Utility of Different Anti-IgG Antibodies in Recognition of Punctate (Powdery) Podocyte Staining by Immunofluorescence in Patients with Diffuse Podocyte Foot Process Effacement
by Alana Dasgupta1, Anjali A Satoskar1, Tibor Nadasdy1, Cherri Bott1, Zaid Abassi2, Sergey V Brodsky1*
1Department of Pathology, Ohio State University Wexner Medical Center, Columbus, OH, USA
2Department of Physiology, Technion – Israel Institute of Technology, Haifa, Israel
*Corresponding author: Sergey V Brodsky, Professor, Department of Pathology, The Ohio State University, 333 W 10th Ave Graves Hall, 4173, Columbus, OH 43210, USA
Received Date: 21 October, 2025
Accepted Date: 03 November, 2025
Published Date: 07 November, 2025
Citation: Dasgupta A, Satoskar AA, Nadasdy T, Bott C, Abassi Z, et al. (2025) Utility of Different Anti-IgG Antibodies in Recognition of Punctate (Powdery) Podocyte Staining by Immunofluorescence in Patients with Diffuse Podocyte Foot Process Effacement. Curr Trends Med Diagn Meth. 3: 111. https://doi.org/10.29011/CTMDM.100111
Abstract
Circulating anti-nephrin autoantibodies recently were recognized as a possible pathogenic factor in patients with steroid-resistant minimal change disease. In a kidney biopsy, these patients have punctate (“powdery”) staining in the podocytes by immunofluorescence. Previously published data described such pattern of staining by using anti-IgG Dako antibody. In the routine practice, different laboratories use different anti-IgG antibodies from a variety of vendors. The aim of this work was to compare the ability to recognize the punctate staining in the podocyte by two different anti-IgG antibodies (Dako and Kent) that are in use in our routine renal pathology practice. Our data indicates that both anti-IgG antibodies recognize the punctate (“powdery) staining in the podocytes in a subset of patients with minimal change disease.
Keywords: Renal pathology; Immunofluorescence; Podocytes; Minimal change disease
Background
Minimal change disease is characterized by prominent podocyte foot process effacement by electron microscopy and the absence of immune complex deposition in the kidney. Nephrin is a transmembrane protein that is expressed in podocytes and is a structural component of the slit diaphragm. A defect in the nephrin gene, NPHS1, is associated with congenital nephrotic syndrome of the Finnish type and causes massive proteinuria. The recent discovery of anti-nephrin autoantibodies in patients with minimal change disease as possible etiologic factor of podocyte injury [1] changed the interpretation of immunofluorescence findings by renal pathologists. While the mechanisms of action of anti-Nephrin autoantibodies is poorly understood, it was suggested that these autoantibodies may cause redistribution of Nephrin in the podocyte. Although punctate (powdery) IgG staining in the podocytes was observed in patients with minimal change disease in the past, the diagnostic significance of this finding was unclear [2]. Recent data from patients with circulating anti-nephrin autoantibodies indicates there is punctate (powdery) staining for IgG in the podocytes [3]. The authors demonstrated a colocalization of this punctate IgG staining in podocytes with staining for nephrin and they hypothesized that this is a marker of anti-nephrin antibodies in a kidney biopsy [3]. In their work, the authors used anti-human IgG antibody from Dako (F0315). However, renal pathology laboratories use different anti-IgG antibodies from different vendors. The goal of this study was to compare the utility of two different anti-IgG antibodies that are used routinely in renal pathology practice in recognizing this punctate (powdery) staining in podocytes by immunofluorescence.
Material and Methods
Fifteen kidney biopsies with diagnosis “diffuse podocyte foot process effacement” were randomly selected from the OSUWMC renal pathology database between January 2023 and December 2024. Patients with immune-complex mediated glomerulonephritis (such as membranous glomerulonephritis, lupus nephritis, IgA nephropathy, C3 glomerulonephritis) were excluded.
The following antibodies were used in the study: Dako anti-IgG FITC F(ab)2, F031501-2, 1:20 dilution (Agilent Technologies, Santa Clara, CA); Kent Anti Human IgG FITC, 101202, 1:40 dilution (Kent Laboratories, Bellingham, WA); R&D Systems anti-Nephrin, AF4269, 1:200 dilution (R&D Systems, Inc. Minneapolis, MN); Invitrogen secondary AlexaFluor 594, Donkey anti sheep IgG (H+L) cross absorbed, A11016, 1:100 dilution (Invitrogen, Waltham, WA).
