Validation of Augmented Reality-Guided Implant Positioning in Cadaveric Models: A Step Toward Clinical Application
by Javier Orozco-Martínez1*, Tanya Fernández-Fernández1, Elena Aguilera-Jiménez2,3, Amaia Iribar-Zabala4,6, Carla de Gregorio-Bermejo2, Alicia Pose-Díez-de-la-Lastra2,4, Javier Pascau2,4, Mónica García-Sevilla2,4, Lydia Mediavilla Santos1,2,3,5, José Calvo-Haro1,2,3,5, Rubén Pérez Mañanes1,2,3,5
1Servicio de Cirugía Ortopédica y Traumatología, Hospital General Universitario Gregorio Marañón, Madrid, Spain
2Instituto de Investigación Sanitaria Gregorio Marañón, Madrid, Spain
3Advanced Planning and 3D Manufacturing Unit, Hospital General Universitario Gregorio Marañón, Madrid, Spain
4Departamento de Bioingeniería, Universidad Carlos III de Madrid, Leganés, Spain
5Department of Surgery, Universidad Complutense de Madrid, Madrid, Spain
6Digital Health and Biomedical Technologies, Vicomtech Foundation, Spain
*Corresponding author: Javier Orozco Martínez, Orthopaedic Surgery and Traumatology, University Hospital Gregorio Marañón, Madrid, Spain.
Received Date: 17 May, 2025
Accepted Date: 29 May, 2025
Published Date: 31 May, 2025
Citation: Martínez JO, Fernández TF, Jiménez EA, Zabala AI, Bermejo CG, et al. (2025) Validation of Augmented Reality-Guided Implant Positioning in Cadaveric Models: A Step Toward Clinical Application. J Orthop Res Ther 10: 1385. DOi: https://doi.org/10.29011/2575-8241.001385
Abstract
Introduction: Accurate placement of patient-specific implants is essential in orthopaedic oncology, particularly in complex pelvic resections. Augmented reality (AR)-assisted navigation has shown promise in experimental settings, yet its validation in anatomically realistic environments remains limited. This study assesses, for the first time, the precision of a novel AR-based surgical guidance system in human cadaveric specimens for the placement of custom acetabular implants. Materials and Methods: Ten cadaveric hemipelves were used. Preoperative planning was performed using CT imaging and 3D segmentation to design patient-specific implants. A Microsoft HoloLens 2 headset was employed alongside a custom-developed AR application, enabling holographic visualisation of the planned implant over the surgical field. Intraoperative guidance was provided via AR markers and a real-time colour-coded feedback system. Postoperative CT scans were acquired and compared to preoperative plans through 3D registration to quantify positioning accuracy. Results: The mean translational error was 3.79 mm (SD ±2.09 mm) and the mean angular error was 3.73° (SD ±2.59°), both within clinically acceptable thresholds. Compared to previous phantom-based experiments, these errors were significantly greater (p < 0.05), highlighting the influence of anatomical complexity in cadaveric models. The mean implant placement time was 121.5 seconds (95% CI: 59.8–183.2 s). The global surface distance model showed consistent implant-to-bone adaptation, with maximum deviations localised to non-critical areas. Conclusions: This AR-based navigation system proved accurate and feasible for the placement of patient-specific implants in cadaveric specimens. The integrated real-time visual feedback facilitated intraoperative alignment without requiring physical guides or screen-based navigation. These findings support the clinical potential of this technology and warrant further studies in live surgical settings.
Keywords: Augmented Reality; Surgical Navigation; Patient-Specific Implant; Cadaveric Study; Real-Time Guidance; Orthopaedic Oncology
Introduction
Accurate placement of patient-specific implants is a critical objective for ensuring long-term success in orthopaedic surgery, particularly in complex scenarios such as pelvic tumour resections. Proper implant positioning directly impacts the stability, functionality, and longevity of the prosthesis. Traditionally, conventional methods such as manual placement or static navigation have shown limitations in both accuracy and intraoperative adaptability.
