Introduction
The human body functions as a complex interplay of various systems and organs, with recent research shedding light on the intricate relationship between the GM and bone health [1]. The GM, an extensive community of microorganisms in the gastrointestinal tract, has been recognized for its pivotal role in influencing several physiological processes crucial for maintaining overall health [2]. This examination delves into the multifaceted connections between the GM and bone health, exploring the composition and significance of the GM, the fundamentals of bone health, and the emerging evidence pointing towards a mutualistic relationship between the two [3].
Overview of the GM
The GM is a complex and dynamic ecosystem residing in the gastrointestinal tract, comprising trillions of microorganisms, including bacteria, viruses, fungi, and archaea [4,5]. This microbial community is pivotal in maintaining overall health and homeostasis within the human body [6].
Composition
The GM's composition is diverse and varies between individuals. It is shaped by numerous factors, including genetics, diet, age, lifestyle, and environmental exposures [7]. The predominant phyla in the GM include Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria. Each individual's microbiome is unique, resembling a fingerprint that reflects their distinct genetic makeup and life experiences [8–10].
Functions
The functions of the GM are multifaceted. It actively participates in digestion and nutrient absorption, breaking down complex carbohydrates and producing enzymes that the human body cannot generate independently [11,12]. Additionally, the GM contributes to the synthesis of certain vitamins, such as B vitamins and vitamin K, which are essential for various physiological processes [13].
Immune System Regulation
The GM plays a crucial role in training and modulating the immune system. It helps distinguish between harmful pathogens and beneficial microorganisms, contributing to the development of a well-balanced and responsive immune system (figure 1) [14,15]. An imbalanced GM has been associated with immune-related disorders and inflammatory conditions [16].
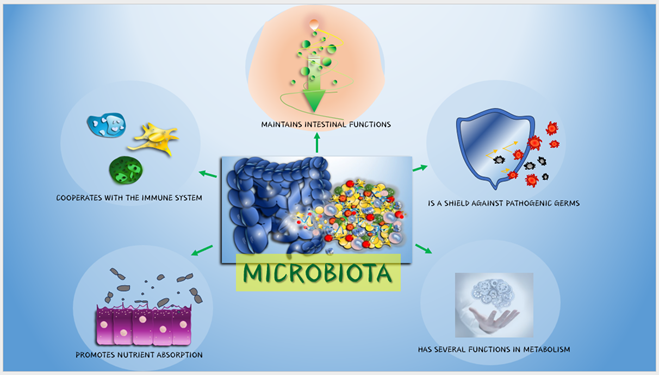
Figure 1: Functions of the GM in the human body.
Metabolism and Energy Homeostasis
The GM influences host metabolism and energy regulation. It can extract additional energy from nutrients, affecting the host's overall energy balance [17]. Imbalances in the GM composition have been linked to conditions such as obesity and metabolic disorders, emphasizing its role in maintaining metabolic homeostasis [18,19].
Influence of External Factors
Various external factors impact the GM. Dietary choices, antibiotic use, lifestyle, and environmental exposures can alter the composition and diversity of the microbiota [20]. A diet rich in fiber and diverse nutrients promotes microbial diversity, contributing to a resilient and healthy GM (figure 2) [21,22].
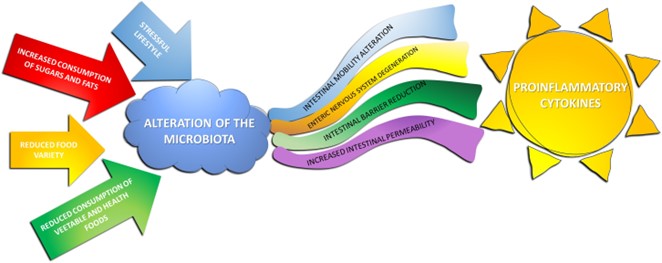
Figure 2: A stressful lifestyle and improper diet can alter GM with gut consequences and inflammatory response by cytokines.
Gut-Brain Axis
An intriguing aspect of the GM is its communication with the central nervous system, forming the gut-brain axis [23]. This bidirectional communication involves neural, hormonal, and immunological pathways, influencing gastrointestinal function and cognitive and emotional processes [24]. Disruptions in the GM have been associated with mental health disorders, highlighting the intricate link between the gut and the brain [25].
Interconnectedness with Overall Health
The GM's impact extends beyond the gastrointestinal system, influencing various aspects of overall health [26]. Imbalances in the GM have been implicated in conditions such as inflammatory bowel disease, allergies, autoimmune disorders, and even systemic diseases like cardiovascular disease [27].
In conclusion, the GM is a fascinating and essential component of human biology, with its intricate interactions influencing various physiological processes. Understanding the composition, functions, and factors influencing the GM is crucial for unlocking its potential to maintain health and prevent a spectrum of diseases [28,29]. Ongoing research continues to unveil the complexities of this dynamic ecosystem, opening avenues for personalized medicine and innovative therapeutic interventions [30].
Bone Health
Bones are the structural foundation of the human body, providing support, protection for vital organs, and serving as a reservoir for essential minerals [31–34]. The maintenance of optimal bone health is crucial for overall well-being, and it involves a dynamic balance between bone formation and resorption throughout one's life [35,36].
Structure and Function
Bones comprise a dense matrix of collagen fibers and mineralized calcium and phosphorus crystals, forming a strong and flexible framework [37–41]. This structure not only supports the body's weight but also protects organs and facilitates movement [42]. Bone marrow, found within bones, is responsible for hematopoiesis, the production of blood cells [43–57].
Bone Cells
Two primary types of cells govern bone health: osteoblasts and osteoclasts. Osteoblasts are responsible for synthesizing and depositing new bone tissue, contributing to bone formation [58]. In contrast, osteoclasts break down and resorb bone tissue, allowing for remodeling and the release of minerals back into the bloodstream [59,60].
Bone Remodeling
Bone remodeling is a continuous and dynamic process that occurs throughout life. It involves the removal of old or damaged bone tissue by osteoclasts and the subsequent replacement with new bone tissue by osteoblasts [61]. This process is crucial for maintaining bone strength, adapting to mechanical stress, and repairing micro-damages within the bone structure [62,63].
Importance of Calcium and Vitamin D
Calcium and vitamin D are pivotal for bone health [64]. Calcium is a fundamental mineral that provides structural integrity to bones, and adequate levels in the bloodstream are necessary for various physiological functions [65]. Vitamin D facilitates the absorption of calcium from the intestines, promoting its incorporation into bones. Calcium and vitamin D deficiency can lead to weakened bones and an increased risk of fractures (figure 3) [66,67].
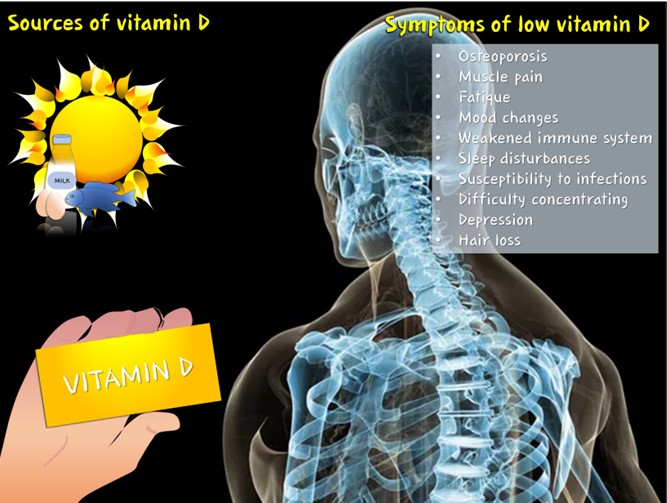
Figure 3: The importance of vitamin D (sources and symptoms of its deficiency).
Interconnection between vitamin K2 and vitamin D
Vitamin K2 intervenes directly in the fixation of calcium in bones. Vitamin K2 delivers vitamin D into the circulation to support the activity of D in calcium fixation [68,69]. In the presence of dysbiosis, the level of vitamin D below 30 ng/mL leads to the certainty of severe osteoporosis and influences bone regeneration, the repair of fractures, in short, in the various areas of medicine and implantology and the process of bone atrophy, which is much more rapid and violent the lower the vitamin D level [70]. In order to be healthy, the vitamin D level must always be above 60 ng/mL on a constant basis, and whenever it falls below 50 ng/mL, the organism suffers severely, and the more the level falls below 50 ng/mL, the more the general condition of the organism suffers [71–73]. As the D level falls, the bones suffer from severe calcium deficiency [74–90].
Factors Affecting Bone Health
Several factors influence bone health throughout one's life. During childhood and adolescence, proper nutrition, physical activity, and adequate calcium intake are critical for achieving optimal peak bone mass [91,92]. Aging, hormonal changes, and lifestyle factors such as smoking, excessive alcohol consumption, and sedentary behavior can negatively impact bone density and increase the risk of conditions like osteoporosis [93].
Osteoporosis
Osteoporosis is a common bone-related condition characterized by reduced bone density and increased susceptibility to fractures [94]. It often occurs in postmenopausal women due to hormonal changes but can affect men and women of all ages [95–97]. Osteoporosis is a major public health concern, emphasizing the importance of preventive measures and interventions to maintain bone health [98].
Exercise and Physical Activity
Weight-bearing exercises, resistance training, and physical activity play a crucial role in maintaining bone health [99–101]. These activities stimulate bone formation, enhance bone density, and improve overall bone strength. Regular exercise also helps maintain joint flexibility and balance, reducing the risk of falls and fractures [102].
Preventive Measures and Treatment
Preventive measures for maintaining bone health include a balanced diet rich in calcium and vitamin D, regular physical activity, and lifestyle modifications [103]. In cases where bone health is compromised, medical interventions such as medications, hormone therapy, and dietary supplements may be recommended [104–108]. Early detection and management of bone-related conditions are essential for preserving bone strength and preventing complications [109–111].
In conclusion, bone health is integral to overall well-being and requires a holistic approach encompassing proper nutrition, physical activity, and lifestyle choices [112–114]. Understanding the intricate processes of bone formation and resorption, along with the factors influencing bone health, empowers individuals to take proactive measures to preserve their skeletal integrity throughout life [115].
GM and Bone Metabolism
The relationship between the GM and bone metabolism is an evolving area of research, uncovering intricate connections between the gastrointestinal tract's microbial inhabitants and the maintenance of skeletal health [116–118]. This section explores how the GM influences nutrient absorption, particularly calcium and vitamin D, and the role of microbial metabolites, such as short-chain fatty acids (SCFAs), in modulating bone metabolism [119].
Nutrient Absorption
One of the crucial contributions of the GM to bone metabolism lies in its influence on nutrient absorption, particularly calcium and vitamin D. Calcium, a fundamental mineral for bone health, is absorbed in the small intestine [120]. The gut helps regulate the solubility and bioavailability of calcium, impacting its absorption [121]. Additionally, vitamin D, synthesized in the skin or obtained through diet, undergoes further activation in the liver and kidneys, processes influenced by the GM [122–124]. Efficient absorption of both calcium and vitamin D is essential for optimal bone mineralization and overall skeletal integrity [125–142].
Microbial Metabolites and Bone Health
The GM's fermentation processes yield various metabolites, with SCFAs emerging as key players in the gut-bone axis [143–147]. SCFAs, including acetate, propionate, and butyrate, are produced through the microbial fermentation of dietary fibers [148,149]. These metabolites not only contribute to energy metabolism but also impact bone cells. Research suggests that SCFAs can influence osteoclast and osteoblast activity, the cells responsible for bone resorption and formation, respectively [150–152]. The balance between these processes is crucial for maintaining bone homeostasis [153].
Specific Bacteria and Bone Health
Recent studies have identified specific bacterial strains associated with positive or negative effects on bone health [154–156]. For instance, some species within the Lactobacillus and Bifidobacterium genera have been linked to increased calcium absorption and improved bone density [157]. On the other hand, dysbiosis, an imbalance in the GM composition, has been associated with conditions like inflammatory bowel disease, which may impact bone health negatively [158–162]. Understanding the role of specific bacteria in bone metabolism offers potential targets for therapeutic interventions [163].
Immune System Modulation
The GM's influence extends to immune system regulation, affecting bone health [164–166]. Inflammatory responses can influence bone metabolism, and the gut microbiota plays a role in modulating immune function [167–169]. Dysregulation of the immune system, often associated with imbalances in the GM, can contribute to conditions such as osteoporosis and rheumatoid arthritis [170–185].
Bile Acids and Bone Health
Bile acids, essential for lipid absorption, also contribute to the gut-bone axis [186–188]. The GM influences bile acid metabolism, and certain bile acids have been implicated in bone metabolism [189–191]. These acids can act as signaling molecules, affecting bone cells and contributing to the regulation of bone homeostasis [192].
Hormonal and Neural Pathways
The gut and bone communication involves intricate hormonal and neural pathways [193–195]. Hormones such as leptin, ghrelin, and serotonin, which play roles in both gut function and bone metabolism, mediate this crosstalk [196–200]. The gut-brain-bone axis reflects the interconnectivity of these systems, emphasizing the holistic nature of bone health [201].
Understanding the dynamic interplay between the GM and bone metabolism holds promise for developing targeted interventions to promote skeletal health [202–204]. As research in this field advances, it may pave the way for innovative therapies and personalized strategies aimed at optimizing the gut-bone axis for enhanced overall well-being [205].
Experimental Evidence of the Impact of the GM on Bone Health
Scientific investigations utilizing both animal models and human trials have provided compelling experimental evidence supporting the notion that the GM significantly influences bone health [206]. These studies have offered insights into the complex interplay between the gut microbiota and bone metabolism, shedding light on mechanisms, microbial metabolites, and potential therapeutic interventions [207–225].
Animal Models
Germ-Free Mice Studies: Experimental evidence often relies on germ-free mice, which are bred and raised in sterile conditions without any exposure to microorganisms [226–228]. Comparisons between germ-free mice and conventionally raised mice have revealed distinct bone density and structure differences. Germ-free mice often exhibit altered bone phenotypes, emphasizing the impact of the absence of a microbiota on skeletal development [229].
Microbiota Transplantation Studies: Transplanting gut microbiota from donor mice to germ-free mice has been a crucial experimental approach [230–232]. These studies demonstrated that the introduction of a diverse and healthy microbiota positively affects bone health in recipients [233–235]. Conversely, transplanting microbiota from diseased or imbalanced donors may lead to compromised bone density and structure [236].
Human Trials
Probiotics and Bone Health: Clinical trials exploring the effects of probiotics on bone health have provided encouraging results [237–239]. Probiotics, which are beneficial microorganisms, can modulate the GM [240–242]. Certain strains of probiotics have been associated with increased calcium absorption and improved bone density in both animal models and human subjects [243].
Short-Term Antibiotic Use and Bone Density: Studies investigating the impact of short-term antibiotic use on GM and bone health have revealed noteworthy findings [244]. Antibiotics, while effective in treating bacterial infections, can disrupt the balance of the gut microbiota. Such disruptions have been linked to reduced bone density and altered bone metabolism in experimental settings [245].
Identification of Specific Bacterial Strains
Akkermansia muciniphila and Bone Health: Research has identified specific bacterial strains associated with positive effects on bone health [246]. For example, Akkermansia muciniphila, a mucin-degrading bacterium, has been linked to enhanced bone density [247]. Experimental studies involving the supplementation of A. muciniphila have demonstrated positive effects on bone health, highlighting the potential role of specific microbes in promoting skeletal integrity [248].
Role of Butyrate-Producing Bacteria: Bacteria that produce SCFAs, particularly butyrate, have garnered attention for their impact on bone metabolism [249]. Experimental evidence suggests that butyrate-producing bacteria contribute to bone health by influencing the activity of osteoblasts and osteoclasts, the cells responsible for bone formation and resorption [250].
Microbial Metabolites
SCFAs and Bone Remodeling: The production of SCFAs by the gut microbiota, especially acetate, propionate, and butyrate, has been linked to bone remodeling [251]. SCFAs can influence osteoclast and osteoblast activity, thus regulating the delicate balance between bone resorption and formation [252–254]. Animal studies have demonstrated that SCFAs contribute to maintaining optimal bone density [255].
Indole and Bone Health: Another microbial metabolite, indole, has been implicated in bone health [256]. Experimental evidence suggests that indole may positively influence bone density by modulating the differentiation and activity of bone cells [257–259]. These findings underscore microbial metabolites' diverse and dynamic role in bone metabolism [260].
In conclusion, a wealth of experimental evidence from both animal models and human trials supports the significant impact of the GM on bone health [261]. These studies have unraveled complex mechanisms, identified specific microbial strains, and highlighted the role of microbial metabolites in influencing bone metabolism [262–264]. This knowledge provides a foundation for further exploration and the development of targeted interventions to optimize the gut-bone axis for improved skeletal health [265].
Exploring the Interconnected Landscape of Interleukins, Periodontitis, and COVID-19: A Comprehensive Analysis
The intersection of interleukins, chronic periodontitis, and COVID-19 unveils a fascinating landscape of genetic intricacies, immune responses, and systemic implications. As we delve into this complex tapestry, it is essential to contextualize the broader understanding of susceptibility and varied responses to the SARS-CoV-2 virus [266–268]. The provided speech emphasizes the multifaceted factors influencing SARS-CoV-2 infection and the highly variable responses observed in individuals, with a particular focus on genetic predisposition, immune defenses, and pre-existing conditions [269].
Genetic Predisposition and SNPs: Unveiling Susceptibility and Severity
The investigation into single nucleotide genetic polymorphisms (SNPs) serves as a genetic gateway to deciphering individual responses to COVID-19. SNPs associated with COVID-19 susceptibility and severity provide a lens through which we can examine the nuanced genetic landscape [270–274]. Genomic variations, especially those pertaining to immune-related genes, play a pivotal role in shaping an individual's response to viral infections [275]. The genetic makeup of hosts significantly influences disease progression, and understanding these genetic nuances is paramount for personalized medicine approaches in managing COVID-19 [276–279]. In the pursuit of unraveling the genetic underpinnings, the study focuses on key interleukin genes, including IL1β, IL1RN, IL6, IL6R, IL10, IFNγ, TNFα, ACE2, SERPIN3, VDR, and CRP [280]. These genes are not only integral to proinflammatory and immunomodulatory responses but are also considered crucial in the progression and complications of COVID-19. The genetic variations in these key genes set the stage for a diverse array of immune responses and contribute to the observed heterogeneity in disease outcomes [281].
