When Clarity Comes at a Cost: Macular Edema Following YAG Laser Capsulotomy: A Structured Study
by Dr. Rajeev Kumar
Opthalmologist, Zulekha hospital, Sharjah, UAE
Received Date: 26 January 2026
Accepted Date: 09 February 2026
Published Date: 12 February 2026
Citation: Rajeev Kumar (2026) When Clarity Comes at a Cost: Macular Edema Following YAG Laser Capsulotomy: A Structured Study. Ophthalmol Res Rep 10: 173. https://doi.org/10.29011/2689-7407.100173
Abstract
Background: Nd:YAG laser posterior capsulotomy is the standard treatment for visually significant posterior capsular opacification (PCO). Although generally safe and effective, macular edema (ME) remains a potentially vision-limiting complication. This study evaluated the incidence, risk factors, clinical course, and management outcomes of ME following Nd:YAG laser posterior capsulotomy. Materials and Methods: This retrospective observational study was conducted at a tertiary care hospital from January 2023 to December 2024. Records of 200 patients who underwent Nd:YAG laser posterior capsulotomy for visually significant PCO were reviewed. Data collected included demographic details, systemic and ocular history, interval since cataract surgery, laser parameters, best-corrected visual acuity, and optical coherence tomography (OCT) findings. ME was defined as an increase in central macular thickness ≥ 30 µm from baseline and/or the presence of intraretinal or subretinal fluid on OCT. Treatment modalities and clinical outcomes were documented. Results: ME developed in 5 patients (2.5 %). Identified risk factors included pre-existing diabetic retinopathy with a dry macula, history of uveitis or other inflammatory ocular diseases, higher cumulative laser energy, and a shorter interval between cataract surgery and capsulotomy. Most cases responded to topical non-steroidal antiinflammatory drugs (NSAIDs) and corticosteroids, with resolution within 3-4 weeks. One patient required intravitreal therapy for persistent edema. Conclusion: ME after Nd:YAG laser capsulotomy is uncommon but clinically significant. Early OCT-based detection, careful patient selection, and optimized laser parameters contribute to favorable visual outcomes.
Keywords: Nd:YAG Lasers; Posterior Capsular Opacification; Macular Edema; Optical Coherence Tomography.
Abbreviations
- BCVA: Best-Corrected Visual Acuity
- CME: Cystoid Macular Edema
- CMT: Central Macular Thickness
- DM: Diabetes Mellitus
- IOP: Intraocular Pressure
- IOL: Intraocular Lens
- ME: Macular Edema
- Nd:YAG: Neodymium-Doped Yttrium Aluminium Garnet
- NSAIDs: Non-Steroidal Anti-Inflammatory Drugs
- OCT: Optical Coherence Tomography
- PCO: Posterior Capsular OpacificationTop of FormBottom of Form
Introduction
Posterior capsular opacification (PCO) is the most frequent long‑term complication of cataract surgery, occurring in up to 20-50% of patients depending on surgical technique, intraocular lens (IOL) material, and postoperative inflammatory response.
Although modern cataract surgery has significantly reduced the incidence of early complications, PCO remains a leading cause of visual decline following an otherwise successful procedure. Patients typically present with decreased visual acuity, glare, halos, and reduced contrast sensitivity, all of which impact functional vision and daily activities [1]. Nd: YAG (neodymium-doped yttrium aluminium garnet) laser posterior capsulotomy is widely accepted as the gold standard treatment for visually significant PCO. The procedure is fast, non-invasive, highly effective, and typically results in the rapid restoration of visual function. Reported complications include transient elevation of intraocular pressure (IOP), pitting of the IOL surface, intraocular inflammation, damage to the anterior hyaloid face, cystoid macular edema (CME), and retinal detachment [2,3]. Among these, macular edema (ME) has the potential to impair central vision substantially. While most patients experience improvement in acuity post-capsulotomy, the development of ME can blunt or reverse these visual gains. The pathophysiology involves inflammatory mediators released after the laser disrupts the posterior capsule, leading to increased vascular permeability in the perifoveal capillaries. Patients with preexisting retinal vulnerability, like diabetes mellitus (DM), epiretinal membranes, or previous episodes of CME, may be at especially high risk [3]. The reported incidence ranges from < 1 % to > 6 %. Given the widespread use of Nd: YAG, this study aims to provide a structured evaluation of the incidence, timing, risk factors, and clinical outcomes of ME following Nd: YAG posterior capsulotomy.
