Very Delayed Complications of Stamey Needle Suspension: A Case Report and Review of the Literature
by Rasadokht Forati1*, Christina M. Mezes2, Majid Mirzazadeh2
1Department of Obstetrics, Gynecology, & Reproductive Sciences, Yale University School of Medicine, New Haven, CT USA
2Department of Urology, Wake Forest Baptist Health, Winston Salem, NC, USA
*Corresponding author: Rasadokht Forati, Department of Obstetrics, Gynecology & Reproductive Sciences, Yale University School of Medicine, New Haven, CT USA
Received Date: 08 September 2025
Accepted Date: 15 September 2025
Published Date: 17 September 2025
Citation: Forati R, Mezes CM, Mirzazadeh M (2025) Very Delayed Complications of Stamey Needle Suspension: A Case Report and Review of the Literature. J Urol Ren Dis 09: 1433. https://doi.org/10.29011/2575-7903.001433
Abstract
Introduction: Surgical treatment of Stress Urinary Incontinence (SUI) has evolved significantly, with needle suspension techniques like the Stamey procedure once widely used. We report a rare, delayed complication over 30 years post-Stamey and review related late outcomes involving synthetic materials.
Methods: We describe a case of a patient presenting over 30 years post-Stamey with chronic lower urinary tract symptoms and recurrent urinary tract infections. A PubMed literature search was then conducted to identify reports of late (>1 year) complications associated with synthetic materials used in the Stamey procedure. Studies involving mesh or other procedures were excluded. Extracted data included presenting symptom, complication type, diagnostic work-up, and management.
Results: The patient was found to have bladder erosion by synthetic suture material, which was removed endoscopically in-office, resolving her symptoms. The literature search identified 24 relevant cases, with time to presentation ranging from 1 to 40 years postoperatively. Presenting symptoms most commonly included pelvic or abdominal pain, urinary tract infections, stress urinary incontinence, and abnormal vaginal discharge. The most frequently reported complications were erosion of synthetic material, vesical calculi formation, and fistulae. Most cases were managed endoscopically. Delays in diagnosis were common due to vague symptoms and a lack of procedural history.
Conclusions: Late complications of the Stamey procedure can present decades after surgery and are often misdiagnosed due to their non-specific symptoms. Clinicians should remain alert to the possibility of retained foreign material in patients with a history of anti-incontinence surgery and persistent lower urinary tract symptoms.
Keywords: Bladder Neck Suspension; Late Complications; Stamey Procedure; Stress Urinary Incontinence; Synthetic Material
Introduction
Surgical treatment of Stress Urinary Incontinence (SUI) has rapidly evolved over the past century. The first effective surgical approach, described by Kelly and Dunn in 1914, involved anterior colporrhaphy with bladder neck plication and became known as the Kelly plication [1]. In 1944, Marshall, Marchetti, and Krantz introduced anterior urethropexy via direct fixation of the bladder neck and urethra to the periosteum of the symphysis pubis [2]. This technique, later known as the MMK procedure, was modified by Burch in 1961 to avoid direct bony fixation, which led to improved outcomes and became the preferred method [3]. In 1959, Armand J. Pereyra introduced the needle suspension as a less invasive alternative to retropubic urethropexy, eliminating the need for an abdominal incision. Subsequent modifications by Raz, Gittes, and Stamey further refined the approach. Stamey emphasized endoscopic visualization to ensure accurate suture placement and avoid bladder injury [2]. He also introduced the use of synthetic materials, notably a Dacron (polyethylene terephthalate) buttress, to avoid suture pull-through and maintain the elevation of the bladder neck over time-marking one of the first uses of synthetic reinforcement in SUI surgery. Needle suspension surgery gained widespread popularity and, by1992, had become the most commonly performed surgery for SUI in the United States, with over 10,000 procedures still being performed annually by 1995 [1]. Despite their early success, needle suspensions eventually fell out of favor due to inferior long-term outcomes [4]. However, as patients who underwent these procedures continue to age, reports of delayed complications-particularly those associated with retained synthetic materials—have emerged. Here, we report a case of a female patient who underwent a Stamey needle suspension and presented with a delayed complication of bladder erosion more than 30 years later. A literature review was then performed to identify and characterize the reported late complications associated with the Stamey procedure to date, with particular focus on urological outcomes related to the use of synthetic materials other than mesh. In doing so, we aim to highlight a clinical pearl for providers of patients presenting with Lower Urinary Tract Symptoms (LUTS) and a remote history of surgery for SUI.