Immune Responses and Lymphocyte Dynamics in COVID-19
Moving beyond genetics, the speech draws attention to the dynamic immune responses observed in COVID-19. Lymphocytes, pivotal players in the immune system, exhibit a notable decline in patients with COVID-19. The immune landscape, as reflected in the alteration of lymphocyte subsets, establishes a significant association with the inflammatory status in COVID-19 [282]. Neutrophils, T-killer cells, T-active cells, T-suppressor cells, and T-CD8+CD38+ cells emerge as crucial actors in the immune response to COVID-19 and COVID-like individuals. The intricate dance of immune cells, especially T-lymphocytes and B-lymphocytes, takes center stage in predicting the severity of COVID-19 and the potential efficacy of therapeutic interventions [283–287]. The study suggests that the levels of these cell subsets could serve as independent predictors of the disease's severity, paving the way for a more nuanced understanding of immune dynamics in COVID-19 [288].
Systemic Implications and Multi-Organ Involvement in COVID-19
As we traverse the systemic implications of COVID-19, the speech illuminates the multi-organ involvement observed in infected individuals, particularly affecting the lungs, kidneys, and heart [289]. Pre-existing comorbidities, including cardiovascular, respiratory, and renal disorders, coupled with severely low vitamin D, extremely high IL-6, and low glomerular filtration rate (eGFR), contribute to the overall complexity of the disease [290].
The immune-mediated responses, characterized by an overexpression of proinflammatory cytokines like IL-6, gain prominence as potential culprits in exacerbating COVID-19 severity [291–295]. Noteworthy is the interconnected web where reduced vitamin D levels, age-related factors, and the presence of metabolic disorders of inflammatory and autoimmune origin intertwine with immune responses, contributing to the observed multi-organ involvement [296,297].
Chronic Periodontitis: A Gateway to Understanding Systemic Dysbiosis
The narrative extends to chronic periodontitis (CP), a multifactorial disease intricately linked to oral health and systemic immune responses (figure 4) [298–300].
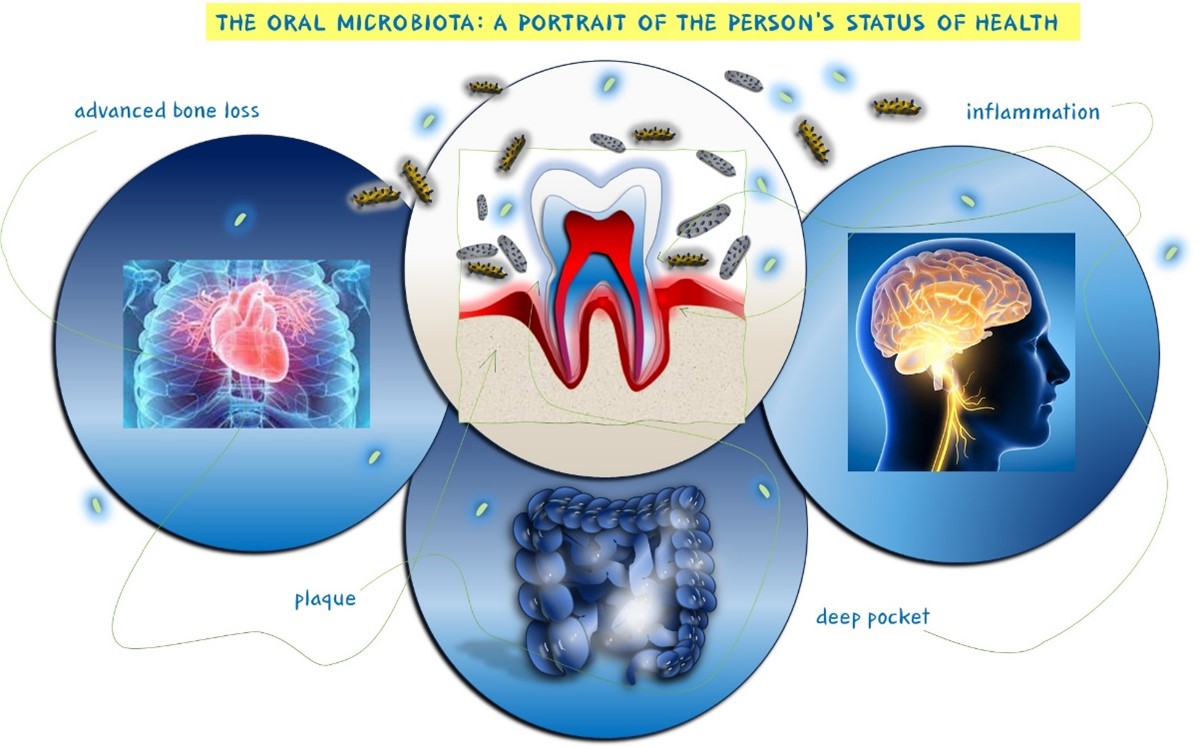
Figure 4: Correlation between oral microbiota and CP, a multifactorial disease related to the overall health of the individual.
CP is not confined to its oral manifestations but is positioned as part of a more complicated systemic disease often co-existing with obesity and metabolic syndrome (MetS) [301]. The association between metabolic syndrome and periodontal inflammation, driven by the elevated presence of lipopolysaccharide (LPS) triggering an immune response, underlines the broader clinical implications of CP [302].
The study bridges the gap between genetics, oral dysbiosis, bacteremia, and CP (figure 5). Specific genetic profiles, particularly those involved in bone metabolism (VDRs, COLIA1) and immune responses (IL-10, TNF-α, IL-1α, 1β, 1RN), play a crucial role in CP susceptibility. The high presence of pro-inflammatory cytokines, including TNF-α, GM-CSF, and IL-6, released from adipose tissue in individuals with MetS and CP, paints a vivid picture of the intricate connections between systemic and oral health [303].
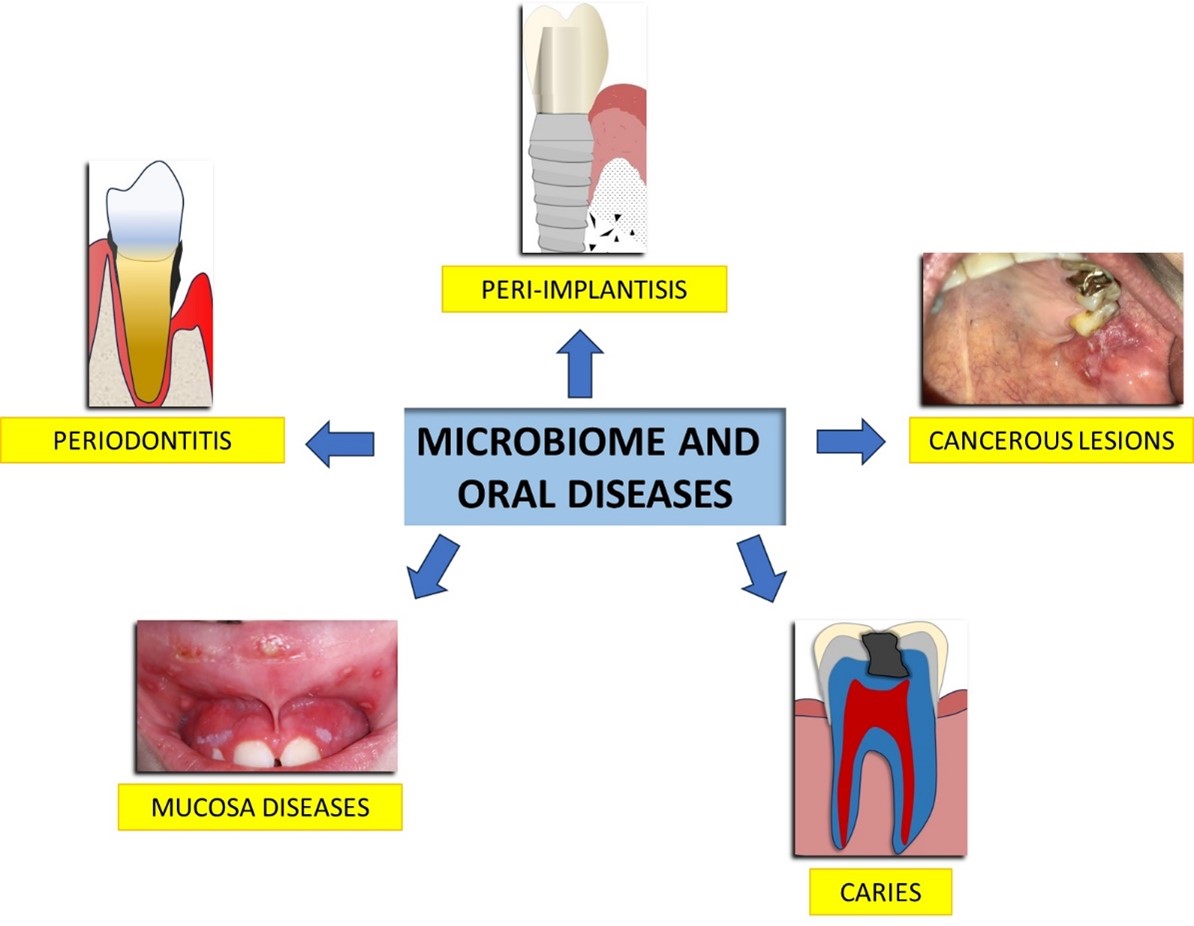
Figure 5: A generic view of possible oral diseases caused by alterations in the microbiome.
Genetic Variants, Dysbiosis, and Therapeutic Strategies
The exploration of genetic variants, particularly in the interleukin-10 gene, sheds light on their association with IL-1α, IL-1β-RN, COLIA1, and VDR genes in chronic periodontitis—a condition often found in patients with COVID-19 [304,305]. This association, coupled with the presence of specific bacterial strains, underscores the complexity of the oral-systemic axis [306–308]. The genetic landscape, oral dysbiosis, and bacteremia collectively contribute to the intricate dance of susceptibility and progression in chronic periodontitis [309]. The speech concludes by proposing a paradigm shift in therapeutic strategies for degenerative and infectious diseases, including COVID-19. Understanding the negative impact of dysbiosis, coupled with insights into immune system dynamics and endocrine balance, emerges as a potential therapeutic strategy [310]. The association between genetic variants of interleukin-10 and other key immune-related genes holds promise for risk assessment in systemic diseases linked to chronic dysbiosis [311–313]. In this evolving understanding landscape, the study makes a significant contribution by systematically observing the correlation between genetic variants and interleukin/cytokine gene polymorphisms in chronic dysbiosis-related conditions [314]. The call for further research, more extensive genetic information, and a representative sample size underscores the need to explore the intricate interplay of genetics, immune responses, and systemic health in the context of COVID-19 and chronic periodontitis [275]
Mechanisms of Interaction between the GM and Bone Cell
The intricate communication between the GM and bone cells involves a variety of mechanisms, signaling pathways, and interactions that contribute to the regulation of bone metabolism [315]. Understanding these mechanisms is essential for unraveling the complex interplay between the microbial inhabitants of the gastrointestinal tract and the cells responsible for bone formation and resorption [316].
Influence on Nutrient Absorption
- The GM plays a pivotal role in nutrient absorption, particularly calcium and vitamin D [317–319]. Calcium is crucial for bone mineralization, and vitamin D facilitates its absorption [320]. Microbial metabolites, such as short-chain fatty acids (SCFAs), modulate calcium solubility, impacting its absorption in the intestines [321].
Production of Microbial Metabolites
- SCFAs, including acetate, propionate, and butyrate, are byproducts of microbial fermentation in the gut [322–324]. These metabolites can directly influence bone cells. For example, butyrate has been shown to stimulate osteoblast activity, promoting bone formation [325].
Regulation of Immune Responses
- The GM influences immune responses, and this modulation can impact bone health. Dysregulation of the immune system may lead to increased inflammation, affecting bone metabolism [326,327]. Proinflammatory cytokines produced in response to an imbalanced GM can influence osteoclast activity, leading to bone resorption [328].
Bile Acid Metabolism
- Bile acids, essential for fat absorption, undergo metabolism by the GM [329–331]. Certain bile acids have been implicated in bone metabolism, acting as signaling molecules that influence bone cells [332]. The microbiome's impact on bile acid metabolism thus indirectly affects bone health [333].
Hormonal Signaling
- Hormonal signaling pathways, including the gut-brain-bone axis, play a crucial role in the crosstalk between the GM and bone cells [334–336]. Hormones such as leptin, ghrelin, and serotonin, involved in both gut function and bone metabolism, mediate communication between these systems [337].
Neural Pathways
- Neural pathways connecting the gut and bone contribute to the bidirectional communication between these systems [338–340]. Sensory nerves in the gut can relay signals to the central nervous system, influencing the release of neurotransmitters that impact bone cells [341].
Regulation of Inflammatory Responses
- The GM modulates inflammatory responses in the gastrointestinal tract, which can extend to affect bone health [342–344]. Chronic inflammation may disrupt the delicate balance between bone formation and resorption. Microbial-derived factors can influence immune cells, leading to altered bone metabolism [345].
SCFAs and Bone Remodeling
- SCFAs, particularly butyrate, have been shown to impact bone remodeling. Butyrate, in particular, can stimulate the differentiation and activity of osteoblasts, promoting bone formation [346–348]. SCFAs may also regulate the receptor activator of nuclear factor-kappa B ligand (RANKL)/osteoprotegerin (OPG) pathway, a key regulator of osteoclast differentiation and activity [349].
Indirect Effects on Hormones and Growth Factors
- The GM can indirectly influence hormones and growth factors involved in bone metabolism [350–352]. For example, modulation of insulin-like growth factor-1 (IGF-1) and transforming growth factor-beta (TGF-β) by the microbiome may impact bone cell function [353].
Modulation of Wnt Signaling Pathway
- The Wnt signaling pathway is critical for bone formation [354–356]. Experimental evidence suggests that the GM can modulate this pathway, influencing the differentiation and activity of osteoblasts and osteocytes [357].
Understanding these mechanisms provides insight into how the GM exerts its influence on bone cells [358–360]. It also highlights the complexity of the gut-bone axis and the potential for targeted interventions aimed at optimizing bone health by modulating the GM. As research in this field progresses, more specific interactions and pathways are likely to be uncovered, paving the way for innovative therapeutic strategies [361].
Factors Influencing the Gut-Bone Axis
The intricate interplay between the gut and bone, often called the gut-bone axis, is influenced by many factors that span dietary choices, lifestyle, medications, and environmental exposures [362]. Understanding these factors is crucial for comprehending how the GM impacts bone health and identifying potential avenues for intervention [363,364].
Diet and Nutrient Intake
- Calcium and Vitamin D: Adequate calcium and vitamin D intake is essential for bone health [365–367]. The GM influences the absorption of these nutrients in the intestines, with a balanced diet supporting microbial diversity and optimal nutrient utilization [368].
- Fiber and Prebiotics: Dietary fibers and prebiotics serve as fuel for beneficial gut bacteria [369–371]. A diet rich in fiber promotes microbial diversity, contributing to a healthier GM, which, in turn, may positively impact bone health [372].
Antibiotic Use
- Antibiotics, while crucial for treating bacterial infections, can significantly impact the GM [373–375]. Broad-spectrum antibiotics may disrupt the microbial balance, potentially affecting bone metabolism. Prolonged or frequent antibiotic use can lead to dysbiosis and altered nutrient absorption [376].
Lifestyle Factors
- Physical Activity: Regular exercise and weight-bearing activities positively influence bone health [377–379]. Physical activity may contribute to a diverse GM, and the gut-bone axis may be modulated through the release of factors influenced by exercise [380].
- Smoking and Alcohol Consumption: Smoking and excessive alcohol consumption have been associated with negative effects on bone health [381–383]. These lifestyle factors can influence the GM composition, potentially impacting bone metabolism [384].
Age and Hormonal Changes
- Bone health is influenced by age-related changes, with hormonal shifts being particularly impactful [385–387]. Postmenopausal women, for example, experience a decline in estrogen levels, leading to increased bone resorption [388–390]. Hormonal changes may also influence the GM, creating a complex interplay between aging, hormones, and bone health [391].
Medications
- Proton Pump Inhibitors (PPIs) and Antacids: Medications that alter gastric acidity, such as PPIs and antacids, may influence calcium absorption in the gut. This can potentially impact bone health over prolonged use [392].
- Antibiotics and Medications Affecting GM: Besides antibiotics, other medications can affect the GM composition [393–395]. For example, certain drugs used in the treatment of inflammatory bowel disease may have implications for both the GM and bone health [396].
Stress and Mental Health
- Stress and mental health conditions can impact the gut-brain axis, influencing the GM and potentially affecting bone metabolism. Chronic stress may contribute to inflammatory responses that could impact bone health [397].
Disease States
- Inflammatory Bowel Disease (IBD): Conditions like IBD, characterized by chronic inflammation in the gastrointestinal tract, can disrupt the GM and impact nutrient absorption [398–400]. This may contribute to compromised bone health [401].
- Celiac Disease: Celiac disease, an autoimmune condition triggered by gluten consumption, can lead to nutrient malabsorption and impact bone density [402–404]. Changes in the GM are also observed in individuals with celiac disease [405].
Genetic Factors
- Individual genetic makeup can influence both the GM composition and bone health [406–408]. Genetic factors may determine how an individual responds to dietary interventions, medications, and other environmental influences that shape the gut-bone axis [409].
Environmental Exposures
- Microbiome Development in Early Life: Early life environmental exposures, including mode of delivery during childbirth and feeding practices in infancy, influence the development of the GM [410–412]. This early microbial imprinting may have long-lasting effects on bone health [413].
Dietary Additives and Preservatives
- Certain additives and preservatives in processed foods may have unintended consequences on the GM [414–416]. If disruptive to microbial balance, these substances may impact nutrient absorption and potentially influence bone metabolism [417].
Understanding these multifaceted factors provides a comprehensive view of the gut-bone axis. Interventions targeting these factors, such as dietary modifications, lifestyle changes, and personalized approaches, promise to optimise the gut-bone axis and promote skeletal health throughout life [418].
Clinical Implications and Therapeutic Interventions
The growing understanding of the interplay between the GM and bone health has significant clinical implications, offering potential avenues for therapeutic interventions to optimise skeletal well-being [419–421]. The following points explore the practical applications of this knowledge and propose potential strategies for clinical management [422].
Probiotics and Prebiotics
- Clinical Applications: Probiotics, live microorganisms with proven health benefits, and prebiotics, substances that promote the growth of beneficial bacteria, represent promising interventions [423–425]. Specific strains of probiotics have shown the potential to positively influence bone health [426].
