Vascular Ehlers-Danlos Syndrome Presenting as Fibromuscular Dysplasia: Four Molecularly Proven Misdiagnoses
by Salma Adham1,2, Michael Frank2, Clarisse Billon3, Francesca Pitocco4,5, Elie Mousseaux4,5, Xavier Jeunemaitre3,5*
1CH Lunel, Service de Médecine Polyvalente, F-34400 Lunel, France
2AP-HP, Hôpital Européen Georges Pompidou, Centre de Référence des Maladies Artérielles Rares, F-75015, Paris, France.
3AP-HP, Hôpital Européen Georges Pompidou, Service de Médecine Génomique des Maladies Rares, DMU BIOPHYGEN, F-75015, Paris, France
4AP-HP, Service de Radiologie Vasculaire, Hôpital Européen Georges-Pompidou, F-75015, Paris, France
5Université Paris Cité, INSERM, PARCC, F-75015, Paris, France
*Corresponding author: Xavier Jeunemaitre, AP-HP, Hôpital Européen Georges Pompidou, Service de, Médecine Génomique des Maladies Rares et DMU BIOPHYGEN, F-75015, Paris, France
Received Date: 18 January 2025
Accepted Date: 23 January 2026
Published Date: 26 January 2026
Citation: Adham S, Frank M, Billon C, Pitocco F, Mousseaux E, et al. (2026). Vascular Ehlers-Danlos Syndrome Presenting as Fibromuscular Dysplasia: Four Molecularly Proven Misdiagnoses. Ann Case Report. 11: 2512. https://doi.org/10.29011/2574-7754.102512
Abstract
Background: Arterial fibromuscular dysplasia (FMD) and vascular Ehlers-Danlos syndrome (vEDS) are non-atherosclerotic arteriopathies affecting medium-sized arteries. Because of overlapping imaging features, vEDS may be misdiagnosed as FMD, with major consequences for management and family screening.
Case Presentation: We report four patients initially diagnosed with FMD who were subsequently found to carry pathogenic COL3A1 variants consistent with vEDS, with a median diagnostic delay of 2.5 years. All patients presented with acute arterial events associated with multiple silent arterial lesions suggestive of FMD. Careful clinical reassessment identified minor features of vEDS and a suggestive family history in three cases. Molecular confirmation enabled cascade testing and identification of several affected relatives.
Conclusions: In patients with FMD-like arterial lesions, the presence of multiple dissections or aneurysms, subtle connective tissue physical signs, or a suggestive family history should prompt COL3A1 testing. Early recognition of vEDS is essential to avoid inappropriate interventions and to guide optimal management and family screening.
Clinical Message: Fibromuscular dysplasia–like arterial lesions may conceal vascular Ehlers-Danlos syndrome. When arterial imaging is associated with multiple events, easy bruising, or a suggestive family history, COL3A1 testing should be considered
Keywords: Fibromuscular Dysplasia; Vascular Ehlers-Danlos Syndrome; COL3A1; Arterial Dissection; Genetic Diagnosis.
Introduction
Arterial fibromuscular dysplasia (FMD) is a non-atherosclerotic, non-inflammatory disease of small- and medium-sized arteries, predominantly affecting middle-aged women. Typical beaded or focal lesions may lead to stenosis, aneurysm formation, or dissection, most commonly involving the renal and extracranial cervical arteries [1]. Management relies on antiplatelet therapy, blood pressure control, and revascularization in selected cases.
Vascular Ehlers-Danlos syndrome (vEDS) is a rare autosomal dominant connective tissue disorder caused by pathogenic variants in COL3A1, resulting in marked fragility of arteries and hollow organs [2]. Major complications usually occur in young adulthood and include arterial rupture or dissection, digestive perforation, and obstetrical complications [3]. In contrast to FMD, the clinical course of vEDS is often severe; invasive arterial procedures are generally avoided, and management is primarily preventive.
Distinguishing between FMD and vEDS is therefore crucial but may be challenging because of overlapping arterial imaging features and the under-recognition of vEDS. We report four patients initially diagnosed with FMD who were later found to have molecularly confirmed vEDS, highlighting key clinical and imaging clues that should prompt reconsideration of the diagnosis.
