The Striking Power of Amniotic Fluid and Its Exosomes Collected at the End of a Normal Pregnancy on Severe Inflammation and Tissue Repair
by Pascal J. Goldschmidt Clermont*, Brock Sevilla, Alex JP. Goldschmidt, Ian A. White
Neobiosis LLC, UF Sid Martin Innovate Biotechnology Institute, Alachua, FL 32615, USA
*Corresponding author: Pascal J Goldschmidt-Clermont, Neobiosis LLC, UF Sid Martin Innovate Biotechnology Institute, Alachua, USA
Received Date: 25 November 2024
Accepted Date: 29 November 2024
Published Date: 02 December 2024
Citation: Clermont PJG, Sevilla B, Goldschmidt AJP, White IA (2024) The Striking Power of Amniotic Fluid and Its Exosomes Collected at the End of a Normal Pregnancy on Severe Inflammation and Tissue Repair. Ann Case Report. 9: 2099. https://doi.org/10.29011/25747754.102099
Abstract
With this short communication, which contains a new case report on diabetic ulcer, we summarize our research progress in treating several complex conditions at a point where currently available treatments were failing to help the affected patients. We review the first case of lower back pain due to severe spondylitis (T12-L1) treated with intravenous injections of a sterile fraction of human purified amniotic fluid (ViX001) obtained from thoroughly screened volunteers at the time of planned c-section at the term of normal pregnancies. Then, we review the first case of recalcitrant diabetic ulcer treated successfully by twice-daily applications of ViX001 directly on the wound and describe another case of diabetic ulcer treated successfully with ViX001. Next, we review the treatment of severe burns with direct application of ViX001 on the burns leading to accelerated improvement of the burn lesions. Finally, we review the first treatment of a patient with advanced pyoderma gangrenosum with ViX001, with significant reduction of pain, inflammation, and observation of tissue regeneration. We conclude our review with speculations on the mechanism of action of ViX001, the importance of extracellular vesicles in the amniotic fluid for its biological activity, and the need for expanding our studies to generate a better understanding of the omics signature of this incredibly beneficial material.
Keywords: Purified Amniotic Fluid; Diabetic Wound; Diabetic Foot Ulcer; Burns; Low Back Pain; Spondylitis; Pyoderma Gangrenosum; Extracellular Vesicles; Exosomes; Inflammation; Wound Healing; Tissue Repair; Regenerative Medicine; Neoangiogenesis; Therapy; ViX001.
Introduction
Recent developments in regenerative medicine highlight the surprisingly powerful therapeutic potential of amniotic fluid (AF) and its extracellular vesicles (also called exosomes). This review examines four articles, [1–4] covering treatments for complex conditions like severe lower back pain (due to spondylitis) [1], recalcitrant diabetic ulcers [2], second- and third-degree burns [3], and pyoderma gangrenosum [4]. The studies focused on the regenerative capabilities of AF-derived products to not only control pain and inflammation, but to also stimulate tissue repair (regeneration), and improve patient outcomes in otherwise refractory cases when standard of care treatments are unproductive.
Although these studies were each single patient case reports, the consistency of the impact of AF on pain, inflammation, and tissue repair observed in all studies, was supportive of a bonafide biological impact rather than a simple placebo effect. Indeed, the impact on severe pain was almost instantaneous; however, onset of anti-inflammatory effects took in some cases a couple of hours and other cases a couple of days, and impact on tissue repair manifested after days to weeks post treatment initiation. In three of the four cases—lower back pain due to spondylitis, recalcitrant diabetic ulcers, and severe burn lesions—there was complete resolution of the problem [1–3].
ViX001 was approved by the Food and Drug Administration for clinical research involving patients. ViX001 is a fraction of human purified amniotic fluid obtained under aseptic conditions from thoroughly screened volunteers at the time of planned c-section at the term of normal pregnancies [4]. Our product ViX001 was generated through a proprietary process and kept in frozen one or two milliliter (1 or 2 mL) vials (protein content was ~1 gram/ liter) [4]. Vials were thawed prior to administration–intravenous injection in the case of spondylitis and direct (topical) application to lesions in the case of burns, diabetic ulcer, and pyoderma gangrenosum–and kept at 34oF between uses (shelf life at 34oF of at least two weeks) [1-4]. ViX001 therapy has received Food and Drug Administration (FDA) approval for an Investigational New Drug (IND) phase I/II human clinical trial to treat the inflammatory component of post-COVID syndrome with intravenous injections.
