An In Vitro Evaluation and Comparison of Commercially Available Needleless Connectors with and Without Anti-Reflux Technology
Josh Hughey1*, S. Matthew Gibson2, Nancy Moureau3, Bob Buzas4
1Nexus Medical, LLC, Lenexa, KS, United States of America
2Vascular Access Consulting, Henderson, KY, United States of America
3PICC Excellence, Inc, Hartwell, GA, United States of America
4Allegheny Health Network Home Infusion, Pittsburgh, PA, United States of America
*Corresponding author: Josh Hughey, Nexus Medical, LLC, 11315 Strang Line Road, Lenexa, KS 66215, United States of America.
Received Date: 20 June, 2023
Accepted Date: 30 June, 2023
Published Date: 05 July, 2023
Citation: Hughey J, Gibson MS, Moureau N, Buzas B (2023) An In Vitro Evaluation and Comparison of Commercially Available Needleless Connectors with and Without Anti-Reflux Technology. Int J Nurs Health Care Res 6: 1439. https://doi.org/10.29011/2688-9501.101439
Abstract
Design, function, and performance vary between negative displacement, positive displacement and pressure-activated antireflux needleless connectors (NCs). Marketing terms used to classify needleless connectors do not adequately describe function and performance, leading to confusion and poor understanding of the correct flush-clamp-disconnect sequence specific to each NC design. We compared commercially available NCs without integrated anti-reflux technology to their exact design counterpart with anti-reflux technology. The goal of this study was to evaluate each NC’s ability to control bi-directional fluid movement caused by mechanical and physiological pressure changes and measure reflux volume. A venous simulator was used to measure and visualize fluid displacement associated with each NCs design upon connection and disconnection of a syringe. A bi-directional flow test was also performed by creating a pressure gradient between two reservoirs which were connected using the NC being tested. The fluid displacement at syringe disconnection was negative for all devices tested. All NCs with integrated anti-reflux technology significantly reduced the reflux volume compared to their design counterparts without anti-reflux technology (P < .0001). The difference in reflux volume was statistically significant (P < .0001) between all NC devices with integrated anti-reflux technology (0.27µL; 95% CI 0.21-0.34µL) compared to all devices without anti-reflux technology (4.15µL; 95% CI 3.76-4.54µL). All NCs without anti-reflux technology failed to demonstrate the ability to control blood reflux and bi-directional fluid movement. These results demonstrate that integrated anti-reflux technology is effective at preventing blood reflux caused by mechanical and physiological pressure changes in a closed IV system.
Keywords: Blood; Biofilm; Catheter-related blood stream infections; Infection control; Vascular access devices; Needleless connectors.
Introduction
Needleless connectors (NCs) were originally introduced as an intravenous (IV) catheter accessory in the early 1990s to prevent needlestick injury among healthcare workers and to maintain closed systems. Half of all post-insertion catheter-related infections are attributed to bacterial colonization of catheter hubs and NCs [1-5]. When refluxed blood contacts IV catheter surfaces, a layer of plasma proteins, which are responsible for platelet adhesion, activation and biomaterial-induced thrombus [6-8] adsorb to the intraluminal surface instantly forming a thin conditioning film [6,9]. This conditioning film sets in motion a series of biological reactions that help form the fibrin mesh that traps blood cells and promotes thrombus formation within the catheter lumen [6,8,10,11] (Figure 1). Bacteria entering through the NC [12,13] can bind to the protein-coated surfaces of the IV catheter and proliferate into biofilm [9,14,15]. Thrombolytics such as tissue plasminogen activators (tPA) commonly used to treat intraluminal catheter occlusions catalyze the conversion of plasminogen to plasmin which cleaves fibrin into fibrin degradation products and dissolves the thrombus [11] (Figure 2). Studies have demonstrated the risk of infection is approximately 3 to 4 times higher when thrombolytics are used to treat intraluminal catheter occlusions [16-18]. This increased infection risk is due to the release of dissolved bacteria-riddled thrombus into the bloodstream during flushing.
