Vascular Ehlers-Danlos and Chronic Intestinal Failure Secondary to Short Bowel Syndrome: A Case Report and Literature Review
by Héctor Solar1*, Martín Alvarez Vidal2, Mariana Ortega1, Franco Badaloni2, Patricio Buquet2, Diego Ramisch2, Gabriel Gondolesi2
1Unidad de Soporte Nutricional, Rehabilitación y Trasplante de Intestino. Hospital Universitario Fundación Favaloro, Buenos Aires, Argentina
2Servicio de Cirugía General. Hospital Universitario Fundación Favaloro, Buenos Aires, Argentina
*Corresponding Author: Héctor Solar, Unidad de Soporte Nutricional, Rehabilitación y Trasplante de Intestino. Hospital Universitario Fundación Favaloro. Buenos Aires, Argentina
Received Date: 01 July 2025
Accepted Date: 08 July 2025
Published Date: 10 July 2025
Citation: Solar H, Vidal MA, Ortega M, Badaloni F , Buquet P, et al. (2025) Vascular Ehlers-Danlos and Chronic Intestinal Failure Secondary to Short Bowel Syndrome: A Case Report and Literature Review. J Surg 10: 11378 https://doi.org/10.29011/2575-9760.011378
Abstract
Introduction: Vascular Ehlers Danlos Syndrome (vEDS) is a rare subtype of Ehlers Danlos syndrome. It is associated with an autosomal dominant mutation in COL3A1, responsible for the synthesis of type III collagen. Clinical manifestations are associated with severe vascular and gastrointestinal complications.
Case presentation: We present a case of a 25 year old male with vEDS and Chronic Intestinal Failure (IF) due to Short Bowel Syndrome (SBS), referred to our center to undergo an intestinal reconstruction surgery, evolving with multiple medical and surgical complications which conditioned a long intensive care and hospital stay.
The multidisciplinary approach made it possible to successfully treat the coexistence of two rare diseases and apply innovative therapies for closure of the abdominal wall.
Results: After 14 months of follow up, the patient has improved his performance, nutritional status and his quality of life. Currently he receives complementary home parenteral nutrition, is fully reintegrated into his social, family and work life and continues his follow up to achieve intestinal sufficiency.
Conclusions: The coexistence of two rare diseases as vEDS and IF-SBS made more difficult the management of this patient. However, the nutritional, medical and surgical interventions were fundamental to achieve the best result.
Keywords: Abdominal Wall Reconstruction; Ehlers Danlos Syndrome; Intestinal Failure; Porcine Acellular Dermal Matrix; Short Bowel Syndrome; Surgical Abdominal Bleeding.
Introduction
Vascular type of Ehlers Danlos syndrome (vEDS), formerly known as EDS type IV, is a rare autosomal dominant disorder caused by structural defects in the pro1 chain type III collagen, encoded by the COL3A1 gene. The diagnosis, in most cases, is performed after catastrophic complications or at postmortem examination. Easy bruising, thin skin with visible veins (over the chest, abdomen and extremities), congenital clubfoot or hip dislocation, characteristic facial appearance (prominent eyes, thin pinched nose, small lips, hollow cheeks and lobeless ears) and rupture of arteries, uterus or intestine are the criteria for the clinical diagnosis. An abnormal synthesis of molecules of type III procollagen in culture of fibroblasts or the identification of a mutation in the gene for type III procollagen (COL3A1) are required to confirm vEDS [1]. The generalized vascular fragility is the most important clinical characteristic, causing excessive bruising, bleeding, severe varicosities and arterial rupture. Most admissions are due to emergency bleedings caused by arterial dissection or rupture involving mild and big size arteries at every possible location from the body. The most common sites of arterial bleeding are thorax or abdomen and are the most frequent causes of death [2]. According to the experience reported by Mayo Clinic, the prevalence of any Gastrointestinal (GI) symptom in vEDS was 47%, being heartburn, nausea, vomiting, retrosternal chest pain and dysphagia the most common upper GI symptoms. Irritable bowel syndrome symptoms, constipation and diarrhea were the most common lower GI symptoms [3].The most severe complications related to GI tract in vEDS are spontaneous intestinal perforation (82% of all the GI complications) mainly involving the colon, especially the sigmoid, followed by perforation of the final ileum and, much more rarely of the stomach, caused basically by gastric volvulus, with a background of gastric ligaments abnormalities or diaphragmatic hernias combined with the rich blood supply of the stomach and its low ability to extend. The mean age of onset is at 24 years old and a mortality rate of up to 12% [4]. This complication was reported in 8% of vEDS in a study published by Pepin et al [1]. Therefore, vEDS has the worst prognosis compared with other EDS types because of the potential risk of fatal vascular and intestinal complications. We aim to report a case of vEDS in a young patient with multiple post surgical complications that required a multidisciplinary approach in a specialized center with management of complex surgical-medical patients.
