Unmasking Metastatic MRSA Infection: A Case Report of Persistent Bacteremia with Multiple Secondary Sites in a Pacemaker Patient
by Emna Fertani¹*, Maria Paola Marino¹, Francesco Donvito², Maria Elena Novielli², Vincenzo Solfrizzi¹, Franco Mastroianni²
¹U.O.C. Medicina Interna e Geriatria Universitaria “C. Frugoni”, Dipartimento Interdisciplinare di Medicina, Università degli Studi di Bari “Aldo Moro”, Bari, Italy
²Geriatric Department, F. Miulli Hospital, Acquaviva delle Fonti (BA), Italy
*Corresponding author: Emna Fertani, U.O.C. Medicina Interna e Geriatria Universitaria “C. Frugoni”, Dipartimento Interdisciplinare di Medicina, Università degli Studi di Bari “Aldo Moro”, Bari, Italy
Received Date: 05 August 2025
Accepted Date: 11 August 2025
Published Date: 13 August 2025
Citation: Fertani E, Marino MP, Donvito F, Novielli ME, Solfrizzi V, Mastroianni F, et al. (2025). Unmasking Metastatic MRSA Infection: A Case Report of Persistent Bacteremia with Multiple Secondary Sites in a Pacemaker Patient. Ann Case Report. 10: 2371. https://doi.org/10.29011/2574-7754.102371
Abstract
Background
Methicillin-resistant Staphylococcus aureus (MRSA) bacteremia poses a significant clinical challenge, particularly in patients with implantable cardiac electronic devices (CIEDs). It is associated with a high risk of metastatic complications such as infective endocarditis, osteomyelitis, and sepsis. Accurate identification of the primary and secondary infection sites is essential for guiding management. We report a case of persistent MRSA bacteremia complicated by septic pulmonary emboli and vertebral osteomyelitis without imaging evidence of device-related infection.
Case Presentation
A 67-year-old man with type 2 diabetes, Charcot foot with chronic osteomyelitis, and chronic kidney disease presented with fever and gastrointestinal symptoms. A dual-chamber pacemaker had been implanted five months prior. Laboratory tests revealed leukocytosis, elevated CRP (30.91 mg/dL), and PCT (31.77 ng/mL). Multiple blood cultures confirmed persistent MRSA bacteremia. Given the device history, infective endocarditis was suspected. However, transthoracic and transesophageal echocardiography were negative. The persistence of bacteremia prompted further investigation for deep-seated infection and the need for aggressive source control. Total-body CT revealed bilateral septic pulmonary emboli and a chronic subdural hematoma. PET/CT showed no uptake at the pacemaker site but revealed hypermetabolism at D11, right ankle/foot, and spleen, suggestive of spondylodiscitis, osteomyelitis, and reactive splenic hypermetabolism. The patient was treated with linezolid, daptomycin, and ceftaroline, with resolution of bacteremia.
Conclusion
This case illustrates the potential for occult metastatic MRSA infection in the absence of echocardiographic or device evidence. Clinicians should maintain a high level of suspicion for hidden sources. Multimodal imaging remains critical in the diagnostic workup and targeted treatment.
Keywords: MRSA bacteremia; Pacemaker; Septic pulmonary emboli; Vertebral osteomyelitis; PET/CT; Charcot foot
Introduction
Methicillin-resistant Staphylococcus aureus (MRSA) bacteremia is a severe bloodstream infection associated with high morbidity and mortality. It can lead to metastatic complications such as infective endocarditis, osteomyelitis, and sepsis, and is frequently associated with healthcare exposures, indwelling devices, and immunocompromised states [1].
In patients with cardiac implantable electronic devices (CIEDs), the risk of endocarditis or device-related infection is significantly increased. As a result, echocardiographic evaluation preferably transesophageal is recommended in all cases of S. aureus bacteremia [2]. However, the absence of vegetations or device involvement on imaging does not exclude metastatic foci, which can be clinically silent and poses diagnostic challenges, especially in elderly or neuropathic individuals [3].
Here, we present a case of persistent MRSA bacteremia in a patient with a pacemaker and no imaging evidence of device infection, ultimately diagnosed with vertebral spondylodiscitis and chronic osteomyelitis of the foot. This case underscores the diagnostic challenges of metastatic MRSA infection in the absence of apparent cardiac involvement. It highlights the role of advanced imaging and combination antimicrobial therapy in successful management.
