Ultrasound Assessment of Volume Status in Participants of High-Altitude Mountaineering Expeditions
by Tomasz Górecki1, Jolanta Cylwik2*
1Jagiellonian University, Collegium Medicum, Faculty of Medicine, Medical Education Unit, Cracow, Poland
2Anesthesiology and Intensive Care Unit, Mazovia Regional Hospital in Siedlce, Poland
*Corresponding author: Jolanta Cylwik, Anesthesiology and Intensive Care Unit, Mazovia Regional Hospital in Siedlce, Poland
Received Date: 12 December, 2023
Accepted Date: 18 December, 2023
Published Date: 21 December, 2023
Citation: Górecki T, Cylwik J (2023) Ultrasound Assessment of Volume Status in Participants of High-Altitude Mountaineering Expeditions. J Community Med Public Health 7: 395. https://doi.org/10.29011/2577-2228.100395
Abstract
Introduction: Acute Mountain Sickness (AMS) refers to a common set of clinical symptoms occurring above an altitude of 2500 m. The severity and frequency of AMS symptoms depends on the acclimatization and other factors, such as the hydration status or medication taken (diuretics). Aim: The aim of this study was to assess volume status in participants of high-altitude mountaineering expeditions by evaluating the Inferior Vena Cava (IVC) and abdominal aorta (Ao). Methods: The study group comprised 100 individuals who underwent the ultrasound evaluation of the Inferior Vena Cava Collapsibility Index (IVC CI) and IVC/Ao index as determinants of volume status. Simultaneously, the validity of taking diuretics to prevent ASM was considered. The end point of the study was to demonstrate differences in ultrasound measurements in subjects with AMS and those without AMS and to assess the impact of taking diuretics on the prevention of AMS. Results: In the study group, no statistically significant differences in the ultrasound evaluation of volume status were revealed between subjects meeting AMS criteria (41% of subjects) and asymptomatic individuals (59% of subjects). In the AMS group, IVC CI amounted to 0.33±0.18 vs. 0.32±0.18 in the group without AMS and the IVC/Ao index was 1.35±0.39 vs. 1.30±0.33, respectively. Diuretics were statistically significantly more often taken in the AMS group, but did not have a significant impact on the occurrence of B-line artifacts (indicating HAPE) in the ultrasound assessment. Conclusions: Ultrasonography appears to be a useful tool for the monitoring of volume status in a high-altitude environment. Presently no reliable data exist that would constitute the normal range to be referred to in the context of a single assessment of hydration. The IVC/Ao index may serve as an alternative for IVC CI in the assessment and monitoring of the hydration status.
Keywords: Acute Mountain Sickness (AMS); Inferior Vena Cava Collapsibility Index (IVC CI); Ultrasound assessment of volume status; High-Altitude Pulmonary Edema (HAPE)
Introduction
It is estimated that approximately 140 million people all over the world live at altitudes above 2500 m. Among those, many populations have developed adaptive mechanisms to function at high altitudes [1]. Some of them may, however, display features of chronic mountain sickness characterized by, for instance, pulmonary hypertension or right ventricular heart failure [2].
Each year, thousands of people worldwide enjoy various forms of mountaineering. With large numbers of high-altitude hikers, trekkers and mountaineers, accurate diagnostics and management of pathologies associated with exposure to high altitudes are increasingly significant. It has been revealed that as many as 77% of deaths caused by High-Altitude Pulmonary Edema (HAPE) or High- Altitude Cerebral Edema (HACE) paradoxically occurred in organized trekking groups. It has also been evidenced that in 70% of participants in high-altitude mountaineering expeditions, who developed symptoms of altitude sickness, the acclimatization process was erroneous [3]. Staying for a longer period at altitudes above 2500 m without a proper acclimatization may lead to the development of symptoms of Acute Mountain Sickness (AMS).
Owing to small and portable devices, field ultrasonography performed at altitude is useful in the diagnosis of AMS, and HAPE in particular [4-7]. Chest ultrasound facilitates the differentiation of the etiology of dyspnea based on artifacts. Ultrasonography may also be applied to the evaluation of volume status and the monitoring of patients’ response to the management of hypovolemia [8,9].
Aim
The aim of this study was to assess volume status in participants of high-altitude mountaineering expeditions by evaluating the Inferior Vena Cava (IVC) and Abdominal aorta (Ao). The additional aim was to determine whether there are differences in the intravascular volume between the group with AMS, the group taking diuretics (as AMS prophylaxis), and the group without AMS symptoms.
Material and Methods
Characteristics of the place of the study and the study group
The study to determine volume status in individuals at high altitudes was carried out in Georgia in the Caucasus, at the base of Mount Kazbek. The study was realized within the framework of the medical project ‘Safe Kazbek’. This is a periodical voluntary project of Polish mountain rescuers aimed at providing indispensable medical assistance for mountaineers in the Caucasus, in particular in the area of Mount Kazbek. The study was conducted from 18 July to 21 August 2019.