Immunofluorescence was performed by using an automated intelliPATH FLX® stainer (Biocare Medical, Pacheco, CA) for both anti-IgG antibodies and manual staining for the anti-nephrin antibody. All immunofluorescence was performed on serial sections of frozen tissue. For anti-IgG antibodies, slides were airdried and fixed in cold acetone for 10 minutes, then rehydrated in phosphate buffer saline (PBS), for 5 minutes and incubated with an anti-IgG antibody for 30 min, rinsed in PBS and coverslip with Aqueous fluorescence mounting medium (cat # S302380-2, Agilent Technologies, Inc, Santa Clara, CA). Staining with anti-nephrin antibody was as following: slides were airdried and fixed in cold acetone for 10 minutes, then rehydrated in phosphate buffer saline (PBS), for 5 minutes and incubated with the anti-nephrin antibody 30 minutes, rinsed in PBS 5 mins, incubated with secondary Anti sheep 594 antibody for 30 minutes, rinsed in PBS and coverslip with Aqueous fluorescence mounting medium (cat # S302380-2, Agilent Technologies, Inc, Santa Clara, CA).
Slides were blindly evaluated by three renal pathologists (AS, SB and AD) for the presence or absence of the punctuate (powdery) stain in the podocytes by using an Olympus BX43 immunofluorescent microscope (Olympus Corporation of the Americas, Center Valley, PA). Interobserver reliability was assessed by using GraphPad Prism 5 software and employing several tests: 1) average of pairwise linearly weighted Cohen k, 2) linearly weighted Fleiss k and 3) Krippendorf k-alpha inter-rated reliability coefficient [4,5]. Descriptive statistics was used to analyze numerical variables, chi-square test was used to analyze differences between categorical variables.
Results
Fifteen kidney biopsies were selected for the study. Patients presented with nephrotic syndrome and 7 (47%) had acute kidney injury on presentation which was attributed to the heavy proteinuria (average serum creatinine at the time of biopsy was 2.1±1.1 mg/dl). The average age of the patients was 53.1±19 y.o., 3 (23%) were African American, 1 (8%) Hispanic and 9 (69%) Caucasian (race records were not available for 2 patients). All the biopsies showed diffuse podocyte foot process effacement by electron microscopy and 3 (20%) had features of Focal Segmental Glomerular Sclerosis (FSGS).
Results of IgG immunofluorescence grading by three different pathologists are presented in Table 1. Each case was stained with two different antibodies and all slides were blindly and independently analyzed. Punctate (powdery) IgG stain with the Dako antibody (Figure 1C) was recognized in 14 out 45 observations (15 cases viewed by 3 pathologists); whereas punctate IgG stain with the Kent antibody (Figure 1D) was seen in 9 out 45 observations (chi-square 1.46, p=0.227). The inter-rater reliability analysis showed that there was high pairwise percentage agreement for both antibodies (88.9%), which was slightly higher for the Kent antibody (91.1% versus 86.7% for the Dako antibody). A similar trend was observed by other statistical methods, where the Kent antibody shows slightly higher inter-rater agreement as compared to the Dako antibody in both Cohen k and Fleiss k analyses (Table 2). In the past, a colocalization of staining with nephrin and with the Dako anti-IgG antibody was demonstrated (3). We confirmed that the Kent anti-IgG antibody showed similar colocalization with nephrin in cases when there is punctate staining in podocytes (Figure 2D-F).
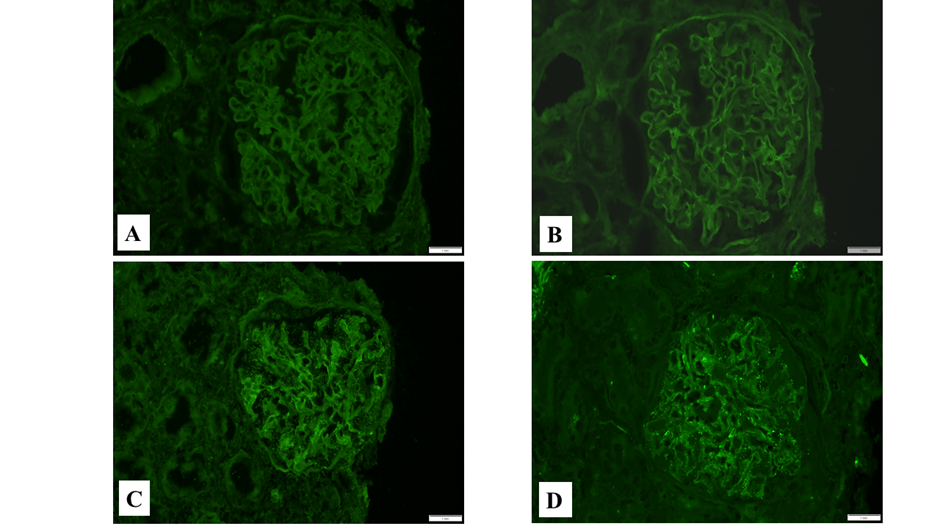
Figure 1: Immunofluorescence staining with different antibodies to IgG. A: Immunofluorescence with the Dako anti-IgG antibody, negative punctate (powdery) stain in podocytes; B: Immunofluorescence with the Kent anti-IgG antibody, negative punctate (powdery) stain in podocytes; C: Immunofluorescence with the Dako anti-IgG antibody, punctate (powdery) stain in podocytes; D: Immunofluorescence with the Kent anti-IgG antibody, punctate (powdery) stain in podocytes.