- Therapeutic Approach: Supplementation with probiotics or including prebiotics in the diet could be explored as a therapeutic approach to modulate the GM and, subsequently, positively impact bone metabolism [427].
Personalized Medicine
- Individualized Approaches: Considering the variability in GM composition among individuals, personalized approaches may be crucial [428–430]. Tailoring interventions based on an individual's microbial profile and genetic makeup could enhance the effectiveness of therapeutic strategies [431].
- Microbiome Analysis: Advanced techniques such as metagenomic sequencing could be employed to analyze an individual's GM composition, allowing for personalized interventions that consider the unique microbial landscape [432].
Dietary Recommendations
Nutrient-Rich Diets: Promoting diets rich in calcium, vitamin D, and prebiotic fibers supports both bone health and a diverse GM [433–435]. Dietary choices that foster microbial diversity can contribute to the production of beneficial metabolites [436].
- Fermented Foods: Including fermented foods in the diet, such as yogurt and kefir, can introduce probiotic microorganisms [437–439]. These foods may contribute to a healthy GM and support bone health [440,441].
Exercise and Physical Activity
- Bone-Strengthening Activities: Encouraging weight-bearing exercises and resistance training can positively impact bone health. Physical activity may influence the GM and contribute to a holistic approach to maintaining skeletal integrity [442].
- Combined Lifestyle Interventions: Integrating exercise regimens with dietary modifications may offer a synergistic effect, addressing both the GM and bone health concurrently [443].
Antibiotic Stewardship
- Awareness of Consequences: Healthcare providers should be mindful of the potential impact of antibiotics on the GM and bone health [444–446]. Antibiotic stewardship programs could emphasize the importance of judicious antibiotic use to minimize unintended consequences on skeletal well-being [447].
- Probiotic Supplementation during Antibiotic Use: Considering probiotic supplementation during and after antibiotic courses may help mitigate disruptions to the GM and support bone health [448].
Clinical Monitoring and Biomarkers
- Biomarkers for Gut-Bone Axis: Developing specific biomarkers that reflect the status of the gut-bone axis could aid in clinical monitoring. Biomarkers may include microbial signatures, metabolite levels, or markers indicative of bone turnover [449].
- Regular Assessments: Routine assessments of bone health, including bone mineral density measurements and biomarker monitoring, could be integrated into clinical practice to identify individuals at risk and tailor interventions accordingly [450].
Patient Education
- Promoting Gut-Bone Health Awareness: Patient education programs can raise awareness about the link between the GM and bone health [451–453]. Empowering individuals with knowledge about lifestyle choices, dietary habits, and the importance of microbial diversity fosters active participation in bone health maintenance [454].
Pharmacological Interventions
- Targeted Medications: Developing pharmacological interventions that specifically target the GM or its metabolic products could open new avenues for therapeutic strategies [455–457]. Research into drugs that modulate microbial activity may provide innovative approaches to influence bone metabolism [458].
Multi-disciplinary Approaches
- Collaboration Across Specialties: A multi-disciplinary approach involving gastroenterologists, endocrinologists, nutritionists, and bone health specialists is essential. Collaboration can facilitate comprehensive assessments, considering both gut and bone health parameters [459].
- Integrated Care Plans: Integrating gut health considerations into existing care plans for conditions like osteoporosis or inflammatory bowel disease can enhance overall patient outcomes [460].
Research and Clinical Trials
- Translational Research: Further translational research is needed to bridge the gap between preclinical findings and clinical applications. Clinical trials exploring the efficacy of GM-targeted interventions on bone health will contribute to evidence-based practices [461].
- Longitudinal Studies: Conducting longitudinal studies that track changes in the GM and bone health over time can provide valuable insights into the causal relationships and long-term effects of interventions [462].
Recognizing the clinical implications of the gut-bone axis opens doors to innovative therapeutic strategies [463]. From personalized medicine to lifestyle interventions, a holistic approach that considers both the GM and bone health holds promise for enhancing patient outcomes and preventing skeletal-related disorders. Continued research and clinical trials are pivotal for translating these insights into effective, tailored clinical interventions [464].
Conclusion
The intricate relationship between the GM and bone health represents a fascinating frontier in biomedical research. As we unravel the mechanisms governing this interplay, opportunities for therapeutic interventions to enhance bone health emerge [465]. Continued research is essential to elucidate the specific bacterial strains, metabolites, and pathways involved in the gut-bone axis. Integrating this knowledge into clinical practice can revolutionize preventive and therapeutic strategies for bone-related conditions, ultimately contributing to enhanced overall health and well-being [466].
References
- Santacroce L, Charitos IA, Ballini A, Inchingolo F, Luperto P, et al. (2020) The Human Respiratory System and its Microbiome at a Glimpse. Biology 9: 318.
- Ballini A, Dipalma G, Isacco CG, Boccellino M, Di Domenico M, et al. (2020) Oral Microbiota and Immune System Crosstalk: A Translational Research. Biology 9: 131.
- Ceci S, Berate P, Candrea S, Babtan A-M, Azzollini D, et al. (2021) The oral and gut microbiota: beyond a short communication. Balneo PRM Res J 12: 405-11.
- Fooks LJ, Gibson GR. (2002) Probiotics as modulators of the gut flora. Br J Nutr 88: S39-49.
- Adjibade M, Davisse-Paturet C, Divaret-Chauveau A, Adel-Patient K, Raherison C, et al. (2022) Enrichment of Formula in Probiotics or Prebiotics and Risk of Infection and Allergic Diseases up to Age 5.5 Years in the Nationwide Etude Longitudinale Française depuis l’Enfance (ELFE) Cohort. J Nutr. 152: 1138-1148.
- Basiri T, Johnson ND, Moffa EB, Mulyar Y, Serra Nunes PL, et al. (2017) Duplicated or Hybridized Peptide Functional Domains Promote Oral Homeostasis. J Dent Res 96: 1162-7.
- Gibson GR, Probert HM, Loo JV, Rastall RA, Roberfroid MB. (2004) Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr Res Rev 17: 259-75.
- Di Paola A, Tortora C, Argenziano M, Marrapodi MM, Rossi F. (2022) Emerging Roles of the Iron Chelators in Inflammation. Int J Mol Sci 23: 7977.
- Li Y, He J, He Z, Zhou Y, Yuan M, et al. (2014) Phylogenetic and functional gene structure shifts of the oral microbiomes in periodontitis patients. ISME J 8: 1879-91.
- Marrapodi MM, Mascolo A, di Mauro G, Mondillo G, Pota E, et al. (2022) The safety of blinatumomab in pediatric patients with acute lymphoblastic leukemia: A systematic review and meta-analysis. Front Pediatr 10.
- Nyrén P, Lundin A. (1985) Enzymatic method for continuous monitoring of inorganic pyrophosphate synthesis. Anal Biochem 151: 504-9.
- Aguilera M, Daddaoua A. (2023) Prebiotics and Probiotics: Healthy Biotools for Molecular Integrative and Modulation Approaches. Int J Mol Sci 24: 7559.
- Balzanelli MG, Distratis P, Lazzaro R, Cefalo A, Catucci O, et al. (2021) The Vitamin D, IL-6 and the eGFR Markers a Possible Way to Elucidate the Lung-Heart-Kidney Cross-Talk in COVID-19 Disease: A Foregone Conclusion. Microorganisms 9: 1903.
- Macpherson AJ, Harris NL. (2004) Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol 4: 478-85.
- Al-Ghazzewi FH, Tester RF. (2014) Impact of prebiotics and probiotics on skin health. Benef Microbes 5: 99-107.
- White JH. (2012) Vitamin D metabolism and signaling in the immune system. Rev Endocr Metab Disord 13: 21-9.
- Bode LM, Bunzel D, Huch M, Cho G-S, Ruhland D, et al. (2013) In vivo and in vitro metabolism of trans-resveratrol by human gut microbiota. Am J Clin Nutr 97: 295-309.
- Malcangi G, Inchingolo AD, Inchingolo AM, Piras F, Settanni V, et al. (2022) COVID-19 Infection in Children and Infants: Current Status on Therapies and Vaccines. Children 9: 249.
- Di Domenico M, Pinto F, Quagliuolo L, Contaldo M, Settembre G, et al. (2019) The Role of Oxidative Stress and Hormones in Controlling Obesity. Front Endocrinol 10: 540.
- Andrews T, Thompson M, Buckley DI, Heneghan C, Deyo R, et al. (2012) Interventions to influence consulting and antibiotic use for acute respiratory tract infections in children: a systematic review and meta-analysis. PloS One 7: e30334.
- Alam Z, Shang X, Effat K, Kanwal F, He X, et al. (2022) The potential role of prebiotics, probiotics, and synbiotics in adjuvant cancer therapy especially colorectal cancer. J Food Biochem 46: e14302.
- Ahmad P, Slots J. (2001) A bibliometric analysis of periodontology. Periodontol 2000. 85: 237-40.
- Alli SR, Gorbovskaya I, Liu JCW, Kolla NJ, Brown L, et al. (2022) The Gut Microbiome in Depression and Potential Benefit of Prebiotics, Probiotics and Synbiotics: A Systematic Review of Clinical Trials and Observational Studies. Int J Mol Sci 23: 4494.
- Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, et al. (2013) The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry 18: 666-73.
- Cryan JF, O’Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, et al. (2019) The Microbiota-Gut-Brain Axis. Physiol Rev 99: 1877-2013.
- Anderson SW, Bazzell AF, Dains JE. (2018) An Integrative Review on the Effect of Prebiotics, Probiotics, and Synbiotics on Infection After Colorectal Cancer Surgery 107: 237-48.
- Kim SC, Ferry GD. (2004) Inflammatory bowel diseases in pediatric and adolescent patients: clinical, therapeutic, and psychosocial considerations. Gastroenterology 126: 1550-60.
- Tamboli CP, Neut C, Desreumaux P, Colombel JF. (2004) Dysbiosis in inflammatory bowel disease. Gut 53: 1-4.
- Asad Salman R, Khudhur Jameel S, Mahdi Shakir S. (2023) Evaluation of the Effects of Probiotics and Prebiotics on the Salmonella typhi Infections. Arch Razi Inst 78: 1115-30.
- Ballini A, Santacroce L, Cantore S, Bottalico L, Dipalma G, et al. (2019) Probiotics Efficacy on Oxidative Stress Values in Inflammatory Bowel Disease: A Randomized Double-Blinded Placebo-Controlled Pilot Study. Endocr Metab Immune Disord Drug Targets 19: 373-81.
- Ahmad P, Asif JA, Alam MK, Slots J. (2000) A bibliometric analysis of Periodontology 2000. Periodontol 2000 82: 286-97.
- Alshamsi M, Mehta J, Nibali L. (2021) Study design and primary outcome in randomized controlled trials in periodontology. A systematic review. J Clin Periodontol 48: 859-66.
- Avila-Ortiz G, Ambruster J, Barootchi S, Chambrone L, Chen C-Y, et al. (2022) American Academy of Periodontology best evidence consensus statement on the use of biologics in clinical practice. J Periodontol 93: 1763-70.
- Ayari H. (2022) The use of periodontal membranes in the field of periodontology: spotlight on collagen membranes. J Appl Biomed 20: 154-62.
- Gargiulo Isacco C, Ballini A, Paduanelli G, Inchingolo AD, Nguyen KCD, et al. (2019) Bone decay and beyond: how can we approach it better. J Biol Regul Homeost Agents 33: 143-154.
- Asha MZ, Khalil SFH. (2020) Efficacy and Safety of Probiotics, Prebiotics and Synbiotics in the Treatment of Irritable Bowel Syndrome: A systematic review and meta-analysis. Sultan Qaboos Univ Med J 20: e13-24.
- Bartold PM. (2018) Lifestyle and periodontitis: The emergence of personalized periodontics. Periodontol 2000 78: 7-11.
- Beck JD, Papapanou PN, Philips KH, Offenbacher S. (2019) Periodontal Medicine: 100 Years of Progress. J Dent Res 98: 1053-62.
- Caffesse RG. (2015) A Latin American perspective of periodontology. Periodontol 2000 67: 7-12.
- Caton JG, Armitage G, Berglundh T, Chapple ILC, Jepsen S, et al. (2018) A new classification scheme for periodontal and peri-implant diseases and conditions - Introduction and key changes from the 1999 classification. J Clin Periodontol 20: S1-8.
- Chambrone L, Wang H-L, Romanos GE. (2018) Antimicrobial photodynamic therapy for the treatment of periodontitis and peri-implantitis: An American Academy of Periodontology best evidence review. J Periodontol 89: 783-803.
- Foster BL, Ao M, Salmon CR, Chavez MB, Kolli TN, et al. (2018) Osteopontin regulates dentin and alveolar bone development and mineralization. Bone107: 196-207.
- Ergoren MC, Paolacci S, Manara E, Dautaj A, Dhuli K, et al. (2020) A pilot study on the preventative potential of alpha-cyclodextrin and hydroxytyrosol against SARS-CoV-2 transmission. Acta Bio Medica Atenei Parm. 91: e2020022.
- Lanteri V, Cossellu G, Farronato M, Ugolini A, Leonardi R, et al. (2020) Assessment of the Stability of the Palatal Rugae in a 3D-3D Superimposition Technique Following Slow Maxillary Expansion (SME). Sci Rep 10: 2676.
- Maspero C, Gaffuri F, Castro IO, Lanteri V, Ugolini A, et al. (2019) Correlation between Dental Vestibular–Palatal Inclination and Alveolar Bone Remodeling after Orthodontic Treatment: A CBCT Analysis. Materials 12: 4225.
- Marchetti E, Tecco S, Caterini E, Casalena F, Quinzi V, et al. (2017) Alcohol-free essential oils containing mouthrinse efficacy on three-day supragingival plaque regrowth: a randomized crossover clinical trial. Trials 18: 154.
- Rosa M, Quinzi V, Marzo G. (2019) Paediatric Orthodontics Part 1: Anterior open bite in the mixed dentition. Eur J Paediatr Dent 20: 80-2.
- Piancino MG, Di Benedetto L, Matacena G, Deregibus A, Marzo G, et al. (2019) Paediatric Orthodontics Part 3: Masticatory function during development. Eur J Paediatr Dent 20: 247-9.
- D’Apuzzo F, Grassia V, Quinzi V, Vitale M, Marzo G, et al. (2019) Paediatric Orthodontics Part 4: SEC III protocol in Class III malocclusion. Eur J Paediatr Dent 20: 330-4.
- Libonati A, Nardi R, Gallusi G, Angotti V, Caruso S, et al. (2018) Pain and anxiety associated with Computer-Controlled Local Anaesthesia: systematic review and meta-analysis of cross-over studies. Eur J Paediatr Dent 19: 324-32.
- Mummolo S, Mancini L, Quinzi V, D’Aquino R, Marzo G, et al. (2020) Rigenera® Autologous Micrografts in Oral Regeneration: Clinical, Histological, and Radiographical Evaluations. Appl Sci 10:5084.
- Tecco S, Mummolo S, Marchetti E, Tetè S, Campanella V, et al. (2011) sEMG activity of masticatory, neck, and trunk muscles during the treatment of scoliosis with functional braces. A longitudinal controlled study. J Electromyogr Kinesiol 21: 885-92.
- Saccomanno S, Quinzi V, D’Andrea N, Albani A, Coceani Paskay L, et al. (2021) Traumatic Events and Eagle Syndrome: Is There Any Correlation? A Systematic Review. Healthcare 9: 825.
- Di Francesco F, Lanza A, Di Blasio M, Vaienti B, Cafferata EA, et al. (2022) Application of Botulinum Toxin in Temporomandibular Disorders: A Systematic Review of Randomized Controlled Trials (RCTs). Appl Sci 12: 12409-12409.
- Rathi S, Chaturvedi S, Abdullah S, Rajput G, Alqahtani NM, et al. (2023) Clinical Trial to Assess Physiology and Activity of Masticatory Muscles of Complete Denture Wearer Following Vitamin D Intervention. Medicina (Mex) 59: 410.
- Minervini G, Franco R, Marrapodi MM, Almeida LE, Ronsivalle V, et al. (2023) Prevalence of temporomandibular disorders (TMD) in obesity patients: A systematic review and meta‐analysis. J Oral Rehabil 50: 1544-53.
- Uzunçıbuk H, Marrapodi MM, Meto A, Ronsivalle V, Cicciù M, et al. (2023) Prevalence of temporomandibular disorders in clear aligner patients using orthodontic intermaxillary elastics assessed with diagnostic criteria for temporomandibular disorders (DC/TMD) axis II evaluation: A cross-sectional study. J Oral Rehabil 51: 500-509.
- Lim JY, Taylor AF, Li Z, Vogler EA, Donahue HJ. (2005) Integrin expression and osteopontin regulation in human fetal osteoblastic cells mediated by substratum surface characteristics. Tissue Eng 11: 19-29.
- Osteoblast- An overview (2022) ScienceDirect Topics [Internet] https://www.sciencedirect.com/topics/materials-science/osteoblast
- Baldi S, Mundula T, Nannini G, Amedei A. (2021) Microbiota shaping - the effects of probiotics, prebiotics, and fecal microbiota transplant on cognitive functions: A systematic review. World J Gastroenterol 27: 6715-32.
- Beerens MW, Ten Cate JM, Van Der Veen MH. (2017) Microbial profile of dental plaque associated to white spot lesions in orthodontic patients immediately after the bracket removal. Arch Oral Biol 78: 88-93.
- Elhennawy K, Manton DJ, Crombie F, Zaslansky P, Radlanski RJ, et al. (2017) Structural, mechanical and chemical evaluation of molar-incisor hypomineralization-affected enamel: A systematic review. Arch Oral Biol 83: 272-81.
- Ballan R, Battistini C, Xavier-Santos D, Saad SMI. (2020) Interactions of probiotics and prebiotics with the gut microbiota. Prog Mol Biol Transl Sci 171: 265-300.
- Balthazar CF, Guimarães JF, Coutinho NM, Pimentel TC, Ranadheera CS, et al. (2022) The future of functional food: Emerging technologies application on prebiotics, probiotics and postbiotics. Compr Rev Food Sci Food Saf 21: 2560-86.
- Dominguez DC. (2004) Calcium signalling in bacteria. Mol Microbiol 54: 291-7.
- Leitão TJ, Cury JA, Tenuta LMA. (2018) Kinetics of calcium binding to dental biofilm bacteria. Das S, editor. PLOS ONE 13: e0191284.
- Barbosa RSD, Vieira-Coelho MA. (2020) Probiotics and prebiotics: focus on psychiatric disorders - a systematic review. Nutr Rev 78:437-50.
