Vasospastic Angina following Coronavirus Disease 2019 in a Korean Male: A Case Report
Taesun Kim1, In Joong Song2†, You Jin Kim2†, Soobin Park2†, Hyunhee Choi1, Kyung Soon Hong1, Kyu Tae Park1*
1Division of Cardiology, Department of Internal Medicine, Chuncheon Sacred Heart Hospital, Hallym University College of Medicine, Chuncheon, Korea
2Division of Cardiology, Department of Internal Medicine, Hallym University College of Medicine, Chuncheon, Korea
†Present Position: Senior student, College of Medicine, Hallym University, Chuncheon, Korea
*Corresponding author: Kyu Tae Park, Division of Cardiology, Department of Internal Medicine, Chuncheon Sacred Heart Hospital, Hallym University College of Medicine, 77 Sakju-ro, Chuncheon 24253, Republic of Korea
Received Date: 02 November 2022
Accepted Date: 07 November 2022
Published Date: 09 November 2022
Citation: Kim T, Song IJ, Kim YJ, Park S, Choi H, et al. (2022) Vasospastic Angina following Coronavirus Disease 2019 in a Korean Male: A Case Report. Ann Case Report. 7: 1028. DOI: https://doi.org/10.29011/2574-7754.101028
Abstract
There is much research on cardiovascular complications associated with coronavirus disease 2019 (COVID-19). However, despite the endothelial dysfunction that can occur after the disease, little is known about COVID-19-related vasospastic angina (VSA). Here, we report the first case of a patient with VSA following COVID-19 in Korea. He reported tight chest pain and dyspnea; however, transthoracic echocardiography, chest computed tomography, and laboratory tests did not reveal abnormalities. He underwent 24-h Holter monitoring, and early-morning electrocardiography showed ST elevation for about 3 min followed by non-sustained ventricular tachycardia for about 5 s. After subsequent recovery of ST change, the patient complained chest pain. We diagnosed him with VSA and prescribed a calcium channel blocker and nitrates, after which his symptoms resolved.
Keywords: Coronavirus disease 2019; Torsade de pointes; Vasospastic angina
Introduction
Various complications of coronavirus disease 2019 (COVID-19) have been observed, [1] including several cardiovascular complications such as myocarditis, pericarditis, heart failure, pulmonary hypertension, acute coronary syndrome, arrhythmia, and venous thromboembolism. [2] In some patients with COVID-19, ST-segment elevation has been observed, occasionally alongside myocardial infarction and non-coronary obstruction. [3] However, reports of vasospastic angina (VSA) in patients with COVID-19 are rare. Here, we present the first case of COVID-19-related VSA in Korea.
Case Description
The patient, a 48-year-old Korean man, had recently recovered from COVID-19 (diagnosed on June 7, 2022), having experienced general weakness but no other specific symptoms. Two weeks after diagnosis with COVID-19, he visited the hospital emergency room with complaints of tight chest pain and dyspnea.
Initial vital signs were stable, with blood pressure of 113/79 mmHg and pulse rate of 50 beats per minute. Physical examination revealed no additional symptoms. There was no family history of cardiovascular disease or sudden cardiac death, nor any recently prescribed medication. Laboratory tests revealed normal levels of cardiac enzymes (creatine kinase-MB, 2.05 ng/mL; troponin I, 4.24 pg/mL). A chest X-ray showed no abnormalities and an electrocardiogram (ECG) showed sinus bradycardia without ST-T changes (Figure 1). Chest computed tomography (CT) showed no pneumonia or other abnormalities. Transthoracic echocardiography for evaluating myopericarditis showed normal left ventricular (LV) systolic function (LVEF 57%) and normal LV wall thickness.