Case Presentation
A 25-year-old male patient with history of clubfoot, hypermobility of hand joints and frequent skin bruising was referred to our intestinal failure unit. As familiar history his mother had a hemorrhagic stroke at 28 years old. She died 15 days post partum due to probable consequences of EDS not being diagnosed. At 13 years old a spontaneous colon perforation was diagnosed and a colostomy was required. Genetic tests were performed and vEDS was diagnosed. Subsequently, the colostomy was closed and a protective ileostomy was performed. A month and a half later, the ileostomy was closed and intestinal continuity was reestablished without complications. At 24 years old, a laparotomy was performed in another center, due to intestinal obstruction requiring resection of 60 cm of the small intestine with primary anastomosis. A new laparotomy was performed 72 hours later due to small intestine perforation and a segmental enterectomy and a jejunostomy approximately at 1m from Treitz angle was performed. Patient was discharged with oral feeding, evolving with high stoma losses (>3000 ml/day). He had weight loss from 59kg to 43kg (27% in 3 months) associated with asthenia, adynamia, dehydration and electrolytes disorders. Home Parenteral Nutrition (HPN) was started and was referred to our center. Upon admission to our hospital, severe malnutrition and Intestinal Failure (IF) secondary to Short Bowel Syndrome (SBS) with anatomy type 1 were diagnosed. A low handgrip strength and low muscle mass measured in an abdominal CT scan at L3 level confirmed the diagnosis of sarcopenia. (Figure 1)
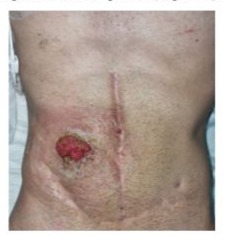
Shows the abdomen by the time of initial assessment. During the follow up the patient improved his medical and nutritional status gaining 14% of body weight, reducing stoma output (<700 ml/day) and improving handgrip strength. So, he was considered a candidate for reconstruction surgery.Seven months after his admission at our center, an autologous gastrointestinal reconstruction surgery was performed. The abdomen was approached through a midline incision, followed by adhesiolysis from the ligament of Treitz to the proximal stoma, and from the distal stoma to the ileocecal valve, as is standard practice in our center. The stoma limbs were transected using a linear stapler device, and the bowel was measured along its antimesenteric border: 58 cm of small bowel from the ligament of Treitz to the proximal transection site, and 15 cm from there to the ileocecal valve, were founded. A two-layer side-to-side anastomosis was performed, and the previous stoma site was resected. Post surgical intestinal length and type were 73 cm and type 3, respectively. Satisfactory hemostasis was achieved, requiring the administration of desmopressin. Upon completion of the procedure, the patient was transferred to the ICU.
The Patient Evolved with the Following Post Surgical Complications
Day 2: during weaning from Mechanical Ventilation (MV), a grade IV evisceration occurred following a coughing episode (Figure 2). A new surgical intervention was performed, revealing multiple bleeding points along the wound edges. An acceptable hemostasis was achieved through tranexamic acid and desmopressin. Due to poor tissue quality, the abdominal wall was closed using a Vicryl mesh. The patient returned to the ICU room, remaining on MV under sedation and analgesia.
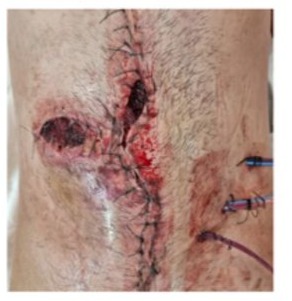
Day 4: the patient presented abdominal wall bleeding, tachycardia and hematocrit drop. Red blood cell transfusions were infused. He evolved with fever secondary to wound infection.
Day 5: As the patient had high nasogastric tube output secondary to ileus, Parenteral Nutrition (PN) was restarted and enteral nutrition was contraindicated. During his hospital stay nitrogen balances were performed every 10 days, showing a protein requirement as high as 3.2g/kg/day. An individualized parenteral nutrition formula was used, and requirements were modified daily according to laboratory work up and clinical condition.
