Wound Healing in Diabetic foot Ulceration by Using Fat-Derived Stromal Vascular Matrix (SVM) Therapy - A Case Series
by Alper Erkin1, Hande Cengiz Açıl2, Havva Sert3, Thomas Eberleın4*
1Department of Cardiovascular Surgery, Wound Clinic, Sakarya University, Faculty of Medicine, Sakarya, Turkey
2Department of Surgical Nursing, Sakarya University, Faculty of Health Sciences, Sakarya, Turkey
3Department of Internal Nursing, Sakarya University, Faculty of Health Sciences, Sakarya, Turkey
4Academy for Certified Wound Management CWM AG, Embrach, Zurich, Switzerland
*Corresponding author: Thomas Eberleın, Academy for Certified Wound Management CWM AG, Embrach, Zurich, Switzerland
Received Date: 16 December 2024
Accepted Date: 20 December 2024
Published Date: 23 December 2024
Citation: Erkin A, Açıl HC, Sert H, Eberleın T (2024) Wound Healing in Diabetic foot Ulceration by Using Fat-Derived Stromal Vascular Matrix (SVM) Therapy - A Case Series. J Surg 9: 11216 https://doi.org/10.29011/2575-9760.011216
Abstract
Some chronic wounds, such as diabetic foot ulcerations, remain constantly resistant to treatment, leading to serious outcomes such as severe infections, limb loss, and even loss of life despite adequate consideration of the underlying disease and causal therapy approaches. Another promising approach involves Mesenchymal Stem Cells (MSCs) isolated from different potential sources. We report on a case series of five cases of refractory diabetic foot lesions that were successfully treated by using a fat-derived Stromal Vascular Matrix (SVM) therapy. SVM is a novel technology that provides sufficient cell counts and viability for effective wound care management. In all cases, the SVM application was resulting in satisfactory wound size reduction up to healing during a follow-up period of about six weeks. To our knowledge, this case series is the first to document outcomes of SVM in wound care. As there currently is insufficient data on the use of SVM in any phase of chronic wound care, further research is needed to determine the optimal timing for its application. Nevertheless, we believe that further observation of this new technology in the treatment of non-healing chronic wounds is absolutely worthwhile.
Keywords: Diabetic foot ulceration; Fat-derived stromal vascular matrix; Mesenchymal stem cells; Wound healing,
Introduction
The main treatment modalities for diabetic ulcers include blood sugar regulation, infection control, offloading applications, debridement, and conventional wound care methods [1]. While these approaches have improved wound healing rates and reduced the costs and complications associated with non-healing wounds, some chronic wounds remain resistant to treatment, leading to serious outcomes such as severe infections, limb loss, and even loss of life [2,3]. Regenerative medicine represents a novel area of research and application for the treatment of chronic wounds that are resistant to conventional therapies. One promising approach involves Mesenchymal Stem Cells (MSCs) isolated from sources such as adipose tissue, bone marrow, and umbilical cord. These cells proliferate and dynamically adapt to their microenvironment through autocrine and paracrine signaling [4,5]. MSCs promote angiogenesis, modulate the inflammatory response, accelerate wound healing processes, and improve the quality of the healing tissue [6,7].
Materials and Methods
Patients underwent surgery in the operating room under spinal anesthesia and sedation. Tumescent anesthesia, approximately 35% more than the planned amount of fat to be harvested, was administered to the donor area. After waiting for an average of 5-10 minutes, the tumescent anesthesia, composed of 500 mL Ringer lactate with 25 mg lidocaine and 1 vial of epinephrine [1:1000], took effect. Using a blunt cannula measuring 20 mL in length, 3 ml in diameter, and with 2 aspiration holes, lipoaspirate was transferred into a closed system bag as milli-fat. To separate the milli-fat from the tumescent anesthesia in the bag, 60 mL of saline solution was added and the mixture was suspended for 5-7 minutes to allow decantation. Once separated, the tumescent anesthesia material was removed using a 3-way stopcock. The remaining washed millifat was refined to a micron level by mechanical processing with knives in two separate stromal vascular fraction cubes(LipocubeTM SVF) (black cubes in Figure 1). The micronized autologous fat was then separated using a patented piston tube with 4 concave gaskets (black piston injector) and processed in the system’s special software-controlled variable speed Celldrive centrifuge for 9 minutes. The ‘Stromal Vascular Matrix’ (SVM) was created by combining the extracellular matrix with the stromal vascular cell collection from the separated fat. This matrix was then injected both intralesionally and perilesionally into the patients’ wounds (Figure 1).
