The Impact and Results of Y-90 Radioembolization in Hepatocellular Carcinoma. Is It an Effective Theraphy?
by Esteban Diaz Serrano1, Teresa Hernandez2, Joan Novo3, Jose Manuel Cordero García4, Sylwia Bilas Sudol1, Javier Salinas5, Isabel Prieto Nieto5*
1MD, General and Digestive Surgery Service, Hospital Universitario La Paz, Madrid, Spain.
2MD, Head of Interventional Radiology Department, Radiology Service, Hospital Universitario La Paz, Madrid, Spain.
3MD, Interventional Radiology Department, Radiology Service, Hospital Universitario La Paz, Madrid, Spain.
4MD, Chief of Nuclear Medicine, Nuclear Medicine Service, Hospital Universitario La Paz, Madrid, Spain.
5MD, PHD, General and Digestive Surgery Service, Hospital Universitario La Paz, Madrid, Spain.
*Corresponding author: Isabel Prieto Nieto, MD, PHD, General and Digestive Surgery Service, Hospital Universitario La Paz, Madrid, Spain.
Received Date: 12 May, 2025
Accepted Date: 19 May, 2025
Published Date: 22 May, 2025.
Citation: Diaz E, Hernandez T, Novo J, Cordero García JM, Bilas S, et al. (2025) The Impact and Results of Y-90 Radioembolization in Hepatocellular Carcinoma. Is It an Effective Theraphy?. J Oncol Res Ther 10: 10284. https://doi.org/10.29011/2574-710X.10284.
Abstract
Background: Hepatocellular carcinoma (HCC) remains a leading cause of cancer-related mortality worldwide, with limited treatment options for patients with unresectable disease. Yttrium-90 (Y-90) transarterial radioembolization (TARE) has emerged as a promising therapy to improve survival and tumor response. Objective: This study aims to evaluate the safety and effectiveness of Y-90 radioembolization in patients with unresectable HCC, focusing on survival outcomes, tumor response, and treatment-related toxicity. Methods: A retrospective, observational study was conducted on 57 patients with unresectable HCC treated with Y-90 radioembolization. Patient selection followed strict inclusion and exclusion criteria, with pre-treatment evaluation including imaging, laboratory tests, and liver function assessments. Survival analysis was performed using Kaplan-Meier curves and Cox regression models. Results: The median overall survival was 14 months (CI95%: 8.3-19.7). Patients with prior surgical resection demonstrated a median survival of 25.2 months compared to 12.3 months for non-resected patients (p=0.11). Median survival for BCLC stage B patients was 20 months, while for stage C patients, it was 11.5 months (p=0.12). The presence of portal vein thrombosis was associated with a median survival of 11.5 months versus 17.7 months in patients without thrombosis (p=0.13). Significant posttreatment increases in bilirubin and liver enzymes were observed at 3 and 6 months. Conclusion: Y-90 radioembolization is a safe and effective treatment for unresectable HCC, offering meaningful survival benefits, particularly in selected patient subgroups. Future research should focus on optimizing patient selection criteria and exploring combination therapies.
Keywords: Yttrium-90; Radioembolization; Hepatocellular Carcinoma; Liver Cancer; Transarterial Radioembolization; Survival Outcomes;
Introduction
Primary and secondary liver tumors are a major cause of mortality, with hepatocellular carcinoma (HCC) accounting for more than 80% of primary tumors originating in the liver. It is the third leading cause of cancer death in the world, with the highest incidence in Africa and Asia [1].
There are numerous therapeutic options for liver tumors that must be tailored to the patient and type of tumor. Thus, they range from surgical resection to other ablative treatments, such as radiofrequency (RF) ablation, microwave ablation, irreversible electroporation (IRE), cryoablation, transarterial chemoembolization (TACE), intensity-modulated radiation therapy (IMRT), stereotactic body radiation therapy (SBRT), and transarterial radioembolization (TARE) with Yttrium 90 (Y-90) [2, 3].
The latter consists of a type of brachytherapy in which microspheres filled with Y-90 are implanted in the tumor parenchyma through the hepatic artery.
The ß radiation emitted by the Y-90 microspheres has its range of action at 2.5 mm around the microsphere, thus producing virtually no damage in the healthy tissue surrounding the tumor tissue. As it loses radioactivity, the Y-90 becomes Zirconium-90, which is residually harmless. Ghoham
The half-life of the microspheres is 64 hours, and therapeutic radiation usually remains for around 14 days.