Material and Method
This retrospective observational study was conducted from January 2023 to December 2024 after obtaining approval from the Institutional Ethics Committee. Medical records of patients who underwent Nd:YAG laser posterior capsulotomy during the study period were reviewed. Adults aged 40 years and above who had undergone uncomplicated phacoemulsification with posterior chamber intraocular lens implantation and had clinically significant PCO causing decreased visual acuity, glare, reduced contrast sensitivity, or difficulty in performing daily activities were included. Patients with DM or a history of uveitis or other inflammatory ocular conditions were included only if the macula was clinically dry at baseline. Additional inclusion criteria were clear ocular media other than PCO, allowing adequate retinal evaluation, and willingness to comply with follow-up. Patients with active macular pathology at baseline (including active diabetic macular edema or neovascular age-related macular degeneration), previous vitrectomy with macular pathology, prior Nd:YAG laser capsulotomy in either eye, complicated cataract surgery (posterior capsule rupture or vitreous loss), known retinal vascular disorders such as retinal vein occlusion, active diabetic retinopathy requiring treatment, use of intravitreal injections, retinal laser therapy, or ocular surgery within the preceding six months, uncontrolled systemic conditions such as DM or hypertension, and those unwilling or unable to complete follow-up were excluded.
A total of 200 patients who fulfilled the study criteria were enrolled. Systemic history, including DM, hypertension, comorbidities, and the interval since cataract surgery, was documented. All patients underwent a comprehensive ophthalmic examination, including best-corrected visual acuity assessment using Snellen’s chart, refraction, IOP measurement with Goldmann applanation tonometry, slit-lamp biomicroscopy of the anterior segment with grading of PCO, and dilated fundus examination using indirect ophthalmoscopy and biomicroscopy.
Optical coherence tomography (OCT) was performed in all patients before capsulotomy to assess central macular thickness, retinal morphology, and the presence of intraretinal or subretinal fluid. A history of diabetic retinopathy and any prior or ongoing treatment for retinopathy was recorded. Anterior segment photography, laser-related parameters, including the number of laser shots and total cumulative energy delivered, were documented for each eye. All procedures were performed by the same experienced ophthalmologist using a Q-switched Nd:YAG laser. The initial laser energy ranged from 1.0 to 1.5 mJ and was titrated up to 1.53.0 mJ as required. An average of 20-40 laser shots was delivered, targeting a capsulotomy size of 3-4 mm. An Abraham capsulotomy lens was used for stabilization and magnification. The laser was applied in a cruciate or circular pattern to achieve a central, clear visual axis. Post-procedure management included topical prednisolone acetate 1% administered four times daily for one week, with IOP-lowering medication prescribed when indicated. Patients were observed for one hour after the procedure to monitor for IOP elevation.
Follow-up examinations were conducted on day 1, week 1, one month, three months, and six months after the procedure. At each visit, best-corrected visual acuity, slit-lamp examination, IOP measurement, and dilated fundus examination were performed. Macular OCT was repeated to assess changes in central macular thickness and to detect CME or intraretinal/subretinal fluid. CME was defined as an increase in central macular thickness (CMT) greater than 30 µm from baseline and/or the presence of cystic intraretinal spaces on OCT. Collected data included demographic characteristics, duration between cataract surgery and capsulotomy, laser energy parameters, pre- and post-procedure best-corrected visual acuity, IOP changes, baseline and follow-up central macular thickness values, and the incidence and timing of CME. Statistical analysis was performed using SPSS software. Continuous variables were expressed as mean ± standard deviation, and categorical variables as frequencies and percentages. Paired t-test or Wilcoxon signed-rank test was used to compare pre- and post-procedure parameters, while the Chi-square test was applied for categorical comparisons. Logistic regression analysis was performed to identify factors associated with the development of CME. A p-value of less than 0.05 was considered statistically significant.