Case Report
A 69-year-old female presented to our institution with an approximately 8-month history of intermittent sharp lower abdominal pain. She also complained of irritative voiding symptoms and her urinalysis was significant for microscopic hematuria. She had previously undergone an unknown ‘bladder tacking’ surgery in the early 1990s. Additional significant surgical history included a total abdominal hysterectomy in 1994, a sacrospinous ligament fixation for Pelvic Organ Prolapse (POP) in 2018, followed by a repeat sacrospinous ligament fixation for recurrent POP in 2019, and finally a laparoscopic sacrocolpopexy for a second recurrence of her POP. Cystoscopy performed at the time of her 2022 surgery was reportedly normal. A contrast Computed Tomography (CT) scan of the abdomen and pelvis was obtained which showed an enhancing mass involving the anterior aspect of the urinary bladder and was read as either a primary urinary bladder neoplastic process or lesion associated with the urachal ligament (Figure 1).
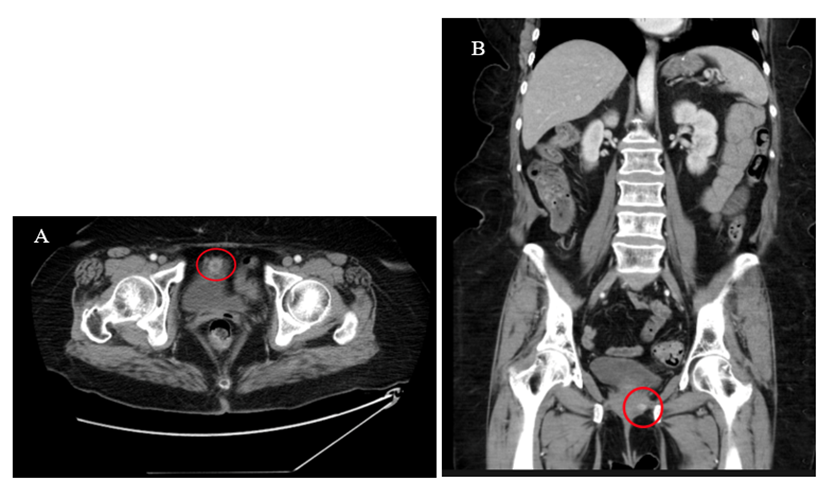
Figure 1: Axial (A) and coronal (B) contrast-enhanced CT images of the abdomen and pelvis demonstrating an enhancing mass along the anterior aspect of the urinary bladder.
Following the CT scan, the patient underwent cystoscopy both with her urogynecologist and her urologist showing a white foreign body on the anterior wall of the bladder measuring 1.5-2 cm in size with overlying stone and granulation tissue. She was referred to our institution for a second opinion. Office cystoscopy was performed with 70-degree lens noting erosion and hyperemia in the right upper part of the bladder without active perforation. A foreign object was seen free floating in the bladder lumen and removed with a grasper (Figure 2).

Figure 2: Foreign body removed with grasper during in-office cystoscopy.
A CT cystogram was obtained at 4 weeks, which confirmed an intact bladder wall without perforation (Figure 3). The patient’s lower urinary tract symptoms were resolved upon her follow-up.
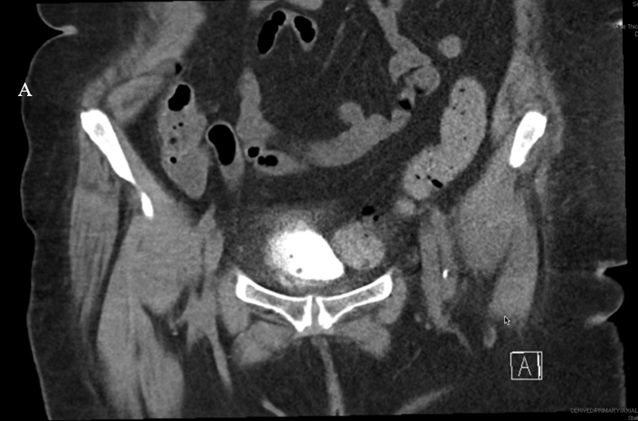
Figure 3: CT cystogram obtained at 4 weeks postoperatively demonstrating an intact bladder wall without evidence of perforation.
