid health benefits-where are we now? Nutr Res Rev 34: 267-302.
- Sahashi Y, Goto A, Takachi R, Ishihara J, Kito K, et al. (2022) Inverse association between fruit and vegetable intake and all-cause mortality: Japan public health center-based prospective study. J Nutr 152: 22452254.
- Fekete M, Csipo T, Fazekas-Pongor V, Fehar A, Szarvas Z, et al. (2023) The effectiveness of supplementation with key vitamins, minerals, antioxidants and specific nutritional supplements in COPD- A review. Nutrients. 15: 2741.
- Chen X, He C, Yu W, Ma L, Gou S, Fu P (2024) Association between dietary carotenoid and biological age acceleration: insights from NHANES 2009-2018. Biogerontology. 26: 24.
- Ahn YJ, Kim H (2021) Lutein as a modulator of oxidative stressmediated inflammatory diseases. Antioxidants 10: 1448.
- Ademowo OS, Oyedode O, Edward R, Conway ME, Griffiths HR, Dias IHK (2024) Effects of carotenoids on mitochondrial dysfunction. Biochem Soc Transactions 52: 65-74.
- Ciccone MM, Cortese F, Gesualdo M, Carbonara S, Zito A, Ricci G, et al. (2013) Dietary intake of carotenoids and their antioxidant and antiinflammatory effects in cardiovascular care. Mediators of Inflammation. Article 782137.
- Wang M, Tang R, Zhou R, Qian Y, Di D (2023) The protective effect of serum carotenoids on cardiovascular disease: a cross-sectional study from the general US adult population. Front Nutr 10: 1154239.
- Sumalla-Cano S, Eguren-Garcia I, Lasarte-Garcia A, Prola TA, Martinez-Diaz R, Elio I (2024) Carotenoids intake and cardiovascular prevention: A systematic review. Nutrients. 16: 3859.
- Obana A, Nakamura M, Miura A, Nozue M, Muto S, Asaoka R (2024) Association between atherosclerotic cardiovascular disease score and skin carotenoids levels estimated via refraction spectroscopy in the Japanese population: a cross-sectional study. Sci Reports 14: 12173.
- Gammone MA, Riccioni G, D’Orazio N (2015) Carotenoids: potential allies of cardiovascular health? Food Nutr Res 59:26762.
- Yao Y, Goh HM, Kim JE (2021) The roles of carotenoids consumption and bioavailability in cardiovascular health. Antioxidants 10: 1978.
- Zhu X, Cheang I, Tang Y, Shi M, Zhu Q, Gao R, et al. (2023) Associations of serum carotenoids with risk of all-cause and cardiovascular mortality in hypertensive adults. J Am Heart Assoc 12: e027568.
- Darvin ME, Sterry W, Lademan J, Vergou T (2011) The role of carotenoids in human skin. Molecules. 16: 10491-10506.
- Zerres S, Stahl W (2020) Carotenoids in human skin. Biochimica et Biophysica Acta (BBA) – Molecular and Cellular Biology. 1865: 158588.
- Michalak M (2022) Plant-derived antioxidants: Significance in skin health and the ageing process. Int J Mol Sci 23: 585.
- Darvin ME, Lademan J, Hagen JV, Lohan SB, Kolmar H, Meinke MC, et al. (2022) Carotenoids in human skin in vivo: Antioxidant and photoprotectant role against external and internal stressors. Antioxidants 11: 1451.
- Metibemu DS, Ogungbe IV (2022) Carotenoids in drug discovery and medicine: Pathways and molecular targets implicated in human disease. Molecules. 27: 6005.
- Madore MP, Hwang J-E, Park J-Y, Ahn S, Joung H, Chun OK (2023) A narrative review of factors associated with skin carotenoid levels. Nutrients. 15: 2156.
- Baswan SM, Klosner AE, Weir C, Salter-Venzon D, Gellenbeck KW, Leverett J, et al. (2021) Role of ingestible carotenoids in skin protection: A review of clinical evidence. Photodermatol Photoimmunol Photomed. 37: 490-504.
- Bavarsad N, Mapar MA, Safaezadeh M, Latiff SM (2020) A doubleblind, placebo-controlled randomized trial of skin-lightening cream containing lycopene and wheat bran extract on melasma. J Cosmet Dermatol 20: 1795-1800.
- Zhang X, Zhou Q, Qi Y, Chen X, Deng J, Zhang Y, et al. (2024) The effect of tomato and lycopene on clinical characteristics and molecular markers of UV-induced skin deterioration: A systematic review and meta-analysis of intervention trials. Critical Rev Food Sci Nutr 64: 6198-6217.
- Varghee R, Emerson A, Vannier B, Doss CGP, Priyadharshini R, Ramamoorthy S, et al. (2025) Substantial effects of carotenoids on skin health: A mechanistic perspective. Phytother Res. 39:4156-4170.