This study aims to assess the effectiveness and safety of the use of radioembolization with Y-90 microspheres in patients with unresectable HCC.
The aim of study was to analyte the safety and effectiveness of Y90 radioembolization in unresectable HCC.
Materials and Methods
We conducted a retrospective, observational study analyzing 57 patients with unresectable HCC, who were treated with radioembolization with Y-90. These patients were included in a database from November 2009 to December 2017, with a maximum follow-up period of 86 months.
One of the materials needed for this treatment is microspheres. At our hospital, we use SIR-Spheres resin microspheres with a median diameter of 32.5 microns and activity of 50 Bq.
The patient selection process includes anamnesis, physical examination, laboratory tests, and the imaging tests required in each case.
Table 1: Inclusion and Exclusion Criteria. |
The inclusion and exclusion criteria are specific and must be met in all cases (Table 1).
*Exceptions include: 1) For patients with hepatocellular carcinoma on hepatic cirrhosis, the criteria established at the Barcelona EASL Conference (coincident radiological diagnosis in two imaging studies – ultrasound, computed tomography [CT] and magnetic resonance imaging [MRI] - for tumors larger than 2 cm in diameter) shall be applied.
**Detection of functional alterations in the clinical period prior to treatment that increase the risk of treatment, specifically: neutropenia less than 1.5/pL, thrombocytopenia less than 25/pL, and bilirubin greater than 2 mg/dL.
Table 1. Grouping of the criteria used in patient screening.
Elevated serum alpha-fetoprotein levels are not a contraindication for treatment.
Treatment Protocol
Pre-treatment Assessment
The attending physician is responsible for ensuring that patients understand and sign the informed consent. In this process, patients will be staged according to their TNM stage; in patients with HCC, the Model for End-Stage Liver Disease (MELD) score will be calculated.
Clinical, Analytical and Imaging Procedures
The following procedures will be reperformed: complete anamnesis and physical examination; blood count, prothrombin activity, activated partial prothrombin time (aPTT) and fibrinogen; aspartate aminotransferase (AST), alkaline phosphatase (ALP), gamma glutamyl transferase (GGT), bilirubin, creatinine, electrolytes, and albumin. Tumor markers (AFP, CEA, CA 19.9 etc) will be determined depending on the tumor type. Additional imaging techniques are also required:
Abdominal CT or MRI: required to calculate the total liver volume and percentage tumor, as well as the characteristics of the lesions.
Positron emission tomography (PET)-CT: useful to assess the functionality and extent of tumor tissue.
Abdominal visceral arteriography (Fig. 1): will include a complete vascular map and confirmation of the specific path the spheres will follow, to redirect the flow if necessary. In such case, coil embolization and occlusion of the different vessels will be applied (gastroduodenal artery, right gastric artery) to prevent gastroduodenal injury (Fig. 2). It is also important to:
- Detect possible arterial variants that supply the liver
- Detect vessels collateral to the gastrointestinal tract and other extrahepatic organs.
- Identify the vessels that supply blood to each liver tumor nodule.
- Assess portal venous flow. Hayley Briody, knight GM.
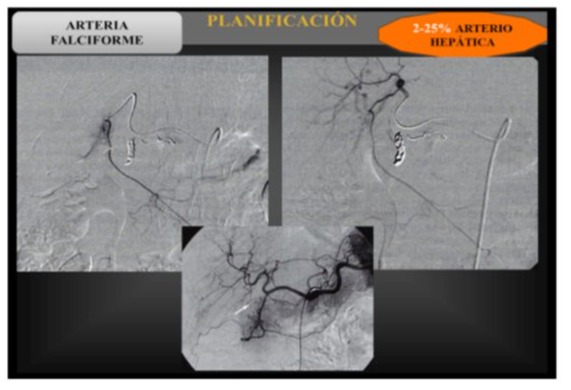
Figure 1: X-ray images of the radioembolization process, showing the anatomy of the hepatic hilum to subsequently inject the coils into the vessel that supplies the tumor lesion.