Methods
A literature search was conducted in PubMed with the assistance of a medical librarian to identify studies reporting delayed complications associated with the Stamey procedure. Search terms included combinations of keywords and MeSH terms related to the Stamey procedure, long-term or delayed complications, relevant anatomic sites (e.g., bladder, urethra, periurethral or paravaginal tissues), and types of synthetic materials (e.g., sutures, bolsters) (Table 1). Articles were included if they described complications presenting one year or more after the original surgery and specifically involved synthetic materials used in the Stamey procedure, particularly those affecting urological structures. Exclusion criteria included studies focused on mesh-related complications, other surgical procedures (e.g., Marshall-Marchetti-Krantz, Raz, or Pereyra), unspecified time to presentation, or inclusion of male participants. Data were independently extracted from each eligible study, including symptomatology, time to presentation, diagnostic evaluation, type of complication, and management approach.
|
PubMed (NCBI) |
(Stamey[tiab] OR "needle suspension"[tiab] OR "neck suspension"[tiab]) |
|
[3/20/2025] |
AND |
|
[86 records returned] |
("Postoperative Complications"[mesh] OR "adverse effects"[sh] OR "Long Term Adverse Effects"[Mesh] OR adverse effect*[tiab] OR adverse event*[tiab] OR adverse outcome*[tiab] OR complication*[tiab] OR exposure[tiab] OR erosion[tiab] OR perforation*[tiab] OR obstruction*[tiab] OR fistula*[tiab]) |
|
AND |
|
|
("Urinary Bladder"[Mesh] OR "Urethra"[Mesh] OR bladder[tiab] OR urethra[tiab] OR lower urinary[tiab] OR paravagina*[tiab] OR periurethral[tiab] OR vagina*[tiab] OR vesical[tiab] OR vesicula*[tiab]) |
|
|
AND |
|
|
(foreign bod*[tiab] OR foreign material*[tiab] OR suture*[tiab] OR bolster*[tiab] OR buttress[tiab] OR tack*[tiab] OR Dacron[tiab] OR synthetic material*[tiab] OR silicone[tiab]) |
Table 1: Database Searches.
Results
The initial search yielded 86 articles, of which 15 met the inclusion and exclusion criteria and were included in the review. Among these, there were nine case reports and six case series for a total of 24 included cases (Table 2). Complications following the Stamey procedure were reported to occur anywhere from 1 to 40 years postoperatively in case reports, whereas the case series documented complications within a range of 1 to 12 years. The majority of patients presented between 1 and 5 years after surgery (Figure 4). The symptoms with which patients presented varied widely, including LUTS, dysuria, hematuria, pelvic pain, abdominal pain, SUI, and abnormal vaginal discharge (Figure 5). Five cases discussed the formation of vesical calculi,[5-8]. and another 3 described the development of fistulas (Figure 6) [5,9,10]. Erosion was the most commonly reported complication, noted in 13 cases [8,10-16]. Suture migration was discussed in 3 reports [13,14,17]. while 5 articles highlighted a general foreign body reaction [8,14,16-18]. Complications were most commonly managed via endoscopic removal of the buttress and sutures, with additional cases treated using open, laparoscopic, or transvaginal approaches (Figure 7).
|
Journal |
Author (Year) |
PMID |
Article type |
Symptom(s) |
Time to presentation |
Synthetic material |
Diagnostic testing |
Complication(s) |
Management |
|
BMJ Case Reports |
Salfity et al. (2021) |
34020989 |
Case report |
Recurrent UTIs, abdominal pain, dysuria, SUI |
40 years |
Dacron buttress |
CT, flexible cystoscopy, MRI |
Bladder erosion, stone formation around foreign material |
Cystoscopic removal of foreign material, Holmium laser |
|
BMJ Case Reports |
O’Callaghan et al. (2014) |
25239995 |
Case report |
Recurrent urinary sepsis, passage of air and faeculent material per-urethra |
20 years |
Suture |
CT, flexible cystoscopy, diagnostic laparoscopy |
Suture attached to calculi in bladder, enterovesical fistula |
Litholapaxy, laparoscopic repair and suture removal |
|
Obstetrics & Gynecology |
Treszezamsky et al. (2012) |
22270428 |
Case report |
Abdominal and pelvic pain, recurrent abnormal vaginal discharge |
22 years |
Dacron buttress |
CT |
Bladder erosion, suprapubic abscess, and vaginal fistula formation |