Day 6: A new abdominal wall bleeding occurred. Red blood cell and platelet transfusions, desmopressin and tranexamic acid were indicated.
Day 8: A new abdominal exploration was required due to an expanding hematoma at the wound site (Figure 3).
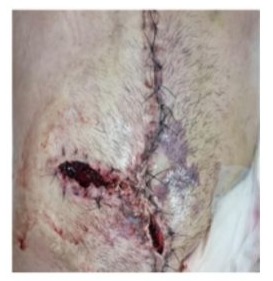
Upon opening the skin, multiple clots were found within the abdominal wall, along with a new tear in the tissue (Figure 4). The initial mesh was removed and replaced with a new one. Once again, meticulous hemostasis was attempted, which proved challenging despite the administration of desmopressin and tranexamic acid. The patient returned to the ICU room and remained on MV. He evolved with hypovolemic shock, requiring fluids, vasopressors and red blood cells and platelets transfusion.
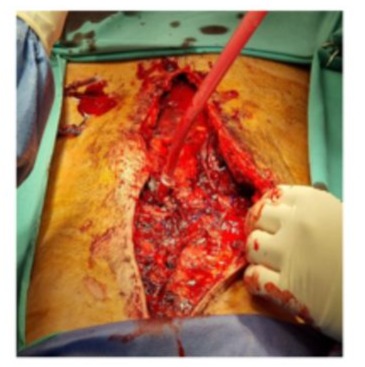
Day 11: A new subcutaneous hematoma developed, tearing through the skin sutures and leading to wound dehiscence. Due to prolonged ileus and abdominal distension an abdominal CT scan was performed. Venous thrombosis of the branch of segment VIII of the portal vein was diagnosed and the presence of free intra-abdominal fluid was reported. The patient was taken back to the operating room. A large number of clots were evacuated and the Vicryl mesh was removed to explore the abdominal cavity.
Packing was performed with vaseline gauze both intra-abdominally and in the subcutaneous tissue and the patient was returned to the ICU (Figure 5).
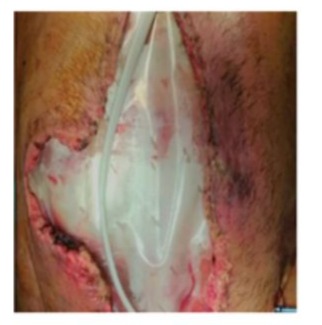
Day 13: The packing was removed in the operating room. An attempt was made to close the fascia with a Vicryl mesh, but this proved impossible due to poor tissue quality, so the mesh was left unfixed. A layer of vaseline gauze and a film dressing with non adherent pad were applied over it. In the following days, sugar was used to aid in wound healing. Dressing changes were performed at the bedside without further bleeding episodes (Figure 6). During this period the patient experienced progressive abdominal wall loss (tissue retraction) and continued on MV under sedation and analgesia. Prophylaxis with enoxaparin was started.
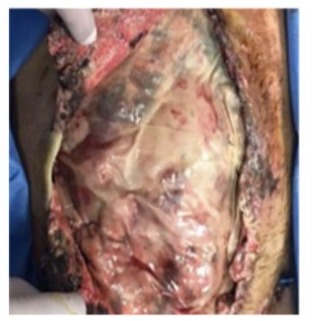
Day 21: A severe coughing during the weaning from MV produced again a grade IV evisceration (Figure 7).
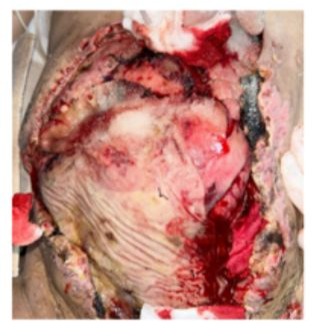
Necrosis of the abdominal wall left an open abdomen. A change in wound-management strategy was therefore undertaken. At the bedside the surgical site was superficially cleansed, vaseline-impregnated gauze was placed over the wound, and a continuous vacuumassisted suction system was applied (Figure 8- 9).
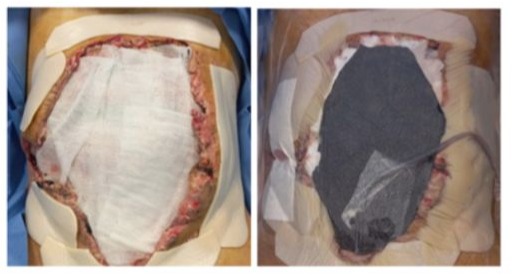
This negative-pressure dressing was changed every 5–7 days, resulting in marked improvement of the wound (Figure 10).