In this context, augmented reality (AR) has emerged as a disruptive technology by enabling real-time visualisation of anatomical models superimposed onto the surgical field. This innovation enhances surgical precision by improving implant alignment and optimising its positioning relative to surrounding bone structures [1,2]. AR refers to a technology that overlays computer-generated virtual content—such as high-definition holograms onto the existing physical environment [3], enhancing the user's perception of reality [4,5]. The use of surgical navigation systems based on optical tracking systems (OTS) and 3D patient-specific instruments (PSIs) [6,7] has been shown to substantially reduce errors in PSI placement compared to manual positioning [7-9].
However, while AR-assisted guidance for PSIs, particularly in cutting guides, has been extensively studied, its potential for prosthesis implantation remains an emerging field of research [8]. Previous studies in the field of maxillofacial surgery have explored the use of guidance systems for dental implant positioning, reporting positive outcomes [10-12]. To date, the use of AR for guiding implant placement remains an emerging field, particularly in cadaveric settings, where published evidence is extremely limited.
Approximately 15% to 20% of all primary bone tumors are located in the pelvis, with the incidence of hemipelvectomy estimated at 1 per 1 million cases annually [13]. The complex anatomy of the hip and pelvis poses significant challenges for reconstruction, even for experienced surgeons [14,15]. Type II pelvic resections, as classified by Enneking and Dunham [16], involve the periacetabular region, further increasing the complexity of prosthesis implantation.
In periacetabular reconstructions, achieving precision in the intraoperative placement and orientation of custom-made prostheses remains a significant challenge due to its critical impact on the stability and functional outcomes of the reconstruction [6]. In 1978, Lewinnek et al. reported in their series of 300 hip prostheses that the dislocation rate was 1.5% for cup orientation within a "safe zone" of 15°±10° of anteversion and 40°±10° of inclination, whereas dislocation rates increased to 6.1% outside this range [17].
Subsequent studies have continued to analyse this "safe zone," consistently confirming an inclination of approximately 40°±10°, though anteversion values show greater variation among authors, ranging from 15° (Lewinnek et al.) to 30° (McCollum et al.18), and 40º 19, with a tolerance of ±10°. While acetabular orientation is not the sole factor influencing hip instability [19], ensuring proper implantation within this "safe zone" remains critically important. Another critical factor to consider is the centre of rotation of the femoral head, which can be accurately replicated through proper 3D planning and implant guidance. According to widely accepted standards for osteotomies, we define optimal PSI placement accuracy as an angular deviation of less than 3° and a mean distance error below 2 mm [20-23].
While previous experimental phases of our study validated the feasibility and accuracy of an augmented reality (AR)-based guidance system in bio models (phantoms), In a previous study, we developed and validated an augmented reality (AR)-based surgical navigation system that enables real-time visualisation of the planned implant position using the HoloLens 2 headset [24].
This system was initially validated using 3D-printed biomimetic hemipelvis models, achieving a mean angular error of 1.70° and a mean translational error of 1.75 mm. AR marker-associated errors included a mean translational deviation of 1.07 mm and a rotational error of 0.86°. However, the transition from simulation to clinical reality requires more stringent validation and testing in cadaveric models is a critical step before clinical implementation.
In this study, we present the results of a new phase in which the same navigation system was applied with the aim of assessing whether the accuracy observed in synthetic models is maintained in a more realistic setting, incorporating biological tissues and anatomical variability-ultimately aiming to enhance surgical precision and workflow.
Materials and Methods
Ten cadaveric hemipelves were used, each undergoing preoperative planning based on computed tomography (CT) imaging. Segmented 3D models of the pelvis and the patient-specific implant were generated using the 3D Slicer software. Each hemipelvis was labelled with Roman numerals (I to X) using metal clips to ensure accurate traceability. The implants were designed following the same protocol established in our previous study.
A custom acetabular prosthesis was created, maintaining the patient’s original centre of rotation. The implant included three screw fixation points targeting the ilium, ischium, and pubis. To enable augmented reality (AR)-based navigation, a dedicated socket was integrated into the prosthesis for the placement of an AR marker.
Surgical planning was performed based on the intended osteotomy planes, which served as references for implant design. All components were 3D printed: the healthy bone segments were fabricated from acrylonitrile styrene acrylate (ASA), a durable thermoplastic used to replicate cortical bone; the AR markers were printed in black and white polylactic acid (PLA); and the implant itself was printed in a rigid, radiopaque 10k resin. The choice of materials was strategically made to optimise segmentation in postoperative CT scans, thereby enabling accurate quantitative analysis of implant positioning.