IV antibiotics, surgical abscess drainage and debridement of necrotizing subcutaneous tissue |
|
Int Urol Nephrol. |
Gregorakis et al. (2006) |
16868695 |
Case report |
Pelvic pain, bladder irritative symptoms |
19 years |
Suture and buttress |
Cystoscopy |
Bladder erosion by nylon suture, inflamed edematous mucosa |
Cystoscopic removal of suture and Dacron buttress |
|
Int Urogynecol J Pelvic Floor Dysfunct |
Smith & Rovner (2006) |
15965575 |
Case series (1) |
Vaginal discharge, LUTS, recurrent SUI |
12 years |
Bolster |
VUD, KUB, cystoscopy |
Vaginal sinus tract |
Vaginal dissection, bolster and suture removal |
|
-2 |
Vaginal discharge, pelvic pain, pyuria |
11 years |
Bolster |
CT, cystoscopy, VUD |
Vaginal sinus tract |
Transvaginal exploration and bolster removal, placement of autologous pubovaginal sling |
|||
|
-3 |
UTI, pelvic pain, LUTS |
9 years |
Bolster |
MRI, VUD, cystoscopy |
Bladder erosion by |
Endoscopic removal of calcified shell, transvaginal exploration, removal of bolster, and bladder wall repair, placement of autologous pubovaginal sling |
|||
|
-4 |
UTI, LUTS, recurrent SUI |
11 years |
Bolster |
VUD, cystoscopy |
Periurethral inflammation secondary to bolster |
Urethral dissection, removal of bolster, and placement of autologous pubovaginal sling |
|||
|
Obstet Gynecol. |
Giles and Davila (2005) |
15863578 |
Case report |
Vaginal discharge, abdominal draining sinus, SUI |
18 years |
Synthetic material |
Fistulogram |
Vagino-abdominal fistula, abscess around synthetic material |
Surgical excision of abscess and track and open removal of suture and bolster |
|
Hinyokika Kiyo |
Komai et al. (2004) |
15148775 |
Case report |
Voiding pain in lower abdomen |
16 years |
Stamey cuff |
Not reported. |
Vesical calculi with Stamey cuff |
Transurethral cystolitholapaxy, open cystotomy cuff removal |
|
Int Urol Nephrol. |
Athanasopoulos et al. (2002) |
12549629 |
Case series (1) |
Recurrent UTIs, pelvic pain |
18 months |
Suture and bolster |
US, bladder scan, KUB, cystoscopy |
Bladder erosion of calcified foreign body |
Attempted but unsuccessful endoscopic litholapaxy, foreign body removal via endoscopic knife |
|
-2 |
Recurrent UTIs, pelvic pain |
14 months |
Suture and bolster |
Bladder US, KUB, cystoscopy |
Bladder erosion by calcified nylon suture |
Endoscopic suture and bolster removal |
|||
|
Int Urol Nephrol. |
Nabi et al. (2001) |
12092654 |
Case report |
Abdominal pain, dysuria |
3 years |
Suture and pledget |
US, X-ray, CT, cystoscopy |
Suture and pledget migration leading to foreign body granuloma |
Cystoscopic resection of inflammatory tissue and removal of foreign body |
|
Int Urogynecol J Pelvic Floor Dysfunct. |
Dwyer et al. (1999) |
10207762 |
Case series (1) |
Recurrent SUI, UTI, pelvic pain |
20 months |
Suture and cuff |
Cystoscopy |
Bladder erosion of suture, edematous inflamed bladder mucosa |
Cystoscopic removal of suture and cuff |
|
-2 |
Recurrent SUI |
2 years |
Suture |
Cystoscopy |
Bladder erosion of suture |
Cystoscopic removal of suture |
|||
|
-3 |
Recurrent SUI, voiding pain |
5 years |
Suture |
Cystoscopy |
Bladder erosion of suture |
Cystoscopic removal of suture |
|||
|
-4 |
Pelvic pain |
1 year |
Suture |
US, cystoscopy |
Bladder erosion of suture |
Cystoscopic removal of suture |
|||
|
Int Urogynecol J Pelvic Floor Dysfunct. |
Biyani and Upsdell (1998) |
9849764 |
Case report |
Dragging sensation over left iliac fossa and groin |
7 years |
Suture and bolster |
US, cystoscopy |
Calcified cuff attached to suture in bladder wall |
Cystolithopaxy and endoscopic removal of cuff |
|
Br J Urol. |
Weiss and Cohen (1992) |
1308659 |
Case report |
Dysuria, hematuria |
4 years |
Suture and buttress |
IV urogram, cystoscopy |
Bladder erosion of Dacron buttress and nylon suture |
Endoscopic resection of buttress and stitch |
UTI, urinary tract infection; SUI, stress urinary incontinence; LUTS, lower urinary tract symptoms; CT, computed tomography; MRI, magnetic resonance imaging; US, ultrasound; IV, intravenous; VUD, videourodynamics
*This article did not provide individual case-level data and was therefore excluded from quantitative analyses.
Table 2: A Summary of the Existing Literature Relating to Delayed Complications of the Stamey Procedure.