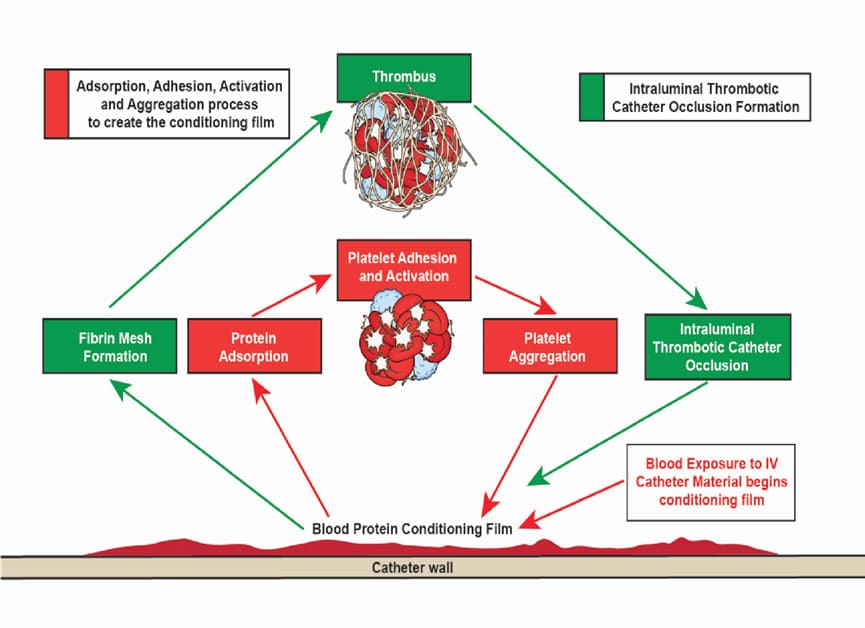
Figure 1: Foreign body response following blood contact to IV catheter material leading to intraluminal thrombotic catheter occlusions. Abbreviations: IV, intravenous.
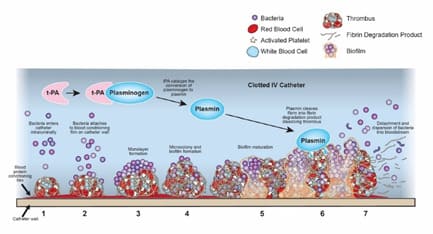
Figure 2: Illustration depicting how bacteria within an occluded IV catheter are dislodged by tPA when a clot is dissolved, thereby allowing the microbes to enter the bloodstream. Abbreviations: tPA, tissue plasminogen activator.
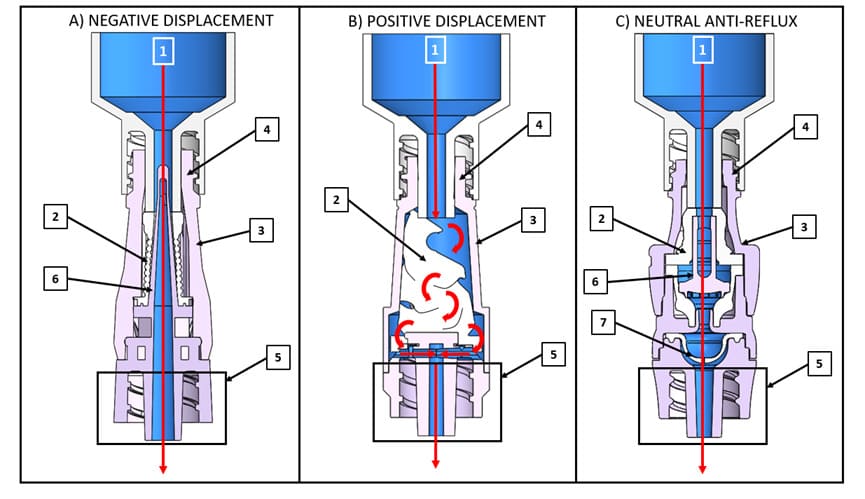
The external design of NCs is similar but there are significant internal differences among the available devices that affect function, performance, and patient safety. For example, all NCs allow for bi-directional fluid movement, defined as the ability to administer or withdraw fluids or medication for infusion and aspiration [20], but not all NCs contain an internal mechanism to control when and how much fluid movement occurs. Commercially available NCs are classified as negative displacement, positive displacement, neutral, or pressure activated anti-reflux devices according to the internal mechanism for fluid displacement by the device [19] (Table 1). It is important that clinicians know which type of NC they are using so that they can employ the appropriate flushing, clamping, and disconnection sequence. However, the 8th edition of the Infusion Therapy Standards (Standards) cautions that there are no established criteria from device regulatory agencies that can help clinicians determine whether a commercial NC is assigned to a particular category [19]. Given this information, it is not surprising that in a survey of 4,000 healthcare workers, 544 responded reporting that over 47% did not understand the correct method to flush and clamp a catheter with the NC or within multiple types of NCs used by their institution [20].