- Ito N, Seki S, Ueda F (2018) The protective role of astaxanthin for UVinduced skin deterioration in healthy people: a randomized, doubleblind, placebo-controlled trial. Nutrients.10: 817.
- Groten K, Marini A, Grether-Beck S, Jaenike T, Ibbotson SH, Moseley H, et al. (2019) Tomato phytonutrient balance UV response: results from a double-blind, randomized, placebo-controlled study. Skin Pharmacol Physiol 32: 101-108.
- So B, Kwon KH (2023) Nutritional approaches of the changing consumer after the pandemic: Sustainable potential of phytoene and phytofluene for photoprotection and skin health. Sustainability. 15: 4416.
- Mapelli-Brahm P, Melendez-Martinez AJ (2021) The colorless carotenoids phytoene and phytofluene: sources, consumption, bioavailability and health effects. Curr Opin Food Science, 41: 201209.
- Baswan SM, Marini A, Klosner AE, Jaenike T, Leverett J, Murray M, et al. (2020) Orally administrated mixed of carotenoids protect human skin against ultraviolet A-induced skin pigmentation: a doubleblind, placebo-controlled, randomized clinical trial. Photodermatol Photoimmunol Photomed. 36: 219-225.
- Zmitek K, Zmitek J, Butina MR, Hristov H, Pagacnik T, Pravst I (2020) Dietary lutein supplementation protects against ultraviolet-radiationinduced erythema: results of a randomized, double-blind, placebocontrolled study. J Funct Foods 75: 104265.
- Stevens GA, Bennett JE, Hennocq Q, Lu Y, De-Regil LM, Rogers L, et al. (2015) Trends and mortality effects of vitamin A deficiency in children in 138 low-income and middle-income countries between 1991-2013: a pooled analysis of population-based surveys. Lance Glob Health 3: e528-e536.
- Hughes DA (1999) Effects of carotenoids on human immune function. Pro Nutr Soc 58: 713-718.
- Bas TG (2024) Bioactivity and bioavailabilit of carotenoids applied in human health: Technological advances and innovation. Int J Mol Sci 25: 7603.
- Anjani G, Ayustaningwarno F, Eviana R (2022) Critical review on the immunomodulatory activities of carrot’s β-carotene and other bioactive compounds. J Funct Foods 99: 105303.
- Chew BP, Park JS (2004) Cartenoids action in the immune response. J Nutr 134: 257S-261S.
- Bohn T, Balbuena E, Ulus H, Iddir M, Wang G, Crook N, et al. (2023) Carotenoids in health as studies by omics-related endpoints. Adv Nutr 14: 1538-1578.
- Santos MS, Meydani SN, Leka L, Wu D, Fotouhi N, Meydani M, et al. (1996) Natural killer cell activity in elderly men is enhance by betacarotene supplementation. Am J Clin Nutr 64: 772-777.
- Santos MS, Gaziano JM, Leka LS, Beharka AA, Hennekens CH, Meydani SN (1998) Beta-carotene-induced enhancement of natural killer cell activity in elderly men: an investigation of the role of cytokines. Am J Clin Nutr 68: 164-170.
- Kramer TR, Burri BJ (1997) Modulated mitogenic proliferative responsiveness of lymphocytes in whole-blood cultures after lowcarotene diet and mixed-carotenoid supplementation in women. Am J Clin Nutr 65: 871-875.
- Watzl B, Bub A, Briviba K, Rechkemmer G (2003) Supplementation of a low-carotenoid diet with tomato or carrot juice modulates immune functions in healthy men. Ann Nutr Metab 47: 255-261.
- Kim JH, Chang MJ, Choi HD, Youn Y-K, Kim JT, Oh JM, et a. (2011) Protective effects of Hawmatococcus astaxanthin on oxidative stress in health smokers. J Med Food 4: 1469-1475.
- Pocovi-Gerardino G, Correa-Rodizuez M, Callejas-Rubio J-L, RiosFernandez R, Martin-Amada M, Cruz-Caparros M-G, et al. (2021) Beneficial effects of Mediterranean diet on disease activity and cardiovascular risk in systemic lupus erythematosus patients: a crosssectional study. Rheumatology (Oxford). 60: 160-169.
- Medina-Garcia M, Baeza-Morales A, Martinez-Peinado P, PascualGarcia S, Pujalte-Satorre C, Martinez-Espinosa RM, et al. (2025) Carotenoids and their interaction with the immune system. Antoxidants 14: 1111.
- Kulczynski B, Sidor A Brzozowska A, Gramza-Michlowska A (2024) The role of carotenoids in bone health – A narrative review. Nutrition 119: 112306.
- Sahni S, Hannan MT, Blumberg J, Cupples LA, Kiel DP, Tucker KL (2009) Inverse association of carotenoid intakes with 4-year change in bone mineral density in elderly men and women: the Framingham Osteoporosis Study. Am J Clin Nutr 89: 416-424.