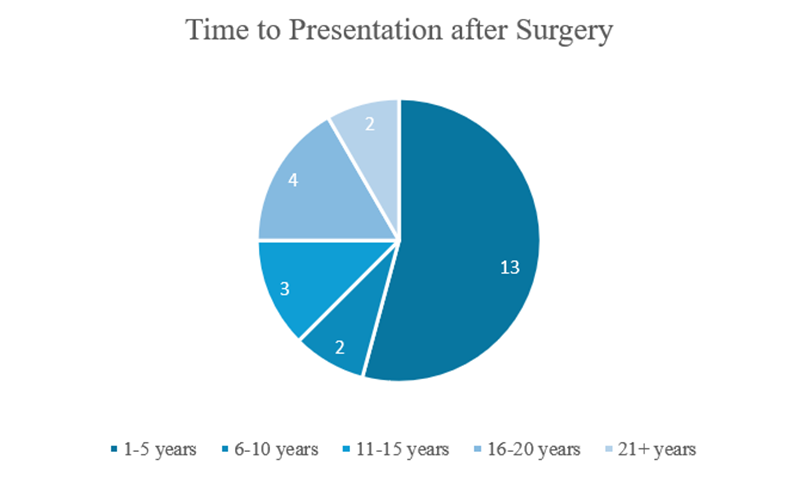
Figure 4: Time to presentation following the Stamey procedure. Among 24 total cases, 13 (54%) presented within 1–5 years postoperatively; 2 cases (8%) presented at 6–10 years; 3 cases (12%) at 11–15 years; 4 cases (17%) at 16–20 years; and 2 cases (8%) presented more than 20 years postoperatively.
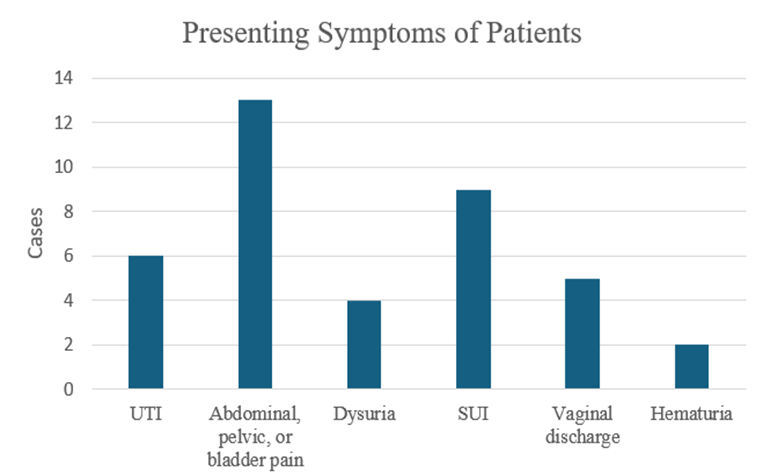
Figure 5: Reported postoperative symptoms following the Stamey procedure. The most commonly reported symptom was abdominal, pelvic, or bladder pain (13), followed by Stress Urinary Incontinence (SUI, 9), Urinary Tract Infection (UTI, 6), vaginal discharge (5), dysuria (4), and hematuria (2).
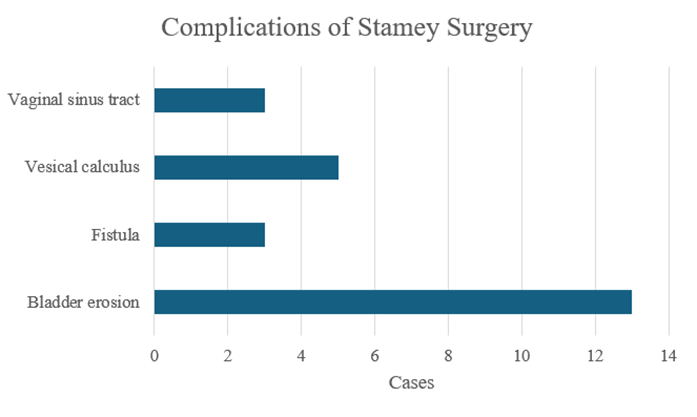
Figure 6: Reported complications following the Stamey procedure. Bladder erosion was the most frequently reported complication (13), followed by vesical calculus (5). Fistula formation and vaginal sinus tract were each reported in 3 patients.
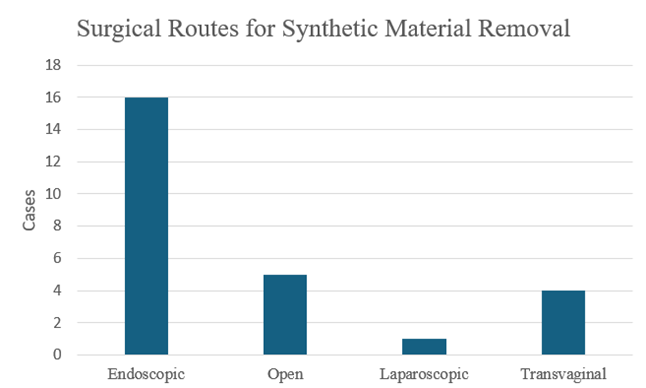
Figure 7: Surgical approaches utilized for suture or buttress removal following the Stamey procedure. Endoscopic removal was the most frequently employed method (16), followed by open (5), transvaginal (4), and laparoscopic approaches (1).