Brief case summaries
1. Lower Back Pain: A 65-year-old man with debilitating low thoracic spondylitis (T12-L1) and end plate inflammation experienced significant relief from bi-weekly intravenous injections of ViX001 (one ml/injection) [1]. The patient reported a 50% reduction in pain within the first month and resolution of the pain and inflammation at the end of three months (confirmed by MRI scans showing decreased inflammation of the T12-L1 joint). The patient experienced a notable eighteen-month improvement in symptoms before resurgence of pain; However, after followup, reported that the pain was managed without further need for ViX001 treatment. This case highlights the potential for ViX001based therapies to alleviate chronic pain and inflammation in conditions resistant to standard treatments.
2. Diabetic Ulcers: A recalcitrant diabetic ulcer in a 79-year-old patient responded remarkably to twice-daily topical applications of ViX001, leading to complete healing within 60 days [2]. The rapid resolution of pain and inflammation, as well as the concentric healing process, suggests that ViX001 extracellular vesicles can accelerate wound healing, particularly for cases where conventional treatments have failed.
We had the opportunity to reproduce this successful treatment with a larger and deeper diabetic ulcer that was resistant to conventional therapy and was threatening the patient to cause osteomyelitis, and per his physician report the patient was at risk for below-the-knee amputation. The patient was a 60-year-old man who had developed, two years prior to his accident, type-2 diabetes mellitus confirmed by fasting glycemia above 125 mg/dl and hemoglobin A1c results repeatedly above 6.4%. The patient was moving a refrigerator that fell on him, nailing him to the ground with the refrigerator on top of him. The corner of the refrigerator injured the external aspect of his left ankle (Figure 1).
After signing an informed consent, applications of 200 microliters of ViX001 on top of the crater of the ulcer were initiated every other day, without the need for prior debridement of the ulcer. The ViX001 product was left in place while the patient was holding his ankle strictly still for 15 minutes. Next the ulcer was covered with a sterile Band-Aid. Immediately after the initial application, the ankle discomfort resolved. A dried blood clot that was covering the ulcer partially detached and came off with removal of the Band-Aid (Figure 1). Surrounding inflammation regressed, tissue regeneration was detected at the bottom of the ulcer crater and the edge of the ulcer started contracting. After one month of applications, we could detect small patches of capillaries (neoangiogenesis) at the bottom of the ulcer while cells continued to fill the crater.
After two months, the ulcer was less than half of its original size, and after 2.5 months the ulcer was healed, applications were discontinued. Two months later, the ulcer scar remained intact.
3. Severe burn lesions: A 24-year-old woman was referred to our group after sustaining full-thickness burns on her right posterior thigh and partial-thickness burns on her lower legs and back [3]. Her injuries resulted from an explosion while attending a 4th of July party where fireworks were ignited. One rocket flew at a horizontal angle into the crowd, hitting the patient and exploded. She was promptly rushed to the local emergency room. The injuries to the right leg were cleaned and bandaged and she was sent home with an antibiotic cream and wrap. Pain management was with hydrocodone. The next day, the patient was unable to move her leg due to severe pain (score 6-10/10 and 10/10 during cleaning of the burns).
On day three following the accident, the patient was referred to the burn unit where her legs were debrided. Physicians of the burn unit estimated her total recovery time to be greater than 2 months and skin grafting options were entertained for the full thickness burn lesions. Instead, at the request of the patient, she was treated with ViX001 (4 ml) administered topically over 2 days. Forty-five minutes following application, her pain decreased from 10/10 to 2/10, making it easier to clean the affected area, improving sleep, and allowing more range of motion and autonomy, without the use of opioids. Accelerated recovery time allowed the patient to return to work sooner, avoiding painful and costly skin grafting.
4. Pyoderma Gangrenosum: A 78-year-old patient with advanced pyoderma gangrenosum, a rare and dreadful inflammatory skin condition, showed significant improvement after receiving every third-day applications of ViX001 [4]. Within 30 minutes of the first application, the patient’s pain decreased dramatically, and visible healing of the ulcers occurred over several weeks. This case demonstrates the anti-inflammatory and regenerative potential of ViX001, even for the worst kind of skin lesions.
