Weil’s disease: Severe Leptospirosis Cases
by Hernández Pantoja Lizbeth1*, Chora Hernández Luis David1, Piñón Villagómez Saraí1, Rangel Molina Edith1, Yañez Dueñas María Fernanda1, Cristóbal Negrete María Dolores2, Carranza Madrigal Jaime1, Zavala Álvarez Elsa Daniela1, Gómez Suárez Víctor Hugo2
1Department of Internal Medicine, Morelia General Hospital “Dr. Miguel Silva”, National Autonomous University of Mexico (UNAM), Morelia, Michoacan, Mexico
2Department of Nephrology, Morelia General Hospital “Dr. Miguel Silva”, National Autonomous University of Mexico (UNAM), Morelia, Michoacan, Mexico
*Corresponding author: Lizbeth Hernández Pantoja, department of Internal Medicine, Morelia General Hospital “Dr. Miguel Silva”, National Autonomous University of Mexico (UNAM), Morelia, Michoacan, Mexico
Received Date: 28 July 2025
Accepted Date: 01 August 2025
Published Date: 08 August 2025
Citation: Lizbeth HP, David CHL, Sarai PV, Edith RM, Fernanda YDM, et al. (2025). Weil’s disease: Severe Leptospirosis Cases. Ann Case Report. 10: 2359. https://doi.org/10.29011/2574-7754.102359
Abstract
Leptospirosis is a zoonotic disease prevalent in tropical climates, causing around one million cases and 60,000 deaths annually. In the Americas, the highest prevalence is seen in the USA, Colombia, Brazil, and Mexico, where the case fatality rate exceeds 12%. The disease can present in a mild (anicteric) form or icteric as Weil’s disease, a severe form characterized by jaundice, acute kidney injury, and hemorrhagic symptoms.
This report describes two cases of Weil’s disease. The first was a 26-year-old man with fever, jaundice, abdominal pain, vomiting, epistaxis, hepatosplenomegaly, and acute kidney injury requiring dialysis. Laboratory findings included hyperbilirubinemia, elevated CPK, amylase, lipase, and severe thrombocytopenia. He recovered after early antibiotic treatment. The second case was a 39-yearold man with similar symptoms, in addition to melena, coffee grounds vomiting. Despite renal replacement therapy, he died. Both cases were confirmed positive for Leptospira interrogans antibodies by TMA.
These cases highlight the need to consider leptospirosis in patients with acute, nonspecific symptoms such as fever, vomiting, abdominal pain, jaundice, and multiorgan failure. Weil’s disease is a rare but life-threatening manifestation requiring prompt diagnosis and treatment.
Keywords: Leptospirosis; Weil’s disease; Jaundice; Thrombocytopenia; Renal replacement therapy; Multiorgan failure.
Introduction
Leptospirosis is an example of infectious diseases that are transmitted by animals or vectors and is considered one of the most widespread and prevalent in nature. It is caused by the bacterium Leptospira spp.
In 1886, Adolph Weil described a febrile illness with jaundice, splenomegaly, renal failure, and conjunctivitis in individuals with outdoor jobs and water exposure, later named “Weil’s disease” [1,2]. In 1907, Stimson identified the causative organism, a spirochete found in the kidneys of a deceased patient, naming it Spirochaeta interrogans due to its question mark shape [3]. Initially considered an occupational disease, evidenced by a 1927 report of coal miners infected [4] it is now recognized as a zoonotic disease.
Leptospires are obligate aerobic spirochetes [5], 6-20 µm long and about 0.1 µm in diameter, with hooked ends and endoflagellar mobility. Their structure includes a cytoplasmic membrane, a peptidoglycan cell wall, and an outer lipid bilayer membrane [6]. They are classified by genotype and serovars: 21 species exist, 9 pathogenic, 5 intermediate, and the rest non-pathogenic [2].
Leptospirosis is globally distributed, with higher incidence in tropical regions. It affects both industrialized and developing countries, with sub-Saharan Africa, Latin America, the Caribbean, South and Southeast Asia, and Oceania showing the highest morbidity and mortality [2]. In the Americas, the most affected countries include the U.S., Colombia, Brazil, and Mexico. High mortality is linked to delayed diagnosis, limited healthcare infrastructure, and possibly to strain virulence or host immune response [5]. The disease is often underdiagnosed due to symptom overlap with illnesses like dengue, influenza, Hantavirus, and rickettsiosis [5]. In Mexico, the first reported cases were initially mistaken for yellow fever in Yucatán [7]. Clinical presentations range from mild, nonspecific symptoms (fever, headache, myalgia, and uveitis) to severe forms with jaundice, kidney damage, pulmonary hemorrhage, or myocarditis, which can lead to death [5].