|
Type of NC |
Description |
|
|
Negative displacement [21- 23] |
• • |
Allows blood to reflux into the catheter lumen during syringe or administration set disconnection Require a clamping sequence of flush, clamp and remove |
|
Positive displacement [21- 23] |
• • |
Allows blood to reflux into the catheter lumen during syringe or administration set connection Requires a clamping sequence of flush, remove and clamp |
|
Neutral [21, 24-26] |
• • |
Allows blood to reflux into the catheter lumen during syringe or administration set disconnection Requires a clamping sequence of flush, clamp and remove |
|
Anti-reflux [21, 24-26] |
• • |
Integrated system prevents unintentional blood reflux caused by mechanical and physiological pressure changes Does not require a specific clamping sequence and contains a normally closed pressure activated anti-reflux diaphragm in the fluid pathway, which automatically opens and closes based upon infusion pressure |
Table 1: Classification of needleless connectors (NCs) by internal mechanism for fluid displacement by the device.
NCs that produce low amounts of blood reflux and can control bi-directional fluid flow have been shown to reduce the risk of infection and catheter-related complications [23-27]. Thus, NC performance may be measured based on the amount of blood movement that refluxed into the catheter [24,25,27]. Additionally, studies have demonstrated that NCs without bi-directional flow control are associated with unintended complications, such as intraluminal thrombotic catheter occlusions and central line associated bloodstream infections (CLABSI) that negatively impact patient safety [28-31]. Furthermore, an experimental study revealed a correlation between blood reflux volume and intraluminal thrombotic catheter occlusion rates [32]. Based on this study, it is reasonable to hypothesize anti-reflux technology may optimize catheter function and reduce consequences associated with blood reflux including delayed treatment [33], device replacement [34], infection [16-18], and increased cost [28,29,33,34].
According to the Standards, there is limited evidence to support the superiority of one type of NC over another, particularly regarding the ability to prevent internal contamination [19]. Instead, clinicians are urged to evaluate the latest evidence when making decisions about the choice of NC. In the absence of guidelines and standardized classification criteria, deciphering the marketing terms (Table 2) used by NC manufacturers is problematic and can make it challenging for clinicians to integrate NCs into practice. The aim of this study is to add to the existing body of knowledge by using in vitro methods to evaluate the performance of commercially available NCs without integrated anti-reflux technology compared to their exact design counterpart with anti-reflux technology based on reflux volume and their ability to control bi-directional fluid movement.
|
|
|
|
Term |
Definition |
|
(1) Fluid pathway [22,23] |
Path by
which fluid flows through an NC. If the fluid flows through the middle of the
elastomeric septum, the NC will have negative displacement while if the fluid
flows between the outer housing and elastomeric septum, the NC will have
positive displacement. |
|
Direct fluid pathway [5,23,24,35]:
Found in negative displacement and neutral anti-reflux NCs, a straight
fluid pathway allows fluid to flow directly through the pre-slit elastomeric
septum resulting in laminar flow profiles. |
|
|
Indirect fluid pathway [5,36]:
Found in positive displacement NCs, a fluid pathway required to flow around
a compressed elastomeric septum resulting in turbulent flow profiles and dead
space (areas with slow or no moving fluid that cannot be effectively flushed. |
|
|
Priming volume: Volume of fluid required to fill engaged
NC to eliminate air (associated with all types of NC). |
|
|
(2) Elastomeric septum (often
referred as centerpiece) [22,23] |
Luer-activated
flexible silicone septum compresses to create a fluid pathway. Negative
displacement and anti-reflux NCs have pre-slit elastomeric septums, while positive
displacement NCs have no slit or a partial slit on the lateral side of the
elastomeric septum that opens the fluid pathway between the elastomeric
septum and inlet housing. |
|
(3) Inlet housing |
Housing
that connects the female luer to the male luer lock of NC providing an
airtight seal for the fluid pathway. |
|
(4) Female Luer lock |
Found on
the proximal end of NCs and is compatible with all ISO/ANSI male luer lock
connectors. |
|
(5) Male Luer lock |
ISO/ANSI
standard male luer lock connector on distal end of NC to safely attach to any
ISO/ANSI female IV connector |
|
(6) Internal cannula [22] |
Rigid
component within elastomeric septum that creates a direct fluid pathway when
activated by a male luer lock. |
|
(7) Pressure-activated anti-reflux
diaphragm [19,24,37,38] |
An
internal pressure activated mechanism designed to reduce blood reflux. This
normally closed three-position diaphragm opens toward the patient when fluid
pressure is greater than venous pressure and opens in reverse based on
aspiration pressure preventing unintentional blood reflux into the patient’s
IV catheter and provides bi-directional flow control. |
|
Bi-directional flow control [24]: Ability
of an NC to control the mechanical and physiological pressure changes within
a closed IV system therefore preventing unintentional backward flow and
uncontrolled blood reflux into the patient’s IV catheter. |
|
Table 2: Common terms and definitions associated with NC design and function.