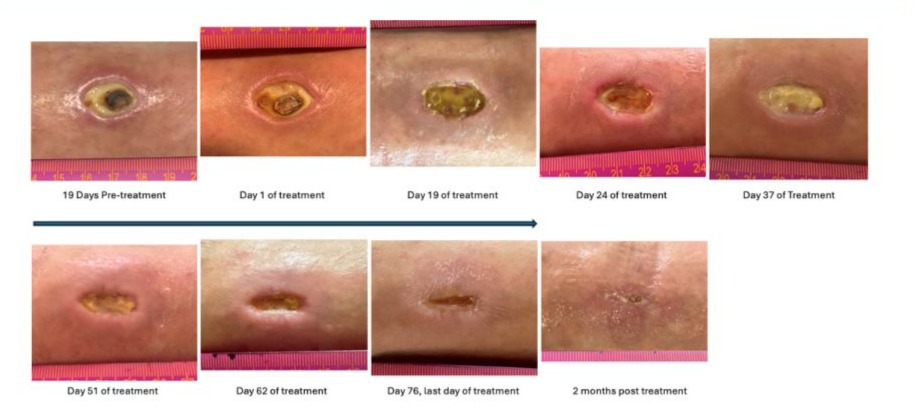
Figure 1: Evolution of the diabetic ulcer for our case report with every other day applications of an aseptic fraction of our human purified amniotic fluid product, ViX001. The upper left picture shows the wound after three months of standard treatment that did not promote healing of the ulcer. The next picture is 19 days later, the day of the first application of ViX001 (200 microliters) showing that if anything, the crater had further enlarged. The next six pictures show the progressive shrinking of the ulcer, which is filling with cells fed by capillaries indicating skin regeneration and contraction of the wound. After 76 days of treatment (11 weeks) the wound had closed, and treatment was discontinued. The last picture obtained two months after treatment discontinuation shows further progression of the skin healing.
Conclusion
The collective evidence from these five case reports highlights the transformative potential of purified amniotic fluid (ViX001) in the treatment of challenging situations where current therapies are limited in their effectiveness: chronic inflammatory disorders like pyoderma gangrenosum [4] diabetic ulcers [2] and spondylitis [1], or acute injuries like severe burns [3]. Further research and clinical trials are warranted to validate these findings, optimize dosing strategies, and explore broader applications of purified amniotic fluid-based therapies in regenerative medicine.
In all cases, ViX001 demonstrated potent anti-inflammatory effects, reducing pain nearly instantly, and inflammation in conditions such as pyoderma gangrenosum, diabetic ulcers, severe burns, and lower back pain [1–4]. ViX001 also promotes tissue regeneration, as evidenced by the healing of diabetic ulcers and severe burn lesions. Perhaps most importantly, whether ViX001 was applied topically or injected intravenously, ViX001 was welltolerated across all case reports, with no significant adverse events reported, even in patients with complex medical histories.
Is it surprising that the ViX001 fraction of purified amniotic fluid has these exceptional anti-inflammatory and regenerative properties? Not really. Human amniotic fluid has been refined over at least seven million years of evolution [5]. We have described pregnancy as a unique case of heterochronic parabiosis, where the fetus is basically “plugged-in” to the maternal body via the placenta that is anchored into the uterus of the mother, with the umbilical cord connecting the placenta to the baby to allow for the formidable pregnancy machinery to happen and function [5]. Not only does amniotic fluid support the development of the fetus, but it is also utilized by the mother to support and enhance her physiology in response to the rigors of pregnancy and childbirth.
Such a connection is complicated because 50% of the genes of the fetus are inherited from the father of the baby, and the resulting antigens theoretically are exposed to rejection by the immune system of the mother. The maternal and fetal circulations are selectively connected and interact primarily through the exchange of plasma and exosomes, but not cells (nearly insignificant quantities of intact cells are exchanged between mother and fetus). These exosomes, produced by the placenta, amnion, and fetal kidneys, carry essential molecules such as lipids, proteins, complex carbohydrates, microRNAs (miRs), and Klotho that support fetal development and maternal adaptation [6].
The amniotic fluid is a pink opalescent liquid that surrounds and protects the developing fetus during pregnancy [7]. It is produced early in pregnancy from maternal (plasma) and fetal sources that include the placenta, the coelomic fluid, and fluid filtered through the amniotic membrane [6,8,9]. Later in pregnancy, it is further modified though swallowing by the fetus and absorption through the gastrointestinal tract and excretion through the kidneys [6,10]. It is also aspirated through the airways where it mixes with lung secretions. It plays several essential roles in fetal development and overall pregnancy health [6,7,11,12]. It prepares the lungs for birth and initial contact with ambient oxygen in air. It also fills the intestine, covers the skin, the eyes, fills upper airways, and thus it is no surprise that it possesses unique protective properties for these tissues [6,13].