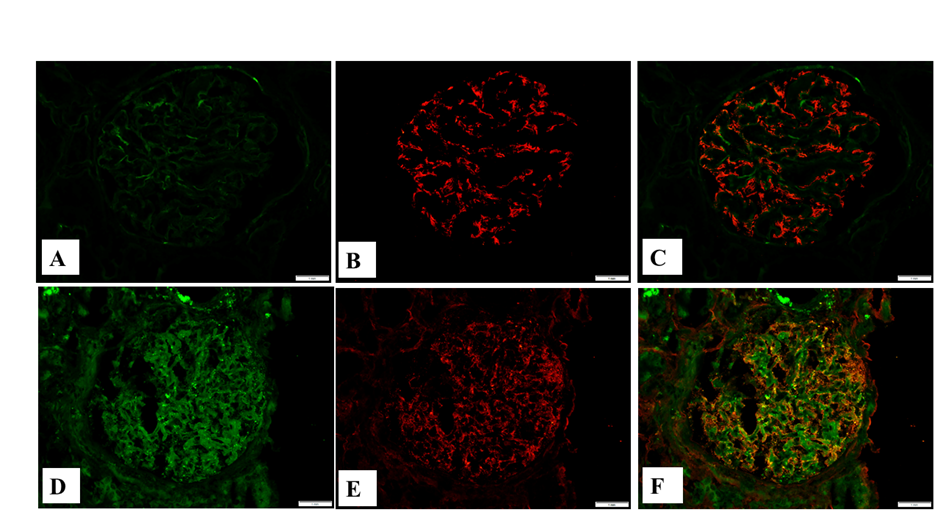
Figure 2: Immunofluorescence staining with the Kent antibody to IgG and nephrin. A: Immunofluorescence with the Kent anti-IgG antibody, negative punctate (powdery) stain in podocytes; B: Immunofluorescence with anti-nephrin antibody, mild diffuse stain in podocytes; C: Combined stain with the Kent anti-IgG (green) and anti-nephrin (red) antibodies; D: Immunofluorescence with the Kent anti-IgG antibody, punctate (powdery) stain in podocytes; E: Immunofluorescence with anti-nephrin antibody, distorted granular stain in the podocytes; F: Combined stain with the Kent anti-IgG (green) and anti-nephrin (red) antibodies, colocalization of both antibodies (yellow).
Discussion
Emerging evidence suggest an important role of circulating anti-nephrin autoantibodies in the pathogenesis of minimal change disease [1]. Renal pathologists recognized punctuate IgG staining in podocytes already in the past [6], but only recently has this pattern of staining been associated with anti-nephrin antibodies.
Renal pathologists use different antibodies for the routine practice; therefore, it was not clear if other anti-IgG antibodies can demonstrate this punctate staining in the podocytes. In the seminal paper by Watts, et al. [3], a Dako anti-IgG antibody was used, while in our renal pathology laboratory we use a Kent anti-IgG antibody.
Results of our study show that the Dako anti-IgG antibody has a slight edge over the Kent anti-IgG antibody: positive punctate staining in the podocytes was recognized more often than with the Kent anti-IgG antibody, even though these differences were not statistically significant (Table 1). Such a discrepancy could be related to different dilutions of these antibodies. While we routinely use the Kent antibody in 1:40 dilution, the Dako antibody requires 1:20 dilution for immunofluorescence. Nevertheless, there was a good inter-rater agreement for both anti-IgG antibodies between three different pathologists. The agreement was slightly better with the Kent anti-IgG antibody than with the Dako anti-IgG antibody, which is most likely due to the routine use of the former antibody in the practice (Table 2). The Kent anti-IgG antibody is also colocalized with nephrin staining in the podocytes when the punctate IgG staining is present (Figure 2); similarly, to the extensively studied colocalization of staining for nephrin with the Dako anti-IgG antibody [3].