A dedicated augmented reality navigation application was developed for the HoloLens 2 headset (Microsoft Corporation, WA, USA) to guide the surgeon throughout the prosthesis placement procedure. The user interface allowed toggling the visibility of the virtual prosthesis in relation to the AR marker placed in the supraacetabular region and adjusting the object's transparency. The planned prosthesis was displayed as a semi-transparent white hologram to facilitate visual alignment.
A second marker, attached directly to the physical prosthesis, enabled real-time tracking of its position. During manipulation, the system provided continuous visual feedback on positioning accuracy using a colour-coded system: red indicated a deviation greater than ±7.5 mm, orange represented an error up to ±7.5 mm, and green signified alignment within ±2.5 mm of the planned position (Figure 1).
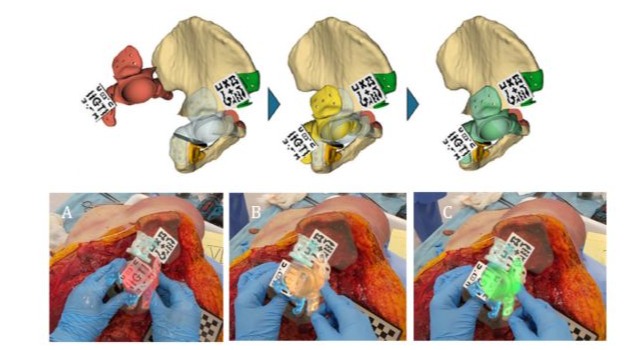
Figure 1: Prosthesis positioning system utilising a colour-coded feedback mechanism. As the implant approaches the planned alignment, the colour of the marker transitions from red to yellow and finally to green, indicating that an acceptable position has been achieved. (A) Red signifies a positioning error greater than 7.5 mm and is classified as incorrect. (B) Yellow indicates alignment within the osteotomies but with a deviation of up to 7.5 mm from the planned target—acceptable but suboptimal. (C) Green corresponds to optimal alignment with an error of less than 2.5 mm. Images were captured through the AR head-mounted display (HMD).
Following the design and fabrication phase, the experimental procedures were carried out by two orthopaedic surgeons, supported by two senior oncologic orthopaedic surgeons and a team of bioengineers. An ilioinguinal approach was performed to fully expose the periacetabular region. The AR marker was then manually placed in the supraacetabular area to enable holographic projection through the HoloLens 2 headset. The acetabular osteotomy was subsequently performed using patient-specific cutting guides. Once resection was complete, a second AR marker was attached to the physical prosthesis, allowing it to be visualised in augmented reality, superimposed over the planned target position.
This visualisation was enhanced by the colour-coded feedback system previously described, which provided real-time information on positioning accuracy (red–orange–green). The implants were then fixed in place using screws, followed by a postoperative computed tomography scan for each hemipelvis (I–X) to enable segmentation and analysis (Figures 2 & 3).
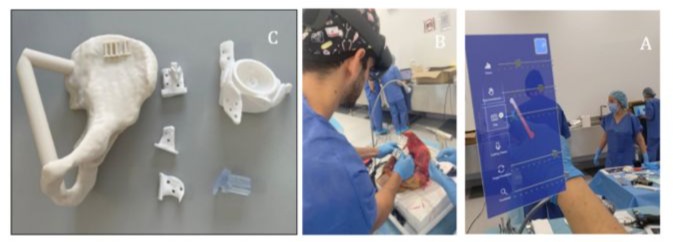
Figure 2: (A) Materials used in the study: Cadaveric hemipelvis bio model fabricated from acrylonitrile styrene acrylate (ASA); patient-specific cutting guides (PSIs) and custom-made prosthesis, both 3D printed in rigid radiopaque 10k resin, along with a removable AR marker socket designed for intraoperative tracking and alignment of the prosthesis. (B) Intraoperative image. (C) Custom-designed software interface integrated into the system, allowing the user to toggle visual elements (bone, target prosthesis, and actual prosthesis) and adjust transparency settings for improved visualisation. Images captured via the AR head-mounted display (HMD).