Case Presentation
A 67-year-old male presented to the emergency room with fever, abdominal pain, diarrhea, and vomiting that had been ongoing for more than 72 hours. His medical history was notable for type 2 diabetes mellitus treated with insulin, complicated by diabetic retinopathy, peripheral neuropathy, and Charcot foot deformity. He also had a history of recurrent soft tissue abscesses and chronic osteomyelitis involving the right foot, which had previously required amputation of the first and third metatarsals. Other comorbidities included first-stage obesity, mixed dyslipidemia, stage 3 chronic kidney disease (KDOQI classification), and chronic heart failure with preserved ejection fraction (HFpEF), likely secondary to hypertensive cardiomyopathy. Additionally, he had a history of thrombosis of the right small saphenous vein, for which he had been on oral anticoagulation since February 2025, and a dual-chamber pacemaker was implanted the same month due to complete atrioventricular block.
At admission to our Geriatrics department, the patient was alert, oriented, and cooperative. He had no facial dysmorphisms, rash, or peripheral edema. Oxygen saturation was 95% on room air. Heart rate was regular at 76 bpm, and blood pressure was 100/50 mmHg. Auscultation of the lungs revealed diffusely decreased breath sounds. The abdomen was notably distended due to adiposity but remained soft, non-tender, with hypoactive bowel sounds. The right foot exhibited structural deformity consistent with known Charcot arthropathy and was warm to the touch, without erythema, overt signs of cellulitis, or visible ulceration.
Initial bloodwork revealed marked leukocytosis (WBC 13.93 × 10⁹/L), severe hyperglycemia (glucose 498 mg/dL), hyponatremia (Na⁺ 121 mmol/L), acute renal failure (serum creatinine 3.93mg/dl and eGFR 25 mL/min/1.73m²) and elevated inflammatory markers, including C-reactive protein (CRP) 26.63 mg/dL (n.v. <0.50mg/dL) and procalcitonin (PCT) 31.77 ng/ mL (n.v. 0.05 ng/mL). Blood cultures drawn on days 1, 2, 6, 7, 10, 12, and 15, and partially on day 19 yielded methicillin-resistant Staphylococcus aureus (MRSA), confirming sustained MRSA bacteremia.
Given the presence of an intracardiac device and persistent MRSA bacteremia, both transthoracic and transesophageal echocardiography (TTE and TEE) were promptly performed. These showed no evidence of valvular vegetations or leadassociated infection. Nevertheless, the ongoing positivity of blood cultures raised concern for disseminated opportunistic infections. A contrast-enhanced full-body CT scan revealed multiple bilateral pulmonary nodules consistent with septic emboli (Figure 1), as well as a chronic right subdural hematoma and a hyperdense focus in the right frontal region. Whole-body 18F-FDG PET/CT (Figures 2,3) demonstrated no abnormal uptake at the pacemaker pocket or along the leads, but revealed increased metabolic activity in the D11 vertebral body (SUV max 12.9), and in the right talus, tarsal bones, and surrounding soft tissues of the lateral malleolus (SUV 7.8) findings suggestive of vertebral osteomyelitis (spondylodiscitis) and chronic osteomyelitis, respectively. Additionally, a reversed liver-to-spleen metabolic gradient (spleen SUV 5.4) was observed, likely reflecting reactive inflammatory changes.
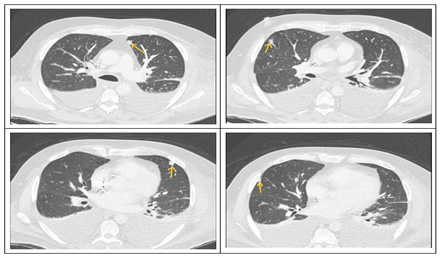
Figure 1: CT scan revealed multiple subcentimeter-sized pseudonodular formations in the expanded lung parenchyma, some of which are triangular, suspicious for septic emboli.