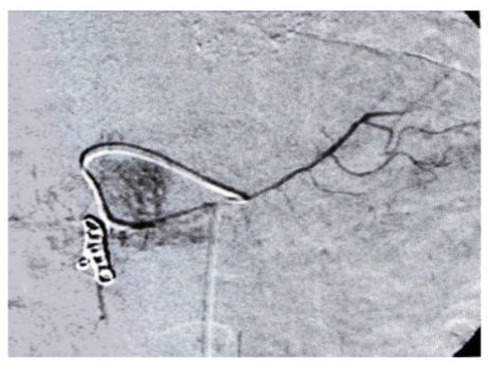
Figure 2: X-ray images taken during radioembolization planning. Injection of coils into the gastroduodenal artery to prevent pancreatitis and duodenal perforation.
As mentioned above, this study of the microsphere route is performed by administering a dose of 75-150 MBq of Tc99m-MAA (macroaggregated albumin) via the catheter in the position where the radioactive compound is to be injected. Images are then taken and activity is measured in the liver, gastroduodenal and pulmonary regions, thus evaluating the shunts: ratio between the count in the lung area and the total counts in the liver and lung area.
Treatment Planning
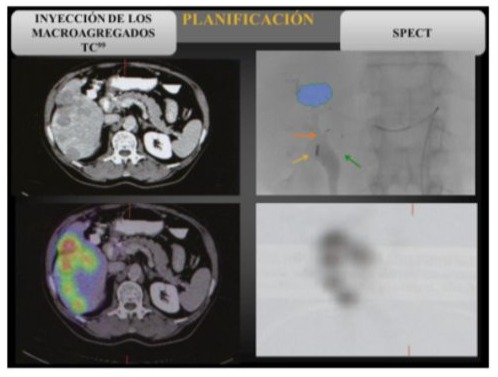
Figure 3: SPECT-CT images for planning with Y90.
Dose Calculation
We chose the body surface method to calculate the radiation dose applied to each patient.
Body Surface Method:
In this method, the activity of the isotope to be administered is calculated based on the patient’s body surface area (BSA) and the proportion of tumor tissue relative to the liver volume. The BSA is calculated from the patient’s weight and height, using standard nomograms. The percentage of liver volume replaced by tumor is calculated from CT measurements [2, 4].
In those with a lung shunt between 10-15%, the activity will be reduced by 20%, between 15-20% the reduction will be 40%, and if the shunt exceeds 20%, it is a contraindication for treatment due to the risk of toxicity [5].
The risk of reflux of the microspheres into the gastrointestinal tract should also be assessed, considering this factor a contraindication.
Treatment
The Y-90 microspheres are administered in a slow bolus infusion. If disease is present in both lobes, it can be treated in a single session or in two separate sessions, in which the left and right lobes would be treated sequentially at two visits 30 days apart, or even 6 weeks apart for greater safety.
Results
The cohort of our study was 57 patients with a median age of 69 years (range 41-88), 80.7% (n: 46) of whom were men and 19.3% women. The etiology of the HCC was Hepatitis C Virus (HCV) infection in 70,2% (n: 40), alcohol in 24,6% (n: 14) and cryptogenic in 5,3% (n: 3) of the cases. The lobar involvement was the right lobe in 61,4% (n: 35), left lobe in 10,5% (n: 6) and bilobar in 28,1% (n: 16) of the cases.
The patients were categorized as Child A in 71,9% (n: 41), Child B in 21,1% (n: 12) and Child C in 7,1% (n: 4) of the cases, and in the Barcelona Liver Cancer Staging (BCLC) they were categorized as B in 56,1% (n: 32) and C in 43,9% (n: 25) of the cases. The previous treatments that patients received included Resection in 17,5% (n: 10), Radiofrequency in 22,8% (n: 13), Chemoembolization in 26,3% (n: 15), Sorafenib in 31,6% (n: 18) and Transplantation in 1,8% (n: 1). 24,6% (n: 14) of the patients had Portal vein thrombosis, 22,8% (n: 13) had ascites and 8,8% (n: 5) had extrahepatic disease now of initiating the Y-90 treatment.