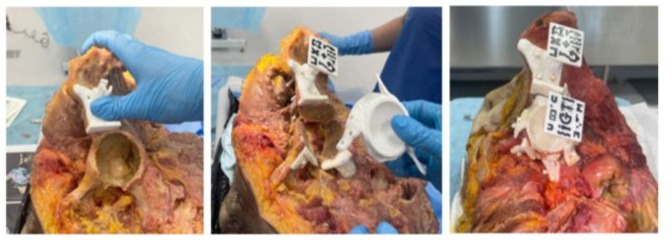
Figure 3: Manual positioning of the supraacetabular guide (Left); implant guidance (Centre) and final fixation (Right). AR markers were 3D printed using black and white polylactic acid (PLA) filament.
The evaluation considered both the final position and spatial orientation of the prosthesis. Post-experimental segmentation included bone, implant, screws, and AR markers. A comparative analysis was then performed between the planned configuration and the postoperative outcome, as well as against results from the equivalent experiment conducted on ten artificial bio models.
The analysis process involved registering the 3D models using the 'Model Registration' module in 3D Slicer, based on the Iterative Closest Point (ICP) algorithm. This procedure generated a transformation matrix between the planned and postoperative hemipelvis models, which was also applied to the postoperative implant model to unify all elements within a common coordinate system.
For implant analysis, the centre of mass was defined as the centre of the hemisphere forming the acetabular portion of the implant. This centre was calculated programmatically from a point cloud derived from the implant geometry. Similarly, implant orientation was defined relative to the normal vector of the hemispherical plane (Z-axis). Using the 'Model Registration' module in 3D Slicer, both the planned and postoperative hemipelvis models were aligned via the Iterative Closest Point (ICP) algorithm. The resulting transformation matrix was applied to the implant models to place all elements within a shared coordinate system.
Based on these definitions, three primary metrics were calculated:
- defined as the Euclidean distance between the centres of mass of the planned and the actual implanted prosthesis.
- defined as the angle between the normal vector of the planned acetabular plane and that of the postoperative implant plane.
- obtained through a comparison between the 3D surfaces of the planned and the implanted prostheses. This model integrates both linear and angular deviations, providing a comprehensive estimate of overall positioning error. The method involves comparing the postoperative and planned implant models by calculating, for each point on the postoperative surface, the distance to its closest point on the planned surface. This process is repeated across the entire surface to generate a deviation map representing the minimum achievable discrepancy between both geometries. The results were visualised as three-dimensional heat maps.
Lastly, since the registration protocol and placement of the supraacetabular AR marker remained consistent with previous studies, the same AR marker-associated error values were applied in this cadaveric phase. These values were obtained by fusing preoperative and postoperative CT scans, using the centre of the supraacetabular marker as the origin of the reference coordinate system. The x- and y-axes lay within the plane of the marker, while the z-axis was defined as the outward-facing normal vector.
In our preliminary study, the mean global translational error (Tx, Ty, Tz) was 1.07 mm (95% CI: 0.82–1.32 mm; SD: 0.68 mm), and the mean global rotational error (Rx, Ry, Rz) was 0.86° (95% CI: 0.55°–1.17°; SD: 0.84°). Both metrics remained within sub-millimetre and sub-degree thresholds. These values were deemed applicable to the present study, as no modifications were made to the registration process or the marker design.
Results
Translational and rotational errors in prosthesis placement were calculated for the ten cadaveric procedures performed (Table 1). The mean translational error was 3.79 mm (SD ±2.09 mm), while the mean rotational error was 3.73° (SD ±2.59°). The maximum recorded deviations reached 8.61 mm in translation and 8.33° in rotation. Nevertheless, in most cases, the errors remained within clinically acceptable thresholds (<5 mm and <5°).