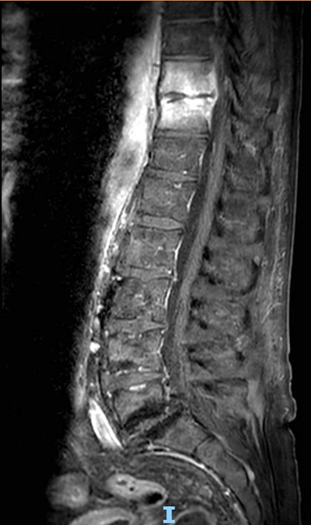
Figure 2: MRI of the thoracolumbar spine (T10-T11) showing vertebral signal abnormalities with T1 hypointensity, long-TR hyperintensity, and post-contrast enhancement involving the adjacent disc consistent with spondylodiscitis. A thin enhancing epidural collection (~3 mm thick, ~26 mm long) is seen in the anterior spinal canal, extending into the neural foramina. Bilateral paravertebral soft tissue enhancement, more evident on the left, shows a small fluid collection (~20 × 4 mm) suggestive of early abscess formation.
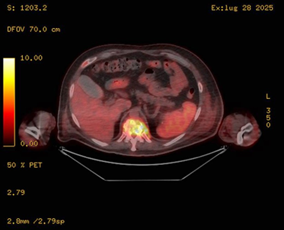
Figure 3: Coronal fused 18F-FDG PET/CT image showing intense hypermetabolic activity at the D11 vertebral body (SUV max 12.9), consistent with spondylodiscitis. Notably, the spleen shows increased uptake relative to the liver (inverted liverspleen gradient), suggestive of reactive/inflammatory splenic hypermetabolism.
The differential diagnosis initially prioritized infective endocarditis (IE), given the presence of a cardiac implantable electronic device (CIED) and sustained MRSA bacteremia. According to the modified Duke criteria [4], a definite diagnosis of IE requires either two major criteria, one major and three minor criteria, or five minor criteria. In our case, persistent bacteremia constituted one major criterion; however, there was no evidence of valvular vegetation or pacemaker lead involvement on imaging studies. Furthermore, PET/CT imaging revealed no abnormal metabolic uptake around the pacemaker generator or leads, making CIEDrelated endocarditis unlikely. Given the absence of a second major criterion and insufficient minor criteria, the diagnosis of IE was not supported. As a result, attention shifted toward alternative sources of infection. The primary diagnostic challenge laid in the discordance between persistent MRSA bacteremia and the absence of device-related infection, which delayed identification of the actual infectious burden.
Ultimately, advanced imaging studies including MRI of the thoracolumbar spine (Figure 4), plain radiography of the right foot, and whole-body 18F-FDG PET/CT revealed two likely sources contributing to the persistent bacteremia: spondylodiscitis at the D10–D11 levels with associated epidural and paravertebral abscesses, and chronic osteomyelitic changes in the right foot. The spinal infection was characterized by vertebral involvement with adjacent epidural enhancement and paravertebral soft tissue abscesses, consistent with hematogenous seeding. The right foot, previously affected by Charcot neuroarthropathy and prior partial amputation, showed progression of osteolysis involving the remaining tarsal structures.
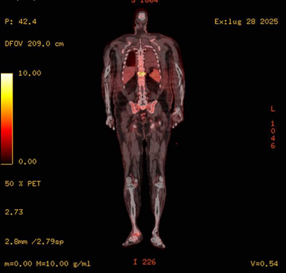
Figure 4: Axial fused 18F-FDG PET/CT image at the thoracolumbar level showing increased FDG uptake at D11 vertebral body, consistent with spondylodiscitis. Inferiorly, partial visualization of the right ankle reveals focal hypermetabolic activity involving bone and soft tissue, suggestive of distal osteomyelitis.