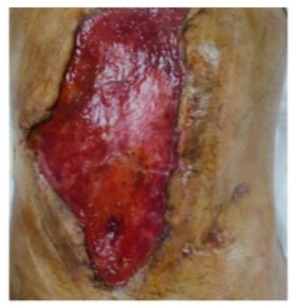
Day 27: a ventilator associated pneumonia was diagnosed, evolving with acute respiratory distress syndrome. Prolonged antibiotic treatment and MV were required. As a complication of MV a pneumothorax by barotrauma occurred and a chest tube was inserted.
Day 41: a percutaneous tracheostomy was performed.
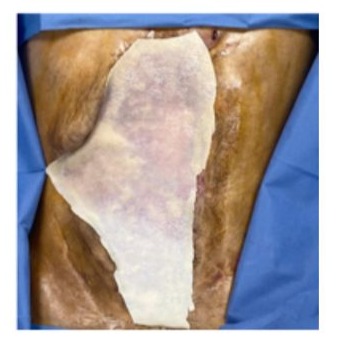
Day 67: the abdominal wall defect had decreased markedly in size and was covered by granulation scar tissue. The next challenge was to achieve epithelialization of this new tissue. Autologous skin grafts were ruled out because of the high risk of bleeding. In agreement with the Plastic-surgery service, a porcine acellular dermal matrix was selected and placed over the non-epithelialized areas (Figure 11). A negative-pressure dressing was applied over the matrix and removed after one week, after which the wound was irrigated daily with a saline–chlorhexidine solution.
Results
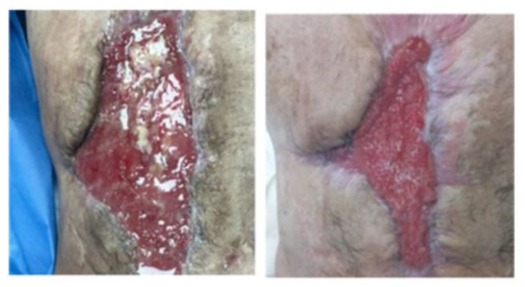
After 90 days on MV the patient was weaned off and decannulated. Swallowing test was performed ruling out risk of bronchoaspiration and oral diet was started. After 93 days in ICU, he was transferred to the general ward, and was discharged on postoperative day 113 with a severe critical illness polyneuropathy in motor kinesic rehabilitation (Figures 12-13). Due to early satiety and the impossibility to achieve his nutritional requirements he required PN 7 days a week.
Outpatient follow-up is ongoing for wound surveillance (Figure 14- 15).
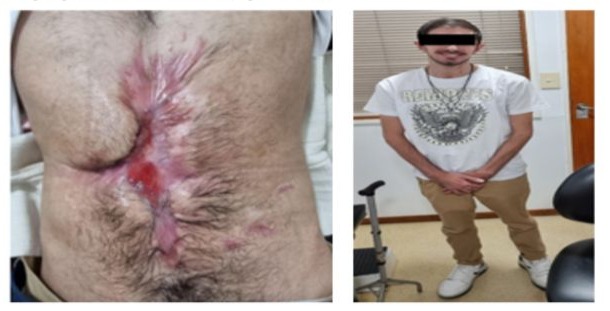
Currently the patient has improved his nutritional and performance status, has returned to his work activities, and is on HPN 3 days a week, restoring oral diet without complications.