- Xu J, Song C, Song X, Zhang X, Li X (2017) Carotenoids and risk of fracture: a meta-analysis of observational studies. Oncotarget. 8: 2391-2399.
- Hayhoe RPG, Lentjes MAH, Mulligan AA, Luben RN, Khaw K-T, Welch AA (2017) Carotenoid dietary intakes and plasma concentrations are associated with heel bone ultrasound attenuation and osteoporotic fracture risk in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk cohort. Br J Nutr 117: 1439-1453.
- Regu GM, Kim H, Kim YJ, Paek JE, Lee G, Chang N, Kwon O (2017) Association between dietary carotenoid intake and bone mineral density in Korean adults aged 30-75 years using data from the fourth and fifth Korean national health and nutrition examination surveys (2008-2011). Nutrients. 16: 1025
- Cao W-T, Zeng F-F, Li B-L, Lin J-S, Liang Y-Y, Chen Y-M (2018) Higher dietary carotenoid intake associated with lower risk of hip fracture in middle-aged and elderly Chinese: A matched case-control study. Bone. 111: 116-122.
- Charkos TG, Liu Y, Oumer KS, Vuong AM, Yang S (2020) Effects of β-carotene intake on the risk of fracture: a Bayesian meta-analysis. BMC Musculoskeletal Disorders 21: 711.
- Kan B, Guo D, Yuan B, Vuong AM, Jiang D, Zhang M, et al. (2021) Dietary carotenoid intake and osteoporosis: the National Health and Nutrition Examination Survey, 2025-2018. Arch Osteoporos 17: 2.
- Zheng Y, Zhou W, Zhang J, Lan T, Zhang R (2025) Association between dietary carotenoid intake and vertebral fracture in people aged 50 years and older: a study based on the national health and nutrition examination survey. Arch Osteoporos 20: 39.
- Bhatt T, Patel K (2020) Carotenoids: Potent to Prevent Diseases Review. Nat Prod Bioprospecting 10: 109-117.
- Davinelli S, Ali S, Solfrizzi V, Scapagnini G, Corbi G (2021) Carotenoids and cognitive outcomes: A meta-analysis of randomized intervention trials. Antioxidants 10: 223.
- Liu X, Dhana K, Furtado JD, Agarwal P, Aggrarwal NT, Tangney C, et al. (2021) Higher circulating α-carotene was associated with better cognitive function: an evaluation among the MIND trial participants. J Nutr Sc 10: e64.
- Yuan C, Chen H, Wang Y, Schneider JA, Willen WC, Morris MC (2021) Dietary carotenoids related to risk of incident Alzheimer dementia (AD) and brain AD neuropathology: a community-based cohort of older adults. Am J Clin Nutr 113: 200-208.
- Morris MC, Wang Y, Barnes LL, Bennett DA, Dawson-Hughes B, Booth SL (2018) Nutrients and bioactives in green leafy vegetables and cognitive decline: Prospective study. Neurology. 90: e214-e222.
- Wang L, Zhao T, Zhu X, Jiang Q (2023) Low blood carotenoid status in dementia and mild cognitive impairment: A systematic review and meta-analysis. BMC Geriatrics. 23:195.
- Flieger J, Forma A, Flieger W, Flieger M, Gawlik PJ, Dzierzynski E, et al. (2024) Carotenoid supplementation for alleviating the symptoms of Alzheimer’s disease. Int J Mol Sci 25: 8982.
- Christimann G, Rocha G, Sattler JAG (2025) Bioactive compounds and dietary patterns in Alzheimer‘s disease. J Alzheimer‘s Dis 104: 597-610.
- Govind B, Stephen KN, Keerthana S, Perumal S, Thirumurugan M (2025) Recent advances on therapeutic mechanism and potential of flavonoids and carotenoids: A focus on Alzheimer‘s and Parkinson’s disease. In Bioactive Ingredients for Healthcare Industry. 2: pp. 75107.
- Wilson DM, Cookson MR, Den Bosch LV, Zetterberg H, Holtzman DM, et al. (2022) Hallmarks of neurodegenerative diseases. Cell. 186: 693714.
- Kabir MT, Rahman MH, Shah M, Jamiruddin MR, Basak D, et al (2022) Therapeutic promise of carotenoids as antioxidants and anti-inflammatory agents in neurodegenerative disorders. Biomed Pharmacother 146:112610.
- Manochkumar J, Doss CGP, El-Seedi HR, Efferth T, Ramaoorthy S (2021) The neuroprotective potential of carotenoids in vitro and in vivo. Phytomedicine. 91:153676.
- Gandla K, Babu AK, Unnisa A, Sharma I, Singh LP, et al. (2023) Carotenoids: Role in neurodegenerative diseases remediation. Brain Sci 13: 457.