The study group comprised representatives of both sexes (113 people), participants of both commercial and privately organized expeditions to climb Mount Kazbek (5047 m). The ambulatory (Figures 1 and 2) in which ultrasound examinations were performed was situated at an altitude of 3650 m.

Figure 1: Ambulatory of the ‘Safe Kazbek’ project.

Figure 2: Interior of the ambulatory of the ‘Safe Kazbek’ project.
Exclusion criteria from the study included: lack of consent for the ultrasound diagnostic examination (4 cases), individuals who remained above 2500 m longer than 7 days (7 cases), individuals with the history of Chronic Obstructive Pulmonary Disease (COPD), pulmonary hypertension (2 cases). In total, 100 subjects qualified for the study.
Ultrasound examination protocol
The ultrasound examination was preceded by a physical examination (HR, BP, SpO2, body mass, height, BMI), subjective assessment of AMS according to the Lake Louise AMS Questionnaire (Table 1) and anamnesis (comorbidities, taken medication, acclimatization process) [10]. Data were recorded in a unified form. Ultrasound examinations were performed with a mobile Philips ultrasound unit (Lumify model), with a S4-1 phased array transducer (1-4 MHz) connected to the tablet (10.1 inch).
|
Symptoms |
Severity |
Score |
|
Headache |
- none at all - mild headache - moderate headache - severe headache, incapacitating |
0 1 2 3 |
|
Gastrointestinal symptoms |
- good appetite - poor appetite or nausea - moderate nausea or vomiting - severe nausea and vomiting, incapacitating |
0 1 2 3 |
|
Fatigue and/or weakness |
- not tired or weak - mild fatigue/weakness - moderate fatigue/weakness - severe fatigue/weakness, incapacitating |
0 1 2 3 |
|
Dizziness/light- headedness |
- no dizziness/light-headedness - mild dizziness/light-headedness - moderate dizziness/light-headedness - severe dizziness/light-headedness, incapacitating |
0 1 2 3 |
|
Difficulty sleeping |
- sleep as well as usual - sleep not as well as usual - waking many times, poor sleep - insomnia |
0 1 2 3 |
|
A total score >3 indicates AMS. Notice: AMS diagnosis is primarily based on the clinical picture. Score systems (for instance the Lake Louis score) serve to assess the severity of AMS symptoms. |
||
Table 1: The Lake Louise AMS Questionnaire.
Ultrasound examinations were performed (depending on the possibilities) with patients in the supine position, with the transducer placed in the substernal projection, below the xiphoid process. The Inferior Vena Cava (IVC) and abdominal aorta (Ao) were assessed in the long axis of the anterior midline (Figures 3 and 4).
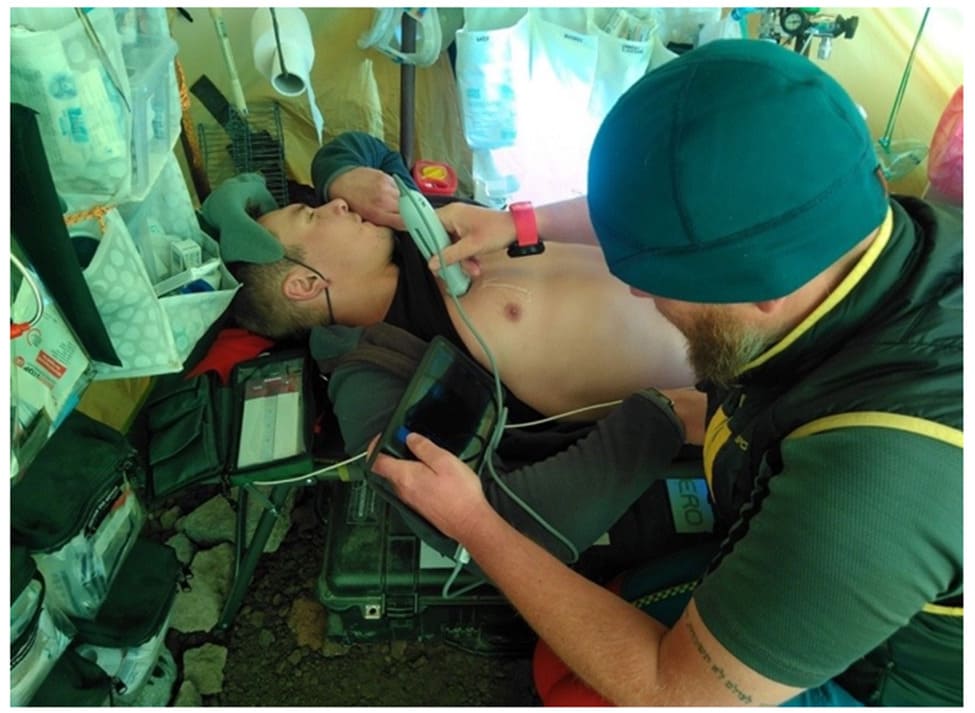
Figure 3: Ultrasound examination in the ambulatory.