Case Presentation
First Case: A 57-year-old man was referred following rupture of a digestive artery aneurysm. He had previously been classified as having FMD based on a history of arterial hypertension and the presence of FMD-like lesions of the right renal artery associated with a cortical defect of the right kidney (Table 1). Updated imaging revealed multiple additional arterial abnormalities, including an aneurysm of the celiac trunk, ectasia of the hepatic and splenic arteries, ectasia of both iliac arteries, and dissection of the right external iliac artery. Both internal carotid arteries displayed a dysplastic appearance, with an associated aneurysm on the right side (Figure 1A). In addition to a history of spontaneous bruising, clinical examination revealed thin, translucent skin and a facial appearance suggestive of vEDS. Genetic testing, performed three years after the initial vascular work-up, identified a heterozygous nonsense variant in COL3A1 (p.Arg562Ter). Cascade genetic testing subsequently identified the same variant in one of his sisters (Figure 2A).
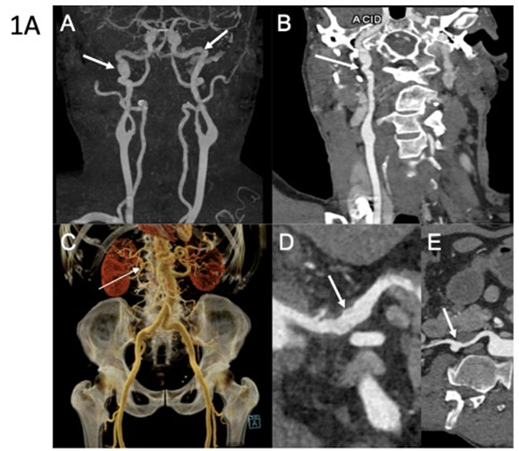
Figure 1A: Arterial imaging of patient 1 on computed tomography angiography (CTA). CTA in a 60-year-old man showing multiple vascular abnormalities. Coronal 3D maximum intensity projection (MIP) reconstruction (A) demonstrates a string-ofbeads appearance of the carotid arteries (arrow). Curved planar reformation (B) shows FMD-like lesions of the right carotid artery (arrow). Coronal volume-rendered image (C) reveals an aneurysm of the right renal artery (arrow). Curved planar reformations of the celiac trunk (D) and the right renal artery (E) show arterial irregularities (arrow) and aneurysmal dilation (arrow). Molecular diagnosis of vascular Ehlers-Danlos syndrome was established after imaging.
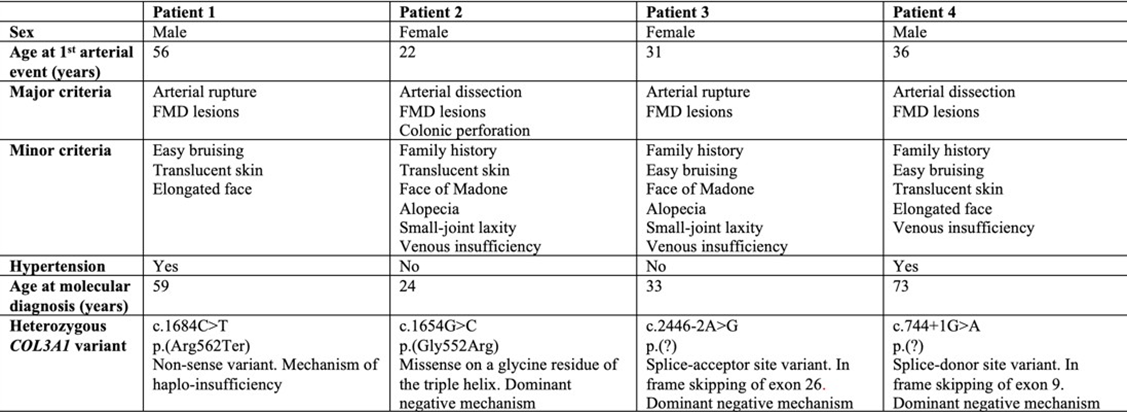
Table 1: Demographic, clinical and genetic characteristics of the patients.
