The Impact of Leiomyomas on Pregnancy and Delivery: Current Perspectives
by Oksana Melnyk
Specialist Obstetrician Gynecologist, Medcare Women and Children Hospital Dubai UAE
Corresponding Author: Oksana Melnyk, Specialist Obstetrician Gynecologist, Medcare Women and Children Hospital Dubai UAE
Received Date: 17 October 2025
Accepted Date: 25 October 2025
Published Date: 27 October 2025
Citation: Melnyk O (2025) The Impact of Leiomyomas on Pregnancy and Delivery: Current Perspectives, Gynecol Obstet Open Acc 9: 251. https://doi.org/10.29011/2577-2236.100251
Abstract
Uterine leiomyomas, commonly known as fibroids, are the most frequent benign smooth muscle tumors of the uterus and present a complex clinical scenario when encountered during pregnancy. Their prevalence is increasing with delayed childbearing, and they can significantly influence maternal and fetal outcomes. During pregnancy, leiomyomas may enlarge under hormonal influence and are associated with complications such as pain from red degeneration, abnormal placentation, spontaneous abortion, malpresentation, and preterm labor. The presence, size, and location of fibroids play pivotal roles in determining the degree of maternal and fetal risk. In relation to delivery outcomes, leiomyomas increase the likelihood of cesarean section due to obstructed labor, lower uterine segment distortion, or fetal malposition, and they may also contribute to postpartum hemorrhage and uterine atony. Managing leiomyomas during pregnancy remains challenging; conservative management is generally preferred to avoid surgical risks, with emphasis on analgesia, hydration, and monitoring for complications. Myomectomy during pregnancy is reserved for select cases where intractable pain or rapid tumor growth threatens pregnancy continuation. Postpartum or interval myomectomy may be considered based on future fertility goals. Future research is required to better elucidate the molecular pathways driving fibroid growth in pregnancy, assess the safety of minimally invasive or pharmacological approaches during gestation, and establish evidence-based guidelines for optimal timing and mode of delivery. In conclusion, leiomyomas can adversely impact pregnancy and delivery, necessitating personalized management strategies that balance maternal safety with fetal well-being.
Keywords: Leiomyoma, Myomectomy, Pregnancy, Uterine Neoplasms
Introduction
Uterine leiomyomas, also known as fibroids or myomas, are non-cancerous growths that develop in the myometrium [1]. The occurrence of leiomyomas is influenced by factors such as age, race, and the techniques used for detection. By the age of 50, it is estimated that between 50% and 80% of individuals will develop a leiomyoma [2]. Although many women with leiomyomas do not experience symptoms, those who do often report menstrual issues like heavy bleeding and painful periods, as well as pelvic discomfort and pressure. Other symptoms can include urinary problems or constipation. Occasionally, the degeneration of specific leiomyomas can lead to sudden abdominal pain [3, 4]. They can negatively impact fertility and are linked to early pregnancy complications [5, 6] and negative obstetric outcomes, including preterm labor, placenta previa, intrauterine growth restriction (IUGR), a higher likelihood of cesarean delivery, and postpartum haemorrhage [7, 8].
Medical treatment, iron supplementation, blood transfusion, interventional radiology, and surgery are treatment options based on symptoms, pregnancy possibility, and desire to maintain the uterus [2]. One-third of hysterectomies worldwide are due to uterine leiomyoma. The annual treatment cost is $3.5–10.3 billion in the United States and $348 million in Germany [9]. Despite severe symptoms and high treatment costs, risk factors for uterine leiomyoma remain unclear. Obesity, nulliparity, hypertension, late menopause, early menarche, family history, and aging are identified risk factors [2]. A previous study showed elevated emotional distress, depression, and anxiety among leiomyoma patients, affecting their quality of life [10]. Given leiomyoma's health effects, research on their impact on pregnancy and delivery is needed. This review examined the effects of leiomyomas on reproductive outcomes, with a focus on pregnancy and delivery outcomes.