The baseline clinic characteristics are shown in table 3 and the liver tests results during the periods of pretreatment and after 1, 3 and 6 months of Y90 radioembolization treatment are shown in table 4.
|
SEX |
Men: 46 (80.7%) |
|
Women: 11 (19.3%) |
|
|
ETIOLOGY |
HCV: 40 (70.2%) |
|
Alcohol: 14 (24.6%) |
|
|
Cryptogenic: 3 (5.3%) |
|
|
LOBAR INVOLVEMENT |
Right: 35 (61.4%) |
|
Left: 6 (10.5%) |
|
|
Bilobar: 16 (28.1%) |
|
|
CHILD STAGE |
A: 41 (71.9%) |
|
B: 12 (21.1%) |
|
|
C: 4 (7.1%) |
|
|
BLCL |
B: 32 (56.1%) |
|
C: 25 (43.9%) |
|
|
NUMBER OF TREATMENTS |
1: 40 (70.2%) |
|
2: 12 (21.2%) |
|
|
3: 4 (7%) |
|
|
4: 1 (1.8%) |
|
|
PREVIOUS TREATMENTS |
Resection: 10 (17.5%) |
|
Radiofrequency: 13 (22.8%) |
|
|
Chemoembolization: 15 (26.3%) |
|
|
Transplantation: 1 (1.8%) |
|
|
Sorafenib: 18 (31.6%) |
|
|
PORTAL VEIN THROMBOSIS |
14 (24,6%) |
|
ASCITIS |
13 (22,8%) |
|
HP SHUNT % Median (Range) |
7,5 (0-20) |
|
ALBUMIN (mg/dl) at baseline Median (range) |
4 (2,6-4,8) |
|
INR at baseline Median (range) |
1,1 (0,84-2,1) |
|
AFP* (ng/ml) at baseline Median (range) |
12,6 (1,5-91914) |
|
EXTRAHEPATIC DISEASE |
5 (8,8%) |
|
*AFP: Alpha-Fetoprotein |
|
Table 3: Baseline clinical characteristics.
|
VALUES |
Median (Range) |
|||
|
TEST |
PRETREATMENT |
1 MONTH |
3 MONTHS |
6 MONTHS |
|
Bilirrubin (mg/dl) |
0,81 (0,2-6) |
1,02 (0,3-11,2) |
1,3 (0,47-24) |
1,4 (0,24-17,2) |
|
AST (U/L) |
53,5 (18-496) |
47 (20-635) |
76 (17-1397) |
62 (22-2139) |
|
ALT (U/L) |
36 (12-436) |
42 (13-289) |
43 (16-869) |
46 (13-1225) |
|
GGT (U/L) |
112 (29-1558) |
236 (36-1195) |
161 (32-1331) |
181 (52-1507) |
|
ALP (IU/L) |
134 (53-1058) |
153 (41-580) |
164 (60-542) |
143 (75-495) |
|
AST: Aspartate Aminotransferase, ALT: Alanine Aminotransferase, GGT: Gamma-Glutamil Tranferase, ALP: Alkaline Phosphatase. |
||||
Table 4: Main liver function tests results during the periods of pretreatment and after 1, 3 and 6 months of treatment with Y90.
Survival Analysis
Since the first treatment of Y90 radioembolization, the median overall survival (OS) was 14 months (CI95%: 8.3-19.7%) (Fig. 4). The median survival rate according to the Child stage was 17,3 months in Child A patients while Child B was 9,5 months, but there was no statistically significant difference (p=0,42). Patients with BCLC B had a median survival of 20 months while patients with BCLC C had a median survival of 11.5 months (p=0.12) (Fig 5).
Also, patients with prior resection to the treatment had a median survival of 25.2 months compared to those who did not, which had a median survival of 12.3 months (p=0.11) (Fig 7). No statistically significant differences were found in other previous treatments, such as radiofrequency, chemoembolization or sorafenib. One patient of the cohort was treated with hepatic transplantation prior to the Y-90 treatment.
Patients with portal vein thrombosis had a median survival 11.5 months while patients who did not have it had a median survival of 17.7 months (p=0.13)
No statistically significant differences were observed in variables like gender, etiology of HCC, presence of ascites during treatment, lobar involvement (right, left or bilobar) or hepatopulmonary shunt percentage.