Another critical role of the amniotic fluid is to surround and support the formation of the nervous system, from differentiation of the ectoplasm to neurulation and maturation of the brain and spinal cord [14]. It is also the source of the original cerebrospinal fluid [15]. Hence, it will be instructive to study the impact of amniotic fluid on the development of neurodegenerative disorders and perhaps their prevention or regression. We recently had the opportunity to evaluate the impact of exosomes produced by Schwann cells (SCE) obtained from healthy donors on the progression of amyotrophic lateral sclerosis (ALS) in a severely affected patient [16] using a protocol approved by the FDA. It will be instructive to compare the effectiveness of SCE versus amniotic fluid exosomes because of the involvement of the latter exosomes in the development of the nervous system.
Across these studies, a recurring theme is the ability of exosomes, whether derived from amniotic fluid or other sources, to mediate significant therapeutic effects through modulation of pain, inflammation, promotion of tissue repair, and stabilization of disease progression or regression [5,16–20] Notably, evidence indicates that the therapeutic effects of amniotic fluid are primarily mediated by exosomes [18,20–23]. Antounians et al. demonstrated that, when compared to the rest of the secretome from rat amniotic fluid stem cells, only small EVs (exosomes) specifically mediated the beneficial effects, highlighting their pivotal role in regenerative processes [20].
These vesicles appear to exert their effects both locally (e.g., paracrine effect at sites of tissue injury) [19,21,22]. and systemically (e.g., endocrine effect via intravenous circulation) [22,24] [23], underscoring their versatility in treating a wide range of conditions. These myriad uses of exosomes coupled with low immunogenicity and ease of obtainment necessitate further study investigating their efficacy, optimal dosing, and long-term outcomes [21].
To further unlock the therapeutic potential of exosomes of amniotic fluid (ViX001), comprehensive multi-omic studies are essential to elucidate their signature molecular contents and mechanisms of action. By employing proteomic, genomic, and metabolomic analyses, researchers can identify the specific bioactive molecules within exosomes that are responsible for their formidable antiinflammatory and regenerative effects. Understanding these molecular signatures will not only shed light on the pathways through which ViX001 exosomes exert their effects but also pave the way for the development of targeted therapies with enhanced efficacy.
Acknowledgements: We extend our sincere gratitude to our patients and their families for their unwavering support and participation in these studies. Their contribution was invaluable to our research. We also thank Neobiosis LLC for their exceptional work in manufacturing the proprietary purified amniotic fluid fraction (ViX001) used in our case studies. Additionally, we are grateful to the United States Army for supporting and funding our team member, Brock Sevilla, through the Career Skills Program.
References
- Goldschmidt-Clermont PJ, Wolf AL, Goldschmidt AJ, White IA. (2023) First Case of Debilitating Lower Back Pain Induced by Severe Inflammation of Lumbar Joints, Healed by Intravenous Infusions of Purified Amniotic Fluid. Ann Case Rep.8:1509.
- Goldschmidt-Clermont PJ, White IA. (2023) First Case of Accelerated Healing of a Recalcitrant Diabetic Ulcer Using Purified Amniotic Fluid. Ann Case Rep. 8:1463.
- Kweh MF, Parks M, Salerno A, Vaughn NE, White IA. (2023) Topical Application of Purified Amniotic Fluid Accelerated Healing of FullThickness Burns, Negating the Need for Skin Grafts: A Case Report. J Wound Manag Res. 19:53-58.
- Goldschmidt-Clermont PJ, Plasencia K, Goldschmidt AJ, White IA. (2023) First Case of Severe Pain and Inflammation Reduction by Application of Purified Amniotic Fluid on Active Lesions of a Patient with Pyoderma Gangrenosum. Ann Case Rep. 8:1566.
- Goldschmidt-Clermont PJ, Hubinont C, Goldschmidt AJP, DiFede DL, White IA. (2021) Pregnancy, a unique case of heterochronic parabiosis and peripartum cardiomyopathy. Front Biosci Landmark Ed26:666672.
- Underwood MA, Gilbert WM, Sherman MP. (2005) Amniotic fluid: not just fetal urine anymore. J Perinatol Off J Calif Perinat Assoc. 25:341348.