- Bej E, Cesare P, d’Angelo M, Volpe AR, Castelli V (2024) Neuronal cell rearrangement during aging: Antioxidant compounds as a potential therapeutic approach. Cells.13: 1945.
- Kudo J, Watanabe K, Sasaki M, Ushida Y, Matsuzaka M, et al. (2025) Serum carotenoids concentrations are associated with enlarged choroid plexus, lateral ventricular volume, and perivascular spaces on magnetic resonance imaging: A large cohort study. Academic Radiology. 32: 4797-4806.
- Gao Y, Liu K, Zhu J (2023) Glymphatic system: an emerging therapeutic approach for neurological disorders. Front Mol Neurosci 16: 1138769.
- Crowe-White KM, Phillips TA, Ellis AC (2019) Lycopene and cognitive function. J Nutr Sci 8: e20.
- Zhu NW, Yin ZL, Lin R, Fan ZL, Chen SJ, et al. (2020) Possible mechanisms of lycopene amelioration of learning and memory impairment in rats with vascular dementia. Neural Regen Res 15: 332341.
- Wang J, Shen Y, Li M, Li T, Shi D, et al. (2023) Lycopene treatment attenuates D-galactose-induced cognitive decline by enhancing mitochondrial function and improving insulin signaling in the brains of female CD-1 mice. J Nutr Biochem 118:109361.
- Iyer S, Bhat I, Sheshappa MB (2024) Luetin and the underlying neuroprotective promise against neurodegenerative diseases. Mol Nutr Food Res 68: e2300409.
- Jayakanthan M, Manochkumar J, Efferth T, Ramamoorthy S (2024) Lutein, a versatile carotenoid: Insight on neuroprotective potential and recent advances. Phytomedicine. 135:156185.
- Power R, Coen RF, Beatty S, Mulcahy R, Moran R, et al. (2018) Supplemental retinal carotenoids enhance memory in healthy individuals with low levels of macular pigment in a randomized, doubleblind, placebo-controlled clinical trial. J Alzheimer’s Dis 61: 947-961.
- Cho KS, Shin M, Kim S, Lee SB (2018) Recent Advances in studies of the therapeutic potential of dietary carotenoids in neurodegenerative diseases. Oxidative Med Cell Longevity. 16: 4120458.
- Wingo TS, Liu Y, Gerasimov ES, Vattathil SM, Wynne ME, et al. (2022) Shared mechanisms across the major psychiatric and neurodegenerative diseases. Nature Communications. 13:4314.
- Anderson E, Crawford CM, Fava M, Ingelfinger J, Nikayin S, et al. (2024) Depression-understanding, identifying, and diagnosing. N Engl Med 390: e41.
- Kulkarni J (2022) Why depression in women is so misunderstood. Nature. 608: S54.
- McCarron RM, Shapiro B, Rawles J, Luo J (2021) Depression. Ann Intern Med 174:ITC65-80.
- Ge H, Yang T, Sun J, Zhang D (2020) Associations between dietary carotenoid intakes and the risk of depressive symptoms. Food Nutr Res 28:64.
- Bardinet J, Pouchieu C, Chuy V, Helmer C, Etheve S, et al. (2023) Plasma carotenoids and risk of depressive symptomatology in a population-based cohort of older adults. J Affective Disorders 339: 615-623.
- Yu Q, Xue F, Li Z, Li X, Ai L, et al. (2022) Dietary intake of carotenoids and risk of depressive symptoms: A systematic review and metaanalysis. Antioxidants. 11: 2205.
- Rasmus P, Kozlowska E (2023) Antioxidant and anti-inflammatory effects of carotenoids in mood disorders: An overview. Antioxidants 12: 676.
- Peng C, Fang MS (2024) Association of dietary antioxidant intake with depressive risk and all-cause mortality in people with prediabetes. Sci Reports 14: 20009.
- Zhang W, Cheng Z, Lin H, Fu F, Zhan Z (2024) Serum carotenoid levels inversely correlate with depression symptoms among adults: Insights from NHANES data. J Affective Disorders 362: 869-876.
- Lu JH, Zhong WW, Tan YL, Zhuo L, Luo GZ (2025) Association of dietary and plasma lutein+ zeaxanthin with depression in US adults: findings from NHANES. Asia Pac J Clin Nutr 34:153-164.
- Li X, Lan Y (2025) Association between higher dietary lycopene intake and reduced depression risk among American adults: evidence from NHANES 2007-2016. Front Nutr 12: 1538396.
- Yen CH, Chang PS, Ghiu CJ, Huang YY, Lin PT (2021) β-Carotene status is associated with inflammation and two components of metabolic syndrome in patients with and without osteoarthritis. Nutrients. 13: 2280.
- Takayanagi Y, Obana A, MutoS, Asaoka R, Tanito M, et al (2021) Relationships between skin carotenoid levels and metabolic syndrome. Antioxidants. 11: 14.