Clinical Cases
Patient 1
26-year-old male, originally from Michoacán and resident of Jalisco, Mexico, systems technician, single. Relevant medical information: smoking (3.6 packs/year since age 17), previous alcohol and methamphetamine use (last use 4 years ago). One week before the onset of symptoms, he had contact with manure and non-potable water while working in a barn in Jalisco, Mexico. No other medical history.
The patient presented with acute symptoms lasting seven days, characterized by fever, headache, asthenia, adynamia, abdominal pain, non-bloody and non-mucous diarrhea (three episodes per day for two days), nausea, and vomiting. Two days after the onset of symptoms, the patient developed jaundice, progressive generalized weakness and inability to ambulate. On admission, the patient presented with hypotension (BP 104/50 mmHg), tachycardia (HR 139 bpm), normal respiratory rate (RR 18 rpm), and oxygen saturation of 98% on 21% FiO₂. Physical examination revealed jaundice, hepatosplenomegaly, and significant bilateral lower limb edema (graded as +++/++++). During hospitalization, the patient required supplemental oxygen via nasal cannula without the need for ventilator support. He developed spontaneous epistaxis, managed with nasal packing by the otorhinolaryngology team, along with ecchymosis on both upper limbs, pruritus, and choluria.
Initial labs showed leukocytosis (14.8 × 10³/µL) with neutrophilia, hemoglobin 14.3 g/dL, and severe thrombocytopenia (59 × 10³/ µL). Liver function tests revealed hyperbilirubinemia (BT 12.4 mg/ dL; BD 9.68 mg/dL), elevated AST (346 U/L), ALT (84 U/L), LDH (619 U/L), and markedly elevated amylase (1125 U/L) and lipase (204 U/L). By day 6 of hospitalization, bilirubin levels peaked (BT 40.3 mg/dL), while transaminases slightly decreased. Severe thrombocytopenia persisted. Renal function worsened by day 4 of hospitalization, with creatinine rising to 8.11 mg/dL, urea 179 mg/dL, BUN 83.6 mg/dL, and CK 8237 U/L, indicating acute kidney injury and rhabdomyolysis. Abdominal ultrasound and CT showed no biliary obstruction or pancreatic abnormalities. MRCP confirmed hepatomegaly with preserved intra- and extrahepatic biliary tracts, and mild bilateral pleural and pericardial effusions without hemodynamic compromise. Additional tests: negative for viral hepatitis (A, B, C), autoimmune markers, blood cultures, TORCH profile, and stool studies.
Due to progressive acute kidney injury and persistent metabolic acidosis, the patient required renal replacement therapy. Based on clinical findings (marked hyperbilirubinemia with mild elevation of transaminases, acute renal failure, and hemorrhagic signs), leptospirosis was suspected. The diagnosis was confirmed by a positive microscopic agglutination test for Leptospira interrogans (antibody titer 1:10,240). Empirical treatment with doxycycline and cefotaxime was initiated. Upon confirmation of the diagnosis, treatment was continued with a third-generation cephalosporin. The patient showed favorable clinical progress until discharge, subsequently attending a follow-up consultation with complete clinical remission and recovery of renal function.
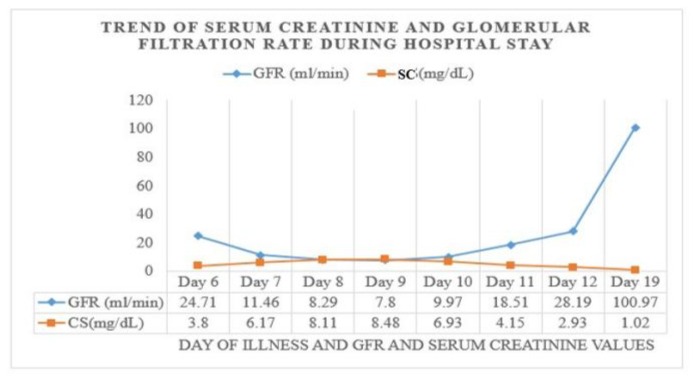
Figure 1: Trend of Biochemical Parameters during Hospitalization: Graphical representation of the trend of serum creatinine values detected during hospitalization and the calculated glomerular filtration rate. Showing worsening of renal function on the fourth day of hospital stay, 9th day of onset of clinical picture.