Observations and Results
200 patients who underwent Nd: YAG laser posterior capsulotomy for visually significant PCO were included in the study. The mean age of the study population was 56.3 ± 7.2 years (range: 40-70 years). There was a male predominance, with 130 males (65%) and 70 females (35%). Out of the 200 patients, 5 patients (2.5%) developed ME following Nd: YAG laser capsulotomy, while 195 patients (97.5%) did not show any evidence of ME during the follow-up period. The mean time to detect ME was 2 to 3 weeks post-procedure, as confirmed by OCT. Table 1 shows the baseline characteristics and ME outcome of the subjects.
|
Parameter |
Value |
|
Total Patients |
200 |
|
Age Range (years) |
40-70 |
|
Mean Age ± SD |
56.3 ± 7.2 |
|
Gender Distribution |
|
|
Male |
130 (65%) |
|
Female |
70 (35%) |
|
ME After YAG Capsulotomy |
|
|
Developed ME |
5 patients (2.5%) |
|
Did Not Develop ME |
195 patients (97.5%) |
|
Mean Time to ME Detection |
2 to 3 weeks |
|
Mean CMT |
|
|
Baseline CMT |
240 to 280 microns |
|
CMT in ME cases |
30 microns above the normal value. |
|
Interval Between Cataract Surgery and YAG |
3 months after cataract surgery. |
|
Mean Total Laser Energy Used |
20-80 mJ. |
Table 1: Baseline Characteristics and ME Outcomes of the Study Population.
The mean total laser energy used ranged from 20 to 80 mJ, with an average of 20-40 laser shots per procedure. Eyes developing ME had received relatively higher cumulative laser energy. The mean interval between cataract surgery and capsulotomy in ME cases was approximately 3 months to 5 years.
Laser parameters used for Capsulotomy
The baseline CMT in the study population ranged from 240 to 280 microns, which was considered within normal limits. In patients who developed ME, OCT revealed a mean increase in CMT of ≥ 30 microns from baseline, along with the presence of cystic intraretinal spaces, consistent with CME. Patients who did not develop ME showed no statistically significant change in CMT during follow-up. The mean total cumulative laser energy used during capsulotomy ranged from 20 to 80 mJ, with an average of 20-40 laser shots per procedure. Patients who developed ME were noted to have received higher cumulative laser energy compared to those without.
ME after YAG Capsulotomy
In patients who developed ME, the interval between cataract surgery and Nd:YAG laser capsulotomy ranged from approximately three months to five years, suggesting that a shorter postoperative interval may represent a potential risk factor for the development of ME. In contrast, patients with a longer duration between cataract surgery and capsulotomy demonstrated a lower incidence of ME. Most patients showed an improvement in best-corrected visual acuity (BCVA) following Nd:YAG capsulotomy. However, eyes that developed ME exhibited a temporary reduction or plateau in visual recovery, which correlated with an increase in CMT on OCT. Following appropriate management, visual acuity improved in all affected cases.
All five patients who developed ME were initially treated with topical non-steroidal anti-inflammatory drugs (NSAIDs) and topical corticosteroids. Four patients demonstrated complete resolution of ME within an average period of three to four weeks, as evidenced by normalization of CMT and resolution of cystic intraretinal changes on OCT. One patient exhibited persistent ME and required intravitreal injection therapy, after which significant anatomical and functional improvement was observed. Figure 1a and 1b show the OCT images of ME after capsulotomy.
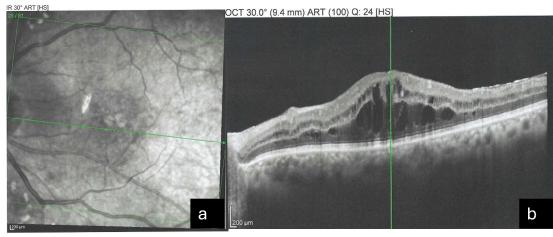
Figure 1a and 1b: OCT Images after Capsulotomy with ME.
ME Resolved after Treatment
Transient elevation of IOP was noted in a small number of patients during the immediate post-procedure period, which was successfully managed with topical IOP-lowering medications. No cases of retinal detachment, persistent uveitis, or visually significant IOL pitting were observed during the follow-up period. Figure 2a and 2b show the OCT images after treatment.