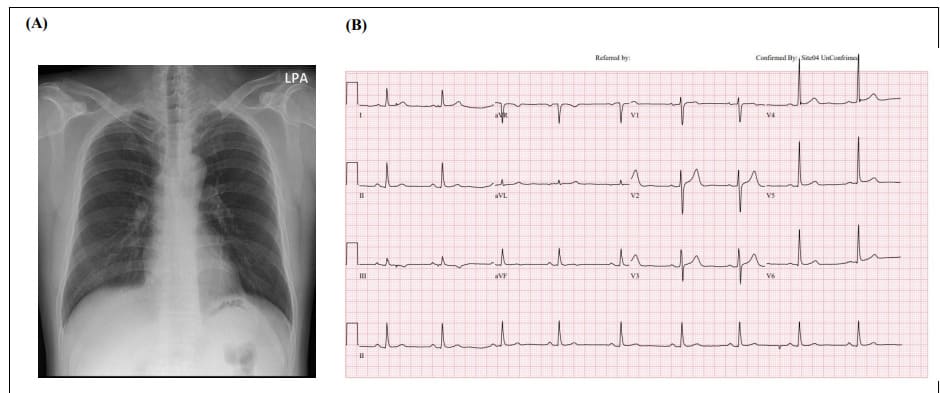
Figure 1: Chest X-ray and ECG and on first day of hospitalization. (A) Chest X-ray showed no cardiomegaly or active lung lesion. (B) ECG, electrocardiogram. ECG showed sinus bradycardia, normal axis, and 55 beats per minute without ST-T changes.
The patient underwent 24-h Holter monitoring for symptoms associated with chest discomfort and presyncope. On the second day of hospitalization, early-morning ECG showed sudden ST elevation for about 3 min followed by non-sustained ventricular tachycardia (NSVT) including torsade de pointes for ~5 s; his ST-T levels then recovered (Figure 2). Twenty minutes after the ECG changes, during recovery from the ST elevation, he reported further chest pain. Coronary CT was performed later that day and showed no significant stenosis in the coronary arteries (Figure 3A). Additionally, cardiac magnetic resonance imaging (MRI) was performed to investigate for myocarditis or pericarditis and showed no major abnormalities in cardiac structure, perfusion, or regional motion (Figure 3B, C). Thus, the patient was diagnosed with VSA and prescribed a calcium channel blocker (CCB) and nitrates as vasodilators. Symptoms improved following the prescription, and there has been no further report of chest pain from the patient since.
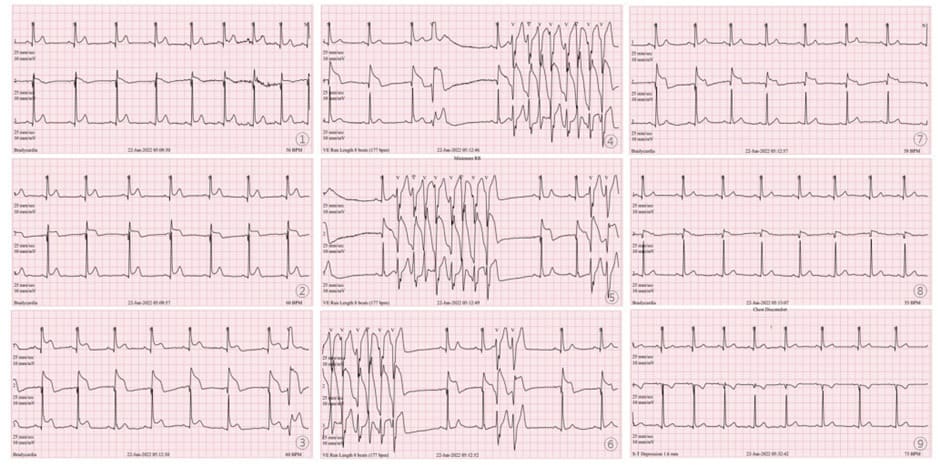
Figure 2: Early-morning 24-h Holter monitoring on second day of hospitalization. Serial ST Changes were observed. Sudden ST elevation for ~3 min followed by non-sustained ventricular tachycardia for about ~5 s; ST elevation and arrhythmia then recovered.
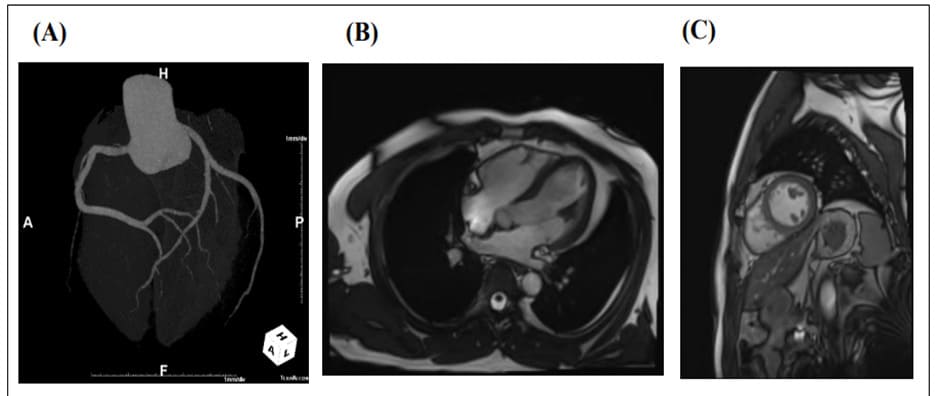
Figure 3: Coronary CT angiography on second day of hospitalization and cardiac MRI on June 29. (A) Coronary CT angiography showed no significant stenosis of the coronary arteries. (B) Four-chamber cine MRI image. (C) Short-axis cine MRI image. All showed no specific abnormalities.