Comparison with the Previous Experimental Study on Biomodels (Phantoms)
To assess the impact of the experimental environment on procedural accuracy, results obtained in cadaveric specimens were compared with those previously recorded in 3D-printed bio models, using the same augmented reality (AR)–based planning and navigation protocol. The analysis revealed statistically significant differences in both translational and angular errors. In the phantom models, the mean translational error was 1.75 mm, compared to 3.79 mm in the cadaveric group (p = 0.029; 95% CI: 0.25–3.82 mm). Similarly, the mean angular error was 1.70° in biomodels versus 3.73° in cadavers (p = 0.045; 95% CI: 0.05–4.01°) (Table 1).
|
Case |
Phantom Angular Error (°) |
Cadaver Angular Error (°) |
Phantom Distance Error (mm) |
Cadaver Distance Error (mm) |
|
I |
1,27 |
6,36 |
1,17 |
4.58 |
|
II |
3,6 |
1,36 |
1,2 |
1.42 |
|
III |
0,41 |
4,16 |
1,81 |
4.4 |
|
IV |
1,45 |
2,54 |
2,53 |
4.33 |
|
V |
1,39 |
1,29 |
0,86 |
3.27 |
|
VI |
2,46 |
8,33 |
1,43 |
4.45 |
|
VII |
0,24 |
2,68 |
2,17 |
3.3 |
|
VIII |
2,2 |
6,95 |
1,04 |
8.61 |
|
IX |
2,28 |
1,53 |
3,45 |
1.94 |
|
X |
1,68 |
2,07 |
1,88 |
1.62 |
|
Mean |
1,698 |
3,73 |
1,75 |
3,79 |
|
Deviation |
±0,995 |
±2,589 |
±0,798 |
±2,087 |
|
p - value |
0,045 |
0,029 |
Table 1: Angular (°) and translational (mm) errors in the phantom and cadaver groups, along with the comparative analysis.
These results suggest greater accuracy in the artificial setting, likely due to the structural stability of synthetic materials, absence of soft tissues, and predictable bone geometry in phantoms. In contrast, cadaveric specimens present real anatomical complexity, surface irregularities, and intraoperative handling challenges that contribute to the observed deviations. This comparison underscores the importance of validating navigation systems in clinically representative conditions before progressing to patient applications.
Global Distance Model Analysis
The global distance model revealed a homogeneous distribution across most contact surfaces between the implant and host bone, with the largest deviations observed in the pubic fixation region. These localised discrepancies did not affect critical areas essential for implant stability. A uniform visual threshold of 7.5 mm was established across all 3D heat map comparisons (Figure 4).
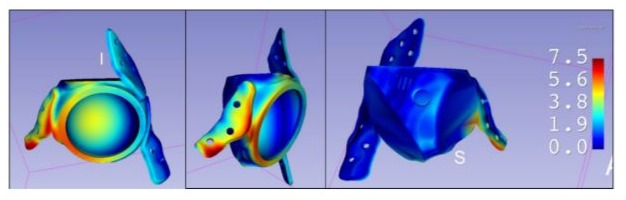
Figure 4: To facilitate interpretation and comparison across cases, a uniform colour scale was established with a fixed range from 0 mm (no error) to 7.5 mm (maximum represented deviation). This visualisation allows for rapid identification of areas with the greatest deviation and assessment of whether these affect regions critical to the implant’s stability or functionality.
Timing
The mean implant placement time was 121.5 seconds (95% CI: 59.8–183.2 s), as shown in Table 2. This compares to a previously recorded mean of 82.8 seconds in phantom models, reflecting the influence of real anatomical conditions, although the difference was not statistically significant (p = 0.26).
|
Case |
Cadaver positioning time (seconds) |
Biomodels positioning time (seconds) |
|
I |
53 |
150 |
|
II |
66 |
100 |
|
III |
231 |
175 |
|
IV |
278 |
53 |
|
V |
12 |
23 |
|
VI |
190 |
25 |
|
VII |
124 |
5 |
|
VIII |
125 |
120 |
|
IX |
78 |
125 |
|
X |
58 |
52 |
|
Mean |
121,5 |
82,8 |
|
Deviation |
86,2 |
59.01 |
|
p-value |
0,26 |
Table 2: Implant guidance time to reach the desired position, assisted by augmented reality (AR).