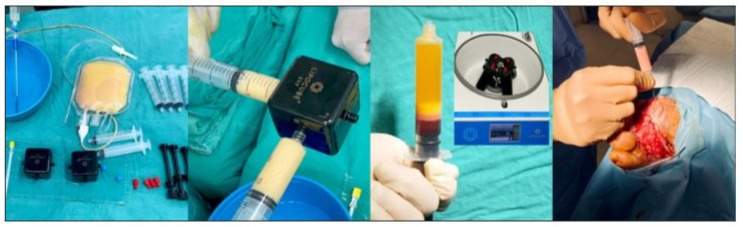
Figure 1: Procedure and intralesional injection (1A: Preparation of SVF kit 1B: Mechanical digestion procedure 1C: Isolation of SVM 1D: Intralesional injection).
Case Presentation
Case 1
A 56-year-old male patient was admitted to the Wound Care Clinic at Sakarya University Training and Research Hospital with an infected wound, including necrotic areas on the back of his left foot and left first toe. The patient had a four-year history of a foot ulcer located at the first metatarsal head of the left foot. He also had a medical history of diabetes mellitus and hypertension. Upon admission, his body temperature was 37.1°C, the erythrocyte sedimentation rate was 115 mm/h, and CRP value was 40.9 mg/L. Revascularization was deemed unnecessary after an ankle-brachial index of 0.9 was determined. A minor amputation was performed, and tissue cultures were taken. E. coli has been identified in the deep tissue culture, and the patient was treated with trimethoprim/sulfamethoxazole according to the antibiogram for 28 days. During the wound care follow-up, NPWT (negative pressure wound therapy) was applied for 2 months. When the granulation tissue progression slowed, fat-derived Stromal Vascular Matrix (SVM) therapy was administered. Before the procedure, the patient’s erythrocyte sedimentation rate was 61 mm/h, and CRP was 11 mg/L. The wound measured 13 cm x 6 cm x 2 cm (156 cm³) at the time of application. Following the SVM treatment, follow-up care included hydrocolloid-based passive wound dressings and offloading. The wound was photographed and assessed at 1-week, 3-week, and 6-week follow-ups. By the end of the sixth week, the wound volume had reduced to 2 cm³, achieving a 98.7% success rate (Figure 2).
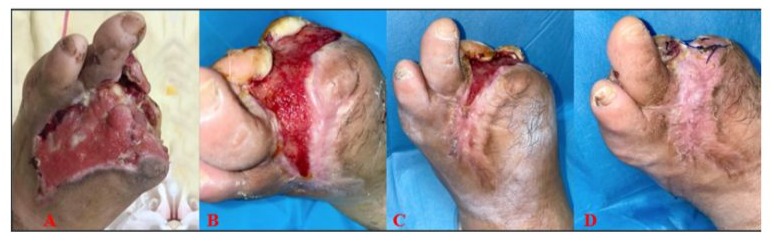
Figure 2: A: Before Application; B: Week 1; C: Week 3; D: Week 6.
Case 2
A 49-year-old male patient was admitted to our clinic with a wound on the back of his right foot following a recommendation for major amputation at an external center. The patient had been diabetic for about 20 years and was an active smoker. He had a history of below-knee amputation on the left lower extremity and was diagnosed with diabetes mellitus and thromboangiitis obliterans. He also had hypertension and chronic renal failure and was undergoing hemodialysis three times a week. The patient’s ankle-brachial index was 0.3, and he had a history of an unsuccessful peripheral revascularization attempt. Upon admission, his body temperature was 36.7°C, his erythrocyte sedimentation rate was 49 mm/h, and the CRP value was 65.1 mg/L. Wound care was managed with sequential debridement, and Amoxicillin/Clavulanic acid (oral) was prescribed due to the detection of Proteus mirabilis in the antibiogram. Antibiotic treatment was continued for 21 days, after which the CRP decreased to 8 mg/L and sedimentation rate to 27 mm/h. During the wound care followup, instillation NPWT (negative pressure wound therapy) was applied for 2 months. By the end of this treatment, the wound size was measured as 12 cm (length) x 6 cm (width) x 0.5 cm (depth), totaling 36 cm³. Since the wound size had not reduced despite NPWT, fat-derived mechanical Stromal Vascular Matrix (SVM) was applied. After the SVM procedure, follow-up care included passive wound dressings containing calcium alginate and offloading. The wound was photographed and evaluated at 1-week, 3-week, and 6-week follow-ups (Figure 3). Complete closure was achieved by the end of the sixth week.