- Moore AE, Dulnoan D, Voong K, Ayis S, Mangelis A, et al. (2023) The additive effect of vitamin K supplementation and bisphosphonate on fracture risk in post-menopausal osteoporosis: a randomised placebo controlled trial. Arch Osteoporos 18: 83.
- Barbuti RC, Schiavon LL, Oliveira CP, Alvares-DA-Silva MR, Sassaki LY, et al. (2020) Gut Microbiota, Prebiotics, Probiotics, And Synbiotics In Gastrointestinal And Liver Diseases: Proceedings Of A Joint Meeting Of The Brazilian Society Of Hepatology (Sbh), Brazilian Nucleus For The Study Of Helicobacter Pylori And Microbiota (Nbehpm), And Brazilian Federation Of Gastroenterology (FBG). Arq Gastroenterol 57: 381-98.
- Knapen MHJ, Braam L a. JLM, Teunissen KJ, Van’t Hoofd CM, Zwijsen RML, et al. (2016) Steady-state vitamin K2 (menaquinone-7) plasma concentrations after intake of dairy products and soft gel capsules. Eur J Clin Nutr 70: 831-6.
- Berding K, Cryan JF. (2022) Microbiota-targeted interventions for mental health. Curr Opin Psychiatry 35: 3-9.
- Bertelsen RJ, Jensen ET, Ringel-Kulka T. (2016) Use of probiotics and prebiotics in infant feeding. Best Pract Res Clin Gastroenterol 30: 39-48.
- Biswasroy P, Pradhan D, Sahu DK, Sahu A, Ghosh G, et al. (2021) Recent Advances in Clinical Utility of Probiotics in Gastrointestinal Tract Disorders. Curr Pharm Biotechnol 22: 1559-73.
- Lanteri V, Cossellu G, Farronato M, Ugolini A, Leonardi R, et al. (2020) Assessment of the Stability of the Palatal Rugae in a 3D-3D Superimposition Technique Following Slow Maxillary Expansion (SME). Sci Rep 10: 2676.
- Sammartino G, Marenzi G, Howard CM, Minimo C, Trosino O, et al. (2008) Chondrosarcoma of the Jaw: A Closer Look at its Management. J Oral Maxillofac Surg 66: 2349-55.
- Mummolo S, Tieri M, Tecco S, Mattei A, Albani F, et al. (2014) Clinical evaluation of salivary indices and levels of Streptococcus mutans and Lactobacillus in patients treated with Occlus-o-Guide. Eur J Paediatr Dent 15: 367-70.
- Di Spirito F, Contaldo M, Amato A, Di Palo MP, Pantaleo G, et al. (2022) COVID ‐19 vaccine and oral lesions: Putative pathogenic mechanisms. Oral Dis 28: 2639-40.
- Di Spirito F, Amato A, Di Palo MP, Ferraro GA, Baroni A, et al. (2022) COVID-19 Related Information on Pediatric Dental Care including the Use of Teledentistry: A Narrative Review. Children 9: 1942.
- Lo Giudice A, Leonardi R, Ronsivalle V, Allegrini S, Lagravère M, et al. (2021) Evaluation of pulp cavity/chamber changes after tooth-borne and bone-borne rapid maxillary expansions: a CBCT study using surface-based superimposition and deviation analysis. Clin Oral Investig 25: 2237-47.
- Rosa A, Miranda M, Franco R, Guarino MG, Barlattani A, et al. (2016) Experimental protocol of dental procedures In patients with hereditary angioedema: the role of anxiety and the use of nitrogen oxide. ORAL Implantol 9:49-53.
- Sammartino G, Gasparro R, Marenzi G, Trosino O, Mariniello M, et al. (2017) Extraction of mandibular third molars: proposal of a new scale of difficulty. Br J Oral Maxillofac Surg 55: 952-7.
- Camerota L, Ritelli M, Wischmeijer A, Majore S, Cinquina V, et al. (2019) Genotypic Categorization of Loeys-Dietz Syndrome Based on 24 Novel Families and Literature Data. Genes 10: 764.
- Favia G, Tempesta A, Limongelli L, Crincoli V, Piattelli A, et al. (2015) Metastatic Breast Cancer in Medication-Related Osteonecrosis Around Mandibular Implants. Am J Case Rep 16: 621-6.
- Pisano M, Romano A, Di Palo MP, Baroni A, Serpico R, et al. (2023) Oral Candidiasis in Adult and Pediatric Patients with COVID-19. Biomedicines 11: 846.
- Dinoi MT, Marchetti E, Garagiola U, Caruso S, Mummolo S, et al. (2016) Orthodontic treatment of an unerupted mandibular canine tooth in a patient with mixed dentition: a case report. J Med Case Reports. 10: 170.
- Lajolo C, Gioco G, Rupe C, Patini R, Rizzo I, et al. (2021) Patient perception after oral biopsies: an observational outpatient study. Clin Oral Investig 25: 5687-97.
- Del Amo FSL, Yu S-H, Sammartino G, Sculean A, Zucchelli G, et al. (2020) Peri-implant Soft Tissue Management: Cairo Opinion Consensus Conference. Int J Environ Res Public Health 17: 2281.
- D’Esposito V, Lecce M, Marenzi G, Cabaro S, Ambrosio MR, et al. (2020) Platelet‐rich plasma counteracts detrimental effect of high‐glucose concentrations on mesenchymal stem cells from Bichat fat pad. J Tissue Eng Regen Med 14: 701-13.
- Crincoli V, Anelli MG, Quercia E, Piancino MG, Di Comite M. (2019) Temporomandibular Disorders and Oral Features in Early Rheumatoid Arthritis Patients: An Observational Study. Int J Med Sci 16:253-63.
- Crincoli V, Scivetti M, Di Bisceglie MB, Pilolli GP, Favia G. (2008) Unusual case of adverse reaction in the use of sodium hypochlorite during endodontic treatment: a case report. Quintessence Int Berl Ger 1985 39: e70-73.
- Norris V, Chen M, Goldberg M, Voskuil J, McGurk G, et al. (1991) Calcium in bacteria: a solution to which problem? Mol Microbiol 5: 775-8.
- Bock PM, Telo GH, Ramalho R, Sbaraini M, Leivas G, et al. (2021) The effect of probiotics, prebiotics or synbiotics on metabolic outcomes in individuals with diabetes: a systematic review and meta-analysis. Diabetologia 64: 26-41.
- Rose RK. (2000) The role of calcium in oral streptococcal aggregation and the implications for biofilm formation and retention. Biochim Biophys Acta 1475: 76-82.
- Rose RK, Dibdin GH, Shellis RP. (1993) A quantitative study of calcium binding and aggregation in selected oral bacteria. J Dent Res 72: 78-84.
- Brüssow H. (2019) Probiotics and prebiotics in clinical tests: an update. F1000Research. 2019;8:F1000 Faculty Rev-1157.
- Buhaș MC, Candrea R, Gavrilaș LI, Miere D, Tătaru A, et al. (2023) Transforming Psoriasis Care: Probiotics and Prebiotics as Novel Therapeutic Approaches. Int J Mol Sci 24: 11225.
- Burlingame B. (2014) Prebiotics and probiotics and the specialized UN agencies. J Clin Gastroenterol. 48 Suppl 1:S1.
- Cairoli E, Zhukouskaya VV, Eller-Vainicher C, Chiodini I. (2015) Perspectives on osteoporosis therapies. J Endocrinol Invest 38: 303-11.
- Butt UD, Lin N, Akhter N, Siddiqui T, Li S, et al. (2021) Overview of the latest developments in the role of probiotics, prebiotics and synbiotics in shrimp aquaculture. Fish Shellfish Immunol 114: 263-81.
100. Calero CDQ, Rincón EO, Marqueta PM. (2020) Probiotics, prebiotics and synbiotics: useful for athletes and active individuals? A systematic review. Benef Microbes 11: 135-49.
101. Cang W, Wu J, Ding R, Wang W, Li N, et al. (2022) Potential of Probiotics as an Adjunct for Patients with Major Depressive Disorder. Mol Nutr Food Res 66: e2101057.
102. Sieverts M, Obata Y, Rosenberg JL, Woolley W, Parkinson DY, et al. (2022) Unraveling the effect of collagen damage on bone fracture using in situ synchrotron microtomography with deep learning. Commun Mater 3: 1-13.
103. Rivero-Calle I, Cebey-López M, Pardo-Seco J, Yuste J, Redondo E, et al. (2019) Lifestyle and comorbid conditions as risk factors for community-acquired pneumonia in outpatient adults (NEUMO-ES-RISK project). BMJ Open Respir Res 6: e000359.
104. Cho Y-D, Kim W-J, Ryoo H-M, Kim H-G, Kim K-H, et al. (2021) Current advances of epigenetics in periodontology from ENCODE project: a review and future perspectives. Clin Epigenetics13: 92.
105. Cirelli JA, Fiorini T, Moreira CHC, Molon RS de, Dutra TP, et al. (2021) Periodontal regeneration: is it still a goal in clinical periodontology? Braz Oral Res 35: e09.
106. Clark-Roberton D. (2019) Periodontology from an undergraduate perspective. Br Dent J 227: 597-8.
107. Dannewitz B, Holtfreter B, Eickholz P. (2021) [Periodontitis-therapy of a widespread disease]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 64: 931-40.
108. Eaton KA, West NX, Wilson NHF, Sanz M. (2022) European Federation of Periodontology Survey of Postgraduate and Specialist Training in Europe in 2020. Eur J Dent Educ Off J Assoc Dent Educ Eur 26: 361-7.
109. Li Y-J, Ou J-J, Li Y-M, Xiang D-X. (2017) Dietary Supplement for Core Symptoms of Autism Spectrum Disorder: Where Are We Now and Where Should We Go? Front Psychiatry 89: 155.
110. Capurso L, Morelli L. (2014) 7th Probiotics Prebiotics & New Foods. Foreword. J Clin Gastroenterol 1:S2.
111. Cardoso BB, Amorim C, Silvério SC, Rodrigues LR. (2021) Novel and emerging prebiotics: Advances and opportunities. Adv Food Nutr Res 95: 41-95.
112. Carpay NC, Kamphorst K, de Meij TGJ, Daams JG, Vlieger AM, et al. (2022) Microbial effects of prebiotics, probiotics and synbiotics after Caesarean section or exposure to antibiotics in the first week of life: A systematic review. PloS One 17: e0277405.
113. Carpi RZ, Barbalho SM, Sloan KP, Laurindo LF, Gonzaga HF, et al. (2022) The Effects of Probiotics, Prebiotics and Synbiotics in Non-Alcoholic Fat Liver Disease (NAFLD) and Non-Alcoholic Steatohepatitis (NASH): A Systematic Review. Int J Mol Sci 23: 8805.
114. Cauli O. (2020) New Effects of Prebiotics, Probiotics, and Symbiotics. Curr Clin Pharmacol 15: 172-3.
115. Rautava S, Kainonen E, Salminen S, Isolauri E. (2012) Maternal probiotic supplementation during pregnancy and breast-feeding reduces the risk of eczema in the infant. J Allergy Clin Immunol. 130: 1355-60.
116. Canalis E, Pash J, Varghese S. (1993) Skeletal growth factors. Crit Rev Eukaryot Gene Expr 3:155-66.
117. Cerdó T, Ruíz A, Suárez A, Campoy C. (2017) Probiotic, Prebiotic, and Brain Development. Nutrients 9: 1247.
118. Ceresola ER, Ferrarese R, Preti A, Canducci F. (2018) Targeting patients’ microbiota with probiotics and natural fibers in adults and children with constipation. Eur Rev Med Pharmacol Sci 22: 7045-57.
119. Fukushima A, Aizaki Y, Sakuma K. (2012) Short-chain fatty acids increase the level of calbindin-D9k messenger RNA in Caco-2 cells. J Nutr Sci Vitaminol 58: 287-91.
120. Lucas S, Omata Y, Hofmann J, Böttcher M, Iljazovic A, et al. (2018) Short-chain fatty acids regulate systemic bone mass and protect from pathological bone loss. Nat Commun 9: 55.
121. Chen C, Wang J, Li J, Zhang W, Ou S. (2023) Probiotics, Prebiotics, and Synbiotics for Patients on Dialysis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J Ren Nutr Off J Counc Ren Nutr Natl Kidney Found 33: 126-39.
122. Chenhuichen C, Cabello-Olmo M, Barajas M, Izquierdo M, Ramírez-Vélez R, et al. (2022) Impact of probiotics and prebiotics in the modulation of the major events of the aging process: A systematic review of randomized controlled trials. Exp Gerontol 164: 111809.
123. Coleman JL, Hatch-McChesney A, Small SD, Allen JT, Sullo E, et al. (2022) Orally Ingested Probiotics, Prebiotics, and Synbiotics as Countermeasures for Respiratory Tract Infections in Nonelderly Adults: A Systematic Review and Meta-Analysis. Adv Nutr Bethesda Md 13: 2277-95.
124. Colombo D, Rigoni C, Cantù A, Carnevali A, Filippetti R, Fet al. (2023) Probiotics and Prebiotics Orally Assumed as Disease Modifiers for Stable Mild Atopic Dermatitis: An Italian Real-Life, Multicenter, Retrospective, Observational Study. Med Kaunas Lith 59: 2080.
125. Vimercati L, De Maria L, Quarato M, Caputi A, Gesualdo L, et al. (2021) Association between Long COVID and Overweight/Obesity. J Clin Med 10: 4143.
126. Inchingolo F, Patano A, Inchingolo AM, Riccaldo L, Morolla R, et al. (2023) Analysis of Mandibular Muscle Variations Following Condylar Fractures: A Systematic Review. J Clin Med 12: 5925.
127. Inchingolo AD, Malcangi G, Inchingolo AM, Piras F, Settanni V, et al. (2022) Benefits and Implications of Resveratrol Supplementation on Microbiota Modulations: A Systematic Review of the Literature. Int J Mol Sci 23: 4027.
128. Minetti E, Dipalma G, Palermo A, Patano A, Inchingolo AD, et al. (2023) Biomolecular Mechanisms and Case Series Study of Socket Preservation with Tooth Grafts. J Clin Med 12: 5611.
129. Malcangi G, Inchingolo AD, Inchingolo AM, Piras F, Settanni V,et al. (2022) COVID-19 Infection in Children and Infants: Current Status on Therapies and Vaccines. Children 9: 249.
130. Inchingolo F, Ballini A, Cagiano R. (2015) Immediately loaded dental implants bioactivated with platelet-rich plasma (PRP) placed in maxillary and mandibular region. Clin Ter 166: E146-52.
131. Fanali S, Tumedei M, Pignatelli P, Inchingolo F, Pennacchietti P, et al. (2021) Implant primary stability with an osteocondensation drilling protocol in different density polyurethane blocks. Comput Methods Biomech Biomed Engin 24: 14-20.
132. Inchingolo F, Tatullo M, Abenavoli FM, Marrelli M, Inchingolo AD, et al. (2011) Non-Hodgkin lymphoma affecting the tongue: unusual intra-oral location. Head Neck Oncol 3:1.
133. Inchingolo F, Tatullo M, Abenavoli FM, Marrelli M, Inchingolo AD, et al. (2010) Non-syndromic multiple supernumerary teeth in a family unit with a normal karyotype: case report. Int J Med Sci 7: 378–84.
134. Marrelli M, Tatullo M, Dipalma G, Inchingolo F. (2012) Oral Infection by Staphylococcus Aureus in Patients Affected by White Sponge Nevus: A Description of Two Cases Occurred in the Same Family. Int J Med Sci 9: 47-50.
135. Inchingolo F, Tatullo M, Abenavoli FM, Marrelli M, Inchingolo AD, et al. (2011) Oral Piercing and Oral Diseases: A Short Time Retrospective Study. Int J Med Sci 8: 649-52.
136. Isacco CG, Ballini A, De Vito D, Nguyen KCD, Cantore S, et al. (2021) Rebalancing the Oral Microbiota as an Efficient Tool in Endocrine, Metabolic and Immune Disorders. Endocr Metab Immune Disord - Drug Targets 21: 777-84.
137. Dipalma G, Inchingolo AM, Malcangi G, Ferrara I, Viapiano F, et al. (2023) Sixty-Month Follow Up of Clinical MRONJ Cases Treated with CGF and Piezosurgery. Bioengineering 10: 863.
138. Dolci C, Cenzato N, Maspero C, Giannini L, Khijmatgar S, et al. (2023) Skull Biomechanics and Simplified Cephalometric Lines for the Estimation of Muscular Lines of Action. J Pers Med 13: 1569.
139. Boccellino M, Di Stasio D, Dipalma G, Cantore S, Ambrosio P, et al. (2019) Steroids and growth factors in oral squamous cell carcinoma: useful source of dental-derived stem cells to develop a steroidogenic model in new clinical strategies. Eur Rev Med Pharmacol Sci 23: 8730-40.
140. Inchingolo F, Inchingolo AM, Malcangi G, De Leonardis N, Sardano R, et al. (2023) The Benefits of Probiotics on Oral Health: Systematic Review of the Literature. Pharmaceuticals 16: 1313.
141. Inchingolo F, Inchingolo AD, Palumbo I, Trilli I, Guglielmo M, , et al. (2024) The Impact of Cesarean Section Delivery on Intestinal Microbiota: Mechanisms, Consequences, and Perspectives—A Systematic Review. Int J Mol Sci 25: 1055.
142. Inchingolo AM, Inchingolo AD, Viapiano F, Ciocia AM, Ferrara I, et al. (2023) Treatment Approaches to Molar Incisor Hypomineralization: A Systematic Review. J Clin Med 12:7194.
143. Fischer RG, Lira Junior R, Retamal-Valdes B, Figueiredo LC de, Malheiros Z, et al. (2020) Periodontal disease and its impact on general health in Latin America. Section V: Treatment of periodontitis. Braz Oral Res 34: e026.
144. Floyd PD, Ide M, Palmer RM. (2014) Clinical guide to periodontology: reconstructive periodontal treatment. Br Dent J 216: 511-8.
145. Genco RJ. (2014) Commentary: the evolution of periodontology: science always wins. J Periodontol. 85: 1308-12.
146. Gholami L, Asefi S, Hooshyarfard A, Sculean A, Romanos GE, et al. (2019) Photobiomodulation in Periodontology and Implant Dentistry: Part 1. Photobiomodulation Photomed Laser Surg 37: 739-65.