- Kimura Y, Hata J, Shibata M, Honda T, Sakata S, et al. (2024) Skin carotenoid score and metabolic syndrome in general Japanese population: the Hisayama study. Internet J Obesity 48: 1465-1471.
- Sun Q, Fan Z, Yao F, Zhao X, Jiang M, et al. (2024) Association of dietary and circulating antioxidant vitamins with metabolic syndrome: an observational and Mendelian randomization study. Front Endocrinol 15:1446719.
- Ortega-Regules AE, Martinez-Thomas JA, Schurenkamper-Carrillo K, de Parrodi CA, Lopez-Mena ER, et al. (2024) Recent advances in the therapeutic potential of carotenoids in preventing and managing metabolic disorders. Plants. 13:1584.
- Bouayed J, Vahid F (2024) Carotenoid pattern intake and relation to metabolic status, risk and syndrome, and its components-divergent findings from the ORISCAV-LUX-2 survey. British J Nutr 132: 50-66.
- Marceline G, Machate DJ, de Cassia Freitas K, Hiane PA, Maldonade IR, et al. (2020) β-Carotene: Preventive role for type 2 diabetes mellitus and obesity: A review. Molecules. 25: 5803.
- Jiang YW, Sun ZH, Tong WW, Yang K, Guo KQ, et al. (2021) Dietary intake and circulating concentrations of carotenoids and risk of type 2 diabetes: A dose-response meta-analysis of prospective observational studies. Adv Nutr 12:1723-1733.
- Leh HE, Sopian MM, Abu Bakar MH, Lee LK (2021) The role of lycopene for the amelioration of glycaemic status and peripheral antioxidant capacity among the type 2 diabetes patients: a casecontrol study. Ann Med 53: 1059-1065.
- Coronel J, Pinos I, Amengual J (2019) β-Carotene in obesity research: Technical considerations and current status of the field. Nutrients. 11: 842.
- Anzar CA, Joseph MV, Vadiraj SR, Prasad CP, Eranimose B, (2023) Safety assessment of lutein and zeaxanthin supplementation and its effects on blood glucose levels, kidney functions, liver functions, and bone health-randomized, double-blind, placebo-controlled clinical study. MedRxiv.
- Mounien L, Tourniaire F, Landrier JF (2019) Anti-obesity effect of carotenoids: Direct impact on adipose tissue and adipose tissuedriven indirect effects. Nutrients.11:1562.
- Yao N, Yan S, Guo Y, Wang H, Li X, et al. (2021) The association between carotenoids and subjects with overweight or obesity: a systematic review and meta-analysis. Food Funct 12: 4768-4782.
- Eroglu A, Al’Abri IS. Kopec RE, Crook N, Bohn T (2023) Carotenoids and their health benefits as derived via their interactions with the gut microbiome. Adv Nutr 4: 238-255.
- Hashemi D, Fard MV, Mohammadhasani K, Barati M, NattaghEshtivani E (2024) Carotenoids improve obesity and fatty liver disease via the gut microbiome. A Narrative Review. Food Sci Nutr 13: e70092.
- Tarshish E, Hermoni K, Muizzudin N (2023) Effect of Lumenato a tomato derived oral supplement on improving skin barrier strength. Skin Res Tech 29: e13504.
- Stanescu C, Chiscop I, Mihalache D, Popa F, Tamas C (2025) Skin aging and carotenoids: a systematic review of their multifaceted protective mechanisms. Nutrients. 17: 2596.
- Zhuang C, Yuan J, Du Y, Zeng J, Sun Y, et al. (2022) Effects of oral carotenoids on oxidative stress: A systematic review and metaanalysis of studies in the recent 20 years. Front Nutr 9: 754707.
- Shanaida M, Mykailenko O, Lysiuk R, Hudz N, Balwierz R, et al. (2025) Carotenoids for antiaging: Nutraceutical, pharmaceutical, and cosmeceutical applications. Pharmaceuticals.18: 403.
- Ermakov IV, Ermakova M, Sharifzadeh M, Gorusupudi A, Farnsworth K, et al. (2018) Optical assessment of skin carotenoid status as a biomarker of vegetable and fruit intake. Arch Biochem Biophys 646: 46-54.
- Radtke MD, Pitts SJ, Jahns L, Firnhaber G, Loofbourrow BM, et al. (2020) Criterion-related validity of spectroscopy-based skin carotenoid measurements as a proxy for fruit and vegetable intake: A systematic review. Adv Nutr 11:1282-1299.
- Radtke MD, Poe M, Stookey J, Pitts SJ, Moran NE, et al. (2021) Recommendations for the use of the Veggie Meter® for spectroscopybased skin carotenoid measurements in the research setting. Res Method Study Design 5: nzab104.