Leiomyomas and pregnancy
The incidence of leiomyomas during pregnancy is reported to range between 0.1% and 10.7% of all pregnancies. According to a study by De Vivo et al., 71.4% of leiomyomas increased in size during the first and second trimesters, and 66.6% continued to grow between the second and third trimesters. They are more commonly seen in pregnant individuals who are over 35 years old, nulliparous, or of African American descent. While many pregnancies with leiomyomas proceed without issues, the presence of leiomyomas does raise the risk of complications. Rapid leiomyoma growth during pregnancy can lead to significant pain. However, regression of leiomyomas after childbirth has been observed in 72% of women, with a volume reduction of more than 50% between early pregnancy and 3–6 months postpartum. This regression was less common in women who had a miscarriage or used progestins after delivery, whereas mode of delivery (including cesarean), use of other hormonal contraceptives, or breastfeeding did not significantly affect regression. For women with leiomyomas considering pregnancy, evaluation should include a pelvic examination and ultrasound to assess the size and location of the leiomyoma (Figure 1). In patients undergoing assisted reproductive techniques, a saline infusion sonogram before conception is useful for identifying submucosal leiomyomas; alternatively, office hysteroscopy can be performed to evaluate the endometrial cavity. Once pregnant, understanding the leiomyomas' position in relation to the placenta and cervix is important for anticipating potential placental complications.
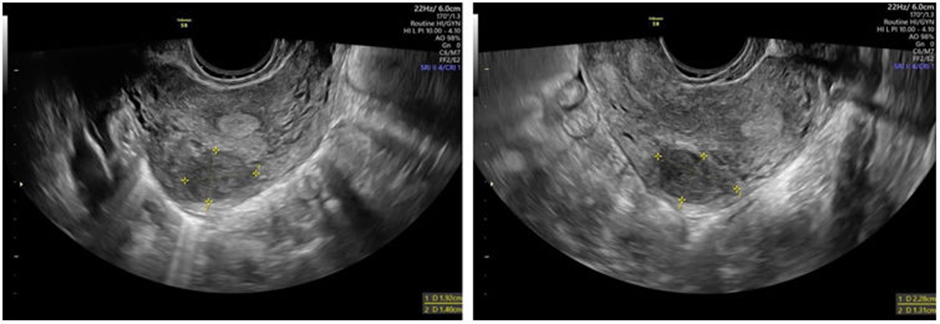
Figure 1: Transvaginal ultrasound exam [11]
Leiomyomas are known to negatively impact pregnancy and prenatal outcomes, with complications influenced by their size, type, number, and location [8]. Larger leiomyomas (>3–10 cm) are linked to increased risks of preterm rupture of membranes, malpresentation, placenta previa, postpartum hemorrhage (PPH), and cesarean delivery [12-15]. Retroplacental leiomyomas, especially those over 4 cm, are associated with placental abruption, vaginal bleeding, fetal malpresentation, and lower birth weights [16]. Submucosal leiomyomas significantly raise the risks of miscarriage and placental abruption, while intramural types correlate with higher rates of PPH and cesarean sections [17, 18]. Multiple leiomyomas (≥2) are associated with higher incidences of preterm birth, breech presentation, cesarean delivery, placenta previa, and intraoperative hemorrhage [19, 20]. However, studies have found no significant increase in adverse outcomes with a higher number of leiomyomas, possibly due to population differences and varied criteria in fibroid assessment [21, 22].
Complications occur in roughly 10–40% of pregnancies involving fibroids [7, 23]. The change in size of uterine leiomyomas during pregnancy remains debatable. A retrospective study of 107 pregnant women with uterine leiomyomas showed that leiomyoma volume generally decreased during pregnancy, with larger ones showing more frequent volumetric decrease than smaller ones from the second to third trimesters [24]. However, a longitudinal sonographic assessment of 137 uterine leiomyomas in 72 pregnant women indicated that most leiomyomas showed no change in diameter and exhibited no clinical signs during pregnancy [25]. A widely acknowledged perspective was that leiomyoma size increased significantly in the first trimester, showed slower growth during midpregnancy, and decreased in late pregnancy [26], which was speculated to be modulated by circulating levels of estrogen, progesterone, and human chorionic gonadotropin (HCG) during pregnancy [27, 28].