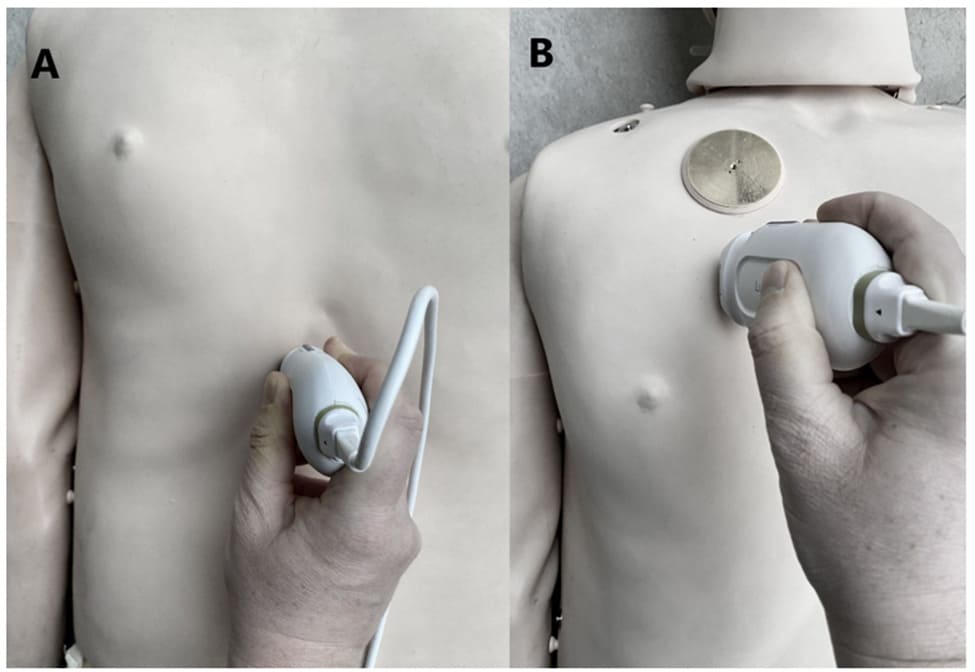
Figure 4: Placement of the transducer in ultrasound assessments a) IVC, Ao assessment, b) lung assessment.
Volume status was assessed by measuring the end-inspiratory and end-expiratory IVC diameter (below the hepatic venous confluence), simultaneously calculating the IVC CI value [(IVC on expiration – IVC on inspiration) /IVC on expiration]. The IVC CI was compared with the IVC assessment and the measurement of Ao taken in the substernal projection, in the long axis about 0.5-1.0 cm from the celiac trunk takeoff (IVC on expiration/Ao) [11-14]. Lungs were evaluated consistent with the BLUE protocol (upper, lower BLUE point, PLAPS point). During lung ultrasound, the presence of B-line artifacts was assessed that might be associated with the development of noncardiogenic HAPE. In justified cases (severe HAPE), lung examinations were performed in the sitting position.
In ambiguous cases and/or questionable measurements, the collected material was transferred via data tele-transmission to a consulting physician in Poland. Images and video material (cineloops) were transferred via the JiveX PACS system (EMD Company).
Statistical analyses
The collected data were analyzed statistically using IBM SPSS Statistics 25.0 software. The software was employed to work out the study group characteristics and analyze basic descriptive statistics, including the Kolmogorov-Smirnov normality test. To compare quantitative data in two groups, Student’s t-test was used for independent variables or Mann-Whitney U test. One-way analysis of variance was performed to compare quantitative data in more than two groups. Pearson’s correlation coefficient was used to establish correlations between quantitative variables. To compare two groups for nominal data, Pearson’s chi-squared test was used, or Fisher’s exact test when the expected number was smaller than 5. The level of significance was α=0.05.