|
Pathologist 1 |
Pathologist 2 |
Pathologist 3 |
||||
|
Dako |
Kent |
Dako |
Kent |
Dako |
Kent |
|
|
Case 1 |
1 |
1 |
0 |
0 |
1 |
0 |
|
Case 2 |
1 |
1 |
1 |
1 |
1 |
0 |
|
Case 3 |
1 |
1 |
1 |
1 |
1 |
1 |
|
Case 4 |
1 |
0 |
1 |
0 |
0 |
0 |
|
Case 5 |
1 |
1 |
1 |
1 |
1 |
1 |
|
Case 6 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Case 7 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Case 8 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Case 9 |
0 |
0 |
0 |
0 |
1 |
0 |
|
Case 10 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Case 11 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Case 12 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Case 13 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Case 14 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Case 15 |
0 |
0 |
0 |
0 |
0 |
0 |
|
0 – absent; 1 – punctate (powdery) staining in the podocytes |
||||||
Table 1: Assessment of IgG staining in the glomeruli by three nephropathologists.
|
Average |
Pairwise |
Pairwise |
Pairwise |
||
|
path 1 & 3 |
path 1 & 2 |
path 2 & 3 |
|||
|
Average Pairwise Percent Agreement, % |
Overall |
88.9 |
86.7 |
93.3 |
86.7 |
|
Dako |
86.7 |
86.7 |
93.3 |
80 |
|
|
Kent |
91.1 |
86.7 |
93.3 |
93.3 |
|
|
Average Pairwise Cohen's Kappa |
Overall |
0.706 |
0.661 |
0.831 |
0.627 |
|
Dako |
0.689 |
0.7 |
0.842 |
0.526 |
|
|
Kent |
0.724 |
0.595 |
0.815 |
0.762 |
|
|
Fleiss' Kappa |
Overall |
0.708 |
|||
|
Dako |
0.689 |
||||
|
Kent |
0.722 |
||||
|
Krippendorff's Alpha |
Overall |
0.711 |
|||
|
Dako |
0.696 |
||||
|
Kent |
0.728 |
Table 2: Inter-rated reliability analysis.
The main deficiency of our study is its retrospective manner and inability to correlate with serum data in patients. However, our main goal was to investigate whether the routinely used anti- IgG antibody is capable to detect the punctate staining in the podocytes, and this goal was successfully achieved.
In conclusion, both routinely used anti-IgG antibodies are capable of recognizing the punctate (powdery) pattern of staining in podocytes by immunofluorescence on frozen tissue. However, the sensitivity of this IF pattern of staining does not appear to be high [7]; therefore, commercially available sensitive laboratory tests will be needed to confirm primarily the absence but also the presence of circulating anti-nephrin autoantibodies.
References
- Hengel FE, Dehde S, Lassé M, Zahner G, Seifert L, et al. (2024) Autoantibodies Targeting Nephrin in Podocytopathies. N Engl J Med 391: 422-433.
- Lal P (2006) Atlas of nontumor pathology. Non-neoplastic kidney disease. First series fascicle 4. Vivette D. D'Agati, J. Charles Jennette, and Fred G. Silva. Washington (DC): American Registry of Pathology in collaboration with the Armed Forces Institute of Pathology; 2005, 721 pp, $179. Human pathology 37: 1122.
- Watts AJB, Keller KH, Lerner G, Rosales I, Collins AB, et al. (2022) Discovery of Autoantibodies Targeting Nephrin in Minimal Change Disease Supports a Novel Autoimmune Etiology. J Am Soc Nephrol 33: 238-252.
- Jen KY, Olson JL, Brodsky S, Zhou XJ, Nadasdy T, et al. (2013) Reliability of whole slide images as a diagnostic modality for renal allograft biopsies. Hum Pathol 44: 888-894.
- Marzi G, Balzano M, Marchiori D (2024) K-Alpha Calculator-Krippendorff's Alpha Calculator: A user-friendly tool for computing Krippendorff's Alpha inter-rater reliability coefficient. MethodsX 12: 102545.
- Furness P (2006) Atlas of Nontumor Pathology: Non-Neoplastic Kidney Diseases. Nature Clinical Practice Nephrology 2: E1.
- Inoki Y, Ichikawa Y, Sakakibara N, Kimura Y, Tanaka Y, et al. (2024) Co-localization of IgG with Nephrin in Immune-Mediated Idiopathic Nephrotic Syndrome: TH-OR95. Journal of the American Society of Nephrology 35: 10.1681/ASN.2024d54w4s2j.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.