The “Hanging Garden” Method of Incidental Durotomy Repair: An Illustrative Case Report and Technique Description
by Anna Martin1, