Variable Expressivity and Incomplete Penetrance in Monogenic Diabetes
by Shiga Rappai Chirayath1*, Idris Mohammed1, Doaa Mohamed Taha2, Aljazi Al-Maraghi3, Khalid Fakhro3,4, Khalid Hussain1
1Division of Endocrinology and Diabetes, Sidra Medicine Doha, Qatar
2Department of General Pediatrics, Sidra Medicine Doha, Qatar
3Department of Human Genetics, Sidra Medicine Doha, Qatar
4Department of Genetic medicine, Weill Cornell Medicine, Doha, Qatar
*Corresponding author’s: Shiga Rappai Chirayath, Department of Endocrinology and Diabetes, Sidra Medicine, Member of Qatar Foundation, PO Box 26999, Doha- Qatar
Received Date: 03 December 2025
Accepted Date: 09 December 2025
Published Date: 11 December 2025
Citation: Chirayath SR, Mohammed I, Taha DM, Al-Maraghi A, Fakhro K, et al. (2025). Variable Expressivity and Incomplete Penetrance in Monogenic Diabetes. Ann Case Report. 10: 2472. https://doi.org/10.29011/2574-7754.102472
Abstract
We present a case of monogenic diabetes which demonstrates marked incomplete penetrance and variable expressivity. A 3-monthold child presented with transient neonatal diabetes mellitus and was found to carry a heterozygous variant in the ABCC8 gene (variant identified - c.4136G>T,p.Arg1379Leu). Her mother was also found to have the same ABCC8 heterozygous variant but has always been euglycemic with no hypoglycaemia or hyperglycaemia. Hence two individuals with the same genotype expressed a highly variable phenotype ranging from transient neonatal diabetes mellitus to normoglycemia.
Keywords: Monogenic diabetes; Incomplete penetrance; ABCC8 gene mutation; Variable expressivity
Introduction
Monogenic Diabetes (MD) accounts for about 1-5% of all diabetes cases with different subtypes such as neonatal diabetes (NDM), maturity onset diabetes of young (MODY) and syndromic forms such as Wolfram syndrome and Wolcott-Rallison syndrome [1]. Mendelian diseases, though individually rare, collectively represent a significant portion of the global population, affecting more than 5%. It encompasses more than 6000 rare phenotypes including MD. The phenomenon unique to Mendelian diseases is incomplete penetrance with variable expressivity [1]).
Penetrance refers to the proportion of individuals who carry a specific genotype and exhibit the expected clinical phenotype associated with that genotype. In other words, it is the likelihood or probability that a person with a particular genetic variant will express the associated trait or disease. If everyone with a particular genotype presents with clinical symptoms by a particular age, then it is said to be fully penetrant, whereas if it falls below this, it is said to be reduced or incomplete penetrance. The severity of the phenotype caused by a particular genotype can vary among affected individuals (wide variation in terms of severity and age of onset).
This phenomenon is defined as variable expressivity displayed by that particular genotype. A said genotype can vary both in the severity of presentation and in level of penetrance across the population. This variable expressivity and penetrance are different from pleiotropy. Pleiotropy implies that different variants of a particular gene cause multiple phenotypes [2].
Case presentation
3-month-old female infant, second child of non-consanguineous parentage, full term birth by normal vaginal delivery (birth weight 3kg) presented in severe diabetic ketoacidosis (DKA). She presented to the emergency room with a Glasgow coma scale of 3, blood glucose was 31mmol/L, beta hydroxy butyrate 5.2mmol/L, venous pH 6.9, bicarbonate 4.2mmol/L and glycated hemoglobin (HbA1c)12.5%. She was critically ill and required intubation and mechanical ventilation. The working diagnosis was neonatal diabetes considering the age of onset, presenting in severe DKA. The DKA was managed as per the ISPAD (International Society for pediatric and Adolescent Diabetes) protocol. DKA resolved within 8 hours and she was extubated in around 24 hours. The child was initially initiated on insulin Detemir 1 unit once a day and bolus insulin Lispro 0.5 units pre feeds if blood glucose was >14mmol/L. She was discharged on detemir 2units twice a day and bolus insulin pre feeds as correction bolus with target blood sugar 14mmol/L.
Diagnostic Assessment
Chromosome position | Gene | Inheritance | Variant identified | Effect | Poly-Phen-2 prediction score | CADD score | GERP score | pLI score |
11:17417461 | ABBC8 | Dominant/ heterozygous | c.4136G>T,p. Arg1379Leu | Missense | 1 | 51 | 5.06 | 1.89E- 14 |
CADD: Combined Annotation Dependent Deletion; GERP: Genomic Evolutionary Rate Profiling; pLI: probability of being loss of function intolerant. | ||||||||
Table 1: Variant identified by WGS with clinical relevance to the patient phenotype.
Using whole genome sequencing (WGS) the patient and the mother (aged 28years) were found to carry a heterozygous variant in ABCC8 gene (variant c.4136G>T, Arg1379Leu) on exon 11. In silico analysis based on PolyPhen-2, CADD, GERP and pLI, predicted that this mutation was likely to be pathogenic (Table 1 summarises the variant details detected by WGS). The father and healthy sibling were negative for this finding. Meng Li et al demonstrated the same variant in a proband after the proband was diagnosed early onset type 2 diabetes mellitus (T2DM) at the age of 16 years. Functional studies revealed that the variant was located on the nucleotide binding domain 2 of the ABCC8 gene. The hyperglycaemia was the possible result of the variant impacting the ATPase activity [3]).The variant Arg1379Leu has been reported before with the phenotype of transient neonatal diabetes in an infant who was 42 days of age at the time of presentation with DKA . The mother was negative for the variant and the father was unavailable for testing [4].