Leiomyomas and delivery outcomes
The biological connection between complications during pregnancy, labor, or delivery and uterine leiomyoma remains uncertain. Some studies have indicated that leiomyomas located behind the placenta or in the lower uterine segment may elevate the risk of delivery complications [29, 30]. Additionally, uterine leiomyomas could potentially reduce the uterus's ability to stretch, create mechanical barriers that limit space, restrict fetal movement, or diminish the strength of contractions [30]. The decreased incidence of rapid labor in women with uterine leiomyomas supports the theory that these growths may disrupt uterine contractions.
Leiomyomas are clearly associated with a higher risk of pregnancy loss. Compared to those without leiomyomas, women with fibroids, regardless of location, have a 1.678-fold increased risk of spontaneous abortion. In assisted reproductive technology (ART) patients, the presence of fibroids is linked to lower ongoing pregnancy and live birth rates, with a relative risk of 0.697, largely due to increased miscarriage rates. The risk of pregnancy loss is influenced by the fibroid’s location; submucosal and intramural fibroids significantly increase miscarriage risk and decrease live birth rates, whereas subserosal fibroids show no meaningful impact [6]. Additionally, having multiple fibroids may further elevate the risk of miscarriage [31].
Leiomyomas are also connected to a higher chance of fetal malpresentation. Women with fibroids have an odds ratio of 3.98 for breech presentation compared to those without, which raises the likelihood of cesarean delivery and associated maternal complications [7]. Furthermore, they may contribute to preterm labor, especially when they are large, numerous, or located near the placenta. While some studies report an increased risk of preterm labor in these cases, findings are inconsistent regarding their effect on actual preterm birth. A 2009 meta-analysis by Olive and Pritts found no significant difference in preterm delivery rates between women with fibroids and those without, across all fibroid locations [6].
Management of leiomyomas during pregnancy
Treatment of leiomyomas during pregnancy remains controversial, with two main approaches (Figure 2): the classical conservative method, which defers myomectomy to 3–6 months postpartum due to concerns about severe bleeding and potential hysterectomy, and the evolving approach of performing cesarean myomectomy (CM) during delivery [32]. Conservative treatment is associated with risks such as miscarriage, placental dysfunction, and preterm birth, particularly in cases involving submucous or intramural leiomyomas. Studies, including those by Barinov et al., have explored conservative strategies like using the Arabin pessary and progesterone to reduce preterm birth [33]. However, cesarean myomectomy (CM) is gaining acceptance, showing comparable outcomes to cesarean section (CS) alone in terms of febrile morbidity and hospital stay, though CM is associated with longer operative times, slightly higher blood transfusion rates, and a small drop in hemoglobin levels [34]. Studies by Zhao et al. and Kwon et al. indicated that leiomyoma size and lower uterine segment location are independent risk factors for hemorrhage, not CM itself [20, 35]. Surgical teams are advised to adopt meticulous techniques and involve multidisciplinary experts to minimize complications [36]. While CM may increase risk in some situations, especially with large, multiple, posterior, or submucosal leiomyomas, surgical intervention may be necessary for severe cases, such as fibroid degeneration, persistent pain, organ compression, or signs of fetal compromise. Myomectomy during pregnancy is generally timed at 14–16 weeks for lower segment leiomyomas and 18–20 weeks for fundal or corporeal leiomyomas. Recent evidence favors laparoscopic over laparotomic myomectomy due to benefits like improved visualization, reduced postoperative pain, and faster recovery, though concerns remain over pneumoperitoneum, CO₂ effects, and bleeding. Despite these concerns, misconceptions about laparoscopy's safety persist, often due to limited data, though when performed correctly with proper monitoring, it is considered safe and effective.