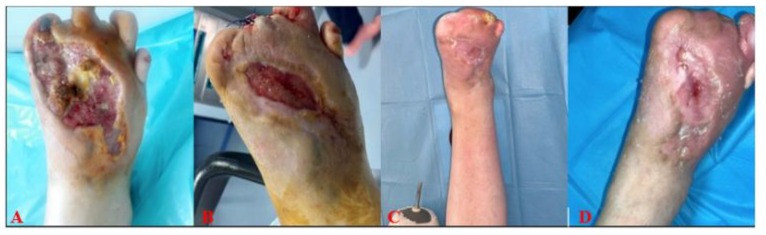
Figure 3: A-Before Application; B-Week 1; C-Week 3; D-Week 6.
Case 3
A 61-year-old male patient, who had been followed for recurrent diabetic foot ulcers since 2018, presented to our clinic in November 2020 with an active diabetic foot infection. Upon admission, his body temperature was normal, but his CRP value was 147 mg/L, and erythrocyte sedimentation rate was 120 mm/h. Given the patient’s history of diabetes mellitus and chronic renal failure, requiring hemodialysis 3 times a week due to diabetic nephropathy, and an ankle-brachial index of 0.8, revascularization was not considered necessary. Radiological evaluation revealed osteomyelitis, leading to surgical intervention on the bone tissue in the operating room. This was followed by wound care with sequential debridement. When Enterococcus faecalis was identified in the microbial detection from the bone, Amoxicillin/Clavulanic acid treatment was initiated based on the antibiogram, and antibiotic therapy continued for 6 weeks. During the wound care follow-up, the wound was treated with silver gauze dressing and instillation NPWT (negative pressure wound therapy) for 2 months.
Due to a delay in wound healing, NPWT was discontinued after two months, and stromal vascular matrix (SVM) treatment was initiated. Prior to the procedure, the CRP value was 76.3 mg/L, and the sedimentation rate was 70 mm/h. The wound measured 13 cm x 8 cm x 2 cm (208 cm³) before SVM application. Following the SVM procedure, the patient was managed with passive wound dressings on the base of hydrocolloid. However, recurrent infections developed due to the patient’s reduced compliance with offloading and wound care protocols. As Acinetobacter baumannii was detected in the microbiological diagnostics, TMP/SMX treatment was administered for 14 days. The patient’s wound care continued with silver-containing gauze dressings and regular debridement. Despite these challenges, wound size reduction was observed, and by the sixth week, the open defect was reduced to 5 cm³, achieving a 97.5% reduction in wound size (Figure 4).
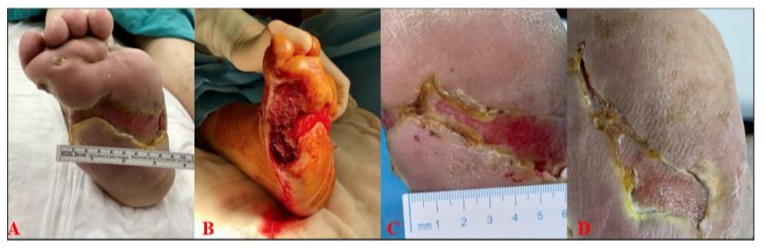
Figure 4: A-Before Application; B-Week 1; C-Week 3; D-Week 6.
Case 4
A 62-year-old male patient was admitted to our clinic with a diabetic foot ulcer. The patient had a history of diabetes mellitus and hypertension. Infection markers were within normal limits, and the ankle-brachial index was 0.9, so no vascular intervention was performed. Initial treatment involved the use of a hydrocolloid passive dressing. Due to slow granulation tissue development, NPWT (negative pressure wound therapy) was applied. However, after two months with no significant reduction in wound size, stromal vascular matrix (SVM) therapy was initiated. Prior to the procedure, the patient’s erythrocyte sedimentation rate was 66 mm/h, and CRP was 15 mg/L. The wound size was measured at 7 cm x 5 cm x 1 cm (35 cm³) before SVM application. Following the SVM treatment, the patient was managed with hydrocolloid-containing passive wound dressings and offloading measures. By the end of 6 weeks, the wound size had reduced, achieving a 97.5% success rate (Figure 5).
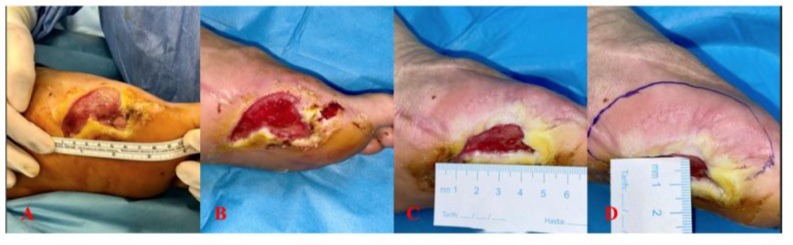
Figure 5: A-Before Application; B-Week 1; C-Week 6 (detail); D-Week 6.