Discussion
This study represents a significant step in the validation of augmented reality (AR)-based surgical navigation systems, as it is one of the first cadaveric investigations focused exclusively on prosthesis placement using AR. It provides a unique experimental validation by applying, for the first time, an AR system specifically for implant positioning in cadaveric specimens, incorporating real-time intraoperative visual feedback.
A key contribution of this work is the integration of a colour-coded feedback system that guides the surgeon during implant placement, enabling real-time adjustments before final fixation. Unlike earlier phases of our research conducted on synthetic models, the use of cadaveric specimens in this study allowed for evaluation of the system’s accuracy under anatomically realistic conditions, including the presence of soft tissues and natural variability in bone geometry.
The results confirm that AR-based navigation maintains a high level of accuracy even within the complex and less predictable anatomical context of human cadaveric specimens. The mean translational error was 3.79 mm, and the mean angular error was 3.73°, both within the thresholds generally considered acceptable for pelvic implants in orthopaedic oncology. Although these errors were higher than those observed in phantom models, the increase is attributable to cadaver-specific factors such as the presence of soft tissues, bone surface irregularities, and reduced intraoperative visibility [24]. Statistical analysis showed these differences to be significant (p = 0.029 for translation; p = 0.045 for rotation), underscoring the importance of validating navigation systems under realistic anatomical conditions prior to clinical implementation.
Despite advances in guided surgery, studies validating the use of AR in cadaveric environments remain scarce. The meta-analysis by Takács et al. (2023) [25] confirmed that AR systems can achieve clinically acceptable accuracy under in vitro conditions, although still slightly inferior to static surgical guides or robotic systems. In a context more closely aligned with our study, Tabernée Heijtmeijer et al. (2024) [26] applied AR-based navigation in human cadavers for the placement of zygomatic implants, reporting errors comparable to those observed in our work (2.43 mm at the entry point and 5.80° in angular deviation), thereby reinforcing the robustness of our findings in anatomically realistic scenarios.
The safe margins for acetabular orientation described in the literature are generally defined as deviations of less than 5 mm in translation and 5° in rotation, particularly when aiming to preserve the original centre of rotation in Enneking and Dunham type II resections [16,17]. Our results, even in cadaveric specimens, largely remained within these thresholds, further supporting the clinical potential of the proposed AR navigation system.
Compared to studies in AR-assisted dental and otologic surgery, our results demonstrate comparable levels of accuracy. For instance, Mai et al. [11] and Jiang et al. [12] reported angular errors ranging from 2° to 5° in dental implant placement using AR systems. In the field of otology, Lui et al. [27] achieved a notable improvement in precision using projected AR, reducing centre-to-centre distance errors from 9mm to 1.9mm. In our case, both angular and linear errors remained within or below these reported ranges, with the added advantage that our system provides real-time visual feedback to the surgeon during the procedure an innovation not previously described.
It is also important to consider the additional error introduced by the registration accuracy of the AR marker system. In our setup, this contributed an added translational error of 1.07mm and a rotational error of 0.86°, values that are comparable to those reported by Lui et al. [27] using an optical projection system. This level of system stability is essential for ensuring reliability and repeatability, and it provides a solid foundation for future integration into clinical operating environments.
The mean implant placement time in cadaveric specimens was 121.5 seconds, compared to 82.8 seconds in phantom models. This difference reflects the greater anatomical complexity of the cadaveric environment, although the durations remained within clinically acceptable limits. As noted by Sun et al. [28], AR systems can significantly reduce surgical time by eliminating exclusive reliance on anatomical landmarks and reducing the need for continuous visual checks on external monitors.
Moreover, the integration of AR devices involves a relatively low technological cost compared to optical navigation systems or surgical simulators [29,30], which could facilitate broader clinical adoption. In addition to its application in oncological pelvic reconstructions, this system could be adapted for other high-precision surgical scenarios, such as revision arthroplasties or implant placement in anatomically challenging regions.
Finally, it is worth emphasising that cadaveric validation represents a critical step before transitioning to patient-based studies. While dynamic surgical factors such as bleeding or tissue traction cannot be fully replicated, cadaveric models preserve true three-dimensional anatomy and the challenges associated with complex structural handling, thereby providing a validated and reliable environment for testing these technologies.