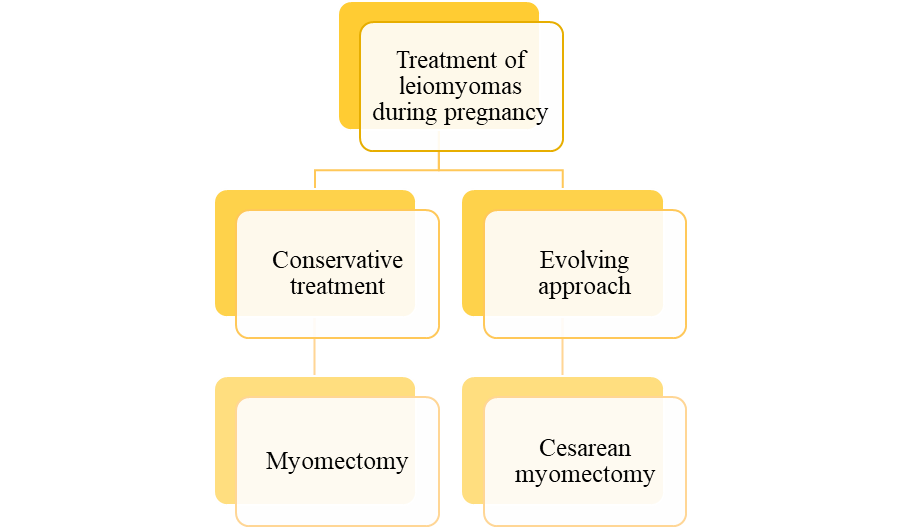
Figure 2: Treatment of leiomyomas during pregnancy
Future directions and research needs
Promising future directions in leiomyoma research involve elucidating the complex genetic and molecular mechanisms of these tumors to refine the understanding of how they impact pregnancy and delivery outcomes, thereby facilitating the development of targeted and less invasive therapies [37]. Further investigations into the predisposing genetic factors for leiomyoma formation could yield preventive interventions and more personalized management strategies, particularly for women planning conception or undergoing assisted reproductive technologies [38]. There remain significant gaps around the optimal timing and indications for surgical or medical intervention during pregnancy, the safety and efficacy of emerging image-guided and minimally invasive procedures, and the need for robust prospective studies to clarify the impact of leiomyoma subtypes and their location on obstetric outcomes. Additionally, research should address care inequities and social determinants of health that influence access to high-quality leiomyoma treatment, ensuring evidence-based approaches are equitably delivered across diverse patient populations. Collectively, future work should prioritize individualized therapeutic approaches, comprehensive risk stratification, and real-world implementation studies that integrate advances in diagnostics, genomics, and care delivery to improve reproductive health for women affected by leiomyomas.
Conclusion
Current perspectives underscore that while most leiomyomas remain asymptomatic throughout pregnancy, their presence markedly elevates the risk of adverse maternal and neonatal outcomes, particularly in association with larger, submucosal, or multiple fibroids. Complications such as preterm labor, malpresentation, dysfunctional and obstructed labor, increased cesarean delivery rates, postpartum hemorrhage, and fetal growth restriction are more commonly seen in affected pregnancies, underscoring the importance of diligent antenatal surveillance and individualized management strategies. Timely diagnosis, multidisciplinary planning, and readiness for obstetric intervention are crucial in optimizing delivery outcomes and minimizing potential sequelae for both mother and child, thereby reaffirming the necessity for high-risk pregnancy protocols in women with leiomyomas. Ongoing research, improved imaging modalities, and emerging minimally invasive therapies hold promise for refining risk stratification and treatment interventions, ultimately contributing to safer reproductive trajectories for this population.
Declarations (Please fill in the below statements)
- CRediT authorship contribution statement
- Declaration of Competing Interest
- Acknowledgments
- Funding sources
- Data availability statement
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article
Funding
The authors received no financial support for the research, authorship, and/or publication of this article
References
- Stewart EA (2015) Clinical practice. Uterine fibroids. The New England journal of medicine 372: 1646-55.