At the time of presentation (day 1), the patient exhibited highgrade fever, gastrointestinal symptoms, and markedly elevated inflammatory markers (CRP >30 mg/dL, PCT >30 ng/mL), raising suspicion for sepsis of unknown origin. Given his diabetic status, chronic osteomyelitis, and renal impairment, empiric broadspectrum coverage was started with meropenem, adjusted for stage IV chronic kidney disease. This was intended to provide coverage for multidrug-resistant Gram-negative bacilli, anaerobes, and enteric pathogens, particularly given the abdominal complaints. At this stage, no Gram-positive coverage was included beyond meropenem’s limited spectrum. Blood cultures on days 1-2 resulted positive for MRSA (Table 1), prompting a switch from meropenem to teicoplanin on day 3 along with ceftriaxone, which was chosen for its MRSA coverage and safety in renal impairment. Despite dual therapy, blood cultures remained positive between days 6 and 15, with only partial improvement in CRP and PCT. Persistent bacteremia and concern for deep-seated infection prompted escalation on day 14 to linezolid, with discontinuation
of teicoplanin. However, a subsequent blood culture on day 15 was again positive for MRSA, raising concern for reduced susceptibility or inadequate tissue penetration. Following the full-body CT scan on day 19, triple therapy with linezolid, daptomycin, and ceftaroline was initiated to enhance bactericidal activity and address heterogeneous MRSA subpopulations or compartmentalized foci. Upon initiation of the triple antibiotic therapy, blood cultures gradually cleared, and inflammatory markers continued to decline (CRP from 14.96 to 7.94 mg/dL; PCT from 31.77 to 0.17 ng/mL), in line with clinical improvement. Subsequent blood cultures on days 21 and 24 were negative, indicating gradual clearance of the bacteremia. This stepwise approach highlights the complexity of managing persistent MRSA bacteremia, necessitating escalation beyond guideline-recommended monotherapy, especially in the context of metastatic foci and prior antibiotic exposure.
|
ANTIBIOTICS DRUG |
MIC |
||
|
Benzylpenicillin |
R |
>0,25 |
|
|
Cefoxitin Screen |
Pos |
||
|
Ceftaroline |
S |
0,5 |
|
|
Clindamycin |
S |
<=0,12 |
|
|
Daptomycin |
S |
0,25 |
|
|
Erythromycin |
S |
1 |
|
|
Fusidic Acid |
S |
<=0,5 |
|
|
Gentamicin |
R |
>8 |
|
|
Inducible Clindamycin Resistance |
Neg |
||
|
Levofloxacin |
I |
<=0,12 |
|
|
Linezolid |
S |
1 |
|
|
Oxacillin |
R |
>2 |
|
|
Rifampicin |
S |
=0,03 |
|
|
Teicoplanin |
S |
<=0,5 |
|
|
Tetracycline |
S |
<=1 |
|
|
Tigecycline |
S |
<=0,12 |
|
|
Trimethoprim/ Sulfamethoxazole |
S |
<=10 |
|
|
Vancomycin |
S |
1 |
|
|
Isolated Bacteria: Staphylococcus Aureus MRSA |
|||
|
R: Resistant, S: sensitive, I: intermediate, Neg: negative, MIC: Minimum Inhibitory Concentration. |
|||
Table 1: Antibiogram summarizing the susceptibility pattern of the S. Aureus isolates.
Follow-up and Outcomes
The patient was closely monitored throughout his hospitalization. Clinical parameters, including vital signs, glycemic control, and inflammatory markers, gradually improved. He demonstrated good adherence to the antibiotic regimen (combination therapy with daptomycin, linezolid, and ceftaroline), without significant adverse effects.
Discussion
This case highlights the diagnostic and therapeutic challenges of persistent MRSA bacteremia, particularly in a patient with a CIED but no evidence of infective endocarditis or device pocket infection. This case shows the need for a high index of suspicion for metastatic seeding, even when the typical hallmarks of endocarditis are absent. In such scenarios, advanced imaging plays a critical role. While transthoracic and transesophageal echocardiography were non-revealing, whole-body FDG PET/CT and CT imaging enabled the identification of multiple secondary infection sites, guiding therapeutic decisions.
There is no general consensus on whether the decision not to remove the pacemaker was based on the lack of clinical, microbiological, or imaging evidence of CIED infection. The American Heart Association recommends that in patients with S. aureus bacteremia and a CIED but no signs of device infection, device removal is not routinely indicated [2,5]. However, this must be approached with caution, as these patients are at increased risk of relapse and mortality. Some observational studies suggest a possible survival benefit with empirical extraction [6], but device removal should remain individualized [7].
An essential limitation of this case lies in the diagnostic capacity of PET/CT imaging. While PET/CT is valuable in detecting metastatic and deep-seated infections, it may fail to identify very early infections at the pacemaker pocket or lead sites. These early localizations may not yet be metabolically active or radiographically apparent at the time of imaging [8]. Therefore, a rigorous and close follow-up after initial positive and subsequent negative blood cultures is essential to exclude device involvement definitively.