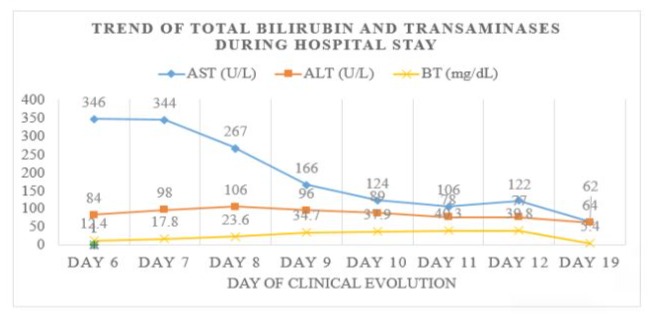
Figure 2: Trend of Biochemical Parameters during Hospitalization: Graphical representation of the trend of serum AST, ALT and total bilirubin values detected, showing a progressive increase in bilirubin presenting a maximum of 40.3 mg/dl on day 6 of hospitalization, 11th day of onset of symptoms.
Patient 2
A 39-year-old male patient, resident of Michoacán, Mexico, farmer, with a history of chronic use of NSAIDs for mechanical low back pain. He was admitted with acute symptoms characterized by asthenia, adynamia, hyporexia, fever, and abdominal pain predominantly in the right hypochondrium. Four days after the onset of clinical symptoms, he presented jaundice, coffee grounds vomiting, melena, spontaneous epistaxis, dyspnea on exertion, and weakness in the lower extremities. On admission, hypotension (88/53 mmHg), 83 bpm, 17 rpm, and oxygen saturation of 96% were documented, progressing to acute respiratory failure and loss of alertness, requiring invasive mechanical ventilation. Laboratory findings: leukocytosis with neutrophilia, severe thrombocytopenia, hyperbilirubinemia (up to 27 mg/dl, direct bilirubin 18.3 mg/dl), hypertriglyceridemia (426 mg/dl), elevated amylase (711 U/L) and lipase (132 U/L), and acute kidney injury with progressive oliguria to anuria, elevated creatinine (maximum value 14.10 mg/ dl) and urea (232 mg/dl), for which renal replacement therapy was initiated. He was admitted to the ICU.
Hepatic and biliary ultrasound showed cholelithiasis without signs of inflammation; abdominal tomography revealed interstitial edematous pancreatitis (Baltazar C), hepatomegaly, and bilateral pleural effusion.
Given the suspected differential diagnosis of cholangitis and lithiasic cholecystitis as triggers of pancreatitis, cholecystectomy with biliary drainage was performed.
Due to the biochemical findings and clinical picture, leptospirosis was suspected as another differential diagnosis, so doxycycline was started and antibodies against Leptospira were requested, subsequently confirming the disease (antibody titer by MAT 1:10240). However, the patient died on day 16 after the onset of clinical symptoms, secondary to complications from invasive mechanical ventilation and severe sepsis.
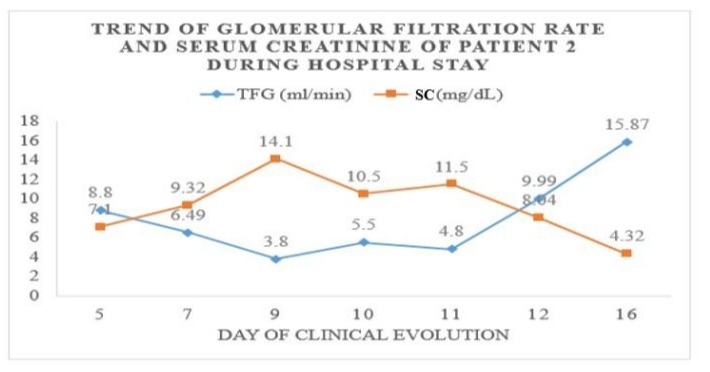
Figure 3: Trend of Biochemical Parameters during Hospitalization: Graphical representation of the trend of serum creatinine values detected during hospitalization and the calculated glomerular filtration rate. Showing worsening of renal function on the 9th day of onset of the clinical picture.