- Radtke M, Pitts SJ, Jahns L, Firnhaber G, Loofbourrow B, et al. (2020) Examining the validity of spectroscopy-based skin carotenoid measurements as a proxy for fruit and vegetable consumption. Curr Dev Nutr 4: nzaa041_029.
- Amoah I, Cairncross C, Rush E (2023) Vegetable-enriched bread: Pliot and feasibility study of measurement of changes in skin carotenoid concentrations by reflection spectroscopy as a biomarker of vegetable intake. Food Sci Nutr 11: 3376-3384.
- Wu Q, Cherry CW, Pitts SJ, Laska MN, Craft N, et al. (2025) A reflection-spectroscopy measured skin carotenoid score strongly correlates with plasma concentrations of all major dietary carotenoids species except for lycopene. Nutr Res133:127-137.
- Hwang JE, Park JY, Jung MH, Eom K, Moon HS, et al. (2023) Evaluation of a commercial device based on reflection spectroscopy as an alternative to resonance Ramman spectroscopy in measuring skin carotenoid levels: Randomized control trials. Sensors. 23: 7654.
- Ahn S, Hwang J-E Kim YJ, Eom K, Jung MH, Moon HS, et al. (2024) Examination of the utility of skin carotenoid status in estimating intakes of carotenoids and fruits and vegetables: A randomized, parallelgroup, controlled feeding trial. Nutrition. 119:112304.
- May K, Pitts SJ, Stage VC, Kelley CJ, Burkholder S, et al. (2020) Use of the Veggie Meter as a tool to objectively approximate fruit and vegetable intake among youth for evaluation of preschool and schoolbased interventions. J Hum Nutr Diet 33: 869-875.
- Martinelli S, Acciai F, Tasevska N, Ohri-Vachaspati P (2021) Using the Veggie Meter in elementary schools to objectively measure fruit and vegetable intake: a pilot study. Methods Protocols. 4: 33.
- Kurotani K, Ohkawara K, Takimoto H (2024) Parent-child skin carotenoid level and vegetable intake relationships in users of children’s cafeterias in Japan. Front. Nutr 11:1388233.
- Caparello G, Groccia GD, Ceraudo F, Cesario M, Bonofiglio R, et al. (2023) Association between skin carotenoid score measured with Veggie Meter and adherence to the Mediterranean diet among adolescents from Southern Italy. Nutrients.15: 4920.
- Hosseinzadeh M, Shriver L, Buehler C, Wideman L, Liernes E (2023) Association between self-reported fruit and vegetable intake and skin carotenoids among pregnant women. J Nutr Ed Behav 55: 46.
- Schweitzer A, Brown R, Monroe-Lord L (2023) Measurements of skin carotenoid status by Veggie Meter in urban adults. Innovation Aging. 7:761-762.
- Sisson SB, Kasahara E, Casperson S, Pitts SJ, Reese J, et al. (2025) Association of Veggie Meter-Assessed Skin Carotenoids and Dietary Intake Among Indigenous Families: The Indigenous Supported Agriculture “Go Healthy” Study. Curr Dev Nutr 9: 107521.
- Pitts SJ, Moran NE, Laska MN, Wu Q, Harnack L, et al. (2023) Reflection spectroscopy-assessed skin carotenoids are sensitive to change in carotenoid intake in a 6-week randomized feeding trial in a racially/ethnically diverse population sample. J Nutr 153: 1133-1142.
- Johnston E, Dudzik J, Chen JY, Adams J, Kulkarni A, et al. (2025) Correlation between Veggie Meter and self-reported carotenoid, fruit and vegetable intake among older adults: Results from bringing the diabetes prevention program to geriatric populations. Circulation. 151: S1: P114.
- Obana A, Asoka R, Takayanagi Y, Gohto Y (2023) Inter-device concordance of Veggie-Meter-A reflection spectroscopy to measure skin carotenoids. J Biophotonics 16: e2023300071.
- Hasin S, Dev DA, Swindle T, Sisson SB, Pitts SJ, et al. (2023) Systemic review of reflection spectroscopy-based skin carotenoid assessment in children. Nutrients.15: 1315.
- Oban A (2025) Measurement of skin carotenoids and their association with disease: A narrative review. BBA Mole Cell Biol Lipids1870: 159612.
- Veggie Meter-Longevity Link Corporation
- Udensi J, Loughman J, Loskutova E, Byrne HJ (2022) Raman spectroscopy of carotenoid compounds for clinical applications- A review. Molecules. 27: 9017.
- Nu Skin RRS technology BioPhontic S3 Scanner
- Pitts ADJ, Johnson NS, Wu Q, Firnhaber GC, Kaur AP, et al. (2021) A meta-analysis of studies examining associations between resonance Raman spectroscopy-assessed skin carotenoids and plasma carotenoids among adults and children. Nutr Rev 80: 230-241.