Materials and methods
Four commercially available NCs without anti-reflux technology (Microclave™, ICU Medical, San Clemente, CA, USA; NIS®6P, Nexus Medical, Lenexa, KS, USA; Bionector®, Vygon, Ecouen, France, and NeutraClear™ CAIR LGL/BD, Teolo, Italy) were compared to their exact design counterpart with anti-reflux diaphragm technology (Neutron™, ICU Medical, San Clemente, CA, USA; TKO®-6P, Nexus Medical, Lenexa, KS, USA; Bionector TKO®, Vygon, Ecouen, France, and NeutroX™, CAIR LGL/BD, Teolo, Italy)(Figure 3).
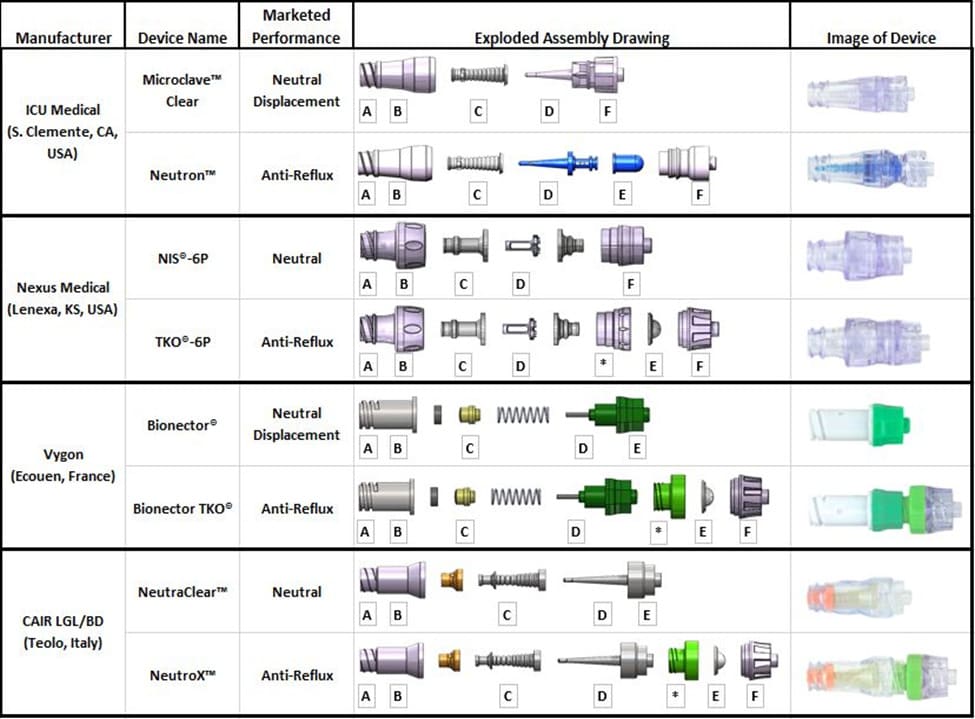
Figure 3: Exploded assembly drawing and image of devices tested; Labeled parts are defined in Table 2; (A) Female Luer (B) Inlet housing (C) Elastomeric septum (D) Internal cannula (E) Pressure-activated anti-reflux diaphragm (F) Male Luer Lock, (*) Coupler component mating inlet and outlet of device.
In vitro testing was conducted by a trained engineer with over 25 years of experience in vascular access and NC systems. Three samples of all devices were tested ten times each for a total of 30 simulations per NC (n=30). Negative and positive fluid displacement represent reflux into the catheter and aspiration towards the patient, respectively. The negative and positive fluid displacement in millimeters were converted to reflux volume using the formula V=πr^2 h, where r is the internal radius of the glass capillary rod and h is the measured fluid displacement.
Bi-Directional Flow Test:The following procedure was used to quantitatively evaluate the ability of each NC to control bidirectional fluid movement caused by pressure changes. Three samples of each device were tested ten times each for a total of 30 simulations per NC. First, the primed unit under test (UUT) was attached to 100 mL bag containing green dyed water. Using a syringe, air was purged from the dye bag and a 24-inch piece of PVC tubing was connected to the UUT. The other end of the PVC tubing was connected to the100 mL dye bag containing clear water. The clear bag was then raised above the green dyed bag level to purge the PVC tubing. After the tubing was purged, the clear bag was lowered 2 inches below simulated insertion site. If the green dyed fluid was seen in the tubing set, the UUT failed to control bi-directional fluid movement. If no green dyed fluid was seen in the tubing set, the UUT was determined to have the ability to control bi-directional fluid movement.