Second Case: A 22-year-old woman was referred after an ischemic stroke secondary to left internal carotid artery dissection, initially attributed to cervical FMD (Table 1). Two months earlier, she had undergone emergency partial colectomy for spontaneous colonic perforation. Clinical examination revealed alopecia, facial features suggestive of vEDS, and venous insufficiency. Vascular imaging confirmed cervical arterial involvement, with a right internal carotid artery aneurysm and a right vertebral artery aneurysm, but also demonstrated additional irregularities of the right renal artery highly suggestive of FMD and all this in the absence of arterial hypertension (Figure 1B). Molecular testing identified a pathogenic COL3A1 variant (p.Gly552Arg), which was subsequently detected in her father and younger brother (Figure 2B).
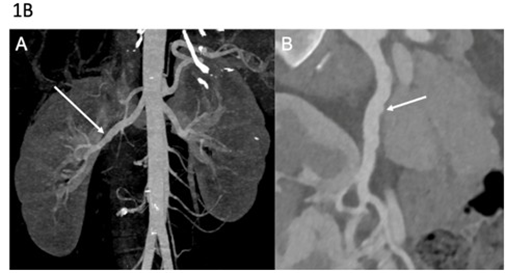
Figure 1B: Arterial imaging of patient 2 on computed tomography angiography (CTA). CTA in a 35-year-old woman showing vascular abnormalities. Coronal 3D MIP image (A) demonstrates irregularities of the right renal artery. Curved planar reformation (B) shows multiple stenoses and arterial irregularities of the right renal artery. Molecular diagnosis of vascular Ehlers-Danlos syndrome was established after imaging.
Third Case: A 31-year-old woman presented with splenic artery rupture complicated by hemorrhagic shock. Computed tomography angiography revealed multiple left renal infarctions associated with FMD-like renal artery stenosis, as well as numerous additional FMDlike arterial lesions (Table 1). These included irregularities of the cervical arteries, dissection and aneurysm of the right internal carotid artery, aneurysm of the right vertebral artery, aneurysmal dissections of the celiac trunk and hepatic artery, and irregular diameters of the iliac and external iliac arteries (Figure 1C). Clinical examination showed extensive spontaneous bruising, keloid scarring, alopecia, a suggestive facial appearance, small-joint hyperlaxity, and temporomandibular joint hypermobility. Molecular analysis identified a pathogenic splice-site variant in COL3A1. Family history revealed the premature death of her paternal grandmother due to arterial rupture at 27 years of age (Figure 2C).
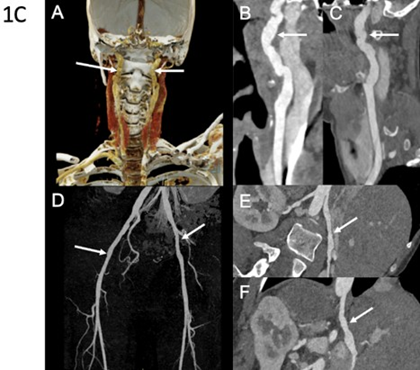
Figure 1C: Arterial imaging of patient 3 on computed tomography angiography (CTA). CTA in a 40-year-old woman with diffuse vascular abnormalities. Coronal color-coded volume-rendered image (A) and curved planar reformations (B and C) show bilateral typical FMD-like lesions of the carotid arteries (arrows). Coronal 3D MIP image (D) demonstrates subtle vascular irregularities of the external iliac arteries (arrows). Curved planar reformations (E and F) show, respectively, multiple stenoses of the right renal artery and the celiac trunk. Molecular diagnosis of vascular EhlersDanlos syndrome was established after imaging.
Fourth Case: A 60-year-old man was referred for suspected familial FMD. His medical history included arterial hypertension secondary to right renal artery dissection at the age of 36, initially diagnosed as FMD (Table 1). Seven years later, he experienced a right internal carotid artery dissection associated with a transient ischemic stroke. Subsequent CT imaging demonstrated additional arterial dissections involving the left renal artery, and the inferior mesenteric artery but ectasia and multiple irregularities and stenosis of the iliofemoral arteries were still suggestive of FMD (Figure 1D). Clinical examination revealed venous insufficiency, spontaneous bruising, thin translucent skin, keloid scarring, and facial features suggestive of vEDS. Molecular testing identified a pathogenic splice-site COL3A1 variant, which was subsequently detected in his daughter, who also exhibited minor signs of vEDS, as well as in several relatives previously diagnosed with FMD (Figure 2D).