As for liver function tests, levels of bilirubin, liver transaminases and gamma-glutamil transferase (GGT) shown statistically significant differences, while the remaining variables, including levels of albumin, INR, and alpha feto protein, did not shown significant differences. All data are summarized in table 5 and 6.
|
Analysis for time to death |
||||
|
Variables |
Median Survival (months). |
95% CI |
p-value (Log Rank) |
|
|
Child Stage |
A |
17,3 |
10,7-24 |
0,42 |
|
B |
9,5 |
5,3-13,7 |
||
|
C |
24 |
0-62,8 |
||
|
BCLC |
B |
20 |
7,4-32,4 |
0,12 |
|
C |
11,5 |
8-15,1 |
||
|
Portal Vein thrombosis |
No |
17,7 |
10,1-25,3 |
0,13 |
|
Yes |
11,5 |
5,6-17,4 |
||
|
Prior resection* |
No |
12,3 |
4,3-20,3 |
0,11 |
|
Yes |
25,2 |
2,2-48,2 |
||
|
Radiofrequency* |
No |
12,5 |
6,8-18,1 |
0,46 |
|
Yes |
17,8 |
2,1-33,4 |
||
|
Chemoembolization* |
No |
12,5 |
5,9-19 |
0,39 |
|
Yes |
17,3 |
0-37,1 |
||
|
Sorafenib* |
No |
12,3 |
10,3-14,2 |
0,69 |
|
Yes |
18,9 |
15,6-22,2 |
||
Table 5: Cualitative analysis for time to death.
|
Variable |
HR |
p-value |
|
|
Bilirrubin |
Pretreatment |
0,875 |
0,547 |
|
1 month |
1,088 |
0,357 |
|
|
3 months* |
1,1 |
0,016 |
|
|
6 months* |
1,173 |
0,001 |
|
|
AST |
Pretreatment |
1,002 |
0,231 |
|
1 month* |
1,003 |
0,006 |
|
|
3 months |
1,001 |
0,346 |
|
|
6 months* |
1,001 |
0,010 |
|
|
ALT |
Pretreatment |
1,001 |
0,538 |
|
1 month |
1,005 |
0,82 |
|
|
3 months |
1 |
0,695 |
|
|
6 months* |
1,001 |
0,017 |
|
|
GGT |
Pretreatment |
1,001 |
0,126 |
|
1 month |
1,001 |
0,160 |
|
|
3 months |
1 |
0,566 |
|
|
6 months* |
1,002 |
0,015 |
|
|
ALP |
Pretreatment |
1,002 |
0,128 |
|
1 month |
1,004 |
0,096 |
|
|
3 months* |
1,006 |
0,002 |
|
|
6 months* |
1,007 |
0,002 |
Table 6: Univariate analysis for time to death.
Figure 4: Overall survival.
Figure 5: Survival curve according to BCLC.
Figure 6: Survival curve according to Prior resection (pretreatment).
Discussion
HCC is the most common cancer of the liver and is the 5th leading cause of cancer-related mortality in men and 7th leading cause in women [7]. Incidence of HCC is expected continue to rise [8].
Most cases of HCC are detected at an advanced stage of the disease. Prognosis depends on the tumor stage, anatomical distribution of the lesions, or degree of liver function, which will determine the response and efficacy of treatments according to the Barcelona Clinic Liver Cancer (BCLC) stage [7]. Without treatment, HCC has a median survival of less than one year [8]. Different alternatives are available for the treatment of liver tumors. Surgical resection of HCC is the treatment of choice [9], with 5-year survival ranging from 40-75% in patients who meet selection criteria and operated with curative intent.
However, only 20% of patients with liver tumors are candidates for surgical resection due to limited hepatic functional reserve. The total residual liver volume should be more than 30%-40% of the original liver volume in order to perform a liver resection safely [6, 10].
In recent decades, local ablation techniques have emerged that seek to overcome the limitations of resection, such as RF, microwave, IRE, cryoablation, TACE and TARE. These techniques can be defined as the direct application of an agent (chemical, thermal or electrical) to specific tumor foci to achieve its eradication or significant tumor destruction [6, 11]. Prior resection appeared to be associated with improved survival outcomes, with a median survival of 25.2 months for patients who had undergone resection compared to 12.3 months for those who had not (p=0.11) This suggests that patients who are initially candidates for surgical intervention but subsequently develop unresectable disease may still derive significant benefit from Y-90 radioembolization. However, other pre-treatment modalities such as radiofrequency ablation, chemoembolization, and the use of Sorafenib did not show statistically significant differences in survival outcomes, indicating that Y-90 can be an effective salvage therapy irrespective of prior treatment history. Nevertheless, as in the study by Sangro et al., we found that previous procedures had no significant effect on survival after radioembolization compared to those with no previous treatments.