Reflux at Syringe Connection and Disconnection Test: A venous simulator was used to observe and measure reflux upon connection and disconnection of a syringe as described previously [21,24,26]. The venous simulator was designed to replicate the peripheral venous pressure in the cephalic vein in the middle third of the forearm (111 mm H2O or 8 mmHg) [39] and consisted of a vertically positioned glass rod and metric ruler within an acrylic stand. The glass rod was connected to a 4-way stopcock whose side ports were connected to a fluid-filled input extension set and output reservoir bag. The primed UUT was connected to the input extension set and the following procedure was performed. Three samples of each device were tested ten times each for a total of 30 simulations per NC. First, a 10 mL syringe filled with green dyed water was attached to the UUT. The stopcock to the glass rod of the simulator was turned to the “OFF” position. Then, a 10 mL syringe plunger was used to purge all air from the UUT, PVC tubing, and stopcock. Next, the stopcock to the reservoir bag was turned to the “OFF” position. The syringe plunger was slowly depressed to allow fluid to fill the glass rod until the fluid level reached 111 mm (equivalent to 8 mmHg of simulated venous pressure) on the metric ruler and marked fluid level. The 10 ml syringe was disconnected from the UUT and the observed fluid displacement was recorded in millimeters. The 10 mL syringe was connected to the UUT again and the plunger was slowly depressed to allow the fluid level to reach approximately 111 mm plus the previously recorded fluid displacement. Once the appropriate level was reached, the syringe was disconnected, and the fluid level was confirmed to be 111 mm. Last the syringe was connected to the UUT and the observed fluid displacement was recorded in millimeters.
Data Analysis
To evaluate differences between a device without anti-reflux technology and its design equivalent with anti-reflux technology, a t-test analysis was performed using GraphPad Prism (GraphPad Software, Inc.). To evaluate differences between all devices with and without anti-reflux technology, a one-way ANOVA analysis was performed using GraphPad Prism (GraphPad Software, Inc., San Diego, CA). A P-value <0.05 was considered statistically significant.
Results
Results from both in vitro tests are summarized in Figure 4.
Bi-Directional Flow Test.Simulation testing demonstrated all devices with anti-reflux technology proved the ability to control bi-directional fluid movement. Conversely, all devices without anti-reflux technology failed to demonstrate the ability to control bi-directional fluid movement. The fluid displacement at syringe connection was positive, displacing fluid away from the IV catheter lumen (which would be towards the patient in a clinical scenario) for all devices tested. The fluid displacement at syringe disconnection was negative for all devices tested. Except for Nexus Medical’s NIS®-6P and TKO-6P (P=.336), the NCs with anti-reflux technology demonstrated significantly reduced positive displacement compared with their counterpart without anti-reflux technology (P<.0001). However, the volume of fluid displacement for the NIS®-6P was significantly less when compared cumulatively to other devices without anti-reflux technology (P<.0001).
Reflux at Syringe Connection and Disconnection Test. All NCs tested demonstrated reflux on disconnection. The reflux volume for devices with anti-reflux technology ranged from 0.04 µL to 0.63 µL. The devices without anti-reflux technology ranged from 1.12 µL to 6.25 µL. The reflux volume for devices with anti-reflux technology was significantly less when compared to their design counterpart without anti-reflux technology (P<.0001). Additionally, the difference in reflux volume was statistically significant (P<.0001) between all devices with anti-reflux technology (0.27 µL; 95% CI 0.21-0.34 µL) compared to all devices without antireflux technology (4.15 µL; 95% CI 3.76-4.54 µL).