- Aguilar SS, Wengreen HJ, Dew J (2015) Skin carotenoid response to a high-carotenoid juice in children: a randomized clinical trial. J Acad Nutr 115: 1771-1778.
- Aguilar SS, Wengreen HJ, Lefevre M, Madden GJ, Gast J (2014) Skin carotenoid: a biomarker of fruit and vegetable intake in children. J Acad Nutr 114: 1174-1180.
- Rerksuppaphol S, Rerksuppaphol L (2006) Effect of fruit and vegetable intake on skin carotenoid detected by non-invasive Raman spectroscopy. J Med Assoc Thai 89: 1206-1212.
- Van Rensburg JA, Wenhold F (2016) Validity and reliability of field resonance Raman spectroscopy for assessing carotenoid status. J Nutr Sci Vitaminol 62: 317-321.
- Zidichouski JA, Mastaloudis A, Poole SJ, Reading JC, Smidt CR (2009) Clinical validation of a noninvasive, Raman spectroscopic method to assess carotenoid nutritional status in humans. J Am Coll Nutr 28: 687,693.
- Toh DWK, Loh WW, Sutanto CN, Yao Y, Kim JE (2021) Skin carotenoid status and plasma carotenoids: biomarkers of dietary carotenoids, fruits and vegetables for middle-aged and older Singaporean adults. Br J Nutr126: 1398-1407.
- Perrone A, Pintaudi AM, Traina A, Carruba G, Attanzio A, et al. (2016) Raman spectroscopic measurements of dermal breast cancer operated patients provide evidence for the positive impact of a dietary regimen rich in fruits and vegetables on body oxidative stress and BC prognostic anthropometric parameters. A five-year study. Oxid Med Cell Longev 2016: 2727403.
- Jahns L, Johnson LK, Conrad Z, Bukowski M, Raatz SK, et al. (2019) Concurrent validity of skin carotenoid status as a concentration biomarker of vegetable and fruit intake compared to multiple 24-recalls and plasma carotenoid concentrations across one year: a cohort study. Nutr J 18:78.
- Sharifzadeh M (2025) Accurate quantification of carotenoids in human skin: Correcting for melanin and hemoglobin interference. Wor Jour Clin Der 2: 01-12.
- Soulat J, Andueza D, Graulet B, Girard CL, Labonne C, et al. (2020) Comparison of the potential abilities of three spectroscopy methods: Near-infrared, mid-infrared, and molecular fluorescence, to predict carotenoid, vitamin and fatty acid contents in cows milk. Foods. 9: 592.
- Lassale C, Gaye B (2024) Addressing global micronutrient inadequacies: enhancing global data representation. Lancet. 12: e1561-e1562.
- Gardner B, Lally P, Wardle J (2012) Making health habitual: the psychology of ‘habit-formation’ and general practice. Br J Gen Practice 62: 664-666.
- Ballard IC, Waskom M, Nix KC, D’Esposito M (2024) Reward reinforcement creates enduing facilitation of goal-directed behaviour. J Cognitive Neurosci 36: 2847-2862.
- Kim YI, Choi Y, Park J (2023) The role of continuous glucose monitoring in physical activity and nutrition management: perspectives on present and possible uses. Physical Activity Nutr 27: 44-51.
- Klonoff DC, Nguyen KT, Xu NY, Gutierrez A, Espinoza JC, et al. (2023) Use of continuous glucose monitors by people without diabetes: An idea whose time has come? J Diabetes Sci Technol. 17: 1686-1697.
- Ehrhardt N, Al Zaghal E (2020) Continuous glucose monitoring as a behaviour modification tool. Clin Diabetes 38:126-131.
- Kruas WE, Janz KF, Powell KE, Campbell WW, Jakicic JM, et al. (2019) Daily step counts for measuring physical activity exposure and its relation to health. Med Sci Sports Exerc 51: 1206-1212.
- Paluch AE, Gabriel KP, Fulton JE, Lewis CE, Schreiner PJ, et al. (2021) Steps per day and all-cause mortality in middle-aged adults in the coronary artier risk development in young adults study. JAMA Network Open. 4: e21224516.
- del Pozo Cruz B, Ahmadi MN, Lee IM, Stamatakis E (2022) Prospective associations of daily step counts and intensity with cancer and cardiovascular disease incidence and mortality and all-cause mortality. JAMA Intern Med 182: 1139-1148.
- Robbins R, Sexis A, Masters LM, Chanko N, Diaby F, et al. (2019) Sleep tracking: A systematic review of the research using commercially available technology. Curr Sleep Med Res 5:156-163.
- Seid A, Fufa DD, Biew ZW (2024) The use of internet-based smartphone apps consistently improved consumer’s healthy eating behaviors: a systematic review of randomized controlled trials. Front Digital Health. 6:1282570.