- Giuliani E, As‐Sanie S, Marsh EE (2020) Epidemiology and management of uterine fibroids. International Journal of Gynecology & Obstetrics 149: 3-9.
- Lippman SA, Warner M, Samuels S, Olive D, Vercellini P, et al. (2003) Uterine fibroids and gynecologic pain symptoms in a population-based study. Fertility and sterility 80: 1488-94.
- Walker CL, Stewart EA (2005) Uterine fibroids: the elephant in the room. Science 308: 1589-92.
- Farquhar C (2009) Do uterine fibroids cause infertility and should they be removed to increase fertility? Bmj 338.
- Pritts EA, Parker WH, Olive DL (2009) Fibroids and infertility: an updated systematic review of the evidence. Fertility and sterility 91: 1215-23.
- Ouyang DW, Economy KE, Norwitz ER (2006) Obstetric complications of fibroids. Obstetrics and Gynecology Clinics 33: 153-69.
- Qidwai GI, Caughey AB, Jacoby AF (2006) Obstetric outcomes in women with sonographically identified uterine leiomyomata. Obstetrics & Gynecology 107: 376-82.
- Soliman AM, Yang H, Du EX, Kelkar SS, Winkel C (2015) The direct and indirect costs of uterine fibroid tumors: a systematic review of the literature between 2000 and 2013. American journal of obstetrics and gynecology 213: 141-60.
- Ghant MS, Sengoba KS, Recht H, Cameron KA, Lawson AK, et al. (2015) Beyond the physical: a qualitative assessment of the burden of symptomatic uterine fibroids on women's emotional and psychosocial health. Journal of Psychosomatic Research 78: 499-503.
- Derme M, Briante M, Masselli G (2024) Uterine leiomyoma in adolescents: A case report and a review of the literature. Clínica e Investigación en Ginecología y Obstetricia 51: 100957.
- Michels KA, Edwards DRV, Baird DD, Savitz DA, Hartmann KE (2014) Uterine leiomyomata and cesarean birth risk: a prospective cohort with standardized imaging. Annals of epidemiology 24: 122-6.
- Martin J, Ulrich ND, Duplantis S, Williams FB, Luo Q (2016) Obstetrical outcomes of ultrasound identified uterine fibroids in pregnancy. American Journal of Perinatology 33: 1218-22.
- Knight JC, Elliott JO, Amburgey OL (2016) Effect of maternal retroplacental leiomyomas on fetal growth. Journal of Obstetrics and Gynaecology Canada 38: 1100-4.
- Lam S-J, Best S, Kumar S (2014) The impact of fibroid characteristics on pregnancy outcome. American journal of obstetrics and gynecology 211: 395. e1- e5.
- Benson CB, Chow JS, Chang‐Lee W, Hill III JA, Doubilet PM (2001) Outcome of pregnancies in women with uterine leiomyomas identified by sonography in the first trimester. Journal of clinical ultrasound 29: 261-4.
- Zhao R, Wang X, Zou L, Li G, Chen Y, et al. (2017) Adverse obstetric outcomes in pregnant women with uterine fibroids in China: a multicenter survey involving 112,403 deliveries. PloS one 12: e0187821.
- Ezzedine D, Norwitz ER (2016) Are women with uterine fibroids at increased risk for adverse pregnancy outcome? Clinical obstetrics and gynecology 59: 119-27.
- Goyal M, Dawood AS, Elbohoty SB, Abbas AM, Singh P, et al. (2021) Cesarean myomectomy in the last ten years; A true shift from contraindication to indication: A systematic review and meta-analysis. European Journal of Obstetrics & Gynecology and Reproductive Biology 256: 145-57.
- Zhao R, Wang X, Zou L, Zhang W (2019) Outcomes of myomectomy at the time of cesarean section among pregnant women with uterine fibroids: a retrospective cohort study. BioMed Research International 2019: 7576934.