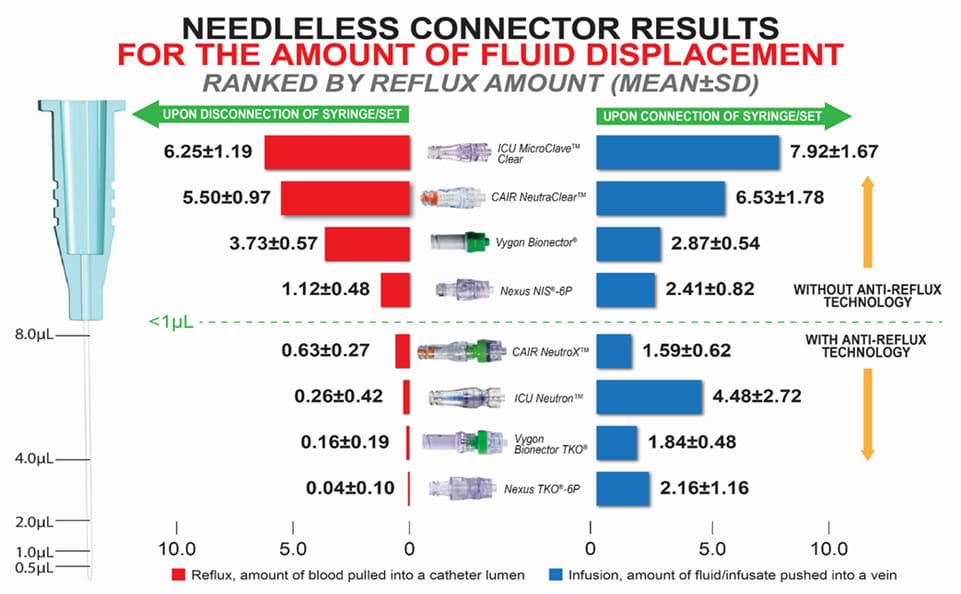
Figure 4: A visual representation of reflux volume into the IV catheter lumen and volume away from the IV catheter lumen during syringe disconnection and connection, respectively.
Discussion
Reducing blood reflux and controlling bi-directional fluid flow has been shown to minimize biofilm formation and the potential for vascular access complications related to NCs. Choosing an appropriate NC is challenging because marketing terms used to classify NCs do not adequately describe function and performance. Furthermore, there are no standardized NC classification criteria and additional head-to-head studies are needed before commercially available NCs can be ranked by superiority. In this study, we report that devices with anti-reflux technology were able to control bi-directional fluid movement unlike their design counterparts without anti-reflux technology. In addition, our in vitro studies showed that differences in reflux volumes for NCs with anti-reflux technology were statistically less than devices without anti-reflux technology.
Studies measuring the clinical performance of NCs with the functional anti-reflux diaphragm design are limited. One study demonstrated increased patency of catheters using pressure activated anti-reflux NCs in comparison to stopcock devices [32]. There are no known studies evaluating the performance of an NC with anti-reflux technology compared to its exact design counterpart without anti-reflux technology. However, there have been several in vitro studies evaluating the performance of NCs. Previous studies evaluated reflux volume using a similar venous simulator and included 4 of the 8 NCs tested here [21,24,26]. Additionally, two studies evaluated bi-directional flow control using similar methods duplicated in this study [21,26]. Finally, Elli et al. evaluated fluid reflux during disconnection of a syringe. That study evaluated 5 of the 8 NCs tested here [25]. The current study results represented in this publication are in general agreement with the trends put forth in the previous in vitro studies.
In the published studies noted above [21,24,26], the average reflux measurement for NCs with and without anti-reflux diaphragm was 0.29 µL and 5.85 µL, respectively. In this study the average reflux for NCs with and without anti-reflux diaphragm was 0.27 µL and 4.15 µL, respectively. Additionally, this study revealed NCs labeled as neutral displacement were shown to reflux at syringe disconnection. This result supports previous studies demonstrating that the term neutral displacement does not appropriately describe the performance of the device.
Furthermore, the Standards define a neutral displacement NC as those devices containing an internal mechanism designed to reduce blood reflux into the vascular access device lumen upon connection and disconnection [19]. Based our current results and evidence from previous studies [21,24-26], it is reasonable to conclude that the only internal mechanism that meets the Standards definition for neutral displacement is the NC with a pressure-activated anti-reflux diaphragm.
Limitations
Many manufacturers fail to provide specific, accurate or clear steps indicating the correct clamping sequences in their instructions for use and sales literature based on an NCs design and function. This lack of clear instruction leads to confusion and poor understanding by the end user bedside clinical staff for proper flush-clamp-disconnect sequence specific to each NC design [20]. With this in mind, the current study did not use a specific clamping sequence applicable to a particular NC design and function.
Conclusions
The importance of reducing the amount of blood reflux coupled with the ability to control bi-directional fluid flow has been demonstrated clinically. The results of this study build upon previous in vitro data that demonstrate control of bi-directional fluid and lower reflux volume flow with pressure-activated anti-reflux NCs. It is reasonable to hypothesize anti-reflux technology may optimize catheter function and reduce consequences associated with blood reflux, however larger clinical trials are needed.