Discussion
EDS was described in 1901 and 1908 by Dane Edward Ehlers and French Henri-Alexandre Danlos respectively as a condition in which joints’ hypermobility coexists with spontaneous hematomas and fragile skin [5, 6]. It is a heterogeneous group of connective tissue disorders caused by alterations in collagen synthesis due to mutations in the collagen coding genes, resulting in quantitative (reduced amounts of collagen type III) or qualitative abnormalities in collagen I,III and V (structurally abnormal type of collagen with altered electrophoretic mobility). Clinically is characterized by skin hyperextensibility and fragility, easy bruising, widened atrophic scarring, joint hypermobility and general fragility of the connective tissues with rupture of vascular and internal organs [7]. It also has been reported a large proportion of GI symptoms and abdominal vascular complications [3]. The Villefranche classification in 1998, recognized 6 clinical subtypes according to major and minor criteria: classical type (I,II), the hypermobility type (III), the vascular type (IV), The kyphoscoliotic type (VI), the arthrochalasia type (VIIa+b) and the dermatosparaxis type (VIIc) [8]. In 2017 EDS was reclassified in thirteen categories according to new identified causative genes [9-10]. The prevalence of EDS is estimated between 1:1000 and 1:25,000, of which the classic, hypermobility and vascular types are most common.vEDS prevalence is estimated at 10 per million inhabitants and the median life expectancy is around 50 years [4,11-13] . vEDS is the most severe subtype of EDS and has the worst prognosis because of the risk of potentially fatal vascular and intestinal complications [12]. According to data published by Pepin,
most deaths resulted from arterial dissection or rupture involving thoracic, abdominal vessels and central nervous system hemorrhage [1]. Bowel rupture and sepsis were the most frequent GI causes of death. We report the case of a patient with a rare disease as vEDS with multiple postsurgical complications. As a consequence of them, his medical-surgical and nutritional management became more complex because of the development of another rare disease as IF-SBS. In this setting, he required a comprehensive approach in a multi and interdisciplinary center with the expertise to manage both rare diseases and their complications.In our patient, bleeding was the earliest and more severe complication despite studies of haemostasis showing a normal bleeding time, prothrombin time, fibrinogen and platelet count. It was present since the first surgery and involved both the abdominal wall and the abdominal cavity. In EDS, bleeding is associated with fragility of the blood vessels and skin and is caused by defective collagen wickerwork of the reticular layer of the skin. Collagen is an integral part of the extracellular matrix and its role appears to be twofold: to maintain the structural integrity of the blood vessels and to serve as an adsorption platforme for Von Willebrand Factor (vWF) and platelets to initiate primary hemostasis (clot initiating factors) when the integrity of the vessels wall is disrupted. Collagen type I, type III and type IV are in abundance in the subendothelial matrix, however, the interaction with type III collagen is more clinically significant due to its higher content in the subendothelial matrix [14,15]. In vEDS, collagen type III is altered, increasing bleeding [10].
On the other hand, a study published in 2016, reported a high prevalence of over 50% for platelet aggregation disorders in vEDS patients, especially for collagen and epinephrine induce tests, whereas the plasmatic cascade did not show any alterations. Additionally, more than half of the tested subjects showed low vitamin D serum levels, which might affect vascular wall integrity [16].To control this complication in vEDS, the use of recombinant factor VIIa has been reported as well as the use of desmopressin that increases vWF release from the endothelial cells improving platelet-subendothelial adhesion and shortens the bleeding time, and tranexamic acid, a synthetic lysine-analogue antifibrinolytic that competitively inhibits the activation of plasminogen in plasmin inhibiting the dissolution and degradation of fibrin clots by plasmin [17,18]. However, it has been shown to increase thrombus formation in a dose-dependent mode in animal models, in contrast to aprotinin, which inhibits thrombus formation [19,20]. In our patient we used desmopressin and tranexamic acid but the bleeding could not be controlled with any of them and maybe the thrombosis of the branch of the portal vein was associated with the use of the latter. On the other hand, vEDS patients present poor wound healing due to the lack of collagen types resulting in a generalized increased fragility of the tissues.
Suture dehiscence, fistulas and incisional hernias were reported due to excessively poor wound healing [21]. Therefore, a mesh placement is considered indispensable for better postsurgical results [22]. In our patient a Vicryl mesh has been placed twice and due to repeated episodes of bleeding it had to be removed and it was impossible to use a third one because of the poor quality of the tissues and retraction of the muscular planes. The necrosis of the abdominal wall was multifactorial: bleeding, bruising, wound infection and shock. In this setting the result was an important defect of the abdominal wall. A conventional plastic surgery using flaps to close the wall was ruled out due to the high risk of bleeding. The Plastic-surgery service suggested the use of a porcine acellular mesh. These meshes are derived from animal tissues processed to remove cells and immunogenic components, preserving a biocompatible extracellular matrix. Once implanted, they serve as a scaffold that supports host cell infiltration (including fibroblasts, endothelial cells and macrophages), promoting angiogenesis, collagen deposition and tissue regeneration. The mesh gradually integrates into the host tissue with minimal inflammatory response and reduced risk of infection or rejection. Over time some meshes biodegrade, leaving behind functional autologous tissue [23].