CT: Computed tomography; MRI: magnetic resonance imaging.
Discussion
VSA could cause myocardial ischemia due to temporary vasoconstriction of the coronary vessels. It has several distinct characteristics from classical angina: first, chest pain is not necessarily induced by increased oxygen demand on exertional activity and can occur at rest; and second, sublingual administration of nitroglycerin can relieve chest pain (ST-T segment change observable on ECG) [4,5].
Diagnosis of VSA is complex and sophisticated: first, absence of significant stenosis in the coronary arteries is confirmed by coronary computed tomographic angiography or invasive coronary angiography; [6] then, in suspected vasospastic cases, ST-T change is confirmed by electrocardiography and subsequently relieved using sublingual nitrates with continuous ECG monitoring (such as Holter monitoring over 1-2 days) [7].
Several mechanisms are suggested for endothelial dysfunction in COVID-19. Endothelial cells may produce and respond to inflammatory cytokines such as IL-6, TNF, IL-2R, IL-1, and CXCL8, thereby mediating vascular permeability; [8,9] they may also, as non-professional antigen-presenting cells, participate in viral immunity by responding to complement or other immune components. [10,11] In addition, endothelial dysfunction could be induced by reactive oxygen species (ROS) produced in conditions of severe COVID-19, which results in a reduction in NO levels. Oxidative stress could interrupt the steady status of the immune state by dysregulating ROS and NO activity [9]
Several mechanisms are also suggested for VSA pathophysiology. Endothelial dysfunction could cause insufficient production of NO, which is needed for vasodilation; Acetyl choline or ergonovine could be used to provoke vasospasm. [12] Increased contractility of coronary smooth muscle cells could contribute to vasospasm: elevation of intracellular calcium ion levels plays a major role in phosphorylation and dephosphorylation of myosin light chain by myosin light chain kinase and myosin light chain phosphatase, thereby regulating vascular smooth muscle tone; CCBs could be used to interfere with the intracellular calcium ion flux. [13] Oxidative stress could also play an important role in VSA. Miyamoto and colleagues revealed that increased thioredoxin, a marker for oxidative stress, could induce vasospasm. [14] Various other factors may also cause vasospasm, such as low-grade inflammation, magnesium deficiency, genetic polymorphism, and enhanced activity of the autonomic nervous system [15-17].
In the present case, the patient was diagnosed with VSA 2 weeks after developing COVID-19. During hospitalization, 24-h Holter monitoring showed ST elevation followed by NSVT. After about 3 min, ST changes recovered to normal. Since there were no specific abnormalities on his ECG, chest CT, cardiac CT, or cardiac MRI, and ECG showed a definite change from ST elevation to recovery, we diagnosed him with VSA. He was prescribed CCBs and nitrates as vasodilators and did not complain of chest pain thereafter.
Reported cases of COVID-19-related VSA are rare, [18-20] and our report may be the first such case in Korea. As there is much evidence that COVID-19 is associated with endothelial dysfunction, a larger proportion of COVID-19-related VSA would be expected. Difficulty in capturing ST changes using ECG during vasospasms may explain this disparity.
Although there is a possibility of non-obstructive coronary lesions in patients with VSA after recovering from COVID-19, this could be related to significant clinical manifestation including acute coronary syndrome, severe dysrhythmia, and sudden cardiac death. [12,13] There was a case of cardiac arrest that occurred in a patient with VSA who had recovered from COVID-19.
While many cases of myocarditis following COVID-19 have been reported, there are relatively few of VSA. We should consider VSA as a differential diagnosis in patients complaining of chest pain after COVID-19, with appropriate medication prescribed as required.
Ethics Statement: This research was reviewed and approved by the Institutional Review Board of Hallym University Chuncheon Sacred Heart Hospital (IRB No. 2022-09-004). Informed consent was also obtained from the patient.