Acknowledgements
The authors wish to thank Emily Collins for her creation of the assembly drawings and visual representation of results and Aisha Cobbs, PhD, for her medical editorial assistance.
Conflict of interest
Josh Hughey is currently the Director of Quality Assurance and Regulatory Affairs at Nexus Medical, LLC.
S. Matthew Gibson is currently the Chief Executive Officer at Vascular Access Consulting. He has previously received funding as a clinical consultant from Nexus Medical LLC, B Braun, Interrad Medical, Access Vascular Inc, Ethicon, and Adhezion. He has received funding as a clinical researcher from Beaumont Hospital (Royal Oak, Michigan) and Deaconess Research Institute (Evansville, Indiana).
Nancy Moureau is a clinician with PICC Access, Infinity Infusion Nursing and is the Chief Executive Officer at PICC Excellence, Inc., an educational and consulting firm, reporting consulting and speaker fees from 3m, Accuvein, Access Vascular Inc, BBraun, Bedal, Chiesi, Civco, Cleansite, General Electric, Linear Health Sciences, Dale Medical, Javelin Health, Nexus Medical, Parker Laboratories, and Teleflex.
Bob Buzas is currently the Director of Pharmacy Operations at Allegheny Health Network Home Infusion.
Funding sources
Nexus Medical, LLC.
Author Biosketches
- Josh Hughey
- S. Matthew Gibson
- Nancy Moureau
- Bob Buzas
References
- Jarvis WR, Murphy C, Hall KK, Fogle PJ, Karchmer TB, et al. (2009) Health care-associated bloodstream infections associated with negative- or positive-pressure or displacement mechanical valve needleless connectors. Clin Infect Dis 49: 1821-1827.
- Bouza E, Muñoz P, López-Rodríguez J, PérezJ MJ, Rincón C, et al. (2003) A needleless closed system device (CLAVE) protects from intravascular catheter tip and hub colonization: a prospective randomized study. J Hosp Infect 54: 279-287.
- Chernecky C, Waller J (2011) Comparative evaluation of five needleless intravenous connectors. J Adv Nurs 67: 1601-1613.
- Sitges-Serra A (1999) Strategies for prevention of catheter-related bloodstream infections. Support Care Cancer 7: 391-395.
- Jarvis WR (2010) Choosing the Best Design for Intravenous Needleless Connectors to Prevent Bloodstream Infections. In: Infection Control Today.
- Xu LC, Bauer JW, Siedlecki CA (2014) Proteins, platelets, and blood coagulation at biomaterial interfaces. Colloids Surf B Biointerfaces 124: 49-68.
- Farrell DH, Thiagarajan P (1994) Binding of recombinant fibrinogen mutants to platelets. J Biol Chem 269: 226-231.
- Grunkemeier JM, Tsai WB, Alexander MR, Castner DG, Horbett TA (2000) Platelet adhesion and procoagulant activity induced by contact with radiofrequency glow discharge polymers: Roles of adsorbed fibrinogen and vWF. J Biomed Mater Res 51: 669-679.
- Neoh KG, Li M, Kang ET, Chiong E, Tambyah PA (2017) Surface modification strategies for combating catheter-related complications: recent advances and challenges. J Mater Chem B 5: 2045-2067.
- Jaffer IH, Fredenburgh JC, Hirsh J, Weitz JI (2015) Medical deviceinduced thrombosis: what causes it and how can we prevent it. J Thromb Haemost 1: S72-81.
- Baskin JL, Pui CH, Reiss U, Wilimas JA, Metzger ML, et al. (2009) Management of occlusion and thrombosis associated with long-term indwelling central venous catheters. Lancet 374: 159-169.
- Mermel LA (2011) What is the predominant source of intravascular catheter infections? Clin Infect Dis 52: 211-212.
- Safdar N, Maki DG (2004) The pathogenesis of catheter-related bloodstream infection with noncuffed short-term central venous catheters. Intensive Care Med 30: 62-67.
- Katsikogianni M, Missirlis YF (2004) Concise review of mechanisms of bacterial adhesion to biomaterials and of techniques used in estimating bacteria-material interactions. Eur Cell Mater 8: 37-57.
- Sharma D, Misba L, Khan AU (2019) Antibiotics versus biofilm: an emerging battleground in microbial communities. Antimicrob Resist Infect Control 8: 76.
- Rowan CM, Miller KE, Beardsley AL, Ahmed SS, Rojas LA et al (2013) Alteplase use for malfunctioning central venous catheters correlates with catheter-associated bloodstream infections. Pediatr Crit Care Med 14: 306-309.