Discussion
Our case report highlights a very late-presenting complication of the Stamey procedure, an antiquated anti-incontinence surgery utilizing synthetic material such as the Dacron buttress and nylon suture. This narrative review complements our findings by compiling published literature on similar delayed complications. Our literature search revealed that the time to onset of such complications can vary widely, with the earliest reported complication presenting 14 months postoperatively and the latest presenting 40 years postoperatively. This underscores the unpredictable latency with which such complications can arise, and the value of a detailed surgical history in patients presenting with otherwise unexplained LUTS. The delayed complications associated with the Stamey procedure fall into several consistent categories: erosion, calculi formation, and fistulae. Intravesical erosion of synthetic materials was the most frequently reported complication, as was seen in our patient. Patients typically presented with recurrent UTIs, hematuria, dysuria, and/or pelvic pain. Cystoscopic evaluation remains the gold standard for diagnosis; nonetheless, it is important to recognize that a negative cystoscopy does not definitively exclude the presence of a migrated or infected bolster within the bladder or vaginal wall or adjacent pelvic soft tissues [16]. In these cases, imaging modalities like Computed Tomography (CT) or Magnetic Resonance Imaging (MRI) may be used as an adjunctive method of evaluation for unexplained and vague pelvic or urinary symptoms. In our case and others, CT imaging was utilized as an initial diagnostic tool to evaluate vague or chronic irritative bladder symptoms, which then led to definitive diagnosis via cystoscopy.
Calculus formation was another commonly reported delayed complication of the Stamey procedure. As Komai et al. noted, the presence of foreign bodies in the bladder can serve as a nidus for stone formation, also exemplified by the overlying stone and granulation tissue formation on the Dacron buttress in our patient [7]. This is consistent with known risks of encrustation on foreign material in the urinary tract [19,20]. One proposed mechanism of calculus formation is via infection or urinary obstruction, which triggers crystals to form and accumulate on a foreign object, leading to a supersaturated environment in the bladder [21]. This allows crystals to cluster together, grow over time, and eventually develop into a calculus. Similarly, fistula formation in these cases is consistent with established mechanisms of chronic inflammation from non-absorbable implants leading to gradual extrusion into nearby organs [22]. A prospective study with long-term follow-up of 66 patients undergoing the Stamey procedure identified fistula formation as the most frequent complication, occurring in 10 patients (15.2%) at a mean of 15.3 months post-operatively (range: 2-36 months) [23]. The authors attributed this outcome to the proximity of the operative field to the anus and the suture material utilized.
Though rare, suprapubic abscesses and enterovesical fistulas were also reported in our review, reinforcing the fact that complications from permanent materials may involve multi-organ systems and present insidiously [4,9]. These patterns mirror those seen with modern synthetic slings and mesh, where erosion and infection remain well-described long-term risks. Several hypotheses have pointed to the possible mechanism behind the delayed presentation of synthetic material-related complications; however, they remain somewhat theoretical. One explanation is a delayed Foreign Body Response (FBR), a chronic inflammatory process that results in adhesion of synthetic material to surrounding structures, increasing the risk of gradual tissue erosion and perforation [13,14]. Multiple preclinical and clinical studies have demonstrated that synthetic materials, such as polypropylene and Dacron, elicit a persistent FBR, characterized histologically by the presence of macrophages, multinucleated giant cells, dense connective tissue deposition, and progressive fibrosis adjacent to the implanted material [24,25]. An alternative explanation for delayed synthetic material migration is the excessive tension placed on sutures at the time of the initial surgery, resulting in chronic ischemia and progressive tissue necrosis that predisposes to late-stage erosion [13,14]. Management across the reviewed cases was consistently surgical, with nearly all patients requiring some form of intervention to remove synthetic material or address resulting complications.