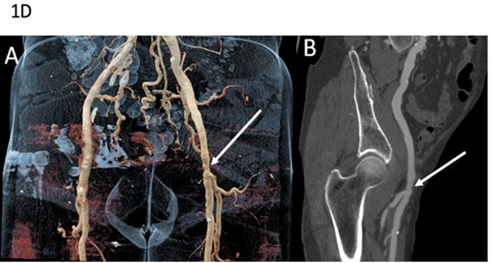
Figure 1D: Arterial imaging of patient 4 on computed tomography angiography (CTA). CTA in a 79-year-old man showing vascular abnormalities. Coronal volume-rendered image (A) depicts ectasia and multiple stenoses of the left external iliac artery (arrow). Curved planar reformation (B) shows alternating areas of relative stenosis and small aneurysms of the left external iliac artery (arrow). Molecular diagnosis of vascular Ehlers-Danlos syndrome was established after imaging.
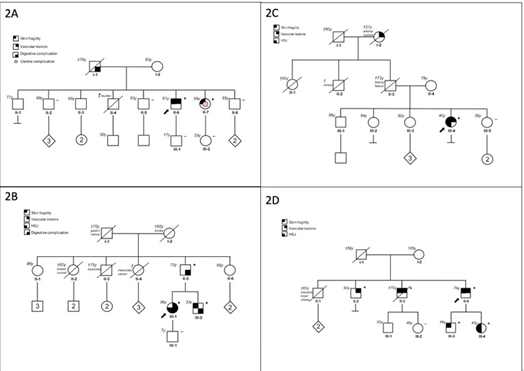
Figure 2A to 2D: Family pedigree of the patients
Discussion
These cases illustrate how vEDS may mimic FMD on arterial imaging, leading to delayed diagnosis and inappropriate management. Although FMD is typically identified in middleaged women presenting with renal artery lesions and hypertension or with cervical artery involvement and ischemic stroke [1], and vEDS is more often diagnosed earlier in life, frequently following severe arterial dissections or ruptures [4], age alone does not reliably distinguish between the two conditions, as illustrated by the four cases reported here. Correct identification of the underlying pathological entity in patients with medium-sized artery lesions is essential, as medical management strategies differ substantially. In particular, while percutaneous angioplasty may be beneficial for stenotic lesions in FMD, intra-arterial procedures and stent placement are generally contraindicated in vEDS because of marked arterial fragility and the associated risk of rupture [5].
The distinction between FMD and vEDS can most often be established through careful assessment of family history, clinical history and examination, and specific arterial imaging features. Although a family history has been reported in up to 10% of patients with FMD, its genetic basis is complex, with no Mendelian inheritance and involvement of multiple susceptibility genes [6]. In contrast, vEDS is an autosomal dominant disorder, and a family history of severe vascular complications is frequently present; however, approximately 50% of cases result from de novo variants [7]. In our series, three of the four patients had a suggestive family history. While physical examination is usually unremarkable in patients with FMD, individuals with vEDS often exhibit minor but characteristic features, including easy bruising, thin translucent skin, and suggestive facial appearance [8]. These signs were present to varying degrees in all four cases, underscoring the importance of thorough clinical examination and physician awareness of vEDS-associated features.
Although the classic “string-of-beads” appearance of the renal or cervical arteries is characteristic of FMD [9], arterial irregularities and pseudoaneurysms may be observed in both conditions. However, patients with vEDS typically exhibit involvement of four to five distinct arterial beds on average [10], particularly affecting visceral and iliofemoral territories, a distribution pattern encountered only in the most severe forms of FMD. Interestingly, a recurrent variant in COL5A1, responsible for classical EhlersDanlos syndrome, has been associated with arterial dissections and multifocal FMD [11].