- Tong XL, Wang L, Gao TB, Qin YG, Qi YQ, Xu YP. (2009) Potential Function of Amniotic Fluid in Fetal Development—Novel Insights by Comparing the Composition of Human Amniotic Fluid with Umbilical Cord and Maternal Serum at Mid and Late Gestation. J Chin Med Assoc. 72:368-373.
- Cho CKJ, Shan SJ, Winsor EJ, Diamandis EP. (2007) Proteomics Analysis of Human Amniotic Fluid. Mol Cell Proteomics. 6:1406-1415.
- Jauniaux E. (2000) Fluid compartments of the embryonic environment. Hum Reprod Update. 6:268-278.
- Beall MH, Van Den Wijngaard JPHM, Van Gemert MJC, Ross MG. (2007) Regulation of Amniotic Fluid Volume. Placenta. 28:824-832.
- Moore TR. (2011) The Role of Amniotic Fluid Assessment in Evaluating Fetal Well-Being. Clin Perinatol. 38:33-46.
- Beall MH, Van Den Wijngaard JPHM, Van Gemert MJC, Ross MG. (2011) Amniotic Fluid Water Dynamics. Placenta. 28:816-823.
- Murphy S, Lim R, Dickinson H, Acharya R, Rosil S, et al. (2007) Human Amnion Epithelial Cells Prevent Bleomycin- Induced Lung Injury and Preserve Lung Function. Cell Transplant. 20:909-924.
- Jang Y, Kim EK, Shim WS, Song KM, Kim SM. (2015) Amniotic fluid exerts a neurotrophic influence on fetal neurodevelopment via the ERK/GSK-3 pathway. Biol Res. 48:44. Chau KF, Springel MW, Broadbelt KG, et al. (2015) Progressive Differentiation and Instructive Capacities of Amniotic Fluid and Cerebrospinal Fluid Proteomes following Neural Tube Closure. Dev Cell. 35:789-802.
- Chau KF, Springel MW, Broadbelt KG, Park H yeon, Topal S, Lun MP, et al. Progressive Differentiation and Instructive Capacities of Amniotic Fluid and Cerebrospinal Fluid Proteomes following Neural Tube Closure. Dev Cell. 2015 Dec;35(6):789–802.
- Goldschmidt-Clermont PJ, Khan A, Jimsheleishvili G, Graham P, Brooks A, Silvera R, et al. Treating amyotrophic lateral sclerosis with allogeneic Schwann cell–derived exosomal vesicles: a case report. Neural Regen Res. 2025 Apr;20(4):1207–16.
- Zhang Y, Yan J, Liu Y, Chen Z, Li X, Tang L, et al. Human Amniotic Fluid Stem Cell-Derived Exosomes as a Novel Cell-Free Therapy for Cutaneous Regeneration. Front Cell Dev Biol. 2021 Jun 21;9:685873.
- Hu P, Yang Q, Wang Q, Shi C, Wang D, Armato U, et al. Mesenchymal stromal cells-exosomes: a promising cell-free therapeutic tool for wound healing and cutaneous regeneration. Burns Trauma. 2019;7:38.
- Del Rivero T, Milberg J, Bennett C, Mitrani MI, Bellio MA. Human amniotic fluid derived extracellular vesicles attenuate T cell immune response. Front Immunol. 2022 Nov 28;13:977809.
- Antounians L, Catania VD, Montalva L, Liu BD, Hou H, Chan C, et al. Fetal lung underdevelopment is rescued by administration of amniotic fluid stem cell extracellular vesicles in rodents. Sci Transl Med. 2021 Apr 21;13(590):eaax5941.
- Wiklander OPB, Brennan MÁ, Lötvall J, Breakefield XO, El Andaloussi S. Advances in therapeutic applications of extracellular vesicles. Sci Transl Med. 2019 May 15;11(492):eaav8521.
- Tannetta D, Dragovic R, Alyahyaei Z, Southcombe J. Extracellular vesicles and reproduction–promotion of successful pregnancy. Cell Mol Immunol. 2014 Nov;11(6):548–63.
- Costa A, Quarto R, Bollini S. Small Extracellular Vesicles from Human Amniotic Fluid Samples as Promising Theranostics. Int J Mol Sci. 2022 Jan 6;23(2):590.
- Lagunas-Rangel FA. Aging insights from heterochronic parabiosis models. Npj Aging. 2024 Aug 17;10(1):38.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.