Common approaches included transurethral or open cystolitholapaxy for bladder stones, cystoscopic or open removal of eroded sutures or buttresses, and in more complex cases, laparoscopic or open fistula repair. In rare cases, the bladder wall was surgically repaired, likely in the setting of large defects [16]. In contrast, our case was managed using a minimally invasive approach through in-office cystoscopy, avoiding the need for general anesthesia. Additionally, there was no visible bladder wall defect, therefore a follow-up cystogram 4 weeks after cystoscopic foreign body removal was performed and showed that the bladder wall had healed spontaneously. This suggests that surgical bladder repair may not be necessary in all cases, especially those with small residual defects and successful removal of foreign material. A recurring theme across the reviewed cases was a significant delay between symptom onset and definitive diagnosis. In many cases, symptoms were vague or misattributed, delaying appropriate diagnosis and management. For instance, LUTS were most often attributed to recurrent UTIs, and despite appropriate antibiotic therapy, infection and associated symptoms were resistant to treatment. Other common but confusable presenting symptoms included pelvic pain and abnormal vaginal discharge that were initially managed as isolated or unrelated issues. In many instances, the true etiology was only uncovered after imaging or cystoscopy revealed erosion, encrustation, or fistula formation related to the original Stamey procedure. In addition, while some patients presented a few years after surgery, a significant proportion did not present until several decades later, sometimes not recalling which specific anti-incontinence surgery they had undergone. In our presented case, the patient recalled a ‘bladder tacking’ surgery many decades prior but was unsure of its name or details. Diagnostic delays were therefore compounded by the long latency period and unfamiliarity with the procedure itself, especially when the original surgery was performed decades earlier and not well-documented in current medical records. Clinicians unfamiliar with historical anti-incontinence procedures may not consider a remote Stamey procedure as a potential source of LUTS unresponsive to the usual diagnostic and treatment algorithm.
This highlights the need for a high index of suspicion in patients with unexplained pelvic or urinary tract symptoms—particularly when they are of an age where prior anti-incontinence surgeries may have involved antiquated techniques or materials. These factors, in conjunction with the rarity of these complications as demonstrated by most relevant literature being case reports, emphasize the profound diagnostic challenge in identifying the true cause of these patients’ symptoms. A systems-level challenge identified by this review is the absence of a standardized framework to categorize very late surgical complications, particularly those occurring more than a decade after the initial procedure. Several cases report “delayed,” “late,” or “long-term” complications of the Stamey procedure, however these terms are not precisely defined to specify the time parameters that constitute a late, as opposed to early, complication. While the International Continence Society (ICS) and International Urogynecology Association (IUGA) have established a classification system for complications related to pelvic floor implants, the temporal component of this system stops at T4 (>12 months postoperatively) [26]. As such, the system fails to capture events occurring 10, 20, or even 40 years post-operatively, as documented in several of the included reports.
This limitation has implications for both clinical recognition and data reporting. Without a consistent category for “very late” complications, these events may be underreported, misclassified, or overlooked in outcomes research. Given the increasing life expectancy of patients and the continued use of synthetic materials in pelvic surgery, there may be a need to revisit existing classification systems to include very delayed presentations to more accurately capture true complication rates of such procedures. We propose defining very late complications as those occurring greater than 5 years postoperatively.This review is inherently limited by its reliance on case reports and small case series, which are subject to publication bias and lack standardized reporting. Therefore, the true incidence and prevalence of late complications following the Stamey procedure remain unknown. Additionally, because most reports lacked detailed surgical records or long-term follow-up, it is unclear whether patient-specific factors (e.g., comorbidities, prior pelvic surgeries, or technique variations) contributed to the delayed presentations, and whether the approaches to management were optimal. Despite these limitations, this review highlights the need for thorough history-taking and knowledge of anti-incontinence procedures of the past.
Conclusion
Our case highlights a very late complication of the Stamey procedure and demonstrates successful management using a minimally invasive approach. To our knowledge, this is the first literature review focused specifically on delayed complications of the Stamey technique. Although rare, clinicians should remain aware of historical pelvic procedures and their potential for late-presenting complications. Ongoing research and improved tracking of long-term outcomes are needed to guide future care.
Acknowledgements: None.
Ethical guidelines: This article does not involve studies with human participants or animals conducted by the authors. Therefore, ethical approval and informed consent were not required.
Conflict of Interest: The authors declare that they have no Conflict of Interest.
References
- Anger JT, Weinberg AE, Albo ME, Smith AL, Kim J-H, Rodríguez LV, Saigal CS (2009) Trends in surgical management of stress urinary incontinence among female Medicare beneficiaries. Urology 74: 283-287.
- Cornella JL, Ostergard DR (1990) Needle Suspension Procedures for Urinary Stress Incontinence: A Review and Historical Perspective. Obstetrical & Gynecological Survey 45: 805-816.
- Dainer M, Hall CD, Choe J, Bhatia NN (1999) The Burch Procedure: A Comprehensive Review. Obstetrical & Gynecological Survey 54: 49-60.
- Glazener CM, Cooper K (2002) Bladder neck needle suspension for urinary incontinence in women. In: The Cochrane Collaboration (ed) Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd, Chichester, UK, p CD003636.