In 1990 these meshes were used successfully to treat burn wounds and currently they have been widely used not only in plastic surgery but in maxillofacial, urogenital and oncology surgery [24]. In our patient the porcine acellular mesh was implanted on day 67 post surgical and, after six months, a complete close of the abdominal wall was achieved.
The other aspect to take into account in our patient’s evolution is that, after intestinal reconstruction surgery, he improved his anatomy, from type 1 to type 3, but remained with SBS and chronic IF.There are non-specific nutritional recommendations for vEDS patients. During his follow up, nutritional strategies were adapted to his clinical condition. The first step, at admission, was to improve his baseline status as prehabilitation for surgery, in order to reduce the risk of complications, prolonged hospitalization, readmissions and even mortality and enhance healing [25, 26]. With individualized HPN, modifications on the diet to avoid high ostomy output and promoting mobilization, the patient was able to improve his nutritional status. In the postoperative period, once hemodynamically stable, PN was restarted and enteral nutrition had to be avoided due to postoperative ileus and frequent surgeries. A large case control study found that EDS patients had a 12.26 higher odds ratio of gastroparesis and motility disorders [27]. Several guidelines recommend a protein intake for critical illness between 1.2 and 2g/kg/day, depending on being in the acute or chronic phase [28-30]. However, patients with open abdomen have a nitrogen loss estimated at 2 g to 4.6 g per liter of abdominal fluid output [31]. Accordingly, we decided to determine a nitrogen balance every ten days considering urine output, abdominal fluids and PN provision. Protein intake was adapted consequently, being his requirements as high as 3.2g/kg/day. Once the patient was discharged, a medical and nutritional monitoring was made every 15 days, reestablishing oral feeding and diminishing HPN requirements to 3 days per week. The patient also had kinesic motor rehabilitation. Currently he is autonomous, has recovered his usual weight and improved functional capacity and is in the process of achieving intestinal rehabilitation.
Conclusion
EDS is a rare and heterogeneous group of heritable disorders that results from defects in the synthesis of collagen.The clinical presentation depends on the gene involved. vEDS manifestations are among the most severe complications of this disease and involve arterial and venous anomalies and GI complications. The coexistence of IF-SBS makes the management of these patients more complicated.The multi and interdisciplinary approach is essential for the successful treatment of these complex patients. Surgical and medical management, nutritional strategies and the use of innovative wound treatment techniques such as porcine acellular dermal matrix, enabled the patient’s recovery and progressive rehabilitation. This case underscores the importance of early and sustained intervention in improving outcomes in complex surgical patients with connective tissue disorders.
Acknowledgments: To our patient and his family for the information and support
Ethical Considerations:The patient authorized the publication of his case and the images for medical purposes.
Conflict of Interest: None
References
- Pepin M, Schwarze U, Superti-Furga A, Byres PH (2000) Clinical and genetic features of Ehlers-Danlos syndrome type IV, the vascular type. N Engl J Med 342: 673-680.
- Paepe AD, Malfait F (2004) Bleeding and bruising in patients with Ehlers-Danlos syndrome and other collagen vascular disorders. British Journal of Haematology 127: 491-500.
- Nelson AD, Mouchli MA, Valentin N (2015) Ehlers-Danlos syndrome and gastrointestinal manifestations: a 20 year experience at Mayo Clinic. Neurogastroenterol Motil 27:1657-1666.
- Menni A, Tzikos G, Sarafis A (2023) Bowel Perforation in Vascular Ehlers-Sanlos syndrome: Case Report and comprehensive Review. J Pers Med 13:1247.
- Danlos HA (1908) Un cas de Cutis Laxa Avec Tumeurs Par Contusion Chronique des Coudes et des Genoux Xanthome Juninile PseudoDiabitique de Mn Hallopeau et Marc de Lipinay. Bull Soc Franc Der Syph 19: 70-72.
- Ehlers, E. Cutis Laxa, Neigung zu Harmorrhagien in der haut, Lockerung Mehrerer Artikulationen. Derm. Zschr. 1901;18:173-175.
- Callewaert B, Malfait F, Loeys B (2008) Ehlers-Danlos syndrome and Marfan syndrome. Best Pract Res Clin Rheumatol 22:165-189.
- Beighton P, De Paeipe A, Steinmann B (1998) Revised Nosology, Villefranche, 1997. Ehlers-Danlos National foundation (USA) and Ehlers-Danlos Suppor Group (UK). Am J Med Genet 77: 31-37.