Results
Characteristics of the study group
The study group featured 100 people (40 females and 60 males), ranging in age from 22 to 60 years. They spent in the base camp on average 2.45 days. Table 2 demonstrates the characteristics of the study group. Ultrasound examination results are presented in Table 3.
|
Variable |
Data |
|
Sex, n (%) |
|
|
Female |
40 (40.0) |
|
Male |
60 (60.0) |
|
Age, M (SD) |
39.35 (9.01) |
|
Height, M (SD) |
172.94 (14.67) |
|
Body mass, M (SD) |
73.05 (17.74) |
|
BMI, M (SD) |
23.47 (3.12) |
|
Day of stay in the base camp, n (%) 1 |
17 (17.0) |
|
2 |
34 (34.0) |
|
3 |
36 (36.0) |
|
4 |
13 (13.0) |
|
Summit attempt, n (%) |
32 (32.0) |
|
SpO2, M (SD) |
83.86 (6.76) |
|
HR, M (SD) |
89.86 (15.69) |
|
systolic BP, M (SD) |
124.30 (13.67) |
|
diastolic BP, M (SD) |
76.90 (8.13) |
|
Diuretics (acetazolamide), n (%) |
21 (21.0) |
|
Legend: M–Mean; SD–Standard Deviation; n–Number; %-Percentage of the sample |
|
Table 2: Characteristics of the study group.
|
N |
M |
Me |
SD |
Sk. |
Kurt. |
Min. |
Max. |
D |
p |
|
|
IVC - INSPIRATION |
100 |
1.45 |
1.46 |
0.52 |
0.15 |
-0.30 |
0.43 |
3.07 |
0.07 |
0.200 |
|
IVC - EXPIRATION |
100 |
2.15 |
2.10 |
0.53 |
-0.01 |
-0.85 |
1.00 |
3.22 |
0.07 |
0.200 |
|
Abdominal aorta diameter |
97 |
1.65 |
1.65 |
0.27 |
0.21 |
-0.36 |
0.99 |
2.36 |
0.07 |
0.200 |
|
IVC/Ao |
97 |
1.32 |
1.33 |
0.36 |
0.24 |
-0.17 |
0.60 |
2.17 |
0.06 |
0.200 |
|
IVC CI |
100 |
0.32 |
0.31 |
0.18 |
0.38 |
-0.42 |
0.02 |
0.77 |
0.08 |
0.165 |
|
M–mean; Me–median; SD–standard deviation; Sk.–skewness; Kurt.–kurtosis; Min.–minimum value; Max–maximum value; D–the Kolmogorov-Smirnov normality test statistic; p–probability value |
||||||||||
Table 3: Ultrasound measurements: descriptive statistics with the normality test.
The IVC CI value in the study group was 0.31 (SD=0.18) and was lower than the normal value provided in literature –0.4-0.75. However, the IVC/Ao index was 1.33 ± 0.36 and was slightly higher than that described in literature –1.2±2.0 (SD=0.17).
It was determined whether the number of days spent in the base camp impacted on the results of IVC and Ao measurements (including IVC CI and IVC/Ao indices). In order to establish the correlation between the number of days spent in the base camp and IVC and Ao, Pearson’s correlation coefficient was used. The analyses did not reveal significant correlations between the number of days spent in the base camp and the measured parameters. Additionally, one-way analysis of variance was performed to compare IVC and Ao and the number of days spent in the base camp (subjects were evaluated on their particular day of stay in the base camp) (Table 4).
|
1 day (n=17) |
2 days (n=34) |
3 days (n=36) |
4 days (n=13) |
|
|
|
|||||
|
M |
SD |
M |
SD |
M |
SD |
M |
SD |
F |
p |
η2 |
|
|
IVC - inspiration |
1.58 |
0.61 |
1.46 |
0.48 |
1.40 |
0.53 |
1.39 |
0.45 |
0.52 |
0.666 |
0.02 |
|
IVC - expiration |
2.17 |
0.63 |
2.10 |
0.50 |
2.16 |
0.54 |
2.21 |
0.45 |
0.16 |
0.923 |
0.00 |
|
Abdominal aorta diameter |
1.71 |
0.31 |
1.67 |
0.30 |
1.64 |
0.23 |
1.60 |
0.27 |
0.47 |
0.703 |
0.01 |
|
IVC/Ao |
1.29 |
0.34 |
1.31 |
0.43 |
1.32 |
0.30 |
1.40 |
0.30 |
0.26 |
0.851 |
0.01 |
|
IVC CI |
0.27 |
0.18 |
0.30 |
0.16 |
0.35 |
0.19 |
0.36 |
0.20 |
1.09 |
0.357 |
0.03 |
|
M–mean; SD–standard deviation; F–ANOVA; p–probability value; η2–effect size |
|||||||||||
Table 4: Comparison of IVC and Ao and the number of days in the base camp.
The performed analyses did not reveal significant differences in mean values of parameters between groups that stayed in the base camp from 1 to 4 days. However, the results indicate a gradual normalization of IVC CI among the subjects within 4 days (0.27-0.36) in relation to the normal value and the increase of the IVC/Ao index (1.29-1.40) above the normal value, which may be associated with the acclimatization and/or the necessity of volume supplementation (oral hydration /diuresis/diuretics).