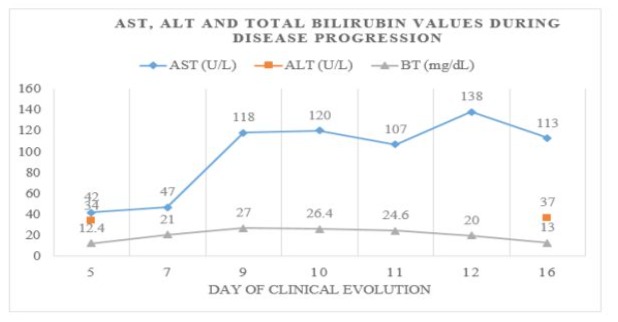
Figure 4: Trend of Biochemical Parameters during Hospitalization: Graphical representation of the trend of serum AST, ALT and total bilirubin values detected during hospitalization, showing a progressive increase in bilirubin presenting a maximum of 27 mg/dl on day 4 of hospitalization, the 9th day of onset of symptoms.
Discussion
Leptospirosis is an emerging zoonotic disease with widespread distribution and epidemic outbreaks worldwide. It is transmitted by direct contact with the urine of infected animals or indirectly through contaminated soil or water. Its prevalence is associated with outdoor activities (recreational wildlife programs, adventure travel, military expeditions) and adverse environmental conditions, such as heavy rainfall or flooding [8-14]. Outbreaks are more frequent in low-income countries with poor housing, sanitation, and animal control [15,16].
This disease can be subclinical in some patients, with no obvious clinical signs. However, when symptomatic, it presents a wide spectrum of manifestations that can be confused with other infectious diseases such as dengue, influenza, Hantavirus, or rickettsiosis [11]. This clinical similarity in the early stages can lead to under diagnosis and underestimation of its prevalence. Therefore, the diagnosis of leptospirosis should be considered in places where these infections coexist, as is the case in our country. The first case reported in Mexico was identified in Mérida, Yucatán, initially diagnosed as yellow fever [7].
In our cases report, both patients were men whose occupation could have represented a risk factor for exposure. In relation to this, a study published in 2015 on the epidemiology of leptospirosis in Mexico reported higher mortality in male patients (61.1%), as well as a higher case fatality rate compared to women [8]. The same study determined an overall case fatality rate for leptospirosis in Mexico of 12.8%, which is considered high compared to that reported in other countries, such as Barbados, Morocco, Germany, Trinidad, and Hawaii. It concluded that 55.7% of the national territory has environmental conditions conducive to the transmission of Leptospira spp [8], with a higher prevalence observed in the southeastern region of the country [1720]. However, the cases presented in this report correspond to the western region of Mexico, which underscores the need to also consider this area as a risk zone that should maintain continuous epidemiological surveillance.
Effective control of leptospirosis is complex because Leptospira spp. can establish symbiotic relationships with certain animal hosts, persisting in the renal tubules and being eliminated in the urine for prolonged periods without causing disease in the host. Rats are the main reservoir involved in the transmission of to humans [2]. Human infection occurs through exposure to infected urine from mammalian hosts, either directly or through contamination of soil or water [11]. We believe that contact with contaminated water was the route of entry for Leptospira infection in our patients; therefore, this history should be carefully evaluated and recorded during the clinical history due to its clinical and epidemiological importance.
The incubation period varies between 2 and 20 days, with an average of 7 to 12 days. Clinically, the disease is divided into two phases: a leptospiremic phase (also called the acute or anicteric phase) and an immune phase [2, 22]. The leptospiremic phase usually lasts between 3 and 9 days [11] and manifests as an acute febrile syndrome accompanied by intense myalgia (particularly in the gastrocnemius, abdominal, and paraspinal muscles) and uveitis. The immune phase is characterized by the appearance of IgM antibodies and the excretion of the pathogen in the urine. However, in clinical practice, the distinction between the two phases can be confusing, given the possible overlap or a brief interval of defervescence between them [2]. Multiple atypical manifestations of leptospirosis in humans have also been documented, including neurological (such as acute disseminated encephalomyelitis, encephalitis, cerebrovascular events, Guillain-Barré syndrome, among others), ocular (optic neuritis), hematological (pancytopenia, hemolytic anemia, thrombotic thrombocytopenic purpura), and gastrointestinal (pancreatitis, cholecystitis), reflecting the wide clinical variability of the disease [2,23]. However, in most cases, this disease can be subclinical, with no obvious manifestations [11].