147. Gholami L, Asefi S, Hooshyarfard A, Sculean A, Romanos GE, et al. Photobiomodulation in Periodontology and Implant Dentistry: Part 2. Photobiomodulation Photomed Laser Surg. 2019;37:766–83.
148. Dalile B, Van Oudenhove L, Vervliet B, Verbeke K. (2019) The role of short-chain fatty acids in microbiota–gut–brain communication. Nat Rev Gastroenterol Hepatol. 16: 461-78.
149. Cooper TE, Khalid R, Chan S, Craig JC, Hawley CM, et al. (2023) Synbiotics, prebiotics and probiotics for people with chronic kidney disease. Cochrane Database Syst Rev 10: CD013631.
150. Cooper TE, Scholes-Robertson N, Craig JC, Hawley CM, Howell M, et al. (2022) Synbiotics, prebiotics and probiotics for solid organ transplant recipients. Cochrane Database Syst Rev 9: CD014804.
151. Coutts L, Ibrahim K, Tan QY, Lim SER, Cox NJ, et al. (2020) Can probiotics, prebiotics and synbiotics improve functional outcomes for older people: a systematic review. Eur Geriatr Med 11: 975-93.
152. Cremon C, Barbaro MR, Ventura M, Barbara G. (2018) Pre- and probiotic overview. Curr Opin Pharmacol 43: 87-92.
153. Den Besten G, Van Eunen K, Groen AK, Venema K, Reijngoud D-J, et al. (2013) The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res 54: 2325-40.
154. Da Silva TF, Casarotti SN, De Oliveira GLV, Penna ALB. (2021) The impact of probiotics, prebiotics, and synbiotics on the biochemical, clinical, and immunological markers, as well as on the gut microbiota of obese hosts. Crit Rev Food Sci Nutr 61: 337-55.
155. Dangi P, Chaudhary N, Chaudhary V, Virdi AS, Kajla P, et al. (2023) Nanotechnology impacting probiotics and prebiotics: a paradigm shift in nutraceuticals technology. Int J Food Microbiol 388: 110083.
156. Darb Emamie A, Rajabpour M, Ghanavati R, Asadolahi P, Farzi S, et al. (2021) The effects of probiotics, prebiotics and synbiotics on the reduction of IBD complications, a periodic review during 2009-2020. J Appl Microbiol 130: 1823-38.
157. MacFabe DF. (2012) Short-chain fatty acid fermentation products of the gut microbiome: implications in autism spectrum disorders. Microb Ecol Health Dis [Internet]. 2012 [cited 2023 Apr 6].
158. Herrera D, Sanz M, Kebschull M, Jepsen S, Sculean A, et al. (2022) Treatment of stage IV periodontitis: The EFP S3 level clinical practice guideline. J Clin Periodontol 24: 4-71.
159. Jepsen K, Sculean A, Jepsen S. (2023) Complications and treatment errors involving periodontal tissues related to orthodontic therapy. Periodontol 2000 92: 135-58.
160. Kantarci A, Stavropoulos A, Sculean A. (2022) Introduction: Vision of Regenerative Periodontology. Dent Clin North Am 66: xi–xiii.
161. Karimbux NY. (2017) Aligning Scope of Practice with Periodontology Education. J Dent Educ 81: 639.
162. Leow NM, Hussain Z, Petrie A, Donos N, Needleman IG. (2016) Has the quality of reporting in periodontology changed in 14 years? A systematic review. J Clin Periodontol 43: 833-8.
163. Manandhar I, Alimadadi A, Aryal S, Munroe PB, Joe B, et al. (2021) Gut microbiome-based supervised machine learning for clinical diagnosis of inflammatory bowel diseases. Am J Physiol Gastrointest Liver Physiol 320: G328-37.
164. Dawood MAO, Abo-Al-Ela HG, Hasan MT. (2020) Modulation of transcriptomic profile in aquatic animals: Probiotics, prebiotics and synbiotics scenarios. Fish Shellfish Immunol 97: 268-82.
165. De Lorenzi-Tognon M, Genton L, Schrenzel J. (2023) [Summary of the 8th Symposium “Feeding the microbiota”: prebiotics and probiotics]. Rev Med Suisse 19: 1149-53.
166. Derikx LAAP, Dieleman LA, Hoentjen F. (2016) Probiotics and prebiotics in ulcerative colitis. Best Pract Res Clin Gastroenterol 30: 55-71.
167. Du X, Xie C, Shi L, Gao H, Yang C, et al. (2020) Probiotics, prebiotics, and synbiotics supplementation in prediabetes: protocol for a systematic review and meta-analysis. Medicine (Baltimore) 99: e19708.
168. Duan D, Chen M, Cui W, Liu W, Chen X. (2022) Application of probiotics, prebiotics and synbiotics in patients with breast cancer: a systematic review and meta-analysis protocol for randomised controlled trials. BMJ Open 12: e064417.
169. Eaimworawuthikul S, Tunapong W, Chunchai T, Yasom S, Wanchai K, et al. (2019) Effects of probiotics, prebiotics or synbiotics on jawbone in obese-insulin resistant rats. Eur J Nutr 58: 2801-10.
170. Arrigoni R, Ballini A, Santacroce L, Cantore S, Inchingolo A, et al. (2022) Another Look at Dietary Polyphenols: Challenges in Cancer Preventionand Treatment. Curr Med Chem 29: 1061-82.
171. Inchingolo F, Pacifici A, Gargari M, Acitores Garcia JI, Amantea M, et al. (2014) CHARGE syndrome: an overview on dental and maxillofacial features. Eur Rev Med Pharmacol Sci 18: 2089-93.
172. Inchingolo F, Tatullo M, Marrelli M, Inchingolo AD, Corelli R, et al. (2012) Clinical case-study describing the use of skin-perichondrium-cartilage graft from the auricular concha to cover large defects of the nose. Head Face Med 8: 10.
173. Inchingolo F, Tatullo M, Abenavoli FM, Marrelli M, Inchingolo AD, et al. (2010) Comparison between traditional surgery, CO 2 and Nd:Yag laser treatment for generalized gingival hyperplasia in Sturge–Weber syndrome: a retrospective study. J Investig Clin Dent 1: 85-9.
174. Coloccia G, Inchingolo AD, Inchingolo AM, Malcangi G, Montenegro V, et al. (2021) Effectiveness of Dental and Maxillary Transverse Changes in Tooth-Borne, Bone-Borne, and Hybrid Palatal Expansion through Cone-Beam Tomography: A Systematic Review of the Literature. Medicina (Mex) 57: 288.
175. Inchingolo AD, Inchingolo AM, Malcangi G, Avantario P, Azzollini D, et al. (2022) Effects of Resveratrol, Curcumin and Quercetin Supplementation on Bone Metabolism—A Systematic Review. Nutrients 14: 3519.
176. Santacroce L, Di Cosola M, Bottalico L, Topi S, Charitos IA, et al. (2021) Focus on HPV Infection and the Molecular Mechanisms of Oral Carcinogenesis. Viruses 13: 559.
177. Inchingolo AD, Patano A, Coloccia G, Ceci S, Inchingolo AM, et al. (2021) Genetic Pattern, Orthodontic and Surgical Management of Multiple Supplementary Impacted Teeth in a Rare, Cleidocranial Dysplasia Patient: A Case Report. Medicina (Mex) 57: 1350.
178. Rapone B, Inchingolo AD, Trasarti S, Ferrara E, Qorri E, et al. (2022) Long-Term Outcomes of Implants Placed in Maxillary Sinus Floor Augmentation with Porous Fluorohydroxyapatite (Algipore® FRIOS®) in Comparison with Anorganic Bovine Bone (Bio-Oss®) and Platelet Rich Plasma (PRP): A Retrospective Study. J Clin Med 11: 2491.
179. Inchingolo F, Santacroce L, Cantore S, Ballini A, Del Prete R, et al. (2019) Probiotics and EpiCor® in human health. J Biol Regul Homeost Agents 33: 1973-9.
180. Inchingolo AD, Inchingolo AM, Bordea IR, Malcangi G, Xhajanka E, et al. (2021) SARS-CoV-2 Disease through Viral Genomic and Receptor Implications: An Overview of Diagnostic and Immunology Breakthroughs. Microorganisms 9: 793.
181. Balzanelli MG, Distratis P, Dipalma G, Vimercati L, Inchingolo AD, et al. (2021) Sars-CoV-2 Virus Infection May Interfere CD34+ Hematopoietic Stem Cells and Megakaryocyte–Erythroid Progenitors Differentiation Contributing to Platelet Defection towards Insurgence of Thrombocytopenia and Thrombophilia. Microorganisms 9: 1632.
182. Inchingolo F, Tatullo M, Abenavoli FM, Marrelli M, Inchingolo AD, et al. (2010) Severe Anisocoria after Oral Surgery under General Anesthesia. Int J Med Sci 7: 314-8.
183. Inchingolo AD, Dipalma G, Inchingolo AM, Malcangi G, Santacroce L, et al. (2021) The 15-Months Clinical Experience of SARS-CoV-2: A Literature Review of Therapies and Adjuvants. Antioxidants. 10:881.
184. Inchingolo AD, Inchingolo AM, Bordea IR, Xhajanka E, Romeo DM, et al. (2021) The Effectiveness of Osseodensification Drilling Protocol for Implant Site Osteotomy: A Systematic Review of the Literature and Meta-Analysis. Materials 14: 1147.
185. Inchingolo AD, Patano A, Coloccia G, Ceci S, Inchingolo AM, et al. (2022) The Efficacy of a New AMCOP® Elastodontic Protocol for Orthodontic Interceptive Treatment: A Case Series and Literature Overview. Int J Environ Res Public Health 19: 988.
186. Edwards PT, Kashyap PC, Preidis GA. (2020) Microbiota on biotics: probiotics, prebiotics, and synbiotics to optimize growth and metabolism. Am J Physiol Gastrointest Liver Physiol 319: G382-90.
187. El-Sayed A, Aleya L, Kamel M. (2021) Microbiota and epigenetics: promising therapeutic approaches? Environ Sci Pollut Res Int 28: 49343-61.
188. Enam F, Mansell TJ. (2019) Prebiotics: tools to manipulate the gut microbiome and metabolome. J Ind Microbiol Biotechnol 46: 1445-59.
189. Favero C, Giordano L, Mihaila SM, Masereeuw R, Ortiz A, et al. (2022) Postbiotics and Kidney Disease. Toxins 14: 623.
190. Fei Y, Chen Z, Han S, Zhang S, Zhang T, et al. (2023) Role of prebiotics in enhancing the function of next-generation probiotics in gut microbiota. Crit Rev Food Sci Nutr 63: 1037-54.
191. Fernandez MA, Marette A. (2017) Potential Health Benefits of Combining Yogurt and Fruits Based on Their Probiotic and Prebiotic Properties. Adv Nutr Bethesda Md 8: 155S-164S.
192. Belli WA, Marquis RE. (1991) Adaptation of Streptococcus mutans and Enterococcus hirae to acid stress in continuous culture. Appl Environ Microbiol 57: 1134-8.
193. Ferrarese R, Ceresola ER, Preti A, Canducci F. (2018) Probiotics, prebiotics and synbiotics for weight loss and metabolic syndrome in the microbiome era. Eur Rev Med Pharmacol Sci 22: 7588-605.
194. Ferro LE, Crowley LN, Bittinger K, Friedman ES, Decker JE, et al. (2023) Effects of prebiotics, probiotics, and synbiotics on the infant gut microbiota and other health outcomes: A systematic review. Crit Rev Food Sci Nutr. 63: 5620-42.
195. Fiocchi A, Pecora V, Dahdah L. (2016) Probiotics, Prebiotics & Food allergy Prevention: Clinical Data in Children. J Pediatr Gastroenterol Nutr 63: S14-17.
196. Liu HR, Ge SH. (2023) [Review on the development of periodontology in China]. Zhonghua Kou Qiang Yi Xue Za Zhi Zhonghua Kouqiang Yixue Zazhi Chin J Stomatol 58: 1205-16.
197. Manresa C, Sanz-Miralles EC, Twigg J, Bravo M. (2018) Supportive periodontal therapy (SPT) for maintaining the dentition in adults treated for periodontitis. Cochrane Database Syst Rev1: CD009376.
198. Menne MC, Seitidis G, Faggion CM, Mavridis D, Pandis N. (2022) Early Optimistic Effect in Periodontology and Implant Dentistry Trials. J Dent Res 101: 30-6.
199. Miron RJ, Zucchelli G, Pikos MA, Salama M, Lee S, et al. (2017) Use of platelet-rich fibrin in regenerative dentistry: a systematic review. Clin Oral Investig 21: 1913-27.
200. Mohammad-Rahimi H, Motamedian SR, Pirayesh Z, Haiat A, Zahedrozegar S, et al. (2022) Deep learning in periodontology and oral implantology: A scoping review. J Periodontal Res 57: 942-51.
201. Andrews RC, Walker BR (1999) Glucocorticoids and insulin resistance: old hormones, new targets. Clin Sci Lond Engl 96:513–23.
202. Ford AC, Harris LA, Lacy BE, Quigley EMM, Moayyedi P (2019) Letter: meta-analysis of prebiotics, probiotics, synbiotics and antibiotics in IBS. Authors’ reply. Aliment Pharmacol Ther. 49:1254–5.
203. Francavilla R, Cristofori F, Indrio F (2016) Indications and Recommendations by Societies and Institutions for the Use of Probiotics and Prebiotics in Paediatric Functional Intestinal Disorders. J Pediatr Gastroenterol Nutr. 63 Suppl 1:S36-37.
204. Frei R, Akdis M, O’Mahony L (2015) Prebiotics, probiotics, synbiotics, and the immune system: experimental data and clinical evidence. Curr Opin Gastroenterol. 31:153–8.
205. Zmora N, Zilberman-Schapira G, Suez J, Mor U, Dori-Bachash M, et al. (2018) Personalized Gut Mucosal Colonization Resistance to Empiric Probiotics Is Associated with Unique Host and Microbiome Features. Cell. 174:1388-1405.e21.
206. Gill H, Prasad J (2008) Probiotics, immunomodulation, and health benefits. Adv Exp Med Biol. 606:423–54.
207. Ornstrup MJ, Harsløf T, Kjær TN, Langdahl BL, Pedersen SB (2014) Resveratrol increases bone mineral density and bone alkaline phosphatase in obese men: a randomized placebo-controlled trial. J Clin Endocrinol Metab. 99:4720–9.
208. Minervini G, Franco R, Marrapodi MM, Fiorillo L, Cervino G, Cicciù M. (2023) Post‐traumatic stress, prevalence of temporomandibular disorders in war veterans: Systematic review with meta‐analysis. J Oral Rehabil. 50(10):1101-1109.
209. Cicciù M, Minervini G, Franco R, Marrapodi MM, Fiorillo L, Cervino G (2023) The association between parent education level, oral health, and oral-related sleep disturbance. An observational cross sectional study. Eur J Paediatr Dent. 24(3):218-223.
210. Minervini G, Franco R, Marrapodi MM, Di Blasio M, Ronsivalle V, et al. (2023) Children oral health and parents education status: a cross sectional study. BMC Oral Health. 23:787.
211. Bollero P, Franco R, Cecchetti F, Miranda M, Barlattani A, et al. (2017) Oral Health and Implant Therapy in Parkinson S Patients: Review. Oral Implantol. 10:105–105.
212. Bollero P, Di Renzo L, Franco R, Rampello T, Pujia A, et al. (2017) Effects of new probiotic mouthwash in patients with diabetes mellitus and cardiovascular diseases. Eur Rev Med Pharmacol Sci. 21:5827–36.
213. Dental Supplement, Minetti E, Palermo A, Savadori P, Barlattani A, et al. (2019) Autologous tooth graft: a histological comparison between dentin mixed with xenograft and dentin alone grafts in socket preservation. J Biol Regul Homeost Agents. 33:189–97.
214. Franco R, Gianfreda F, Miranda M, Barlattani A, Bollero P (2020) The hemostatic properties of chitosan in oral surgery. Biomed Biotechnol Res J BBRJ 4:186–186.
215. Minervini G, Franco R, Marrapodi MM, Di Blasio M, Isola G, et al. (2023) Conservative treatment of temporomandibular joint condylar fractures: A systematic review conducted according to PRISMA guidelines and the Cochrane Handbook for Systematic Reviews of Interventions. J Oral Rehabil. 50:886–93.
216. Contaldo M, Lucchese A, Romano A, Della Vella F, Di Stasio D, et al. (2021) Oral Microbiota Features in Subjects with Down Syndrome and Periodontal Diseases: A Systematic Review. Int J Mol Sci. 22:9251.
217. Caggiano M, Gasparro R, D’Ambrosio F, Pisano M, Di Palo MP, et al. (2022) Smoking Cessation on Periodontal and Peri-Implant Health Status: A Systematic Review. Dent J. 10:162.
218. Campanella V, Libonati A, Nardi R, Angotti V, Gallusi G, et al. (2018) Single tooth anesthesia versus conventional anesthesia: a cross-over study. Clin Oral Investig. 22:3205–13.
219. Quinzi V, Mummolo S, Bertolazzi F, Campanella V, Marzo G, et al. (2020) Comparison of Mandibular Arch Expansion by the Schwartz Appliance Using Two Activation Protocols: A Preliminary Retrospective Clinical Study. J Funct Morphol Kinesiol. 5:61.
220. Farronato D, Pasini PM, Orsina AA, Manfredini M, Azzi L, et al. (2020) Correlation between Buccal Bone Thickness at Implant Placement in Healed Sites and Buccal Soft Tissue Maturation Pattern: A Prospective Three-Year Study. Materials. 13:511.
221. Brambilla E, Locarno S, Gallo S, Orsini F, Pini C, et al. (2022) Poloxamer-Based Hydrogel as Drug Delivery System: How Polymeric Excipients Influence the Chemical-Physical Properties. Polymers. 14:3624.
222. D‘Ettorre G, Farronato M, Candida E, Quinzi V, Grippaudo C. (2022) A comparison between stereophotogrammetry and smartphone structured light technology for three-dimensional face scanning. Angle Orthod. 92:358–63.
223. Crincoli V, Anelli MG, Quercia E, Piancino MG, Di Comite M (2019) Temporomandibular Disorders and Oral Features in Early Rheumatoid Arthritis Patients: An Observational Study. Int J Med Sci. 16:253–63.
224. Crincoli V, Di Comite M, Guerrieri M, Rotolo RP, Limongelli L, et al. (2018) Orofacial Manifestations and Temporomandibular Disorders of Sjögren Syndrome: An Observational Study. Int J Med Sci. 15:475–83.