Acute mountain sickness
AMS developed in 41 individuals. The most frequent symptoms included: headache (49%), sleep difficulties (53%) and fatigue / dyspnea (47%). The least frequent symptoms were: diarrhea (5%), nausea/vomiting (8%) and dizziness (6%). The study group was divided into two subgroups: those with AMS symptoms and without AMS symptoms. To compare quantitative data in the AMS group and the non-AMS group Student’s t-test was used for independent variables, and for nominal data Pearson’s chi-squared test was employed, or Fisher’s exact test (Table 5).
|
Non-AMS group (n=58) |
AMS group (n=41) |
95% CI |
|||||||
|
M |
SD |
M |
SD |
t |
p |
LL |
UL |
d-Cohen |
|
|
Age |
39.71 |
10.29 |
38.85 |
6.90 |
0.49 |
0.623 |
-2.58 |
4.28 |
0.09 |
|
Height |
174.36 |
9.21 |
170.90 |
20.05 |
1.16 |
0.249 |
-2.45 |
9.36 |
0.24 |
|
Body mass |
73.02 |
14.72 |
73.10 |
21.56 |
-0.02 |
0.982 |
-7.28 |
7.11 |
0.00 |
|
BMI |
23.81 |
3.13 |
22.98 |
3.07 |
1.30 |
0.195 |
-0.43 |
2.08 |
0.27 |
|
Day of stay in the base camp |
2.54 |
0.92 |
2.32 |
0.93 |
1.20 |
0.233 |
-0.15 |
0.60 |
0.24 |
|
SpO2 |
84.56 |
4.53 |
82.83 |
9.09 |
1.12 |
0.269 |
-1.38 |
4.85 |
0.26 |
|
HR |
87.42 |
14.65 |
93.37 |
16.62 |
-1.89 |
0.062 |
-12.19 |
0.31 |
0.38 |
|
systolic BP |
126.19 |
13.72 |
121.59 |
13.30 |
1.67 |
0.098 |
-0.87 |
10.07 |
0.34 |
|
diastolic BP |
77.20 |
8.06 |
76.46 |
8.31 |
0.45 |
0.657 |
-2.55 |
4.03 |
0.09 |
|
IVC - INSPIRATION |
1.47 |
0.51 |
1.43 |
0.53 |
0.36 |
0.720 |
-0.17 |
0.25 |
0.07 |
|
IVC - EXPIRATION |
2.17 |
0.53 |
2.12 |
0.53 |
0.50 |
0.621 |
-0.16 |
0.27 |
0.10 |
|
Abdominal aorta diameter |
1.69 |
0.27 |
1.60 |
0.28 |
1.58 |
0.117 |
-0.02 |
0.20 |
0.33 |
|
Declared volume of liquid intake (average number of liters per day - l/24 h) |
2.98 |
0.97 |
2.83 |
1.00 |
0.77 |
0.444 |
-0.24 |
0.55 |
0.16 |
|
IVC/Ao |
1.30 |
0.33 |
1.35 |
0.39 |
-0.68 |
0.497 |
-0.20 |
0.10 |
0.14 |
|
IVC CI |
0.32 |
0.18 |
0.33 |
0.18 |
-0.27 |
0.791 |
-0.08 |
0.06 |
0.05 |
|
M–mean; SD–standard deviation; t–t-test statistic; p–probability value; LL and UL–lower and upper level of confidence interval; d Cohen–effect size |
|||||||||
Table 5: Comparison of the AMS group and non-AMS group as regards anthropometric variables and ultrasound assessed volume status parameters.
The groups did not differ as regards anthropometric variables and clinical parameters, period of stay in the base camp and liquid intake. No statistically significant differences were revealed for IVC, Ao and IVC CI and IVC/Ao indices. This indicates that the ultrasound evaluation of volume status is not significant in differentiating patients with AMS and without AMS, apart from the subjective functional assessment with the Lake Louis score.
Next, the impact of the preventive intake of diuretics on the AMS risk (including HAPE) was analyzed. Statistically significant differences were revealed between the two groups (Table 6). Diuretics were more often taken in the AMS group as compared to the non-AMS group. No statistically significant differences were found, however, as regards the occurrence of B-lines in both groups.
|
Non-AMS |
AMS |
||||||
|
n |
% |
n |
% |
χ2 |
p |
φ |
|
|
Diuretics |
|||||||
|
No |
55 |
93.2 |
24 |
58.5 |
17.54 |
<0.001 |
0.42 |
|
Yes |
4 |
6.8 |
17 |
41.5 |
|||
|
B-lines |
|||||||
|
No |
53 |
89.9 |
35 |
85.4 |
0.543 |
0.07 |
|
|
Yes |
6 |
10.2 |
6 |
14.6 |
|||
|
Summit attempt |
|||||||
|
No |
37 |
62.7 |
31 |
75.6 |
1.85 |
0.174 |
0.14 |
|
Yes |
22 |
37.3 |
10 |
24.4 |
|||
|
n–number; %-percentage in the column; χ2–chi-square test statistic; p–probability value; φ–effect size |
|||||||
Table 6: Comparison of the AMS group and non-AMS group as regards diuretics.