Case 5
A 49-year-old patient was admitted to our clinic with a diabetic foot ulcer. The patient, who had diabetes for about 5 years, had previously undergone a Chopart amputation at an external center. He had a history of smoking and continued to smoke actively. His medical history included diabetes mellitus, thromboangiitis obliterans (Buerger’s Disease), and hypertension. Despite several prior revascularization attempts, the patient’s ankle-brachial index remained at 0.3. During follow-up, various wound care techniques were employed, including passive and active closure methods such as hydrocolloid-containing wound dressings, silver-containing gauze dressings, foam dressings, collagen dressings, and NPWT (negative pressure wound therapy), combined with hyperbaric oxygen therapy. As the wound size did not decrease, a Stromal Vascular Matrix (SVM) application was decided. Prior to the procedure, the wound measured 8 cm x 7 cm x 0.5 cm (28 cm³), with a sedimentation rate of 5 mm/h and a CRP level of 11.3 mg/L. After SVM application, the patient was followed with hydrocolloid-containing passive wound dressings and offloading. The wound was photographed and assessed at 1, 3, and 6-week followups. By the end of 6 weeks, the wound size had reduced significantly, achieving a 98.2% success rate (Figure 6).
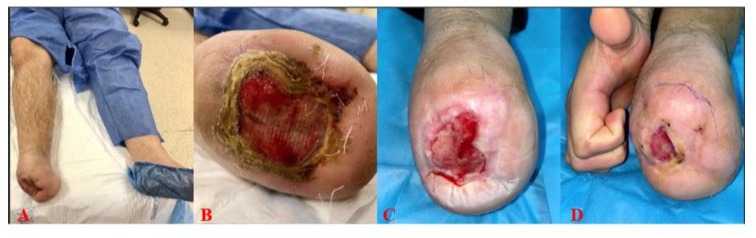
Figure 6: A and B - Before Application; C-Week 3; D-Week 6.
Discussion
Chronic, hard-to-heal wounds that require long term treatment significantly impair patients’ quality of life. Chronic wounds are often referred to as a “silent epidemic” due to extended hospital stays and increased risks of morbidity and mortality [8]. Until recently, advanced dressings were considered adequate for wound care, providing functions such as maintaining moisture balance by absorbing excess exudate, preventing infection, and allowing gas exchange. However, despite technological advancements in modern wound dressings, many chronic wounds take a long time to close, or in some cases, complete closure is not achieved. Skin regeneration is an emerging field that promises more innovative treatment approaches [9]. In this case presentation, we report the outcomes of five cases in which stromal vascular matrix (SVM) was applied. The first case involved a diffuse forefoot infection with accompanying digital cyanosis. After minor amputation and deep tissue debridement, the patient was monitored in the clinic, and the infection was managed with oral antibiotics. SVM was applied due to a halt in epithelialization despite modern wound care methods and meticulous treatment of the underlying disease. Diabetic foot patients with end-stage renal disease present higher rates of amputation and mortality [10-13], but there is no data evaluating the application of adipose-derived stem cells to diabetic foot ulcers in patients undergoing haemodialysis so far. In our study, two of the five cases were haemodialysis patients. The second case had a history of a major amputation of the left lower extremity. A recent study found that 9.1% of 9,549 above-ankle amputees died. Mortality was associated with emergency admission, bilateral operations, age, ASA grade, abnormal ECG, and elevated white cell count or creatinine [14]. To avoid further amputation and prolong the patient’s life expectancy, we used SVM. This patient also had unrevascularized peripheral vascular disease, so SVM was applied both intralesionally and perilesionally, as well as along the arterial trace.
The third case also involved a patient with end-stage chronic renal failure, though without peripheral arterial disease. Both patients showed effective wound size reduction after SVM application. The fourth case had elevated sole pressures, which had hindered wound closure despite using a knee-high, non-removable offloading device. After SVM application, the wound size was reduced by 97.5% at six weeks. Gonzalez-Rey et al. [15] demonstrated that adipose-derived stem cells secrete factors like interleukin-10 and cytokines, which reduce inflammation and help shorten its duration. These growth factors may improve tissue perfusion and have anti-inflammatory effects in patients with thromboangiitis obliterans, in addition to secreting angiogenic factors that promote angiogenesis, enhance wound healing, and improve limb ischemia [16]. These factors are particularly important in treating diabetic foot ulcers. In the fifth case, a diabetic patient with thromboangiitis obliterans was treated with intralesional and perilesional SVM application, resulting in satisfactory wound size reduction during follow-up.