225. Crincoli V, Ballini A, Fatone L, Di Bisceglie MB, Nardi GM, et al. (2016) Cytokine genotype distribution in patients with periodontal disease and rheumatoid arthritis or diabetes mellitus. J Biol Regul Homeost Agents. 30:863–6.
226. Fu Y-S, Chu Q-S, Ashuro AA, Di D-S, Zhang Q, et al. (2020) The Effect of Probiotics, Prebiotics, and Synbiotics on CD4 Counts in HIV-Infected Patients: A Systematic Review and Meta-Analysis. BioMed Res Int. 2020:7947342.
227. Gagliardi A, Totino V, Cacciotti F, Iebba V, Neroni B, et al. (2018) Rebuilding the Gut Microbiota Ecosystem. Int J Environ Res Public Health. 15:1679.
228. Galeana-Patiño CE, Ortiz MI, Cariño-Cortés R, López-Santillán IC, Castro-Rosas J, et al. (2023) Probiotics, as Adjuvant Therapy and Preventive Measure on Progression, and Complications of Head and Neck Cancer. Curr Pharm Biotechnol. 24:1504–14.
229. Eriksson RA, Albrektsson T (1984) The effect of heat on bone regeneration: an experimental study in the rabbit using the bone growth chamber. J Oral Maxillofac Surg Off J Am Assoc Oral Maxillofac Surg. 42:705–11.
230. Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, et al. (2017) Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 14:491–502.
231. Gowen R, Gamal A, Di Martino L, McCormick TS, Ghannoum MA. (2023) Modulating the Microbiome for Crohn’s Disease Treatment. Gastroenterology. 164:828–40.
232. Green M, Arora K, Prakash S (2020) Microbial Medicine: Prebiotic and Probiotic Functional Foods to Target Obesity and Metabolic Syndrome. Int J Mol Sci. 21:2890.
233. Gu Q, Yin Y, Yan X, Liu X, Liu F, et al. (2022) Encapsulation of multiple probiotics, synbiotics, or nutrabiotics for improved health effects: A review. Adv Colloid Interface Sci. 309:102781.
234. Guandalini S, Cernat E, Moscoso D (2015) Prebiotics and probiotics in irritable bowel syndrome and inflammatory bowel disease in children. Benef Microbes 6:209–17.
235. Guevara-Gonzaléz J, Guevara-Campos J, González L, Cauli O (2022) The Effects of Probiotics and Prebiotics on Gastrointestinal and Behavioural Symptoms in Autism Spectrum Disorder. Curr Rev Clin Exp Pharmacol. 17:166–73.
236. Tariq R, Pardi DS, Bartlett MG, Khanna S (2019) Low Cure Rates in Controlled Trials of Fecal Microbiota Transplantation for Recurrent Clostridium difficile Infection: A Systematic Review and Meta-analysis. Clin Infect Dis. 68:1351–8.
237. Iannone LF, Gómez-Eguílaz M, De Caro C (2022) Gut microbiota manipulation as an epilepsy treatment. Neurobiol Dis. 174:105897.
238. Jadhav A, Jagtap S, Vyavahare S, Sharbidre A, Kunchiraman B. (2023) Reviewing the potential of probiotics, prebiotics and synbiotics: advancements in treatment of ulcerative colitis. Front Cell Infect Microbiol. 13:1268041.
239. Jarde A, Lewis-Mikhael A-M, Moayyedi P, Stearns JC, Collins SM, et al. (2018) Pregnancy outcomes in women taking probiotics or prebiotics: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 18:14.
240. Hijova E. (2021) Probiotics and prebiotics, targeting obesity with functional foods. Bratisl Lek Listy. 122:647–52.
241. Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, et al. (2014) Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 11:506–14.
242. Huang L, Guo J, Li W, Jiang M, Wang F, et al. (2019) Probiotics, prebiotics, and synbiotics for the treatment of asthma: Protocol for a systematic review. Medicine (Baltimore). 98:e17840.
243. Ballini A, Gnoni A, De Vito D, Dipalma G, Cantore S, et al. (2019) Effect of probiotics on the occurrence of nutrition absorption capacities in healthy children: a randomized double-blinded placebo-controlled pilot study. Eur Rev Med Pharmacol Sci. 23:8645–57.
244. Lange K, Buerger M, Stallmach A, Bruns T (2016) Effects of Antibiotics on Gut Microbiota. Dig Dis Basel Switz. 34:260–8.
245. Vangay P, Ward T, Gerber JS, Knights D (2015) Antibiotics, pediatric dysbiosis, and disease. Cell Host Microbe 17:553–64.
246. Szajewska H, Konarska Z, Kołodziej M (2016) Probiotic Bacterial and Fungal Strains: Claims with Evidence. Dig Dis Basel Switz. 34:251–9.
247. Das M, Cronin O, Keohane DM, Cormac EM, Nugent H, et al. (2019) Gut microbiota alterations associated with reduced bone mineral density in older adults. Rheumatol Oxf Engl. 58:2295.
248. Duran-Pinedo AE, Frias-Lopez J (2015) Beyond microbial community composition: functional activities of the oral microbiome in health and disease. Microbes Infect. 17:505–16.
249. Park JS, Lee EJ, Lee JC, Kim WK, Kim HS (2007) Anti-inflammatory effects of short chain fatty acids in IFN-gamma-stimulated RAW 264.7 murine macrophage cells: involvement of NF-kappaB and ERK signaling pathways. Int Immunopharmacol. 7:70–7.
250. Park JH, Lee NK, Lee SY (2017) Current Understanding of RANK Signaling in Osteoclast Differentiation and Maturation. Mol Cells. 40:706–13.
251. Dierksen KP, Moore CJ, Inglis M, Wescombe PA, Tagg JR (2007) The effect of ingestion of milk supplemented with salivaricin A-producing Streptococcus salivarius on the bacteriocin-like inhibitory activity of streptococcal populations on the tongue. FEMS Microbiol Ecol. 59:584–91.
252. Jayanama K, Theou O (2020) Effects of Probiotics and Prebiotics on Frailty and Ageing: A Narrative Review. Curr Clin Pharmacol. 15:183–92.
253. Johnson-Henry KC, Abrahamsson TR, Wu RY, Sherman PM. (2016) Probiotics, Prebiotics, and Synbiotics for the Prevention of Necrotizing Enterocolitis. Adv Nutr Bethesda Md. 7:928–37.
254. Kareb O, Aïder M (2019) Whey and Its Derivatives for Probiotics, Prebiotics, Synbiotics, and Functional Foods: a Critical Review. Probiotics Antimicrob Proteins. 11:348–69.
255. Nam JW, Kim MY, Han SJ (2016) Cranial bone regeneration according to different particle sizes and densities of demineralized dentin matrix in the rabbit model. Maxillofac Plast Reconstr Surg. 38:27.
256. Cialiè Rosso M, Stilo F, Squara S, Liberto E, Mai S, et al. (2021) Exploring extra dimensions to capture saliva metabolite fingerprints from metabolically healthy and unhealthy obese patients by comprehensive two-dimensional gas chromatography featuring Tandem Ionization mass spectrometry. Anal Bioanal Chem. 413:403–18.
257. Kaufmann B, Seyfried N, Hartmann D, Hartmann P (2023) Probiotics, prebiotics, and synbiotics in nonalcoholic fatty liver disease and alcohol-associated liver disease. Am J Physiol Gastrointest Liver Physiol. 325:G42–61.
258. Kazemi A, Noorbala AA, Azam K, Eskandari MH, Djafarian K (2019) Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: A randomized clinical trial. Clin Nutr Edinb Scotl. 38:522–8.
259. Keulers L, Dehghani A, Knippels L, Garssen J, Papadopoulos N, et al. (2022) Probiotics, prebiotics, and synbiotics to prevent or combat air pollution consequences: The gut-lung axis. Environ Pollut Barking Essex 1987. 302:119066.
260. Lamont RJ, Koo H, Hajishengallis G (2018) The oral microbiota: dynamic communities and host interactions. Nat Rev Microbiol. 16:745–59.
261. Coretti L, Cristiano C, Florio E, Scala G, Lama A, et al. (2017) Sex-related alterations of gut microbiota composition in the BTBR mouse model of autism spectrum disorder. Sci Rep. 7:45356.
262. Khalesi S, Vandelanotte C, Thwaite T, Russell AMT, Dawson D, Williams SL. (2021) Awareness and Attitudes of Gut Health, Probiotics and Prebiotics in Australian Adults. J Diet Suppl. 18:418–32.
263. Khan FF, Sohail A, Ghazanfar S, Ahmad A, Riaz A, et al. (2023) Recent Innovations in Non-dairy Prebiotics and Probiotics: Physiological Potential, Applications, and Characterization. Probiotics Antimicrob Proteins. 15:239–63.
264. Kim NY, Kim JM, Son J-Y, Ra CH (2023) Synbiotic Fermentation of Undaria pinnatifida and Lactobacillus brevis to Produce Prebiotics and Probiotics. Appl Biochem Biotechnol. 195:6321–33.
265. Inchingolo AD, Di Cosola M, Inchingolo AM, Greco Lucchina A, Malcangi G, et al. (2021) Correlation between occlusal trauma and oral microbiota: a microbiological investigation. J Biol Regul Homeost Agents. 35:295–302.
266. Koletzko S (2016) Probiotics and Prebiotics for Prevention of Food Allergy: Indications and Recommendations by Societies and Institutions. J Pediatr Gastroenterol Nutr. 63 Suppl 1: S9–10.
267. Koopman N, Molinaro A, Nieuwdorp M, Holleboom AG (2019) Review article: can bugs be drugs? The potential of probiotics and prebiotics as treatment for non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 50:628–39.
268. Kumar D, Lal MK, Dutt S, Raigond P, Changan SS, Tiwari RK, et al. (2022) Functional Fermented Probiotics, Prebiotics, and Synbiotics from Non-Dairy Products: A Perspective from Nutraceutical. Mol Nutr Food Res. 66:e2101059.
269. Abbasi J (2021) Study Suggests Lasting Immunity After COVID-19, With a Big Boost From Vaccination. JAMA. 326:376.
270. Nwizu N, Wactawski-Wende J, Genco RJ (2020) Periodontal disease and cancer: Epidemiologic studies and possible mechanisms. Periodontol 2000. 83:213–33.
271. Pandya N (2019) The role of a specialist in periodontology. Br Dent J. 227:626–7.
272. Pannuti CM, Costa FO, Souza NV, Retamal-Valdes B, Costa AA, et al. (2021) Randomized clinical trials in periodontology: focus on outcomes selection. Braz Oral Res. 35:e100.
273. Pini Prato GP, Di Gianfilippo R, Wang H-L (2019) Success in periodontology: An evolutive concept. J Clin Periodontol. 46:840–5.
274. Porter S, Johnson NW, Fedele S (2019) Challenges of the interface of oral medicine and periodontology: Some lessons for the future? Periodontol 2000. 80:225–8.
275. Inchingolo F, Martelli FS, Gargiulo Isacco C, Borsani E, Cantore S, et al. (2020) Chronic Periodontitis and Immunity, Towards the Implementation of a Personalized Medicine: A Translational Research on Gene Single Nucleotide Polymorphisms (SNPs) Linked to Chronic Oral Dysbiosis in 96 Caucasian Patients. Biomedicines. 8:115.
276. Leach ST (2024) Role of Probiotics and Prebiotics in Gut Symbiosis. Nutrients. 16:238.
277. Leis R, de Castro MJ, de Lamas C, Picáns R, Couce ML. (2020) Effects of Prebiotic and Probiotic Supplementation on Lactase Deficiency and Lactose Intolerance: A Systematic Review of Controlled Trials. Nutrients. 12:1487.
278. Li HY, Zhou DD, Gan RY, Huang SY, Zhao CN, Shang A, et al. (2021) Effects and Mechanisms of Probiotics, Prebiotics, Synbiotics, and Postbiotics on Metabolic Diseases Targeting Gut Microbiota: A Narrative Review. Nutrients. 13:3211.
279. Li S, Liu J, Wang Z, Duan F, Jia Z, et al. (2022) The promising role of probiotics/prebiotics/synbiotics in energy metabolism biomarkers in patients with NAFLD: A systematic review and meta-analysis. Front Public Health. 10:862266.
280. Mehar R, Swarnakar S, Lakkakula S, Verma HK, Bhaskar LVKS. (2021) Interleukin-6 gene -174G>C promoter polymorphism reduces the risk of periodontitis in Brazilian populations: A meta-analysis. J Oral Biosci [Internet] 63(4):388-393.
281. Sandoval Torrientes M, Castelló Abietar C, Boga Riveiro J, Álvarez-Argüelles ME, Rojo-Alba S, et al. (2021) A novel single nucleotide polymorphism assay for the detection of N501Y SARS-CoV-2 variants. J Virol Methods.294:114143.
282. Balzanelli MG, Distratis P, Dipalma G, Vimercati L, Catucci O, et al. (2021) Immunity Profiling of COVID-19 Infection, Dynamic Variations of Lymphocyte Subsets, a Comparative Analysis on Four Different Groups. Microorganisms. 9:2036.
283. Rösing CK, Romito GA (2021) Brazilian contribution to evidence-based periodontology. Braz Oral Res. 35:e103.
284. Sanz M, Herrera D, Kebschull M, Chapple I, Jepsen S, et al. (2020) Treatment of stage I-III periodontitis-The EFP S3 level clinical practice guideline. J Clin Periodontol. 47 Suppl 22:4–60.
285. Saravanamuttu R (2015) Periodontology: A little caution. Br Dent J. 218:438.
286. Scannapieco FA (2015) Unanswered Questions in Periodontology. Dent Clin North Am. 59:xiii–xiv.
287. Sculean A (2022) Periodontology 2000: The global voice of Periodontology and Implant Dentistry. Periodontol 2000. 88:7–8.
288. Abu Jabal K, Ben-Amram H, Beiruti K, Batheesh Y, Sussan C, et al. (2021) Impact of age, ethnicity, sex and prior infection status on immunogenicity following a single dose of the BNT162b2 mRNA COVID-19 vaccine: real-world evidence from healthcare workers, Israel, December 2020 to January 2021. Eurosurveillance [Internet]. 26: 6.
289. Inchingolo AD, Gargiulo CI, Malcangi G, Ciocia AM, Patano A, et al. (2022) Diagnosis of SARS-CoV-2 during the Pandemic by Multiplex RT-rPCR hCoV Test: Future Perspectives. Pathogens. 11:1378.
290. Koning R, Bastard P, Casanova JL, Brouwer MC, van de Beek D, et al. (2021) Autoantibodies against type I interferons are associated with multi-organ failure in COVID-19 patients. Intensive Care Med. 47:704–6.
291. Shirmohammadi A, Babaloo A, Maleki Dizaj S, Lotfipour F, Sharifi S, et al. (2021) A View on Polymerase Chain Reaction as an Outstanding Molecular Diagnostic Technique in Periodontology. BioMed Res Int. 2021:9979948.
292. Slots J (2020) Life-threatening pathogens in severe/progressive periodontitis: Focal infection risk, future periodontal practice, role of the Periodontology 2000. Periodontol 2000. 84:215–6.
293. Slots J (2013) Periodontology: past, present, perspectives. Periodontol 2000. 62:7–19.
294. Smith PC, Martínez C, Cáceres M, Martínez J. (2015) Research on growth factors in periodontology. Periodontol 2000. 67:234–50.
295. Theodoro LH, Marcantonio RAC, Wainwright M, Garcia VG (2021) LASER in periodontal treatment: is it an effective treatment or science fiction? Braz Oral Res.35:e099.
296. Balzanelli MG, Distratis P, Lazzaro R, Cefalo A, Catucci O, et al. (2021) The Vitamin D, IL-6 and the eGFR Markers a Possible Way to Elucidate the Lung-Heart-Kidney Cross-Talk in COVID-19 Disease: A Foregone Conclusion. Microorganisms. 9:1903.
297. Inchingolo AM, Patano A, Di Pede C, Inchingolo AD, Palmieri G, et al. (2023) Autologous Tooth Graft: Innovative Biomaterial for Bone Regeneration. Tooth Transformer® and the Role of Microbiota in Regenerative Dentistry. A Systematic Review. J Funct Biomater. 14:132.
298. Tong DC (2017) Surgical management in dentistry: the interdisciplinary relationship between periodontology and oral and maxillofacial surgery. Periodontol 2000. 74:168–75.
299. Woelber JP, Fleiner J, Rau J, Ratka-Krüger P, Hannig C. (2018) Accuracy and Usefulness of CBCT in Periodontology: A Systematic Review of the Literature. Int J Periodontics Restorative Dent. 38:289–97.
300. Yan Y, Zhan Y, Wang X, Hou J (2020) Clinical evaluation of ultrasonic subgingival debridement versus ultrasonic subgingival scaling combined with manual root planing in the treatment of periodontitis: study protocol for a randomized controlled trial. Trials. 21:113.
301. Balzanelli MG, Distratis P, Aityan SK, Amatulli F, Catucci O, et al. (2021) An Alternative “Trojan Horse” Hypothesis for COVID-19: Immune Deficiency of IL-10 and SARS-CoV-2 Biology. Endocr Metab Immune Disord Drug Targets. 22(1):1-5.
- 302. Boerckel JD, Kolambkar YM, Dupont KM, Uhrig BA, Phelps EA, et al. (2011) Effects of protein dose and delivery system on BMP-mediated bone regeneration. Biomaterials 32: 5241–51.
303. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, et al. (2020) COVID-19: consider cytokine storm syndromes and immunosuppression. The Lancet 395: 1033-1034.
304. Li Y, Hintze KJ, Ward RE (2021) Effect of supplemental prebiotics, probiotics and bioactive proteins on the microbiome composition and fecal calprotectin in C57BL6/j mice. Biochimie 185: 43–52.
305. Li Y, Tan Y, Xia G, Shuai J (2023) Effects of probiotics, prebiotics, and synbiotics on polycystic ovary syndrome: a systematic review and meta-analysis. Crit Rev Food Sci Nutr 63: 522-538.
306. Lichtenstein L, Avni-Biron I, Ben-Bassat O (2016) Probiotics and prebiotics in Crohn’s disease therapies. Best Pract Res Clin Gastroenterol 30: 81-8.
307. Liu F, Liu Y, Lv X, Lun H (2023) Effects of prebiotics, probiotics and synbiotics on serum creatinine in non-dialysis patients: a meta-analysis of randomized controlled trials. Ren Fail 45: 2152693.