High altitude pulmonary edema
Individuals presenting HAPE symptoms were identified in the study group. In the ultrasound evaluation pulmonary edema is manifested by the presence of B-line artifacts. Subjects with HAPE symptoms were compared with the remaining study participants as regards IVC, Ao and transcutaneous oxygen saturation (SpO2). To this end, the Mann-Whitney U test was used. The analyses revealed that individuals with B-lines had a lower SpO2 level and a higher IVC CI value as compared to those without the symptoms of HAPE. Differences for other variables were statistically insignificant. Table 7 demonstrates the results of analyses.
|
norm (n = 88) |
B-lines (n = 12) |
||||||||||
|
Average rank |
M |
Me |
SD |
Average rank |
M |
Me |
SD |
Z |
p |
r |
|
|
SpO2 |
52.16 |
84.83 |
85.00 |
4.63 |
34.33 |
76.83 |
80.00 |
13.37 |
-2.02 |
0.043 |
0.20 |
|
IVC - inspiration |
52.56 |
1.49 |
1.54 |
0.52 |
35.38 |
1.19 |
1.19 |
0.41 |
-1.93 |
0.054 |
0.19 |
|
IVC - expiration |
49.92 |
2.14 |
2.10 |
0.54 |
54.75 |
2.22 |
2.10 |
0.40 |
-0.54 |
0.588 |
0.05 |
|
Abdominal aorta diameter |
49.70 |
1.66 |
1.67 |
0.28 |
43.50 |
1.60 |
1.58 |
0.18 |
-0.69 |
0.491 |
0.07 |
|
IVC/Ao |
47.90 |
1.31 |
1.30 |
0.37 |
57.64 |
1.41 |
1.43 |
0.23 |
-1.08 |
0.280 |
0.11 |
|
IVC CI |
47.32 |
0.30 |
0.29 |
0.17 |
73.79 |
0.47 |
0.44 |
0.17 |
-2.96 |
0.003 |
0.30 |
|
M–mean; SD–standard deviation; Me–median; Z–Mann-Whitney U test standardized statistic; p–probability value; r–effect size |
|||||||||||
Table 7: Comparison of subjects without (norm) and with B-lines in relation to IVC and SpO2.
Fisher’s exact test was employed to compare the percentage of individuals who took and those who did not take diuretics in the group with HAPE (B-lines detected in ultrasound images) and without HAPE (absence of B-lines). The analyses did not reveal significant differences between the groups in this respect. In both groups the percentage of individuals taking acetazolamide was similar and amounted to 20-25%. The results are presented in Table 8.
|
without B-lines |
B-lines |
|||||
|
Diuretics |
n |
% |
n |
% |
p |
φ |
|
No |
70 |
79.5 |
9 |
75.0 |
0.712 |
0.04 |
|
Yes |
18 |
20.5 |
3 |
25.0 |
||
|
n–number; %-percentage in the column; p–probability value of Fisher’s exact test; φ–effect size |
||||||
Table 8: Comparison of the HAPE group with non-HAPE group (presence of B-lines) as regards diuretics.
Discussion
Ultrasonography has been employed in high-altitude environments for many years. The majority of studies concern the occurrence of HAPE, evaluation of hemodynamic efficiency and indirect assessment of the optic nerve when HACE was suspected. Many publications have been also devoted to the hydration status assessment, involving the measurements of IVC and calculations based on IVC CI.
AMS constitutes a common set of clinical symptoms that occur at altitudes above 2500 m. The severity and frequency of AMS symptoms depend on the pace of reaching high altitudes, exposure time to high altitudes, physical strain, health status (individuals with the respiratory tract diseases, such as bronchitis and pneumonia, before and during reaching high altitudes are predisposed), hydration status, taken medication (diuretics) or genetic predispositions [15].
At high altitudes in the mountains the human organism has a natural tendency towards a discrete loss of body fluids (decreased appetite, significant physical strain, diuretics). It has been evidenced that dehydration is a predictor of high-altitude physical performance [16,17].
During the ‘Safe Kazbek’ project, it was observed that a relatively large number of individuals took diuretics (e.g., acetazolamide) to prevent AMS. Acetazolamide belongs to the family of sulfonamide carbonic anhydrase inhibitors. It is also a mild diuretic that has been used as an AMS prophylaxis for decades [18]. In our study group, AMS was detected in 41 cases (41%). In the group with AMS, as many as 17 subjects (41.5%) preventively took diuretics. The medication was self-managed, without a prior consultation with and/or suggestion from leaders of organized groups. No statistically significant differences were revealed between the group that took diuretics and the group that did not take such medication who developed HAPE (B-line artifacts) (20.5% and 25.0%, respectively). This may suggest that preventive diuretics do not decrease the risk of developing complications such as AMS, including HAPE.