- Chu F-C, Shao SS-W, Lo L-M, Hsieh Ts-Ta, Hung T-H (2020) Association between maternal anemia at admission for delivery and adverse perinatal outcomes. Journal of the Chinese Medical Association 83: 402-7.
- Liu C-H, Chang W-H, Yeh C-C, Wang P-H (2021) Simultaneous myomectomy during cesarean section p. 397-8.
- Exacoustòs C, Rosati P (1993) Ultrasound diagnosis of uterine myomas and complications in pregnancy. Obstetrics & Gynecology 82: 97-101.
- Hammoud AO, Asaad R, Berman J, Treadwell MC, Blackwell S, et al. (2006) Volume change of uterine myomas during pregnancy: do myomas really grow? Journal of minimally invasive gynecology 13: 386-90.
- Neiger R, Sonek JD, Croom CS, Ventolini G (2006) Pregnancy-related changes in the size of uterine leiomyomas. The Journal of reproductive medicine 51: 671-4.
- Vitagliano A, Noventa M, Di Spiezio Sardo A, Saccone G, Gizzo S, et al. (2018) Uterine fibroid size modifications during pregnancy and puerperium: evidence from the first systematic review of literature. Archives of gynecology and obstetrics 29: 823-35.
- Benaglia L, Cardellicchio L, Filippi F, Paffoni A, Vercellini P, et al. (2014) The rapid growth of fibroids during early pregnancy. PloS one 9: e85933.
- Sarais V, Cermisoni GC, Schimberni M, Alteri A, Papaleo E, et al. (2017) Human chorionic gonadotrophin as a possible mediator of leiomyoma growth during pregnancy: molecular mechanisms. International journal of molecular sciences 18: 2014.
- Davis JL, Ray-Mazumder S, Hobel CJ, Baley K, Sassoon D (1990) Uterine leiomyomas in pregnancy: a prospective study. Obstetrics & Gynecology 75: 41-4.
- Vergani P, Ghidini A, Strobelt N, Roncaglia N, Locatelli A, et al. (1994) Do uterine leiomyomas influence pregnancy outcome? American Journal of Perinatology 11: 356-8.
- Lee HJ, Norwitz ER, Shaw J (2010) Contemporary management of fibroids in pregnancy. Reviews in Obstetrics and Gynecology 3: 20.
- Saccardi C, Visentin S, Noventa M, Cosmi E, Litta P, et al. (2015) Uncertainties about laparoscopic myomectomy during pregnancy: a lack of evidence or an inherited misconception? A critical literature review starting from a peculiar case. Minimally Invasive Therapy & Allied Technologies 24: 189-94.
- Jhalta P, Negi SG, Sharma V (2016) Successful myomectomy in early pregnancy for a large asymptomatic uterine myoma: case report. Pan African Medical Journal 24(1).
- Munro MG, Critchley HO, Broder MS, Fraser IS, (2011) Disorders FWGoM FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. International Journal of Gynecology & Obstetrics113: 3-13.
- Kwon JY, Byun JH, Shin I, Hong S, Kim R, et al. (2021) Risk factors for intraoperative hemorrhage during cesarean myomectomy. Taiwanese Journal of Obstetrics and Gynecology 60: 41-4.
- Than WW, Daud MNM, Jeffree MS, Hayti F (2024) Pregnancy-associated Leiomyomas: What is New? Journal of South Asian Federation of Obstetrics and Gynaecology 16: 29-33.
- Levy G, Hill MJ, Beall S, Zarek SM, Segars JH, et al. (2012) Catherino WH. Leiomyoma: genetics, assisted reproduction, pregnancy and therapeutic advances. J Assist Reprod Genet 29: 703-12.
- Hoy SM (2024) Take an individualized approach when managing women of reproductive age with uterine fibroids. Drugs & Therapy Perspectives 40: 264-8.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.