- Thakarar K, Collins M, Kwong L, Sulis C, Korn C et al. (2014) The role of tissue plasminogen activator use and systemic hypercoagulability in central line-associated bloodstream infections. Am J Infect Control 42: 417-420.
- Alkully T, Hensley S, Khuder S, Luke N, Ruzieh M, et al. (2020) PICC line associated blood stream infections: an analysis of host and device factors. Translation: The University of Toledo Journal of Medical Sciences 8:
- Gorski LA, Hadaway L, Hagle ME, Broadhurst D, Clare S et al. (2021) Infusion Therapy Standards of Practice, 8th Edition. J Infus Nurs 44: S1-S224.
- Hadaway L (2011) Needleless Connectors: Improving Practice, Reducing Risks. Journal of the Association for Vascular Access 16: 20-33.
- Gorzek S, LaDisa JF (2021) Assessment of Reflux From Needleless Connectors: Blinded Comparison of Category Designation to Benchtop Function Using a Venous Simulator. J Infus Nurs 44: 323-330.
- Hadaway L (2012) Needleless connectors for IV catheters. Am J Nurs 112: 32-44; quiz 45.
- Kelly LJ, Jones T, Kirkham S (2017) Needle-free devices: keeping the system closed. Br J Nurs 26: S14-S19.
- Hull GJ, Moureau NL, Sengupta S (2018) Quantitative assessment of reflux in commercially available needle-free IV connectors. J Vasc Access 19:12-22.
- Elli S, Abbruzzese C, Cannizzo L, Lucchini A (2016) In vitro evaluation of fluid reflux after flushing different types of needleless connectors. J Vasc Access 17: 429-434.
- Gibson SM, Primeaux J (2020) Do Needleless Connector Manufacturer Claims on Bidirectional Flow and Reflux Equate to In Vitro Quantification of Fluid Movement? Journal of the Association for Vascular Access 25: 28-36.
- Hadaway LC (1998) Major thrombotic and nonthrombotic complications. Loss of patency. J Intraven Nurs 21: S143-160.
- Steere L, Rousseau M, Durland L (2018) Lean Six Sigma for Intravenous Therapy Optimization: A Hospital Use of Lean Thinking to Improve Occlusion Management. Journal of the Association for Vascular Access 23:42-50.
- Steere L, Ficara C, Davis M, Moureau N (2019) Reaching One Peripheral Intravenous Catheter (PIVC) Per Patient Visit With Lean Multimodal Strategy: the PIV5RightsTM Bundle. Journal of the Association for Vascular Access 24: 31-43.
- Jasinsky LM, Wurster J (2009) Occlusion reduction and heparin elimination trial using an antireflux device on peripheral and central venous catheters. J Infus Nurs 32: 33-39.
- Btaiche IF, Kovacevich DS, Khalidi N, Papke LF (2011) The effects of needleless connectors on catheter-related bloodstream infections. Am J Infect Control 39: 277-283.
- Sansalone A, Vicari R, Orlando F, Dell’Avo A, Giuffrida S, et al. (2023) Needle-free connectors to prevent central venous catheter occlusion at a tertiary cardiac center: A prospective before and after intervention study. J Vasc Access 24: 475-482.
- Ernst FR, Chen E, Lipkin C, Tayama D, Amin AN (2014) Comparison of hospital length of stay, costs, and readmissions of alteplase versus catheter replacement among patients with occluded central venous catheters: Outcomes in CVC Occlusions. J Hosp Med 9: 490-496.
- Helm RE, Klausner JD, Klemperer JD, Flint LM, Huang E (2015) Accepted but unacceptable: peripheral IV catheter failure. J Infus Nurs 38: 189-203.
- Curran E (2016) Needleless connectors: the vascular access catheter’s microbial gatekeeper. J Infect Prev 17: 234-240.
- Macklin D (2010) The Impact of IV Connectors on Clinical Practice and Patient Outcomes. Journal of the Association for Vascular Access 15: 126-139.
- McElroy B (2018) Needleless Connectors: Improving Patient Care While Keeping Health Care Professionals Safe. National Home Infusion Association 24: 20-22
- Hitchcock J (2016) Preventing intraluminal occlusion in peripherally inserted central catheters. Br J Nurs 25: S12-S18.
- Ochsner A, Colp R, Burch GE (1951) Normal blood pressure in the superficial venous system of man at rest in the supine position. Circulation 3: 674-680.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.