The International Climbing and Mountaineering Federation (Union Internationale des Associations d’Alpinisme, UIAA) recommends reaching high altitudes gradually, appropriately extending the process in time, adequate hydration and nutrition as AMS prophylaxis. These recommendations do not include preventive acetazolamide. Taking medication to prevent the symptoms of high altitude should be limited to exceptional situations (e.g., rescue operations, impossibility to reach high altitude slowly).
The ultrasound evaluation of volume status in the AMS group as compared to the non-AMS group did not reveal statistically significant differences, irrespective of the compared parameter (IVC CI, IVC/Ao). In the case of subjects who presented B-line artifacts (HAPE), the IVC CI values were more variable (individuals without B-lines / individuals with B-lines) as compared to the IVC/Ao index. The differences in measurements (although slight) may result from the fact that IVC CI is based on the measurement of the vessel (IVC) the dimension of which depends on many factors (e.g., sex, body mass, age) [19] and also on the quality of ultrasound images obtained and the type of the transducer used. To be calculated, the IVC/Ao index (despite similar dependencies) does not require information concerning body surface. Thus it may serve as an easier and more reliable tool to assess volume status in various clinical conditions in high-altitude medicine. We have been unable to find in available medical literature unequivocally determined normal values for IVC CI and IVC/Ao indices in adults remaining in high-altitude mountainous conditions. We do not have, therefore, a reliable reference point to assess volume status and, for instance, predict the risk of HAPE. The calculated indices (IVC CI and/or IVC/Ao) are much more accurate and useful in assessing responsiveness to the administered fluid therapy than the assessment of volume expansion itself [20-22].
At this point, the study conducted by Pitman JT, et al. [23], devoted to the use of ultrasound in high-altitude mountains to assess intravascular volume and the occurrence of AMS, should be mentioned. Based on the conducted study, the authors provide evidence that subjects with AMS have a higher intravascular volume than asymptomatic individuals. In our study, limited to the ultrasound assessment of volume status with two indices: IVC CI and ICV/Ao, we have not observed any statistically significant differences between individuals with AMS and those without AMS.
A meta-analysis published by Ritchie ND, et al. [24], which offers conclusions concerning acetazolamide for the prevention of AMS, also merits attention. Based on the conducted meta- analysis, the authors concluded that acetazolamide prophylaxis was associated with a 48% relative- risk AMS reduction compared to placebo. This is inconsistent with our results. This incompatibility may result from the fact that our study was based on the subjective assessment of AMS with the Lake Louis score, without considering the impact of side effects of acetazolamide. Additionally, in our study the obtained data did not include the dose/day of the taken diuretics.
Limitations of the Study
The first limitation of the study is the lack of the verification of the Kappa coefficient among researchers who assessed particular ultrasound parameters, in this case IVC CI and IVC/Ao, for high-altitude mountainous conditions. It was not possible to obtain good quality ultrasound images for all subjects (limitations associated with, e.g., obesity or a forced sitting position).
Our study is very likely the first assessment of volume status of this type in a high-altitude environment in which the IVC/Ao index was employed. The IVC/Ao values obtained in our study are similar to the normal values for adults determined at sea level. Establishing the normal value of this index for high altitudes requires further research. The study group was very heterogenous in terms of the acclimatization process, e.g., the pace of reaching high altitudes.
Another limitation concerns the reliability of information given by the participants as regards the declared liquid intake per day and the preventive diuretics taken.
Conclusions
Ultrasonography appears to be a useful tool for the monitoring of volume status in a high-altitude environment. Currently, there is no reliable data regarding the normal value that can be referred to in the context of a single assessment of the hydration status. The employment of the IVC/Ao index may serve as an alternative for IVC CI in the assessment and monitoring of the hydration status. No statistically significant differences were revealed in the ultrasound assessment of volume status between subjects meeting the AMS criteria (subjective functional assessment according to the Lake Louis score) and those without AMS.
Unjustified preventive self-administration of acetazolamide was associated with an increased occurrence of AMS symptoms as compared with the group who did not take diuretics. Taking diuretics did not have a statistical significance for the occurrence of B-line artifacts (HAPE) as compared to individuals who did not take such medication.
References
- Yi X, Liang Y, Huerta-Sanchez E, Jin X, Cuo ZXP, et al. (2010) Sequencing of 50 human exomes reveals adaptation to high altitude. Science 329: 75-78.