In any clinically suggestive situation, COL3A1 sequencing should be performed, as it is highly sensitive, as illustrated by the four cases reported here. Three of the identified COL3A1 variants are predicted to exert a dominant-negative effect on the collagen III triple helix, whereas one variant (p.Arg562Ter) is a nonsense mutation leading to haploinsufficiency, which may account for the milder and later-onset phenotype observed in that patient. In this context, COL3A1 sequencing remains a cost-effective, highyield diagnostic test with direct therapeutic implications. Early diagnosis of vEDS is critical, as it allows avoidance of potentially harmful endovascular procedures, implementation of tailored medical management, and appropriate family screening.
Conclusion
In patients presenting with arterial lesions suggestive of fibromuscular dysplasia, the coexistence of multiple dissections or aneurysms, subtle connective tissue signs, or a family history of arterial rupture or sudden death should prompt consideration of vascular Ehlers-Danlos syndrome. In such situations, COL3A1 testing is essential to establish the correct diagnosis and guide management.
Acknowledgements : We are grateful to patients and their families for the invaluable contribution to our clinical research programs, which is also supported by grants from the vEDS France association and the Association DAVID.
Ethics Approval and Consent to Participate: This case report did not require approval from an institutional ethics committee in accordance with national regulations for non-interventional retrospective case reports.
Consent for Publication: Written informed consent was obtained from all patients (or their legal representatives) for publication of this case report and the accompanying images. Patient anonymity has been preserved, and no identifiable personal data are included.
Conflict of interest: none
References
- Gornik HL, Persu A, Adlam D, Aparicio LS, Azizi M, et al. (2019) First international consensus on the diagnosis and management of fibromuscular dysplasia. Vascular Medicine. 24: 164-189.
- Malfait F, Francomano C, Byers P, Belmont J, Berglund B, et al. (2017) The 2017 international classification of the Ehlers-Danlos syndromes. American Journal of Medical Genetics Part C: Seminars in Medical Genetics. 175: 8-26.
- Shalhub S, Byers PH, Hicks KL, Coleman DM, Davis FM, et al. (2020) A multi-institutional experience in vascular Ehlers-Danlos syndrome diagnosis. Journal of Vascular Surgery. 71: 149-157.
- Adham S, Legrand A, Bruno RM, Billon C, Dalens V, et al. (2022) Assessment of arterial damage in vascular Ehlers-Danlos syndrome: A retrospective multicentric cohort. Frontiers in Cardiovascular Medicine. 9: 953894.
- Buso G, Corvini F, Fusco EM, Messina M, Cherubini F, et al. (2024) Current evidence and future perspectives in the medical management of vascular Ehlers-Danlos syndrome: Focus on vascular prevention. Journal of Clinical Medicine. 13: 4255.
- Georges A, Bouatia-Naji N. (2022) The complex genetic basis of fibromuscular dysplasia, a systemic arteriopathy associated with multiple forms of cardiovascular disease. Clinical Science (London). 136: 1241-1255.
- Legrand A, Devriese M, Dupuis-Girod S, Simian C, Venisse A, et al. (2019) Frequency of de novo variants and parental mosaicism in vascular Ehlers-Danlos syndrome. Genetics in Medicine. 21: 15681575.
- Henneton P, Albuisson J, Adham S, Legrand A, Mazzella JM, et al. (2019) Accuracy of clinical diagnostic criteria for patients with vascular Ehlers-Danlos syndrome in a tertiary referral centre. Circulation: Genomic and Precision Medicine. 12: e001996.
- Savard S, Steichen O, Azarine A, Azizi M, Jeunemaitre X, et al. (2012) Association between two angiographic subtypes of renal artery fibromuscular dysplasia and clinical characteristics. Circulation. 126: 3062-3069.
- Jeunemaitre X, Mousseaux E, Frank M, Adham S, Pitocco F, et al. (2025) Efficacy of irbesartan in celiprolol-treated patients with vascular Ehlers-Danlos syndrome. Circulation. 151: 686-695.
- Richer J, Hill HL, Wang Y, Yang ML, Hunker KL, et al. (2020) A novel recurrent COL5A1 genetic variant is associated with a dysplasiaassociated arterial disease exhibiting dissections and fibromuscular dysplasia. Arteriosclerosis, Thrombosis, and Vascular Biology. 40: 2686-2699.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.