- O’Callaghan JM, Boyd JT, Singh A (2014) A stitch in time: an unusual cause of enterovesical fistula. BMJ Case Rep: bcr2014205853.
- Evans JW, Chapple CR, Ralph DJ, Milroy EJ (1990) Bladder calculus formation as a complication of the Stamey procedure. Br J Urol 65: 580-582.
- Komai Y, Urushibara M, Morimoto S, Sakai K (2004) [Bladder stone formation caused by a Stamey cuff as the nidus indwelt 16 years ago]. Hinyokika Kiyo 50: 203-205.
- Bihrle W, Tarantino AF (1990) Complications of retropubic bladder neck suspension. Urology 35: 213-214.
- Giles DL, Davila GW (2005) Suprapubic-vaginocutaneous fistula 18 years after a bladder-neck suspension. Obstet Gynecol 105: 1193-1195.
- Treszezamsky AD, Goldstein I, Sharyarinejad A, Ascher-Walsh CJ (2012) Suprapubic abscess and vaginal fistula 22 years after bladder neck suspension with Dacron buttresses. Obstet Gynecol 119: 431-433.
- Gregorakis A, Bouropoulos C, Dimitriou D, Rallis G, Vernadakis S, Papadopoulos IN, Kastriotis I (2006) Delayed reaction to the Dacron buttress used in Stamey bladder neck suspension. Int Urol Nephrol 38: 269-272.
- Salfity L, Dekel E, Sahai A, Faure Walker N (2021) Late urological manifestation of stress incontinence surgery. BMJ Case Rep 14: e241660.
- Athanasopoulos A, Liatsikos EN, Perimenis P, Barbalias GA (2002) Delayed suture intravesical migration as a complication of a Stamey endoscopic bladder neck suspension. Int Urol Nephrol 34: 5-7.
- Biyani CS, Upsdell SM (1998) An unusual foreign body in the bladder 7 years after a Stamey endoscopic bladder neck suspension. Int Urogynecol J Pelvic Floor Dysfunct 9: 303-304.
- Weiss RE, Cohen E (1992) Erosion of buttress following bladder neck suspension. Br J Urol 69: 656-657.
- Smith A, Rovner E (2006) Long-term chronic complications from Stamey endoscopic bladder neck suspension: a case series. Int Urogynecol J Pelvic Floor Dysfunct 17: 290-294.
- Nabi G, Hemal AK, Khaitan A (2001) Endoscopic management of an unusual foreign body in the urinary bladder leading to intractable symptoms. Int Urol Nephrol 33: 351-352.
- Richardson DA, Bent AE, Ostergard DR, Cannon D (1984) Delayed reaction to the Dacron buttress used in urethropexy. J Reprod Med 29: 689-692.
- Herout R, Reicherz A, Chew BH, Lange D (2022) Urinary Tract Infections and Encrustation in Urinary Stents. In: Soria F, Rako D, De Graaf P (eds) Urinary Stents. Springer International Publishing, Cham, pp 341-350.
- Bansal A, Yadav P, Kumar M, Sankhwar S, Purkait B, Jhanwar A, Singh S (2016) Foreign Bodies in the Urinary Bladder and Their Management: A Single-Centre Experience From North India. Int Neurourol J 20: 260-269.
- Drach GW (1992) Urinary lithiasis etiology, diagnosis, and medical management. In: Campbell’s Textbook of Urology p 2085.
- Welk B, Dmochowski R, McCarthy K, Keck J, Mourad S, Hashim H (2024) Complications associated with the use of mesh to treat female urinary incontinence and pelvic organ prolapse. Continence 12: 101713.
- Huland H, Bucher H (1984) Endoscopic bladder neck suspension (Stamey-Pereyra) in female urinary stress incontinence. Long-term follow-up of 66 patients. Eur Urol 10: 238-241.
- Kyriakides TR, Kim H-J, Zheng C, Harkins L, Tao W, Deschenes E (2022) Foreign body response to synthetic polymer biomaterials and the role of adaptive immunity. Biomed Mater.
- Dievernich A, Achenbach P, Davies L, Klinge U (2022) Characterization of innate and adaptive immune cells involved in the foreign body reaction to polypropylene meshes in the human abdomen. Hernia 26: 309-323.
- Haylen BT, Freeman RM, Swift SE, et al (2011) An international urogynecological association (IUGA)/international continence society (ICS) joint terminology and classification of the complications related directly to the insertion of prostheses (meshes, implants, tapes) and grafts in female pelvic floor surgery. Neurourology and Urodynamics 30: 2-12.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.