Weil’s disease represents the most severe form of the disease. This syndrome can develop after the acute phase as the second phase of a biphasic disease or present as a single, progressive disease. It is characterized by jaundice, renal failure, and hemorrhage, with a variable clinical course. Both patients presented above met these characteristics. The case fatality rate ranges from 5% to 15%. Platelet counts may be very low and contribute in part to the hemorrhagic diathesis [24]. Acute renal failure occurs in 16% to 40% of cases and is not usually oliguric. Oliguria is a significant predictor of death [25].
In clinical practice, leptospirosis remains a diagnostic challenge, especially in its early stages, due to the nonspecific nature of its clinical manifestations, which can be confused with other infectious diseases such as dengue, influenza, Hantavirus, or rickettsiosis [11]. This clinical similarity contributes to the underestimation of its true prevalence, and its consideration in the differential diagnosis remains limited. Therefore, it is important to consider this entity in patients presenting with acute febrile syndrome, especially when no common etiological agents, icteric syndrome, and multiple organ failure are identified, given that timely diagnosis is associated with a significant reduction in morbidity and mortality. It is essential to maintain a high index of clinical suspicion to initiate treatment in a timely manner. The diagnostic suspicion of leptospirosis should be based on the integration of relevant epidemiological history, compatible clinical manifestations, and suggestive biochemical findings. Biochemical findings may include elevated ESR, mild increases in transaminases, alkaline phosphatase, and abnormal urine analysis with proteinuria, pyuria, and microscopic hematuria, thrombocytopenia, impaired renal function, and hyperbilirubinemia generally disproportionate to other liver function test values (serum concentrations may reach 30-40 mg/dl and take days or weeks to normalize). Creatine phosphokinase and serum amylase may also be elevated [11].
The diagnosis of Weil’s syndrome is usually suspected based on the clinical picture and biochemical findings observed during hospitalization. In our case, both patients presented maximum renal function deterioration on the ninth day of hospitalization, requiring renal replacement therapy in both cases, in addition to hyperbilirubinemia with a progressive increase disproportionate to transaminases, reaching maximum total bilirubin levels of 40.3 mg/dl in patient 1 and 27 mg/dl in-patient 2. Similarly, both patients had elevated amylase and lipase levels; however, only patient 2 met the diagnostic criteria for pancreatitis, an atypical manifestation of leptospirosis.
Therefore, the diagnosis of leptospirosis is based on clinical characteristics, together with a history of exposure to risk factors, in addition to laboratory confirmation by serology or detection of the microorganism or it’s DNA [2]. Our institution does not have molecular testing facilities, so when the diagnosis was suspected, we requested a microscopic agglutination test (MAT) for both patients, which remains the reference method for the serological diagnosis of leptospirosis in humans and animals [26,21]. This technique is based on the detection of specific antibodies by observing agglutination reactions between the patient’s serum and live cultures of Leptospira spp., representative of endemic serovars [21]. Antibody titers ≥ 1:1600 are generally associated with active or recent infection. In some contexts, this value may be considered diagnostic on its own [26,27]. We obtained titers of 1:10240 in both patients in the second week after the onset of clinical symptoms, which allowed us to make the diagnosis.
Antibiotics and supportive therapy are the mainstay of treatment [2]. Recommended antibiotics are penicillin G sodium, ceftriaxone, cefotaxime, and doxycycline. Due to the severity of the cases, we decided to treat them with cefotaxime. The first patient showed complete improvement and regained normal function; however, the second patient died from complications associated with invasive mechanical ventilation and sepsis.
Conclusion
The diagnosis of leptospirosis is based on the correlation between clinical symptoms, epidemiological history of exposure to risk factors, and confirmation by laboratory methods, either through serology, direct detection of the microorganism, or identification of its genetic material (DNA). It is essential not to underestimate this condition in the differential diagnosis, since, although most cases present with mild clinical symptoms, there are severe forms, such as Weil’s disease, which can progress with severe complications and even fatal outcomes. Currently, there are no clinical scoring systems or validated predictive models that can accurately identify which patients will develop severe forms of the disease. Therefore, it is recommended to start antibiotic treatment early, without waiting for confirmatory diagnostic test results. In addition, it is essential to consider that the prevention of leptospirosis depends largely on health measures and environmental control, aspects that must be actively integrated into epidemiological surveillance programs.
Ethical Considerations
The authors have been duly authorized to publish these clinical cases; however, we guarantee that the information contained herein does not allow for the identification of the patients. Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Weil A. (1886). Ueber einer eigenhuemliche, mit Milztumor, Icterus un Nephritis einhergehende, acute Infektionskrankheit. Deutsch Arch Klin Med. 1886: 39.