Author Contributions: Conceptualization: Park KT, Kim TS. Formal analysis: Park KT, Kim TS, Choi HH. Methodology: Park KT, Choi HH, Hong KS. Writing - original draft: Kim TS, Song IJ. Writing - review & editing: Park KT, Kim TS, Song IJ, Kim YJ, Park SB.
Disclosure: The authors have no potential conflicts of interest to disclose.
Acknowledgements: None.
References
- da Rosa MR, Francelino SJLC, Santos SFM, de Oliveira TF, Alcantara CR, et al. (2021) Clinical manifestations of COVID-19 in the general population: systematic review. Wien Klin Wochenschr; 133: 377-382.
- Louis DW, Saad M, Vijayakumar S, Ilyas S, Kokkirala A, Aronow HD (2022) The Cardiovascular Manifestations of COVID-19. Cardiol Clin; 40: 277-285.
- Bangalore S, Sharma A, Slotwiner A, Yatskar L, Harari R, et al. (2020) ST-Segment Elevation in Patients with Covid-19 - A Case Series. N Engl J Med; 382: 2478-2480.
- Prinzmetal M, Kennamer R, Merliss R, Wada T, Bor N (1959) Angina pectoris. I. A variant form of angina pectoris; preliminary report. Am J Med; 27: 375-88.
- Song JK (2018) Coronary Artery Vasospasm. Korean Circ J; 48: 767-777.
- Beltrame JF, Crea F, Kaski JC, Ogawa H, Ong P, et al. (2017) International standardization of diagnostic criteria for vasospastic angina. Eur Heart J; 38: 2565-2568.
- Group JCSJW (2014) Guidelines for diagnosis and treatment of patients with vasospastic angina (Coronary Spastic Angina) (JCS 2013). Circ J; 78: 2779-801.
- Opal SM, van der Poll T (2015) Endothelial barrier dysfunction in septic shock. J Intern Med; 277: 277-293.
- Otifi HM, Adiga BK (2022) Endothelial Dysfunction in Covid-19 Infection. Am J Med Sci; 363: 281-287.
- Pober JS, Merola J, Liu R, Manes TD (2017) Antigen Presentation by Vascular Cells. Front Immunol; 8: 1907.
- Pons S, Fodil S, Azoulay E, Zafrani L (2020) The vascular endothelium: the cornerstone of organ dysfunction in severe SARS-CoV-2 infection. Crit Care; 24: 353.
- Rehan R, Weaver J, Yong A (2022) Coronary Vasospastic Angina: A Review of the Pathogenesis, Diagnosis, and Management. Life (Basel); 12: 1124.
- Yasue H, Mizuno Y, Harada E (2019) Coronary artery spasm - Clinical features, pathogenesis and treatment. Proc Jpn Acad Ser B Phys Biol Sci; 95: 53-66.
- Miyamoto S, Kawano H, Sakamoto T, Soejima H, Kajiwara I, et al. (2004) Increased plasma levels of thioredoxin in patients with coronary spastic angina. Antioxid Redox Signal; 6: 75-80.
- Glueck CJ, Valdes A, Bowe D, Munsif S, Wang P (2013) The endothelial nitric oxide synthase T-786c mutation, a treatable etiology of Prinzmetal's angina. Transl Res; 162: 64-6.
- Miwa K, Igawa A, Miyagi Y, Nakagawa K, Inoue H (1998) Alterations of autonomic nervous activity preceding nocturnal variant angina: sympathetic augmentation with parasympathetic impairment. Am Heart J; 135: 762-71.
- Satake K, Lee JD, Shimizu H, Ueda T, Nakamura T (1996) Relation between severity of magnesium deficiency and frequency of anginal attacks in men with variant angina. J Am Coll Cardiol; 28: 897-902.
- Sumerkan MC, Er Kara A, Dogan GM, Alyan O (2021) Spontaneous, severe, and diffuse coronary vasospasm in a patient with COVID-19. Anatol J Cardiol; 25: E36-E37.
- Aikawa T, Ogino J, Oyama-Manabe N, Funayama N (2022) Vasospastic Angina: A Cause of Post-acute COVID-19 Syndrome. Intern Med; 61: 2693-2695.
- Wang M, Talon A, Saririan M (2021) COVID-19 cardiac arrest due to Prinzmetal's angina in a previously normal heart. Clin Case Rep; 9: e04205.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.