The mean survival in HCC after radioembolization treatment is 7-27 months, depending on the performance status, extent of disease, degree of liver function, and Child-Pugh stage. Child stage A patients have a median survival of 24.4 months compared to a median survival of 16.9 months in Child stage B [6, 15].
The analysis of 57 patients treated with Y-90 provides valuable insights into survival outcomes and the balance of therapeutic benefits versus potential toxic effects. The median overall survival (OS) for the cohort was 14 months, which aligns with previous studies that report a range of 12 to 18 months for similar patient populations [2, 4, 11]. Notably, the survival rates varied significantly based on the Child-Pugh score and the Barcelona Clinic Liver Cancer (BCLC) staging system. Patients classified as Child A had a median survival of 17.3 months, compared to 9.5 months for those classified as Child B, although this difference was not statistically significant. Similarly, BCLC stage B patients had a median survival of 20 months, whereas BCLC stage C patients had a median survival of 11.5 months. When comparing our results in terms of survival, we obtain slightly higher results than those reported in similar studies in BCLC stage B (20 vs Olive-Sasot: 18.6, Hilgard: 16.4, Mazzaferro: 18 months) and similar in BCLC stage C (11.5 vs Olive-Sasot: 8.8, Mazaferro: 13 months). These findings underscore the importance of liver function and cancer stage in determining patient prognosis post-Y-90 treatment.
The Child C group in our study had the highest survival rate, 24 months, perhaps because it had the smallest number of patients or had the least number of previous treatments, so it rests of, statistically speaking, importance. Anyhow, our findings suggest that the Y-90 treatment should be considered not only for endstage disease patients, but also for early stages, as it is suggested by other authors [4].
Analysis comparing HCC patients treated with TACE versus TARE showed a significantly longer time to progression in the TARE group (13.3 vs. 8.4). The response rate was similar: 69% in the TACE group versus 72% in the TARE group, with no significant differences. Overall survival (OS) was 17.4 months in the TACE group versus 20.5 months in the TARE group. In our study, OS at 2 years was 80.8%.
Progression-free survival (PFS) was better with TARE; in our study 41.9% of patients had disease progression, with a mean time of 50 months.
This longer progression time has an advantage for patients on the transplant waiting list, with the rates of transplanted patients included on the waiting list being 87% after treatment with TARE versus 70% after TACE [9, 11]. Thus, Y-90 radioembolization can positively affect survival by improving the transplant rate [7]. In our series, we only had one patient who had previously had a transplant. The presence of portal vein thrombosis (PVT) was associated with a median survival of 11.5 months compared to 17.7 months for patients without PVT (p=0.13). While not statistically significant, this trend highlights the challenging prognosis for HCC patients with PVT and suggests that Y-90 radioembolization might still offer a meaningful survival benefit in this high-risk group. The presence of portal vein thrombosis is not an exclusion criterion, as it occurs in 10% to 40% of HCC at the time of diagnosis. Radioembolization is one of the options available in these patients with portal vein thrombosis [14], which is a poor prognostic factor; OS with thrombosis is 2-4 months compared to 10-24 months for patients without portal vein thrombosis. In our study, there was one patient with portal vein thrombosis who had a survival of 7 months after treatment.
The analysis of liver function tests pre- and post-treatment revealed significant changes in bilirubin, AST, ALT, GGT, and ALP levels, particularly at the 3-month and 6-month marks. The increase in these markers indicates liver stress and potential toxicity, which is an anticipated risk given the nature of Y-90 treatment. Specifically, the increase in bilirubin levels and alkaline phosphatase at 3 months (p=0.016) and 6 months (p=0.001) post-treatment and suggests a need for close monitoring of liver function during follow-up. Despite these increases, the overall clinical management of these patients was feasible, with no reports of severe liver or pulmonary dysfunction directly attributed to the treatment.
Implications for Clinical Practice
This study supports the use of Y-90 radioembolization as a viable treatment option for patients with unresectable HCC, offering a potential extension of survival with manageable toxicity. The selection criteria used in this study, which emphasize good general health (ECOG 0-2), unresectable disease, and specific contraindications (e.g., high lung shunt fraction), are critical in optimizing patient outcomes. These criteria should be rigorously applied in clinical practice to identify the best candidates for Y-90 treatment.