- Knaggs H, Hester SN, Gibb T, Riggs M, Ferguson S, et al. (2025) Global, gender, and age variation in skin carotenoid scores (SCS) quantified by spectroscopy reflects a measure of health and wellness. 2025 10th International Conference on Health & Nutrition, Chicago, IL, USA, Integrative Nutrition. in press.
- Major R, Hester S, Gibb T, Riggs M, Ferguson S, et al. (2025) The correlation between skin carotenoid scores and specific diet and lifestyle choices. 2025 10th International Conference on Health and Nutrition, Chicago, IL, USA. Integrative Nutrition. in press.
- Knaggs, H, Lephart ED (2025) Global Lifetime Non-invasive Spectroscopy-based RRS Quantification of Skin Carotenoids Provides Evidence for Enhancing the Human Healthspan-A Narrative Review.
- Knaggs H, Lephart ED (2025) Impact of lifestyle factors on global skin carotenoid levels quantified by non-invasive spectroscopy-based RRS technology.
- Faffetti E, Mondino E, Di Baldassarre G (2022) COVID-19 vaccine hesitancy in Sweden and Italy: The role of trust in authorities. Scandinavian J Pub Health 50:803-809.
- Okada E, Kurotani K, Takimoto H (2024) Skin carotenoid levels are associated with demographic factors, body size, and fruit and vegetable intake in the Japanese population. Nutrients. 16: 2133.
- Agan S, Rigbee L, Kenche H, Michail S, Khamis HJ, (2011) Distal gut microbiota of adolescent children is different from that of adults. FEMS Microbiol Ecol 77: 404-412.
- Derrien M, Alvarez AS, de Vos WM (2019) The gut microbiota in the first decade of life. Trends Microbiol 27: 997-1010.
- Carson MD, Westwater C, Novince CM (2023) Adolescence and the Microbiome, Implications for healthy growth and maturation. Am J Pathology193:1900-1909.
- Stahl W, Heinrich U, Jungman H, von Larr J, Schietzel M, et al. (1998) Increased dermal carotenoid levels assessed by noninvasive reflection spectrophotometry correlates with serum levels in women ingesting betatene. Nutr Metab128: 903-907.
- Streker M, Proksch E, Kattenstroth JC, Poeggeler B, Lemmnitz G (2025) Comparative assessment of nutraceuticals for supporting skin health. Nutraceuticals. 5:13.
- Babar M, Buzdar JA, Zaheer Z, Nizam-ud-din N, Mustafa G, et al. (2025) Carotenoids as a nutraceutical and health-promoting dietary supplement for human and animals: an update review. Trad Med Res 10:14.
- Ibrahim M, Singh H, Fahim M, Khan S, Khan J, et al. (2025) Nutraceutical interventions for mitigating skin ageing: Analysis of mechanism and efficacy. Curr Pharm Design 31: 2385-2401.
- Hester SN, Major RA, Gibb T, Riggs M, Ferguson S, et al. (2025) Use of spectroscopy to validate effects of a multivitamin on nutrient status. Am. Soc. Nutr. 2 Orlando, Florida, USA, Curr Dev Nutr in press.
- Black HS, Boehm F, Edge R, Truscott TG (2020) The benefits and risks of certain dietary carotenoids that exhibit both anti- and prooxidative mechanisms-A comprehensive review. Antioxidants. 9: 264.
- Ahmadkhaniha R, Yousefian F, Noushin R (2021) Impact of smoking on oxidant/antioxidant status and oxidative stress index levels in serum of university students. J Environ Health Sci Eng19:1043-1046.
- Astori E, Garavaglia ML, Colombo G, Landoni L, Portinaro NM, et al (2022) Antioxidants in Smokers. Nutr Res Rev35: 70-97.
- de Juras AR, Hsu WC, Hu SC (2021) The double burden of malnutrition at the individual level among adults: A nationwide survey in the Philippines. Front Nutr 8:760437.
- World Population Review (2025) Smoking Rates by Country.
- Augimeri G, Lofaro D, Vivacqua A, Barone I, Giordano C, et al (2025) MeDiet Working Group. J Translational Med 23:952.
- Bakhtair S, Azimi S, Mehdipour M, Amini S, Elmi Z, et al. (2015) Effect of cigarette smoke on salivary total antioxidant capacity. J Dent Res Dent Clin Dent Prospects 9: 281-284.
- Adefegha AS (2018) Functional foods and nutraceuticals as dietary interventions in chronic disease; novel perspectives for health promotion and disease prevention. J Dietary Supplements 15: 9771009.
- Arikawa AY, Snyder J, Ross JM, Harris M, Perez D, et al. (2023) Dietary supplement intake is associated with healthier lifestyle behaviors in college students attending a regional university in the Southeast: A cross-sectional study. J Dietary Supplements 20: 870-884.
- John S (2024) Unlocking the power of antioxidants: Protecting cells from damage, supporting immune health, and promoting longevity through nutrient-rich diet. J Nutrition Human Health 8: 235.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.