308. Liu RT, Walsh RFL, Sheehan AE (2019) Prebiotics and probiotics for depression and anxiety: A systematic review and meta-analysis of controlled clinical trials. Neurosci Biobehav Rev 102: 13-23.
309. Balzanelli MG, Distratis P, Lazzaro R, Pham VH, Tran TC, et al. (2022) Analysis of Gene Single Nucleotide Polymorphisms in COVID-19 Disease Highlighting the Susceptibility and the Severity towards the Infection. Diagn Basel Switz 12: 2824.
310. Lin B, Zhao F, Liu Y, Wu X, Feng J, et al. (2022) Randomized Clinical Trial: Probiotics Alleviated Oral-Gut Microbiota Dysbiosis and Thyroid Hormone Withdrawal-Related Complications in Thyroid Cancer Patients Before Radioiodine Therapy Following Thyroidectomy. Front Endocrinol 13: 834674.
311. Liu Y, Zhao Y, Yang Y, Wang Z (2019) Effects of Probiotics, Prebiotics, and Synbiotics on Calcium Homeostasis and Bone Health With Aging: A Systematic Review. Worldviews Evid Based Nurs 16: 478-484.
312. Maguire M, Maguire G (2017) The role of microbiota, and probiotics and prebiotics in skin health. Arch Dermatol Res 309: 411-421.
313. Maguire M, Maguire G (2019) Gut dysbiosis, leaky gut, and intestinal epithelial proliferation in neurological disorders: towards the development of a new therapeutic using amino acids, prebiotics, probiotics, and postbiotics. Rev Neurosci 30: 179-201.
314. Cueno ME, Imai K (2021) Structural Insights on the SARS-CoV-2 Variants of Concern Spike Glycoprotein: A Computational Study With Possible Clinical Implications. Front Genet [Internet] 12: 773726.
315. Boyce BF, Xing L (2007) The RANKL/RANK/OPG pathway. Curr Osteoporos Rep 5: 98-104.
316. Dobson A, Crispie F, Rea MC, O’Sullivan O, Casey PG, et al. (2011) Fate and efficacy of lacticin 3147-producing Lactococcus lactis in the mammalian gastrointestinal tract: Lacticin-producing L. lactis in the gastrointestinal tract. FEMS Microbiol Ecol 76: 602-14.
317. Martín-Peláez S, Cano-Ibáñez N, Pinto-Gallardo M, Amezcua-Prieto C (2022) The Impact of Probiotics, Prebiotics, and Synbiotics during Pregnancy or Lactation on the Intestinal Microbiota of Children Born by Cesarean Section: A Systematic Review. Nutrients 14: 341.
318. Martinez RCR, Bedani R, Saad SMI (2015) Scientific evidence for health effects attributed to the consumption of probiotics and prebiotics: an update for current perspectives and future challenges. Br J Nutr 114: 1993-2015.
319. Martyniak A, Medyńska-Przęczek A, Wędrychowicz A, Skoczeń S, Tomasik PJ (2021) Prebiotics, Probiotics, Synbiotics, Paraprobiotics and Postbiotic Compounds in IBD. Biomolecules 11: 1903.
320. Schmidt TS, Hayward MR, Coelho LP, Li SS, Costea PI, et al. (2019) Extensive transmission of microbes along the gastrointestinal tract. Garrett WS, Nieuwdorp M, Prodan A, O’Toole P, editors. eLife 8: e42693.
321. Paassen NBV, Vincent A, Puiman PJ, Sluis MVD, Bouma J et al. (2009) The regulation of intestinal mucin MUC2 expression by short-chain fatty acids: implications for epithelial protection. Biochem J. 420: 211-9.
322. Marx W, Scholey A, Firth J, D’Cunha NM, Lane M, et al. (2020) Prebiotics, probiotics, fermented foods and cognitive outcomes: A meta-analysis of randomized controlled trials. Neurosci Biobehav Rev 118: 472-484.
323. McFarland LV, Goh S (2019) Are probiotics and prebiotics effective in the prevention of travellers’ diarrhea: A systematic review and meta-analysis. Travel Med Infect Dis 27: 11-19.
324. McFarlane C, Kelly JT, Conley M, Johnson DW, Campbell KL (2023) Consumers’ Perspectives and Experiences of Prebiotics and Probiotics for Gut Health in Chronic Kidney Disease. J Ren Nutr Off J Counc Ren Nutr Natl Kidney Found 33: 116-125.
325. Foley KA, Ossenkopp K-P, Kavaliers M, MacFabe DF (2014) Pre- and Neonatal Exposure to Lipopolysaccharide or the Enteric Metabolite, Propionic Acid, Alters Development and Behavior in Adolescent Rats in a Sexually Dimorphic Manner. Hashimoto K, editor. PLoS ONE 9: e87072.
326. Hawrelak JA, Myers SP (2004) The causes of intestinal dysbiosis: a review. Altern Med Rev J Clin Ther 9: 180-97.
327. Inchingolo AM, Fatone MC, Malcangi G, Avantario P, Piras F, et al. (2022) Modifiable Risk Factors of Non-Syndromic Orofacial Clefts: A Systematic Review. Child Basel Switz 9: 1846.
328. Gahan CG, Driscoll BO, Hill C (1996) Acid adaptation of Listeria monocytogenes can enhance survival in acidic foods and during milk fermentation. Appl Environ Microbiol 62: 3128-32.
329. Merenstein D, Pot B, Leyer G, Ouwehand AC, Preidis GA, et al. (2023) Emerging issues in probiotic safety: 2023 perspectives. Gut Microbes 15: 2185034.
330. Mishra A, Chakravarty I, Mandavgane S (2021) Current trends in non-dairy based synbiotics. Crit Rev Biotechnol 41: 935-952.
331. Mitchell LK, Davies PSW (2022) Pre- and probiotics in the management of children with autism and gut issues: a review of the current evidence. Eur J Clin Nutr 76: 913-921.
332. Monteagudo-Mera A, Rastall RA, Gibson GR, Charalampopoulos D, Chatzifragkou A (2019) Adhesion mechanisms mediated by probiotics and prebiotics and their potential impact on human health. Appl Microbiol Biotechnol 103: 6463-6472.
333. Foster JW, Moreno M (1999) Inducible acid tolerance mechanisms in enteric bacteria. Novartis Found Symp 221: 55-69.
334. Newman AM, Arshad M (2020) The Role of Probiotics, Prebiotics and Synbiotics in Combating Multidrug-Resistant Organisms. Clin Ther 42: 1637-1648.
335. Ng QX, Loke W, Venkatanarayanan N, Lim DY, Soh AYS, et al. (2019) A Systematic Review of the Role of Prebiotics and Probiotics in Autism Spectrum Disorders. Med Kaunas Lith 55: 129.
336. Notay M, Foolad N, Vaughn AR, Sivamani RK (2017) Probiotics, Prebiotics, and Synbiotics for the Treatment and Prevention of Adult Dermatological Diseases. Am J Clin Dermatol 18: 721-732.
337. ten Dijke P, Miyazono K, Heldin CH (1996) Signaling via hetero-oligomeric complexes of type I and type II serine/threonine kinase receptors. Curr Opin Cell Biol 8: 139-45.
338. Mugwanya M, Dawood MAO, Kimera F, Sewilam H (2022) Updating the Role of Probiotics, Prebiotics, and Synbiotics for Tilapia Aquaculture as Leading Candidates for Food Sustainability: a Review. Probiotics Antimicrob Proteins 14: 130-157.
339. Munir MB, Hashim R, Nor SAM, Marsh TL (2018) Effect of dietary prebiotics and probiotics on snakehead (Channa striata) health: Haematology and disease resistance parameters against Aeromonas hydrophila. Fish Shellfish Immunol 75: 99-108.
340. Nath A, Molnár MA, Csighy A, Kőszegi K, Galambos I, et al. (2018) Biological Activities of Lactose-Based Prebiotics and Symbiosis with Probiotics on Controlling Osteoporosis, Blood-Lipid and Glucose Levels. Med Kaunas Lith 54: 98.
341. Gondo K, Tobimatsu S, Kira R, Tokunaga Y, Yamamoto T, et al. (2001) A magnetoencephalographic study on development of the somatosensory cortex in infants. Neuroreport 12: 3227-31.
342. Ojima MN, Yoshida K, Sakanaka M, Jiang L, Odamaki T, et al. (2022) Ecological and molecular perspectives on responders and non-responders to probiotics and prebiotics. Curr Opin Biotechnol 73: 108-120.
343. Olas B (2020) Probiotics, Prebiotics and Synbiotics-A Promising Strategy in Prevention and Treatment of Cardiovascular Diseases? Int J Mol Sci 21: 9737.
344. Oliveira LSD, Wendt GW, Crestani APJ, Casaril KBPB (2022) The use of probiotics and prebiotics can enable the ingestion of dairy products by lactose intolerant individuals. Clin Nutr Edinb Scotl 41: 2644-2650.
345. Pietruszewska W, Barańska M, Wielgat J (2018) Place of phytotherapy in the treatment of acute infections of upper respiratory tract and upper gastrointestinal tract. Otolaryngol Pol Pol Otolaryngol 72: 42-50.
346. Ong TG, Gordon M, Banks SS, Thomas MR, Akobeng AK (2019) Probiotics to prevent infantile colic. Cochrane Database Syst Rev 3: CD012473.
347. Oniszczuk A, Oniszczuk T, Gancarz M, Szymańska J (2021) Role of Gut Microbiota, Probiotics and Prebiotics in the Cardiovascular Diseases. Mol Basel Switz 26: 1172.
348. Ozen M, Dinleyici EC (2015) Foreword: all things considered about probiotics, prebiotics and ıntestinal microbiota in children - from bench to bedside. Benef Microbes 6: 153-7.
349. Imhann F, Bonder MJ, Vila AV, Fu J, Mujagic Z, et al. (2016) Proton pump inhibitors affect the gut microbiome. Gut 65: 740-8.
350. Pai-Dhungat JP-D (2021) Probiotics & Prebiotics. J Assoc Physicians India 69: 11-12.
351. Panelli S, D’Auria E, Papaleo S, Alvaro A, Bandi C, et al. (2022) Biotics in pediatrics: a short overview. Minerva Pediatr 74: 682-687.
352. Paul P, Kaul R, Harfouche M, Arabi M, Al-Najjar Y, et al. (2022) The effect of microbiome-modulating probiotics, prebiotics and synbiotics on glucose homeostasis in type 2 diabetes: A systematic review, meta-analysis, and meta-regression of clinical trials. Pharmacol Res 185: 106520.
353. Linkhart TA, Mohan S, Baylink DJ (1996) Growth factors for bone growth and repair: IGF, TGF beta and BMP. Bone 19: 1S-12S.
354. Pavlidou E, Fasoulas A, Mantzorou M, Giaginis C (2022) Clinical Evidence on the Potential Beneficial Effects of Probiotics and Prebiotics in Cardiovascular Disease. Int J Mol Sci 23: 15898.
355. Pei C, Wu Y, Wang X, Wang F, Liu L (2020) Effect of probiotics, prebiotics and synbiotics for chronic bronchitis or chronic obstructive pulmonary disease: A protocol for systematic review and meta-analysis. Medicine (Baltimore) 99: e23045.
356. Pei M, Wei L, Hu S, Yang B, Si J, et al. (2018) Probiotics, prebiotics and synbiotics for chronic kidney disease: protocol for a systematic review and meta-analysis. BMJ Open 8: e020863.
357. Tabatabaei FS, Torshabi M (2016) Effects of Non-Collagenous Proteins, TGF-β1, and PDGF-BB on Viability and Proliferation of Dental Pulp Stem Cells. J Oral Maxillofac Res 7: e4.
358. Peng M, Tabashsum Z, Anderson M, Truong A, Houser AK, et al. (2020) Effectiveness of probiotics, prebiotics, and prebiotic-like components in common functional foods. Compr Rev Food Sci Food Saf 19: 1908-1933.
359. Peterson CT (2020) Dysfunction of the Microbiota-Gut-Brain Axis in Neurodegenerative Disease: The Promise of Therapeutic Modulation With Prebiotics, Medicinal Herbs, Probiotics, and Synbiotics. J Evid-Based Integr Med 25: 2515690X20957225.
360. Pujari R, Banerjee G (2021) Impact of prebiotics on immune response: from the bench to the clinic. Immunol Cell Biol 99: 255-273.
361. Kojima Y, Ohshima T, Seneviratne CJ, Maeda N (2016) Combining prebiotics and probiotics to develop novel synbiotics that suppress oral pathogens. J Oral Biosci 58: 27-32.
362. Inchingolo AD, Cazzolla AP, Cosola MD, Lucchina AG, Santacroce L, et al. (2021) The integumentary system and its microbiota between health and disease. J Biol Regul Homeost Agents 35: 303-321.
363. Kilian M, Chapple ILC, Hannig M, Marsh PD, Meuric V, et al. (2016) The oral microbiome – an update for oral healthcare professionals. Br Dent J 221: 657-666.
364. Inchingolo AD, Carpentiere V, Piras F, Netti A, Ferrara I, et al. (2022) Orthodontic Surgical Treatment of Impacted Mandibular Canines: Systematic Review and Case Report. Appl Sci 12: 8008.
365. Puri P, Sharma JG, Singh R (2022) Biotherapeutic microbial supplementation for ameliorating fish health: developing trends in probiotics, prebiotics, and synbiotics use in finfish aquaculture. Anim Health Res Rev 23: 113-135.
366. Quigley EMM (2019) Prebiotics and Probiotics in Digestive Health. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc 17: 333-344.
367. Quigley EMM, Gajula P (2020) Recent advances in modulating the microbiome. F1000Research 9: F1000 Faculty Rev-46.
368. Acen EL, Biraro IA, Worodria W, Joloba ML, Nkeeto B, et al. (2021) Impact of vitamin D status and cathelicidin antimicrobial peptide on adults with active pulmonary TB globally: A systematic review and meta-analysis. Hirst JA, editor. PLOS ONE 16: e0252762.
369. Radford-Smith DE, Anthony DC (2023) Prebiotic and Probiotic Modulation of the Microbiota-Gut-Brain Axis in Depression. Nutrients 15: 1880.
370. Rangel-Torres BE, García-Montoya IA, Jiménez-Vega F, Rodríguez-Tadeo A (2022) [Effect of prebiotics, probiotics, and symbiotics on molecular markers of inflammation in obesity.]. Rev Esp Salud Publica 96: e202212090.
371. Rashidi K, Darand M, Garousi N, Dehghani A, Alizadeh S (2021) Effect of infant formula supplemented with prebiotics and probiotics on incidence of respiratory tract infections: A systematic review and meta-analysis of randomized clinical trials. Complement Ther Med 63: 102795.
372. Franco-Robles E (2020) Prebiotics and probiotics: potential benefits in nutrition and health. London: IntechOpen
373. Rashidinejad A, Bahrami A, Rehman A, Rezaei A, Babazadeh A, et al. (2022) Co-encapsulation of probiotics with prebiotics and their application in functional/synbiotic dairy products. Crit Rev Food Sci Nutr 62: 2470-2494.
374. Reid G, Abrahamsson T, Bailey M, Bindels LB, Bubnov R, et al. (2017) How do probiotics and prebiotics function at distant sites? Benef Microbes 8: 521-533.
375. Rohani MF, Islam SM, Hossain MK, Ferdous Z, Siddik MA, et al. (2022) Probiotics, prebiotics and synbiotics improved the functionality of aquafeed: Upgrading growth, reproduction, immunity and disease resistance in fish. Fish Shellfish Immunol 120: 569-589.
376. Isacco CG, Ballini A, De Vito D, Nguyen KCD, Cantore S, et al. (2021) Rebalancing the Oral Microbiota as an Efficient Tool in Endocrine, Metabolic and Immune Disorders. Endocr Metab Immune Disord Drug Targets 21: 777-784.
377. Rovinaru C, Pasarin D (2020) Application of Microencapsulated Synbiotics in Fruit-Based Beverages. Probiotics Antimicrob Proteins 12: 764-773.
378. Roy S, Dhaneshwar S (2023) Role of prebiotics, probiotics, and synbiotics in management of inflammatory bowel disease: Current perspectives. World J Gastroenterol 29: 2078-2100.
379. Sáez-Orviz S, Rendueles M, Díaz M (2023) Impact of adding prebiotics and probiotics on the characteristics of edible films and coatings- a review. Food Res Int Ott Ont 164: 112381.
380. Ballini A, Santacroce L, Cantore S, Bottalico L, Dipalma G, et al. (2018) Probiotics Improve Urogenital Health in Women. Open Access Maced J Med Sci 6: 1845-1850.
381. Salehi-Abargouei A, Ghiasvand R, Hariri M (2017) Prebiotics, Prosynbiotics and Synbiotics: Can They Reduce Plasma Oxidative Stress Parameters? A Systematic Review. Probiotics Antimicrob Proteins 9: 1-11.
382. Samanta S (2022) Potential Impacts of Prebiotics and Probiotics on Cancer Prevention. Anticancer Agents Med Chem 22: 605-628.
383. Sanders ME, Merenstein DJ, Reid G, Gibson GR, Rastall RA (2019) Probiotics and prebiotics in intestinal health and disease: from biology to the clinic. Nat Rev Gastroenterol Hepatol 16: 605-616.
384. Contaldo M, Fusco A, Stiuso P, Lama S, Gravina AG, et al. (2021) Oral Microbiota and Salivary Levels of Oral Pathogens in Gastro-Intestinal Diseases: Current Knowledge and Exploratory Study. Microorganisms 9: 1064.
385. Rong L, Ch’ng D, Jia P, Tsoi KKF, Wong SH, et al. (2023) Use of probiotics, prebiotics, and synbiotics in non-alcoholic fatty liver disease: A systematic review and meta-analysis. J Gastroenterol Hepatol 38: 1682-1694.
386. Rouhani MH, Hadi A, Ghaedi E, Salehi M, Mahdavi A, et al. (2019) Do probiotics, prebiotics and synbiotics affect adiponectin and leptin in adults? A systematic review and meta-analysis of clinical trials. Clin Nutr Edinb Scotl 38: 2031-2037.
387. Rousseaux A, Brosseau C, Bodinier M (2023) Immunomodulation of B Lymphocytes by Prebiotics, Probiotics and Synbiotics: Application in Pathologies. Nutrients 15: 269.
388. Sarao LK, Arora M (2017) Probiotics, prebiotics, and microencapsulation: A review. Crit Rev Food Sci Nutr 57: 344-371.