SVM is a novel technology that provides sufficient cell counts and viability for effective wound care management [17]. To our knowledge, this case series is the first to document outcomes of SVM in wound care. Doubtless, further research is needed to determine the optimal timing for its application, as there is currently insufficient data on the use of SVM in any phase of chronic wound care. In our study, we applied SVM after ensuring that the wound bed and periwound area were infection-free and that conventional wound care methods had been exhausted completely.
Conclusion
In conclusion, SVM cells play a significant role in treating hardto-heal wounds of any etiology, and application along the vascular trace offers additional benefits by promoting angioneogenesis and improving perfusion. Even though we can only present a limited number of observational cases here, we believe that the method is well worthy of a more comprehensive examination and intensive scientific debate.
Acknowledgement
We kindly want to thank Mrs Sina Schüttler for her support in proofreading and finalization.
References
- Tsourdi E, Barthel A, Rietzsch H, Reichel A, Bornstein SR (2013) Current aspects in the pathophysiology and treatment of chronic wounds in diabetes mellitus. Biomed Res Int 2013: 385641.
- Morton LM, Phillips TJ (2016) Wound healing and treating wounds: differential diagnosis and evaluation of chronic wounds. J Am Acad Dermatol 74: 589‐605.
- Powers JG, Higham C, Broussard K, Phillips TJ (2016) Wound healing and treating wounds: chronic wound care and management. J Am Acad Dermatol 74: 607‐625.
- Hsieh JY, Wang HW, Chang SJ, Liao KH, Lee IH, et al. (2013) Mesenchymal stem cells from human umbilical cord express preferentially secreted factors related to neuroprotection,neurogenesis, and angiogenesis. PLoS One 8: 72604.
- Discher DE, Mooney DJ, Zandstra PW (2009) Growth factors, matrices, and forces combine and control stem cells. Science 324: 1673-1677.
- Lee SH, Jin SY, Song JS, Seo KK, Cho KH, et al. (2012) Paracrine effects of adipose-derived stem cells on keratinocytes and dermal fibroblasts. Ann. Dermatol 24: 136-143.
- Jackson WM, Nesti LJ, Tuan RS (2012) Mesenchymal stem cell therapy for attenuation of scar formation during wound healing. Stem Cell Res. Ther 20.
- Ward J, Holden J, Grob M, Soldin M (2019) Management of wounds in the community: Five principles. Br. J. Community Nurs 24: S20-S23.
- Tottoli EM, Dorati R, Genta I, Chiesa E, Pisani S, et al. (2020) Skin Wound Healing Process and New Emerging Technologies for SkinWound Care and Regeneration. Pharmaceutics 12: 735.
- Otte J, van Netten JJ, Woittiez A-JJ (2015) The association of chronic kidney disease and dialysis treatment with foot ulceration and major amputation. J Vasc Surg 62: 406-411.
- Papanas N, Liakopoulos V, Maltezos E, Stefanidis I (2007) The diabetic foot in end stage renal disease. Ren Fail 29: 519-528.
- Ndip A, Lavery LA, Boulton AJM (2010) Diabetic foot disease in people with advanced nephropathy and those on renal dialysis. Curr Diab Rep 10: 283-290.
- Lewis S, Raj D, Guzman NJ (2012) Renal failure: implications of chronic kidney disease in the management of the diabetic foot. Semin Vasc Surg 25: 82-88.
- Ambler GK, Thomas-Jones E, Edwards A, Twine CP (2019) Prognostic Risk Modelling for Patients Undergoing Major Lower Limb Amputation: An Analysis of the UK National Vascular Registry. Eur J Vasc Endovasc Surg 59: 606-613.
- Gonzalez-Rey E, Gonzalez MA, Varela N, et al. (2010) Human adipose-derived mesenchymal stem cells reduce inflammatory and T cell responses and induce regulatory T cells in vitro in rheumatoid arthritis. Ann RheumDis 69: 241-248.
- Lee HC, An SG, Lee HW, et al. (2012) Safety and effect of adipose tissue-derived stem cell implantation in patients with critical limb ischemia: a pilot study. Circ J 76: 1750-1760.
- Tiryaki T, Canikyan S, Kocak P, Cohen S, Sterodimas A, et al. (2021) Adipose-derived Stromal Vascular Matrix (SVM): a new paradigm in regenerative medicine. CellR4 9: e3060
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.