- Rajapakse S. (2022). Leptospirosis: clinical aspects. Clin Med. 22: 147.
- Levett PN. (2001). Leptospirosis. Clin Microbiol Rev. 14: 296-326.
- Buchanan G. (1927). Spirochaetal Jaundice. BMJ. 1: 844.
- Bharti AR, Nally JE, Ricaldi JN, Matthias MA, Diaz MM, et al. (2003). Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis. 3: 757-771.
- Evangelista KV, Coburn J. (2010). Leptospira as an emerging pathogen: a review of its biology, pathogenesis, and host immune response. Future Microbiol. 5: 1413-1425.
- Noguchi H, Klieger J. (1920). Immunological studies with a strain of Leptospira isolated from a case of yellow fever in Mérida, Yucatán. J Exp Med. 32: 627-637.
- Sánchez-Montes S, Espinosa-Martínez DV, Ríos-Muñoz CA, Berzunza-Cruz M, Becker I. (2015). Leptospirosis in Mexico: Epidemiology and Potential Distribution of Human Cases. PLoS One. 10: e0133720.
- World Health Organization. (1999). Leptospirosis worldwide. Wkly Epidemiol Rec. 74: 237-244.
- Levett PN. (2001). Leptospirosis. Clin Microbiol Rev. 14: 296-326.
- Bharti AR, Nally JE, Ricaldi JN, Matthias MA, Diaz MM, et al. (2003). Peru-United States Leptospirosis Consortium. Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis. 3: 757-771.
- Adler B, de la Peña Moctezuma A. (2010). Leptospira and Leptospirosis. Vet Microbiol. 140: 287-296.
- Acha PN, Szyfres B. Leptospirosis. In: Pan American Health Organization (PAHO). Zoonoses and communicable diseases. 1: 580.
- Samrot AV, Sean TC, Bhavya KS, Sahithya CS, Chan-Drasekaran S, et al. (2021). Leptospiral Infection, Pathogenesis and Its Diagnosis-A Review. Pathogens. 10: 145.
- Galloway RL, Schafer R, Stoddard RA. Centers for Disease Control and Prevention. Yellow Book, Chapter 4: Travel-related infections, section on Leptospirosis.
- WHO. (2020). Leptospirosis Burden Epidemiology Reference Group (LERG). Available in online.
- Zavala J, Caballero-Guerrero C, Sánchez-Vázquez I. (1967). Leptospirosis in the state of Chiapas, Mexico (preliminary report). Public Health Mex. 18: 989-998.
- Zavala J, Pinzón J, Flores M, Damián A. (1984). Leptospirosis in the Yucatan. Serological study in humans and animals. Public Health Mex. 26: 254-259.
- Zavala J, Bolio A, Suarez G. (1985). Leptospirosis in Yucatan. Rev Invest Clin. 37: 353-357.
- Gavaldón D, Cisneros M, Rojas N, Moles-Cervantes L. (1988). The importance of human Leptospirosis in Mexico. Detection of antileptospira antibodies in a population of blood donors. Gac Med. Mex. 131: 289-292.
- Haake DA, Levett PN. (2015). Leptospirosis in humans. Curr Top Microbiol Immunol. 387: 65-97.
- Turner LH, Leptospirosis I. (1967). Trans R Soc Trop Med Hyg. 61: 842-55.
- Rajapakse S, Rodrigo C, Balaji K, Fernando SD. (2015). Atypical manifestations of leptospirosis. Trans R Soc Trop Med Hyg. 109: 294302.
- Vinetz JM, Glass GE, Flexner CE, Mueller P, Kaslow DC. (1996). Sporadic urban leptospirosis. Ann Intern Med. 125: 794-98.
- Daher E, Zanetta DM, Cavalcante MB, Abdulkader RC. (1999). Risk factors for death and changing patterns in leptospirosis acute renal failure. Am J Trop Med Hyg. 61: 630-34.
- World Health Organization (WHO). (2003). Human leptospirosis: guidance for diagnosis, surveillance and control. Geneva: WHO; 2003.
- Goris MGA, Leeflang MMG, Loden M, Wagenaar JFP, KlatserPR, et al. (2013). Prospective evaluation of three rapid diagnostic tests for diagnosis of human leptospirosis. PLoS Negl Trop Dis. 7: e2290.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.