389. Sarkar A, Lehto SM, Harty S, Dinan TG, Cryan JF, at al. (2016) Psychobiotics and the Manipulation of Bacteria-Gut-Brain Signals. Trends Neurosci 39: 763-781.
390. Sheyholislami H, Connor KL (2021) Are Probiotics and Prebiotics Safe for Use during Pregnancy and Lactation? A Systematic Review and Meta-Analysis. Nutrients 13: 2382.
391. Ballini A, Signorini L, Inchingolo AD, Saini R, Gnoni A, et al. (2020) Probiotics May Improve Serum Folate Availability in Pregnant Women: A Pilot Study. Open Access Maced J Med Sci 8: 1124-1130.
392. Isacco CG, Ballini A, Vito DD, Inchingolo AM, Cantore S, et al. (2019) Probiotics in Health and Immunity: A First Step toward Understanding the Importance of Microbiota System in Translational Medicine. In: Franco-Robles E, Ramírez-Emiliano J, editors. Prebiotics Probiotics - Potential Benefits Nutr Health [Internet]. IntechOpen.
393. Shu Q, Kang C, Li J, Hou Z, Xiong M, et al. (2024) Effect of probiotics or prebiotics on thyroid function: A meta-analysis of eight randomized controlled trials. PloS One 19: e0296733.
394. Singh A, Alexander SG, Martin S (2023) Gut microbiome homeostasis and the future of probiotics in cancer immunotherapy. Front Immunol 14: 1114499.
395. Sorboni SG, Moghaddam HS, Jafarzadeh-Esfehani R, Soleimanpour S (2022) A Comprehensive Review on the Role of the Gut Microbiome in Human Neurological Disorders. Clin Microbiol Rev 35: e0033820.
396. Campanella V, Syed J, Santacroce L, Saini R, Ballini A, et al. (2018) Oral probiotics influence oral and respiratory tract infections in pediatric population: a randomized double-blinded placebo-controlled pilot study. Eur Rev Med Pharmacol Sci 22: 8034-8041.
397. Casu C, Mosaico G, Natoli V, Scarano A, Lorusso F, et al. (2021) Microbiota of the Tongue and Systemic Connections: The Examination of the Tongue as an Integrated Approach in Oral Medicine. Hygiene 1: 56-68.
398. Sun P, Zhang W, Miao Y, Chen Z (2019) Letter: meta-analysis of prebiotics, probiotics, synbiotics and antibiotics in IBS. Aliment Pharmacol Ther 49: 1253-1254.
399. Swanson KS, Gibson GR, Hutkins R, Reimer RA, Reid G, et al. (2020) The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat Rev Gastroenterol Hepatol 17: 687-701.
400. Szydłowska I, Marciniak A, Malanowska E, Nawrocka-Rutkowska J, Brodowska A (2020) [Probiotics intake as gut-microbiota modulating therapy in an interdisciplinary aspect]. Pol Merkur Lek Organ Pol Tow Lek 49: 279-281.
401. Pacifici A, Pacifici L, Nuzzolese M, Cascella G, Ballini A, et al. (2020) The alteration of stress-related physiological parameters after probiotics administration in oral surgeons with different degrees of surgical experience. Clin Ter 171: e197–e208.
402. Tan Q, Orsso CE, Deehan EC, Kung JY, Tun HM, et al. (2021) Probiotics, prebiotics, synbiotics, and fecal microbiota transplantation in the treatment of behavioral symptoms of autism spectrum disorder: A systematic review. Autism Res Off J Int Soc Autism Res 14: 1820-1836.
403. Tarantino G, Finelli C (2015) Systematic review on intervention with prebiotics/probiotics in patients with obesity-related nonalcoholic fatty liver disease. Future Microbiol 10: 889-902.
404. Thakkar A, Vora A, Kaur G, Akhtar J (2023) Dysbiosis and Alzheimer’s disease: role of probiotics, prebiotics and synbiotics. Naunyn Schmiedebergs Arch Pharmacol 396: 2911-2923.
405. Pacifici L, Santacroce L, Dipalma G, Haxhirexha K, Topi S, et al. (2021) Gender medicine: the impact of probiotics on male patients. Clin Ter 171: e8-e15.
406. Trone K, Rahman S, Green CH, Venegas C, Martindale R, (2023) Synbiotics and Surgery: Can Prebiotics and Probiotics Affect Inflammatory Surgical Outcomes? Curr Nutr Rep 12: 238-246.
407. Tunapong W, Apaijai N, Yasom S, Tanajak P, Wanchai K, et al. (2018) Chronic treatment with prebiotics, probiotics and synbiotics attenuated cardiac dysfunction by improving cardiac mitochondrial dysfunction in male obese insulin-resistant rats. Eur J Nutr 57: 2091-2104.
408. Ugural A, Akyol A (2022) Can pseudocereals modulate microbiota by functioning as probiotics or prebiotics? Crit Rev Food Sci Nutr 62: 1725-1739.
409. Santacroce L, Sardaro N, Topi S, Pettini F, Bottalico L, et al. (2020) The pivotal role of oral microbiota in health and disease. J Biol Regul Homeost Agents 34: 733-737.
410. Uyeno Y, Shigemori S, Shimosato T (2015) Effect of Probiotics/Prebiotics on Cattle Health and Productivity. Microbes Environ 30: 126-32.
411. Vaghef-Mehrabany E, Maleki V, Behrooz M, Ranjbar F, Ebrahimi-Mameghani M (2020) Can psychobiotics “mood” ify gut? An update systematic review of randomized controlled trials in healthy and clinical subjects, on anti-depressant effects of probiotics, prebiotics, and synbiotics. Clin Nutr Edinb Scotl 39: 1395-1410.
412. Valero-Cases E, Cerdá-Bernad D, Pastor J-J, Frutos M-J (2020) Non-Dairy Fermented Beverages as Potential Carriers to Ensure Probiotics, Prebiotics, and Bioactive Compounds Arrival to the Gut and Their Health Benefits. Nutrients 12: 1666.
413. Inchingolo F, Santacroce L, Cantore S, Ballini A, Del Prete R, et al. (2019) Probiotics and EpiCor® in human health. J Biol Regul Homeost Agents 33: 1973-1979.
414. Dorst VJM, Tam RY, Ooi CY (2022) What Do We Know about the Microbiome in Cystic Fibrosis? Is There a Role for Probiotics and Prebiotics? Nutrients 14: 480.
415. Wagenberg CPAV, Horne PLMV, Asseldonk MAPMV (2020) Cost-effectiveness analysis of using probiotics, prebiotics, or synbiotics to control Campylobacter in broilers. Poult Sci 99: 4077-4084.
416. Vandenplas Y (2016) Probiotics and prebiotics in infectious gastroenteritis. Best Pract Res Clin Gastroenterol 30: 49-53.
417. Ballini A, Cantore S, Saini R, Pettini F, Fotopoulou EA, et al. (2019) Effect of activated charcoal probiotic toothpaste containing Lactobacillus paracasei and xylitol on dental caries: a randomized and controlled clinical trial. J Biol Regul Homeost Agents 33: 977-981.
418. Cantore S, Ballini A, Vito DD, Abbinante A, Altini V, et al. (2018) Clinical results of improvement in periodontal condition by administration of oral probiotics. J Biol Regul Homeost Agents 32: 1329-1334.
419. Vaz SR, Tofoli MH, Avelino MAG, Costa PSSD (2024) Probiotics for infantile colic: Is there evidence beyond doubt? A meta-analysis and systematic review. Acta Paediatr 113: 170-182.
420. Venema K (2016) Foreword: probiotics and prebiotics - a field that is alive and kicking. Benef Microbes 7: 1-2.
421. Wasilewski A, Zielińska M, Storr M, Fichna J (2015) Beneficial Effects of Probiotics, Prebiotics, Synbiotics, and Psychobiotics in Inflammatory Bowel Disease. Inflamm Bowel Dis 21: 1674-82.
422. Inchingolo F, Dipalma G, Cirulli N, Cantore S, Saini RS, et al. (2018) Microbiological results of improvement in periodontal condition by administration of oral probiotics. J Biol Regul Homeost Agents 32: 1323-1328.
423. Wawryk-Gawda E, Markut-Miotła E, Emeryk A (2021) Postnatal probiotics administration does not prevent asthma in children, but using prebiotics or synbiotics may be the effective potential strategies to decrease the frequency of asthma in high-risk children - a meta-analysis of clinical trials. Allergol Immunopathol (Madr) 49: 4-14.
424. Weizman Z (2015) The role of probiotics and prebiotics in the prevention of infections in child day-care centres. Benef Microbes 6: 181-3.
425. Wu Z, Nasab EM, Arora P, Athari SS (2022) Study effect of probiotics and prebiotics on treatment of OVA-LPS-induced of allergic asthma inflammation and pneumonia by regulating the TLR4/NF-kB signaling pathway. J Transl Med 20: 130.
426. Signorini L, Leonardis FD, Santacroce L, Haxhirexha K, Topi S, et al. (2020) Probiotics may modulate the impact of aging on adults. J Biol Regul Homeost Agents 34: 1601-1606.
427. Dipalma G, Inchingolo AD, Inchingolo F, Charitos IA, Cosola MD, et al. (2021) Focus on the cariogenic process: microbial and biochemical interactions with teeth and oral environment. J Biol Regul Homeost Agents 35.
428. Xiong R-G, Li J, Cheng J, Zhou D-D, Wu S-X, et al. (2023) The Role of Gut Microbiota in Anxiety, Depression, and Other Mental Disorders as Well as the Protective Effects of Dietary Components. Nutrients 15: 3258.
429. Xu L, Song M, Jiang Y, Li X (2023) Comparative effectiveness of oral antibiotics, probiotics, prebiotics, and synbiotics in the prevention of postoperative infections in patients undergoing colorectal surgery: A network meta-analysis. Int Wound J 20: 567-578.
430. Yadav MK, Kumari I, Singh B, Sharma KK, Tiwari SK (2022) Probiotics, prebiotics and synbiotics: Safe options for next-generation therapeutics. Appl Microbiol Biotechnol 106: 505-521.
431. Inchingolo AD, Malcangi G, Inchingolo AM, Piras F, Settanni V, et al. (2022) Benefits and Implications of Resveratrol Supplementation on Microbiota Modulations: A Systematic Review of the Literature. Int J Mol Sci 23: 4027.
432. Inchingolo F, Hazballa D, Inchingolo AD, Malcangi G, Marinelli G, et al. (2022) Innovative Concepts and Recent Breakthrough for Engineered Graft and Constructs for Bone Regeneration: A Literature Systematic Review. Mater Basel Switz 15: 1120.
433. Yin Y, Li Z, Gao H, Zhou D, Zhu Z, et al. (2024) Microfluidics-Derived Microparticles with Prebiotics and Probiotics for Enhanced In Situ Colonization and Immunoregulation of Colitis. Nano Lett. 24: 1081-1089.
434. Yu T, Zheng Y-P, Tan J-C, Xiong W-J, Wang Y, et al. (2017) Effects of Prebiotics and Synbiotics on Functional Constipation. Am J Med Sci 353: 282-292.
435. Yurtdaş G, Akdevelioğlu Y (2020) A New Approach to Polycystic Ovary Syndrome: The Gut Microbiota. J Am Coll Nutr 39: 371-382.
436. Inchingolo AD, Malcangi G, Semjonova A, Inchingolo AM, Patano A, et al. (2022) Oralbiotica/Oralbiotics: The Impact of Oral Microbiota on Dental Health and Demineralization: A Systematic Review of the Literature. Child Basel Switz 9: 1014.
437. Zagórska A, Marcinkowska M, Jamrozik M, Wiśniowska B, Paśko P (2020) From probiotics to psychobiotics - the gut-brain axis in psychiatric disorders. Benef Microbes 11: 717732.
438. Zaidan N, Nazzal L (2022) The Microbiome and Uremic Solutes. Toxins 14: 245.
439. Zawadzka K, Kałuzińska K, Świerz MJ, Sawiec Z, Antonowicz E, et al. (2023) Are probiotics, prebiotics, and synbiotics beneficial in primary thyroid diseases? A systematic review with meta-analysis. Ann Agric Environ Med AAEM 30: 217-223.
440. Rapone B, Ferrara E, Montemurro N, Converti I, Loverro M, et al. (2020) Oral Microbiome and Preterm Birth: Correlation or Coincidence? A Narrative Review. Open Access Maced J Med Scim 8: 123-132.
441. Inchingolo AM, Malcangi G, Costa S, Fatone MC, Avantario P, et al. (2023) Tooth Complications after Orthodontic Miniscrews Insertion. Int J Environ Res Public Health. 20(2): 1562.
442. Montemurro N, Perrini P, Marani W, Chaurasia B, Corsalini M, et al. (2021) Multiple Brain Abscesses of Odontogenic Origin. May Oral Microbiota Affect Their Development? A Review of the Current Literature. Appl Sci. 11:3316.
443. Corriero A, Gadaleta RM, Puntillo F, Inchingolo F, Moschetta A, et al. (2022) The central role of the gut in intensive care. Crit Care Lond Engl. 26:379.
444. Zepeda-Hernández A, Garcia-Amezquita LE, Requena T, García-Cayuela T (2021) Probiotics, prebiotics, and synbiotics added to dairy products: Uses and applications to manage type 2 diabetes. Food Res Int Ott Ont. 142:110208.
445. Zhang Q, Chen B, Zhang J, Dong J, Ma J, et al. (2023) Effect of prebiotics, probiotics, synbiotics on depression: results from a meta-analysis. BMC Psychiatry.23:477.
446. Zhang S, Han F, Wang Q, Fan F. (2024) Probiotics and Prebiotics in the Treatment of Autism Spectrum Disorder: A Narrative Review. J Integr Neurosci. 23:20.
447. Inchingolo F, Martelli FS, Gargiulo Isacco C, Borsani E, Cantore S, et al. (2020) Chronic Periodontitis and Immunity, Towards the Implementation of a Personalized Medicine: A Translational Research on Gene Single Nucleotide Polymorphisms (SNPs) Linked to Chronic Oral Dysbiosis in 96 Caucasian Patients. Biomedicines. 8:115.
448. Borsani E, Bonazza V, Buffoli B, Nocini PF, Albanese M, et al. (2018) Beneficial Effects of Concentrated Growth Factors and Resveratrol on Human Osteoblasts In Vitro Treated with Bisphosphonates. BioMed Res Int. 2018:4597321.
449. Hemadi AS, Huang R, Zhou Y, Zou J (2017) Salivary proteins and microbiota as biomarkers for early childhood caries risk assessment. Int J Oral Sci. 9:e1.
450. Fine DH, Markowitz K, Furgang D, Fairlie K, Ferrandiz J, et al. (2009) Macrophage inflammatory protein-1alpha: a salivary biomarker of bone loss in a longitudinal cohort study of children at risk for aggressive periodontal disease? J Periodontol. 80:106–13.
451. Zhang T, Gao G, Kwok LY, Sun Z (2023) Gut microbiome-targeted therapies for Alzheimer’s disease. Gut Microbes. 15:2271613.
452. Zhang WX, Shi LB, Zhou MS, Wu J, Shi HY. (2023) Efficacy of probiotics, prebiotics and synbiotics in irritable bowel syndrome: a systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials. J Med Microbiol. 72.
453. Zhang XF, Guan XX, Tang YJ, Sun JF, Wang XK, et al. (2021) Clinical effects and gut microbiota changes of using probiotics, prebiotics or synbiotics in inflammatory bowel disease: a systematic review and meta-analysis. Eur J Nutr. 60:2855–75.
454. Chow LQM, Haddad R, Gupta S, Mahipal A, Mehra R, et al. (2016) Antitumor Activity of Pembrolizumab in Biomarker-Unselected Patients With Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma: Results From the Phase Ib KEYNOTE-012 Expansion Cohort. J Clin Oncol Off J Am Soc Clin Oncol. 34:3838–45.
455. Zheng DW, Li RQ, An JX, Xie TQ, Han ZY, et al. (2020) Prebiotics-Encapsulated Probiotic Spores Regulate Gut Microbiota and Suppress Colon Cancer. Adv Mater Deerfield Beach Fla. 32:e2004529.
456. Zheng HJ, Guo J, Wang Q, Wang L, Wang Y, et al. (2021) Probiotics, prebiotics, and synbiotics for the improvement of metabolic profiles in patients with chronic kidney disease: A systematic review and meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr. 61:577–98.
457. Żółkiewicz J, Marzec A, Ruszczyński M, Feleszko W (2020) Postbiotics-A Step Beyond Pre- and Probiotics. Nutrients. 12:2189.
458. Weaver CM (2015) Diet, Gut Microbiome, and Bone Health. Curr Osteoporos Rep. 13:125–30.
459. Ebersole JL, Nagarajan R, Akers D, Miller CS (2015) Targeted salivary biomarkers for discrimination of periodontal health and disease(s). Front Cell Infect Microbiol. 5:62.
460. Seely KD, Kotelko CA, Douglas H, Bealer B, Brooks AE (2021) The Human Gut Microbiota: A Key Mediator of Osteoporosis and Osteogenesis. Int J Mol Sci. 22:9452.
461. Contaldo M, Itro A, Lajolo C, Gioco G, Inchingolo F, et al. (2020) Overview on Osteoporosis, Periodontitis and Oral Dysbiosis: The Emerging Role of Oral Microbiota. Appl Sci. 10:6000.
462. Bo S, Gambino R, Ponzo V, Cioffi I, Goitre I, Evangelista A, et al. (2018) Effects of resveratrol on bone health in type 2 diabetic patients. A double-blind randomized-controlled trial. Nutr Diabetes. 8:51.
463. Inchingolo AM, Patano A, Piras F, Mancini A, Inchingolo AD, et al. (2023) Interconnection between Microbiota-Gut-Brain Axis and Autism Spectrum Disorder Comparing Therapeutic Options: A Scoping Review. Microorganisms. 11:1477.
464. Tomlinson RE, Silva MJ. (2013) Skeletal Blood Flow in Bone Repair and Maintenance. Bone Res. 1:311–22.
465. Nayak S, Bhad Patil WA, Doshi UH. 2014) The relationship between salivary insulin-like growth factor I and quantitative cervical maturational stages of skeletal maturity. J Orthod. 41:170–4.
466. Li Y, Fu G, Gong Y, Li B, Li W, et al. (2022) BMP-2 promotes osteogenic differentiation of mesenchymal stem cells by enhancing mitochondrial activity. J Musculoskelet Neuronal Interact. 22:123–31.