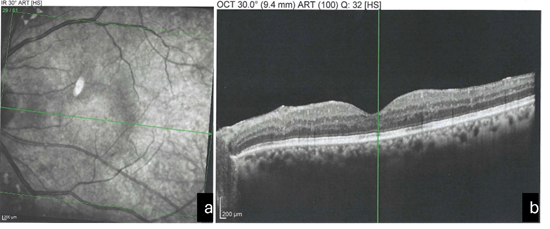
Figure 2a and 2b: OCT Image after Treatment.
Discussion
Nd: YAG laser posterior capsulotomy is an established and highly effective procedure for the treatment of visually significant PCO. Despite its excellent safety profile, several complications have been described, among which ME remains one of the most visually significant [3]. The present study evaluated the incidence, risk factors, and clinical course of ME following Nd: YAG capsulotomy in a real-world clinical setting. In this study, ME developed in 2.5% of patients following Nd: YAG capsulotomy. This incidence is comparable with previously published reports, which have documented rates ranging from less than 1% to approximately 6%. Steinert et al. were among the first to report CME as a complication of Nd: YAG capsulotomy, emphasizing the role of postoperative inflammation in its pathogenesis [3]. Similarly, Ari et al. reported an incidence of CME of approximately 2-3%, aligning closely with the findings of the present study [4]. The variability in reported incidence across studies may be attributed to differences in study design, patient selection, timing of OCT evaluation, and criteria used to define ME. In the current study, ME was strictly defined as an increase in CMT of ≥ 30 µm from baseline or the presence of cystoid changes on OCT, allowing for objective and early detection.
The mean time to detection of ME was 2 to 3 weeks postcapsulotomy, which is consistent with the inflammatory mechanism proposed in the literature. Studies by Raza and Rosen have suggested that laser-induced disruption of the posterior capsule leads to the release of inflammatory mediators such as prostaglandins, resulting in breakdown of the blood-retinal barrier and subsequent fluid accumulation in the macula [5]. This delayed onset further supports the need for follow-up beyond the immediate postoperative period.
A significant finding of this study was the association of ME with pre-existing diabetic retinopathy, even in eyes with a clinically dry macula at baseline. Previous studies by Shah et al. and Benson et al. have demonstrated that diabetic eyes are inherently more susceptible to inflammatory insults, making them vulnerable to post-laser macular changes [6,7]. Our findings reinforce the concept that diabetic patients represent a high-risk group, warranting closer postoperative surveillance. A history of uveitis or other inflammatory ocular conditions was found to be an important risk factor. This observation is supported by Ladas et al., who reported a higher incidence of CME following Nd: YAG capsulotomy in eyes with pre-existing inflammatory pathology [8]. Such eyes likely have a compromised blood–retinal barrier, predisposing them to exaggerated inflammatory responses after laser application.
The role of laser energy in the development of ME has been debated. In the present study, patients who developed ME were exposed to higher cumulative laser energy, suggesting a dosedependent inflammatory effect. This finding is consistent with the observations of Ari et al. and Holweger and Marefat, who reported increased rates of CME and IOP elevation with higher total laser energy [4,9]. These results underscore the importance of using the lowest effective energy to achieve an adequate capsulotomy. Another notable observation was the shorter interval between cataract surgery and Nd: YAG capsulotomy in patients who developed ME. Early capsulotomy coincides with residual postoperative inflammation from cataract surgery, thereby increasing the risk of CME. Several authors, including Winslow and Taylor, have suggested delaying capsulotomy, when possible, to allow complete stabilization of the ocular inflammatory milieu [10].
From a management perspective, most ME cases in this study responded favorably to topical NSAIDs and corticosteroids, with resolution occurring within 3-4 weeks. This favorable response aligns with reports by Henderson et al., who emphasized the effectiveness of topical anti-inflammatory therapy in postprocedural CME [11]. However, the requirement for intravitreal therapy in one patient highlights that some cases may follow a more persistent course, particularly in eyes with multiple risk factors. The use of OCT played a critical role in this study, enabling early detection, objective monitoring, and assessment of treatment response. OCT has been widely endorsed by authors such as Hee et al. for diagnosing and monitoring ME, and its routine use in high-risk patients undergoing Nd: YAG capsulotomy is strongly supported by our findings [12].