- Malfait F, Francomano C, Byres P (2017) The 2017 international classification of the Ehlers-Danlos syndrome. Am J Med Gener C Semin Med Genet 175: 8-26.
- Jesudas R, Chaudhury A, Laukaitis CM (2019) An update on the new classification of Ehlers-Danlos syndrome and review of the causes of bleeding in this population. Haemophilia 25: 558-566.
- The Ehlers Danlos Society
- Paepe AD, Malfait F (2004) Bleeding and bruising in patients with Ehlers-Sanlos syndrome and other collagen vascular disorders. British Journal of Haematology 127: 491-500.
- Allaparthi S, Verma H, Burns DL (2013) Conservative management of small bowel perforation in Ehlers-Danlos syndrome type IV. World J Gastrointest Endosc 5: 398-401.
- Manon-Jensen T, Kjeld NG, Kasdal MA (2016) Colagen-mediated hemostasis. J Thromb Hamost 14: 438-448.
- Springer TA (2014) von Willebrand factor, Jedi knight of the bloodstream. Blood 124:1412-1425.
- Albert B, Hoffjan S, Frauke B (2016) Vascular type Ehlers-Danlos syndrome is associated with platelet dysfunction and low vitamin D serum concentration. Orphanet Journal of Rare Diseases 11:111.
- Faber P, Craig WL, Duncan JL (2007) The successful use of recombinant factor VIIa in a patient with vascular-type Ehlers-Danlos syndrome. Anaesthesiol Scand 51:1277-1279.
- Mast KJ, Nunes ME, Ruymann FB (2009) Desmopressin responsiveness in children with Ehlers-Danlos syndrome associated bleeding symptoms. Br J Haematol 144: 230-233.
- William ng, Jeradt A, Wasowicz M (2015) Tranexamic acid: A clinical Review. Anasthesiology Intensive Therapy 47: 339-50.
- Lindsay H, Lee-Kim YJ, Srivatghs LV (2016) Perioperative hemostatic management in Ehlers-Danlos Syndrome: a report of 2 cases and literature review. J Pediatr Hematol Oncol 38:158-160.
- Castori M, Camerota F, Celletti C (2010) Natural History and Manifestations of the Hypermobility Type Ehlers-Danlos syndrome: A Pilot study on 21 Patients. Am J Med Genet 152: 556-564.
- Girotto JA, Malaisrie SC, Bulkely G (2000) Recurrent ventral herniation in Ehlers-Danlos syndrome. Plast Reconstr surg 106:1520-1526.
- Chen Y, Liu X, Zheng X (2022) Advances on the modification and biomedical applications of acellular dermal matrices.Journal of Leather Science and Engineering 4:19.
- Compton CC, Hickerson W, Nadire K (1993) Acceleration of skin regeneration from cultured epithelial autografts by transplantation to homograft dermis. J Burn Care Rehabil 14: 653-662.
- Weimann A, Braga M, Carli F, Higashiguchi T, Hübner M, et al (2021) ESPEN practical guideline: Clinical nutrition in surgery. Clin Nutr 40: 4745-4761.
- Gillis C, Wischmeyer PE (2019) Pre-operative nutrition and the elective surgical patient: why, how and what? Anaesthesia 74:27-35.
- Shah N, Wall E (2024) Nutritional considerations for hypermobile Ehlers-Danlos syndrome. Practical Gastroenterology 38-50.
- Compher C, Bingham AL, McCall M, Patel J, Rice TW, et al (2022) Guidelines for the provision of nutrition support therapy in the adult critically ill patient: The American Society for Parenteral and Enteral Nutrition. JPEN J Parenter Enteral Nutr 46:12-14.
- Elke G, Hartl WH, Kreymann KG, Adolph M, Felbinger TW, et al (2019) Clinical Nutrition in Critical Care Medicine - Guideline of the German Society for Nutritional Medicine (DGEM). Clin Nutr ESPEN 33: 220275.
- Singer P, Blaser AR, Berger MM, Calder PC, Casaer M, et al (2023) ESPEN practical and partially revised guideline: Clinical nutrition in the intensive care unit. Clin Nutr 42:1671-1689.
- Chabot E, Nirula R (2017) Open abdomen critical care management principles: resuscitation, fluid balance, nutrition, and ventilator management. Trauma Surg Acute Care Open 2: e000063.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.