Treatment and follow-up
The HbA1c after 1.5 months of the initial presentation was 6.2% and the child was off insulin therapy due to frequent hypoglycemic episodes. Dexcom G6 continuous glucose sensor showed time in range (TIR%) as 91%. She was developmentally appropriate for age. Following the whole genome sequencing of the family members, the mother was evaluated with fasting blood glucose and HbA1c and was found to be normoglycemic. Currently our patient is 17 months old, with normal developmental milestones for age and is off insulin from 5 months of age with an HbA1c of 5.5%.
Discussion
The incomplete penetrance and variable expressivity are observed in some cases of MD. This is due to a wide spectrum of factors which can be broadly classified into age, sex, ethnicity, lifestyle, environmental factors (like intrauterine hyperglycemia) and genetic factors (especially genetic modifiers) [1,2]. Figure 1 shows the factors influencing incomplete penetrance and variable expressivity in MD families. Meihang Li et al has shown that less than half of the MD cases have an affected parent which reflects the highly reduced penetrance pattern in MD [1,5]. Incomplete penetrance is a well-documented phenomenon in cases with ABCC8 mutation. P.Klee et al described a novel variant His863Tyr in ABCC8 in a three generation family which led to NDM in a child, asymptomatic T2DM in father and impaired fasting glucose in a the grandmother [6]. Gonsorcikova et al described a family with 6 members having a novel V84I mutation in ABCC8. The proband was a 12 yr old boy with fasting hyperglycemia. Subsequently the twin brother, sister, mother, maternal aunt and grandfather were detected to have fasting hyperglycaemia [7]. Lenfant et al described a case of a 6-year-old girl with juvenile onset diabetes and congenital cataract who was found to have a heterozygous mutation in ABCC8 and homozygous mutation in CRYBB1. Both the healthy mother and sibling were found to be heterozygous for the same ABCC8 mutation suggesting incomplete penetrance [8]. Our patient and her healthy mother have been shown to have the same genotype but variable penetrance which led to a highly variable phenotype which was transient neonatal diabetes and normoglycemia respectively.
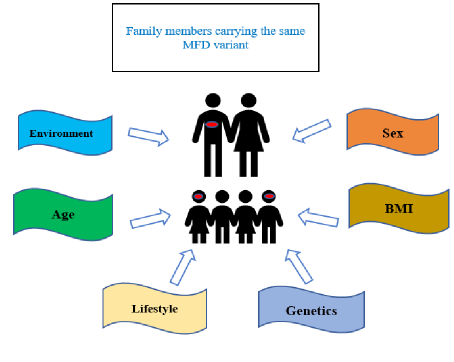
Figure 1: Factors influencing incomplete penetrance and variable expressivity in MD families.
Learning points:
- Heterozygous variants in the ABCC8 gene can present with marked variable penetrance and expressivity for MD (ranging from transient NDM to normoglycemia).
- Genetic testing for NDM is mandatory in patients presenting with diabetes before 6 months.
- The molecular basis of this marked variable penetrance and expressivity for ABCC8 gene mutations needs further study.
Contributors
All authors made individual contributions to authorship.SC and KH was involved in the patient management, manuscript preparation and submission. IM was involved in the genetic studies and literature review. DM was involved in literature review. AA, KF was involved in the genetic studies. KH is the senior author who was involved in the overall supervision in patient care and manuscript finalization.
Funding
No funding was received for this research.
Disclosures
None
Informed patient consent for publication
Signed informed consent obtained directly from the patient’s relatives or guardians.
Data availability statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- Li M, Popovic N, Wang Y, Chen C, Polychronakos C. (2023) ‘Incomplete penetrance and variable expressivity in monogenic diabetes; a challenge but also an opportunity’, Reviews in Endocrine and Metabolic Disorders, 24: 673-684.
- Kingdom R, Wright CF. (2022) ‘Incomplete penetrance and variable expressivity: From clinical studies to population cohorts’, Frontiers in Genetics, 13.
- Li M, Gong S, Han X, Zhang S, Ren Q, et al. (2021) ‘Genetic variants of abcc8 and phenotypic features in Chinese early onset diabetes’, Journal of Diabetes, 13: 542-553.
- Vaxillaire M, Dechaume A, Busiah K, Cavé H, Pereira S, et al. (2007) ‘New abcc8 mutations in relapsing neonatal diabetes and clinical features’, Diabetes, 56(6), pp. 1737–1741.
- Li M, Wang S, Xu K, Chen Y, Fu Q, et al. (2019) ‘High prevalence of a monogenic cause in Han Chinese diagnosed with type 1 diabetes, partly driven by nonsyndromic recessive wfs1 mutations’, Diabetes, 69(1), pp. 121-126.
- Klee P, Bellanné-Chantelot C, Depret G, JP Llano, C Paget, et al. (2012) ‘A novel ABCC8 mutation illustrates the variability of the diabetes phenotypes associated with a single mutation’, Diabetes; Metabolism, 38: 179-182.
- Gonsorcikova L, Vaxillaire M, Pruhova S, Dechaume A, Dusatkova P, et al. (2011) ‘Familial mild hyperglycemia associated with a novel ABCC8-V84I mutation within three generations’, Pediatric Diabetes, 12: 266-269.
- Lenfant C, Baz P, DegavreA, Philippi A, Senee V, et al. (2017) ‘Juvenileonset diabetes and congenital cataract: “double-gene” mutations mimicking a syndromic diabetes presentation’, Genes, 8: 309.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.