Moreover, another significant limitation in managing such cases is the lack of specific, evidence-based guidelines or clear diagnostic criteria for CIED infection outside of definite infective endocarditis. Empirical device removal could be considered the safest approach but may carry procedural risks and adversely affect patient prognosis given the lifesaving nature of the device. Finally, as a single-patient case report, these findings have limited generalizability but importantly highlight the real-world dilemmas clinicians face considering the clinical complexity of patients with persistent MRSA bacteremia including prolonged hospitalization, chronic organ dysfunction such as heart failure and renal insufficiency, diabetes mellitus, and advanced vascular disease which raises significant concerns. This case emphasizes the urgent need for further prospective studies to define clear diagnostic criteria and management algorithms tailored to this vulnerable population.
The patient responded favorably to targeted antimicrobial therapy with clearance of bacteremia confirmed by serial negative blood cultures, supporting a conservative, device-sparing approach in carefully selected cases where clinical, microbiological, and imaging data do not suggest device involvement. However, this strategy requires close monitoring, including repeat blood cultures every 48-72 hours until clearance, followed by clinical reassessment and potential repeat imaging if relapse is suspected [2]. We advocate thorough follow-up with regular clinical review and bloodwork to detect any recurrence or complications.
Clinicians managing persistent MRSA bacteremia in CIED patients should maintain a broad differential diagnosis and consider early advanced imaging, particularly when standard modalities fail to identify a clear source. Conservative management of the CIED may be appropriate in select cases, but close follow-up and interdisciplinary coordination are essential.
Conclusion
This case illustrates that MRSA bacteremia can lead to extensive metastatic infection in the absence of overt endocarditis or CIED pocket involvement. In such scenarios, multimodal imaging including FDG-PET/CT and MRI is invaluable for identifying occult sites of infection.
A conservative approach to CIED management may be reasonable when device infection is not evident, especially when another clear source of bacteremia is established. Targeted antibiotic therapy, informed by imaging and microbiology, was effective in this case. Ultimately, this case reinforces the need for a multidisciplinary and individualized approach in the management of MRSA bacteremia with potential complications.
Informed Consent
Written informed consent was obtained from the patient to publish this report by the journal’s patient consent policy.
Funding Information
The authors acknowledge financial support from F. Miulli Hospital, Acquaviva delle Fonti (BA), Italy, which provided funding for the research and publication of this case report.
Conflict of interest statement
The authors declare that they have no competing interests.
Reference
- Hassoun A, Linden PK, Friedman B. (2017). Incidence, prevalence, and management of MRSA bacteremia across patient populations-a review of recent developments in MRSA management and treatment. Crit Care. 21: 211.
- Baddour LM, Garrigos EZ, Sohail RM, Havers-Borgersen E, Krahn AD, et al. (2024). American Heart Association Council on Lifelong Congenital Heart Disease and Heart Health in the Young (Young Hearts); and Council on Clinical Cardiology. Update on Cardiovascular Implantable Electronic Device Infections and Their Prevention, Diagnosis, and Management: A Scientific Statement From the American Heart Association: Endorsed by the International Society for Cardiovascular Infectious Diseases. Circulation. 149: e201-e216.
- Horino T, Hori S. (2020). Metastatic infection during Staphylococcus aureus bacteremia. J Infect Chemother. 26: 162-169.
- Fowler VG, Durack DT, Selton-Suty C, Athan E, Bayer AS, et al. (2023). The 2023 Duke-International Society for Cardiovascular Infectious Diseases Criteria for Infective Endocarditis: Updating the Modified Duke Criteria. Clin Infect Dis. 77: 518-526.
- Baddour LM, Epstein AE, Erickson CC. (2010). Update on Cardiovascular Implantable Electronic Device Infections and Their Management: A Scientific Statement From the American Heart Association.. Circulation. 121: 458-477.
- Nakajima I, Narui R, Tokutake K. (2021). Staphylococcus Bacteremia Without Evidence of Cardiac Implantable Electronic Device Infection. Heart Rhythm. 18: 752-759.
- Obeid KM, Szpunar S, Khatib R. (2012). Long-term outcomes of cardiovascular implantable electronic devices in patients with Staphylococcus aureus bacteremia. Pacing Clin Electrophysiol. 35: 961-965.
- Brixey AG, Fung A, De Leon AD, Walker CM, Porter KK. (2024). ACR Appropriateness Criteria® Sepsis. J Am Coll Radiol. 21: S292-S309.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.