- Penaloza D, Arias-Stella J (2007) The heart and pulmonary circulation at high altitudes: healthy highlanders and chronic mountain sickness. Circulation 115: 1132-1146.
- https://www.theuiaa.org/mountain-medicine/medical-advice
- Pratali L, Cavana M, Sicari R, Picano E (2010) Frequent subclinical high-altitude pulmonary edema detected by chest sonography as ultrasound lung comets in recreational climbers. Crit Care Med 38: 1818-1823.
- Lim R, Ma IWY, Brutsaert TD, Nysten HE, Nysten CN, et al. (2019) Transthoracic sonographic assessment of B-line scores during ascent to altitude among healthy trekkers. Respir Physiol Neurobiol 263: 14-19.
- Pratali L (2018) Right Heart-Pulmonary Circulation at High Altitude and the Development of Subclinical Pulmonary Interstitial Edema. Heart Fail Clin 14: 333-337.
- Fain SB, Eldridge MW (2017) Exploring new heights with pulmonary functional imaging: insights into high-altitude pulmonary edema. J Appl Physiol (1985) 122: 853-854.
- Fagenholz PJ, Murray AF, Noble VE, Baggish AL, Harris NS (2012) Ultrasound for high altitude research. Ultrasound Med Biol 38: 1-12.
- Gharahbaghian L, Anderson KL, Lobo V, Huang RW, Poffenberger CM, et al. (2017) Point-of-Care Ultrasound in Austere Environments: A Complete Review of Its Utilization, Pitfalls, and Technique for Common Applications in Austere Settings. Emerg Med Clin North Am 35: 409-441.
- Roach RC, Hackett PH, Oelz O, Bärtsch P, Luks AM, et al. (2018) The 2018 Lake Louise Acute Mountain Sickness Score. High Alt Med Biol 19: 4-6.
- Sridhar H, Mangalore P, Chandrasekaran V, Manikam R (2012) Caval Aorta Index and Central Venous Pressure Correlation in Assessing Fluid Status! “Ultrasound Bridging the Gap”. International Scholarly Research Notices 2012: 828626.
- Fields JM, Lee PA, Jenq KY, Mark DG, Panebianco NL, et al. (2011) The interrater reliability of inferior vena cava ultrasound by bedside clinician sonographers in emergency department patients. Acad Emerg Med 18: 98-101.
- Durajska K, Januszkiewicz E, Szmygel Ł, Kosiak W (2014) Inferior vena cava/aorta diameter index in the assessment of the body fluid status - a comparative study of measurements performed by experienced and inexperienced examiners in a group of young adults. J Ultrason 14: 273-279.
- Ragaisyte E, Bardauskiene L, Zelbiene E, Darginavicius L, Zemaityte E, et al. (2018) Evaluation of volume status in a prehospital setting by ultrasonographic measurement of inferior vena cava and aorta diameters. Turk J Emerg Med 18: 152-157.
- Auerbach PS (2012) High Altitude Medicine and Physiology, Wilderness Medicine, 6th Edition. Chapter 1, 2-33.
- Castellani JW, Muza SR, Cheuvront SN, Sils IV, Fulco CS, et al. (2010) Effect of hypohydration and altitude exposure on aerobic exercise performance and acute mountain sickness. J Appl Physiol (1985) 109: 1792-1800.
- Ladd E, Shea KM, Bagley P, Rundell S, Auerbach PS, et al. (2016) Hydration Status as a Predictor of High-altitude Mountaineering Performance. Cureus 8: e918.
- Murdoch D (2010) Altitude sickness. BMJ Clin Evid 2010: 1209.
- Cheriex EC, Leunissen KM, Janssen JH, Mooy JM, van Hooff JP (1989) Echography of the inferior vena cava is a simple and reliable tool for estimation of 'dry weight' in haemodialysis patients. Nephrol Dial Transplant 4: 563-568.
- Duwat A, Zogheib E, Guinot P, Levy F, Trojette F, et al. (2014) The gray zone of the qualitative assessment of respiratory changes in inferior vena cava diameter in ICU patients. Crit Care 18: R14.
- Feissel M, Michard F, Mangin I, Ruyer O, Faller JP, et al. (2001) Respiratory changes in aortic blood velocity as an indicator of fluid responsiveness in ventilated patients with septic shock. Chest 119: 867-873.
- Michard F, Teboul JL (2002) Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence. Chest 121: 2000-2008.
- Pitman JT, Thapa GB, Harris NS (2015) Field Ultrasound Evaluation of Central Volume Status and Acute Mountain Sickness. Wilderness Environ Med 26: 319-326.
- Ritchie ND, Baggott AV, Andrew Todd WT (2012) Acetazolamide for the prevention of acute mountain sickness--a systematic review and meta-analysis. J Travel Med 19: 298-307.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.