Conclusions
This study demonstrates that augmented reality–based surgical navigation enables accurate placement of patient-specific implants in cadaveric models, with errors falling within clinically acceptable thresholds and comparable or even superior to those achieved with other navigation methods, partly due to the immediate visual feedback provided by the colour-coded system. These findings support the clinical applicability of the technology and justify its progression to patient-based studies.
Limitations
Study limitations include the absence of dynamic surgical conditions such as bleeding, soft tissue retraction, or visibility constraints, which can only be assessed in a clinical setting involving live patients. Additionally, the limited sample size of ten cases may affect the generalisability of the findings, although it provides a robust foundation for future studies.
Ethical Guidelines
This study was conducted in accordance with institutional and national ethical standards. All procedures complied with current guidelines for anatomical research.
Conflicts of Interest
The authors declare no conflicts of interest related to this study.
Acknowledgments
This study has been funded by: Project TED2021-132200B-I00 (Proyectos Estratégicos Orientados a la Transición Digital 2021. Agencia Estatal de Investigación. Ministerio de Ciencia e Innovación.).
This study has been funded by Instituto de Salud Carlos III (ISCIII) through the Biomodels and Biobanks Platform and co-funded by the European Union (PT23/00116 to RPM).
Plataforma para el desarrollo de la Fabricación Aditiva (FAB3D) por su soporte’// “We acknowledge support from the PTI FAB3D, Consejo Superior de Investigaciones Científicas (CSIC), Spain.
References
- Mendicino AR, Condino S, Carbone M, F Cutolo, N Cattari, et al. (2022) Augmented Reality as a Tool to Guide Patient-Specific Templates Placement in Pelvic Resections. Annu Int Conf IEEE Eng Med Biol Soc 2022: 3481-3484.
- García-Sevilla M, Moreta-Martinez R, García-Mato D, Lastra APD, Mananes RP, et al. (2021) Augmented Reality as a Tool to Guide PSI Placement in Pelvic Tumor Resections. Sensors 21(23): 7824.
- Mangano FG, Admakin O, Lerner H, Mangano C (2023) Artificial intelligence and augmented reality for guided implant surgery planning: A proof of concept. J Dent 133: 104485.
- Carrozzi A, Chylinski M, Heller J, Hilken T, Keeling DI, de Ruyter K (2019) What’s Mine Is a Hologram? How Shared Augmented Reality Augments Psychological Ownership. J Interact Mark 48(1): 71-88.
- Dolega-Dolegowski D, Proniewska K, Dolega-Dolegowska M, et al. (2022) Application of holography and augmented reality-based technology to visualize the internal structure of the dental root - a proof of concept. Head Face Med 18(1): 12.
- Ackermann J, Liebmann F, Hoch A, Snedeker JG, Farshad M, et al. (2021) Augmented Reality Based Surgical Navigation of Complex Pelvic Osteotomies- A Feasibility Study on Cadavers. Appl Sci 11(3): 1228.
- Hoch A, Liebmann F, Farshad M, Fürnstahl P, Rahm S, Zingg PO (2024) Augmented reality-guided pelvic osteotomy of Ganz: feasibility in cadavers. Arch Orthop Trauma Surg 144(3): 1077-1089.
- Pflugi S, Liu L, Ecker TM, Schumann S, Cullman JL, et al. (2016) A cost-effective surgical navigation solution for periacetabular osteotomy (PAO) surgery. Int J Comput Assist Radiol Surg 11(2): 271-280.
- Mediavilla-Santos L, García-Sevilla M, Calvo-Haro JA, et al. (2022) Validación de los modelos de impresión 3D paciente-específicos para cirugía ortopédica oncológica pélvica. Rev Esp Cir Ortopédica Traumatol 66(5): 403-409.
- Ma L, Jiang W, Zhang B, Qu X, Ning G, et al. (2019) Augmented reality surgical navigation with accurate CBCT-patient registration for dental implant placement. Med Biol Eng Comput 57(1): 47-57.
- Mai HN, Dam VV, Lee DH (2023) Accuracy of Augmented Reality - Assisted Navigation in Dental Implant Surgery: Systematic Review and Meta-analysis. J Med Internet Res 25: e42040.