Limitations and Future Research
The retrospective nature of this study and the relatively small sample size are limitations that should be acknowledged. Future prospective studies with larger cohorts are necessary to confirm these findings and to explore the long-term impacts of Y-90 radioembolization on quality of life and liver function. Additionally, the development of biomarkers to predict response to Y-90 therapy could further refine patient selection and improve outcomes.
Conclusions
Y-90 radioembolization demonstrates promising efficacy and an acceptable safety profile in patients with unresectable HCC. This treatment offers a valuable option for extending survival in a challenging patient population, particularly when prior surgical interventions are no longer viable. The findings of this study demonstrate the efficacy of Yttrium-90 (Y-90) radioembolization in patients with unresectable hepatocellular carcinoma (HCC).
Continued research and careful patient selection will be essential to maximize the benefits of this therapeutic approach.
Ethical Compliance and Conflict of Interest Statement
We confirm that this study was conducted in full accordance with the relevant ethical guidelines and regulations.
Approval was obtained from the appropriate institutional ethics committee prior to the commencement of the research.
Furthermore, the authors declare that there are no conflicts of interest that could have influenced the results or interpretations presented in this manuscript.
References
- Gholam PM, Iyer R, Johnson MS (2019) Multidisciplinary Management of Patients with Unresectable Hepatocellular Carcinoma: A Critical Appraisal of Current Evidence. Cancers 11:873.
- Salem R, Lewandowski RJ, Mulcahy MF, Riaz A, Ryu RK et al. (2010) Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology 138:52-64.
- Lobo L, Yakoub D, Picado O, Ripat C, Pendola F, et al. (2016) Unresectable hepatocellularcarcinoma: radioembolization versus chemoembolization: a systematic review and meta-analysis. Cardiovasc Intervent Radiol 39:1580-1588.
- Knight GM, Gordon AC, Gates V, Talwar A, Riaz A, et al. (2013) Evolution of Personalized Dosimetry for Radioembolization of Hepatocellular Carcinoma. J Vasc Interv Radiol 34:1214-1225.
- Kim HC (2017) Radioembolization for the treatment of hepatocellular carcinoma. Clin Mol Hepatol 23:109-114.
- Choi JW, Kim HC (2022) Radioembolization for hepatocellular carcinoma: what clinicians need to know. J Liver Cancer 22:4-13.
- Hamad A, Aziz H, Kamel IR, Diaz DA, Pawlik TM (2023) Yttrium-90 Radioembolization: Current Indications and Outcomes. J Gastrointest Surg 27:604-614.
- Siegel RL, Miller KD, Jemal A (2019) Cancer Statistics 2019. CA Cancer J Clin 69:7-34.
- Mazzaferro V, Sposito C, Bhoori S, Romito R, Chiesa C, et al. (2013) Yttrium-90 radioembolization for intermediate-advanced hepatocellular carcinoma: a phase 2 study. Hepatology 57:1826-37.
- Wang DS, Louie JD, Sze DY (2019) Evidence-Based Integration of Yttrium-90 Radioembolization in the Contemporary Management of Hepatic Metastases from Colorectal Cancer. Tech Vasc Interv Radiol 22:74-80.
- Ludwig JM, Zhang D, Xing M, Kim HS (2017) Meta-analysis: adjusted indirect comparison of drug-eluting bead transarterial chemoembolization versus (90)Y-radioembolization for hepatocellular carcinoma. Eur Radiol 27:2031-2041.
- Shiina S, Sato K, Tateishi R, Shimizu M, Ohama H, et al. (2018) Percutaneous Ablation for Hepatocellular Carcinoma: Comparison of Various Ablation Techniques and Surgery. Can J Gastroenterol Hepatol 4756147.
- Titano J, Voutsinas N, Kim E (2019) The Role of Radioembolization in Bridging and Downstaging Hepatocellular Carcinoma to Curative Therapy. Semin Nucl Med 49: 189-196.
- Ozkan ZG, Poyanli A, Ucar A, Kuyumky S, Akyuz F, et al. (2015) Favorable survival time provided with radioembolization in hepatocellular carcinoma patients with and without portal vein thrombosis. Cancer Biother Radiopharm 30:132-8.