The present study had a systematic OCT-based evaluation of macular changes before and after Nd:YAG laser posterior capsulotomy, enabling objective quantification of CMT and early detection of both clinical and subclinical ME. The relatively large sample size of 200 patients, including individuals with controlled systemic comorbidities and a dry macula at baseline, enhances the reliability and external validity of the findings. Standardization of the laser procedure, with all capsulotomies performed by a single experienced ophthalmologist using uniform parameters, minimized inter-operator variability and strengthened outcome assessment. Furthermore, the use of well-defined inclusion and exclusion criteria, a clear OCT-based definition of ME, and a structured follow-up protocol improved the reproducibility and interpretability of the results. The study is limited by its retrospective observational design, which is inherently prone to selection bias and limits causal inference. Although the overall sample size was adequate, the small number of patients who developed ME reduced the statistical power for subgroup and multivariate analyses. Being a single-centre study with variable follow-up duration further restricts generalizability and precludes assessment of long-term outcomes. Additionally, the absence of a control group and lack of angiographic or inflammatory biomarker evaluation limit insights into preventive strategies and underlying pathophysiological mechanisms.
Conclusion
The findings of the present study show that ME after Nd:YAG laser posterior capsulotomy is an infrequent but clinically significant complication, with an incidence of 2.5%, and can adversely affect visual outcomes if not promptly detected. Higher risk was observed in eyes with pre-existing retinal vulnerability, shorter intervals between cataract surgery and capsulotomy, and greater cumulative laser energy, although most cases responded well to topical anti-inflammatory therapy. Routine OCT evaluation and careful laser parameter optimization are useful in early detection and reducing visual morbidity.
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- Sinha R, Shekhar H, Sharma N, Titiyal JS, Vajpayee RB (2013) Posterior capsular opacification: A review. Indian J Ophthalmol 61: 371-376.
- Aron-Rosa D, Aron JJ, Cohn HC (1980) Use of the neodymium-YAG laser to open the posterior capsule after lens implantation. Am J Ophthalmol 6: 352-354.
- Steinert RF, Puliafito CA (1985) The Nd:YAG laser in ophthalmology: principles and clinical applications. Surv Ophthalmol 30: 1-34.
- Ari S, Cingü AK, Sahin A, Çinar Y, Çaça I (2012) The effects of Nd:YAG laser posterior capsulotomy on macular thickness, intraocular pressure, and visual acuity. Ophthalmic Surg Lasers Imaging 43: 395400.
- Raza A, Rosen ES (1990) Inflammatory response following Nd:YAG laser capsulotomy. Eye 4 :507-513.
- Shah GR, Gills JP, Durham DG, Ausmus WH (1984) Three thousand Nd:YAG lasers posterior capsulotomies: An analysis of complications. Ophthalmology 91: 511-517.
- Benson WE, Brown GC, Tasman W, McNamara JA (1988) Cystoid macular edema following Nd:YAG laser capsulotomy. Ophthalmology 95: 858-860.
- Ladas JG, Wheeler NC, Morhun PJ, Rimmer SO, Holland GN (2001) Cystoid macular edema after Nd:YAG laser posterior capsulotomy in patients with uveitis. Am J Ophthalmol 132: 373-377.
- Holweger RR, Marefat B (1997) Intraocular pressure change after Nd:YAG capsulotomy. J Cataract Refract Surg 23: 115-121.
- Winslow RL, Taylor BC (1985) Retinal complications following YAG laser capsulotomy. Ophthalmology 92: 785-789.
- Henderson BA, Kim JY, Ament CS, Ferrufino-Ponce ZK, Grabowska A, et al. (2007) Clinical pseudophakic cystoid macular edema: Risk factors for development and duration after treatment. J Cataract Refract Surg 33: 1550-1558.
- Puliafito CA, Hee MR, Reichel E, Schuman JS, Duker JS, et al. (1995) Optical coherence tomography of macular diseases. Ophthalmology 102: 217-229.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.