- Jiang W, Ma L, Zhang B, Fan Y, Qu X, et al. (2018) Evaluation of the 3D Augmented Reality-Guided Intraoperative Positioning of Dental Implants in Edentulous Mandibular Models. Int J Oral Maxillofac Implants 33(6): 1219-1228.
- Jemal A, Siegel R, Xu J, Ward E (2010) Cancer statistics, 2010. CA Cancer J Clin 60(5): 277-300.
- Mayerson JL, Wooldridge AN, Scharschmidt TJ (2014) Pelvic resection: current concepts. J Am Acad Orthop Surg 22(4): 214-222.
- Karaca MO, Özbek EA, Özyıldıran M, Merter A, Basarir K, et al. (2022) External and internal hemipelvectomy: A retrospective analysis of 68 cases. Jt Dis Relat Surg 33(1): 132-141.
- Enneking WF, Dunham WK (1978) Resection and reconstruction for primary neoplasms involving the innominate bone. J Bone Joint Surg Am 60(6): 731-746.
- Lewinnek GE, Lewis JL, Tarr R, Compere CL, Zimmerman JR (1978) Dislocations after total hip-replacement arthroplasties. J Bone Joint Surg Am 60(2): 217-220.
- McCollum DE, Gray WJ (1990) Dislocation after total hip arthroplasty. Causes and prevention. Clin Orthop (261): 159-170.
- Esposito CI, Gladnick BP, Lee Y yu, Lymann S, Wright TM, et al. (2015) Cup Position Alone Does Not Predict Risk of Dislocation after Hip Arthroplasty. J Arthroplasty 30(1): 109-113.
- Jud L, Fotouhi J, Andronic O, Aichmair A, Osgood G, et al. (2020) Applicability of augmented reality in orthopedic surgery - A systematic review. BMC Musculoskelet Disord 21(1): 103.
- Verhey JT, Haglin JM, Verhey EM, Hartigan DE (2020) Virtual, augmented, and mixed reality applications in orthopedic surgery. Int J Med Robot Comput Assist Surg MRCAS 16(2): e2067.
- Bian D, Lin Z, Lu H, Zhong Q, Wang K, et al. (2024) The application of extended reality technology-assisted intraoperative navigation in orthopedic surgery. Front Surg 11: 1336703.
- Bruschi A, Donati DM, Di Bella C (2023) What to choose in bone tumour resections? Patient specific instrumentation versus surgical navigation: a systematic review. J Bone Oncol 42: 100503.
- Javier Orozco Martinez, Tanya Fernandez Fernandez, Amaia Iribar Zabala (2025) Augmented Reality-Assisted Implant Positioning: A Novel Method for Real-Time Precision Placement. J Surg Res 8(2): 241-247.
- Takács A, Hardi E, Cavalcante BGN, et al. (2023) Advancing accuracy in guided implant placement: A comprehensive meta-analysis: Meta-Analysis evaluation of the accuracy of available implant placement Methods. J Dent 139: 104748.
- Tabernée Heijtmeijer S, Glas H, Janssen N, Vosselman N, Visscher SD, et al. (2024) Accuracy of augmented reality navigated surgery for placement of zygomatic implants: a human cadaver study. PeerJ 12: e18468.
- Lui JT, Dahm V, Chen JM, Lin VY, Irish JC, et al. (2023) Using augmented reality to guide bone conduction device implantation. Sci Rep 13(1): 7182.
- Sun P, Zhao Y, Men J, Ma ZR, Jiang HZ, et al. (2023) Application of Virtual and Augmented Reality Technology in Hip Surgery: Systematic Review. J Med Internet Res 25: e37599.
- Kriechling P, Roner S, Liebmann F, Casari F, Fürnstahl P, (2021) Augmented reality for base plate component placement in reverse total shoulder arthroplasty: a feasibility study. Arch Orthop Trauma Surg 141(9): 1447-1453.
- Logishetty K, Western L, Morgan R, Iranpour F, Cobb JP, Auvinet E (2019) Can an Augmented Reality Headset Improve Accuracy of Acetabular Cup Orientation in Simulated THA? A Randomized Trial. Clin Orthop 477(5): 1190-1199.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.