- Chow PKH, Gandhi M, Tan SB, Khin MW, Khasbazar A, et al. (2018) SIRveNIB: Selective internal radiation therapy versus sorafenib in Asia-Pacific patients with hepatocellular carcinoma. J Clin Oncol 36:1913-1921.
- Vouche M, Habib A, Ward TJ, Kim E, Kulik L, et al. (2014) Unresectable solitary hepatocellular carcinoma not amenable to radiofrequency ablation:mmulticenter radiology-pathology correlation and survival of radiation segmentectomy. Hepatology 60:192-201.
- Vilgrain V, Pereira H, Assenat E, Guiu B, Ilonca AD, et al. (2017) Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol 18:1624-1636.
- Chow PKH, Gandhi M, Tan SB, Khin MW, Khasbazar A, et al. (2018) SIRveNIB: Selective internal radiation therapy versus sorafenib in Asia-Pacific patients with hepatocellular carcinoma. J Clin Oncol 36:1913-1921.
- Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, et al. (2010) Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 362:1273-1281.
- Rayar M, Sandri GBL, Houssel-Debry P, Camus C, Sulpice L, et al. (2016) Multimodal Therapy including Yttrium-90 Radioembolization as a Bridging Therapy to Liver Transplantation for a Huge and Locally Advanced Intrahepatic Cholangiocarcinoma. J Gastrointestin Liver Dis 25:401-4.
- Al-Adra DP, Gill RS, Axford SJ, Shi X, Kneteman N, et al. (2015) Treatment of unresectable intrahepatic cholangiocarcinoma with yttrium-90 radioembolization: a systematic review and pooled analysis. Eur J Surg Oncol 41:120-127.
- Boehm LM, Jayakrishnan TT, Miura JT, Zacharias AJ, Johnston FM, et al. (2015) Comparative effectiveness of hepatic artery based therapies for unresectable intrahepatic cholangiocarcinoma. J Surg Oncol 111:213-20.
- Edeline J, Touchefeu Y, Guiu B, et al. (2019) Radioembolization Plus Chemotherapy for First-line Treatment of Locally Advanced Intrahepatic Cholangiocarcinoma: A Phase 2 Clinical Trial. JAMA Oncol 6:51-59.
- Fernandez FG, Drebin JA, Linehan DC, Dehdashti F, Siegel BA, et al. (2004) Five-year survival after resection of hepatic metastases from colorectal cancer in patients screened by positron emission tomography with F-18 fluorodeoxyglucose (FDG-PET). Ann Surg 240:438-450.
- Tournigand C, Andre T, Achille E, Lledo G, Flesh M, e al. (2004) FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 22:229– 237.
- Van Cutsem E, Cervantes A, Adam R, et al. (2016) ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 27:1386-1422.
- Gray BN, Anderson JE, Burton MA, et al. (1992) Regression of liver metastases following treatment with yttrium-90 microspheres. Aust N Z J Surg 62:105-110.
- Hendlisz A, Van den Eynde M, Peeters M, Maleux G, Lam- bert B, et al. (2010) Phase III trial comparing protracted intravenous fluorouracil infusion alone or with yttrium-90 resin microspheres radioembolization for liver-limited metastatic colo- rectal cancer refractory to standard chemotherapy. J Clin Oncol 28:3687–94.
- Van Hazel G, Blackwell A, Anderson J, et al. (2004) Randomised phase 2 trial of SIR-spheres plus fluorouracil/leucovorin chemotherapy versus fluorouracil/leucovorin chemotherapy alone in advanced colorectal cancer. Journal of Surgical Oncology 88:78–85.
- Wasan HS, Gibbs P, Sharma NK, et al. (2017) First-line selective internal radiotherapy plus chemotherapy versus chemotherapy alone in patients with liver metastases from colorectal cancer (FOXFIRE, SIRFLOX, and FOXFIRE-Global): a combined analysis of three multicentre, randomised, phase 3 trials. Lancet Oncol 18:1159-1171.
- Cosimelli M, Golfieri R, Cagol PP, et al. (2010) Multi-centre phase II clinical trial of yttrium-90 resin microspheres alone in unresectable, chemotherapy refractory colorectal liver metastases. Br J Cancer 103:324-331.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.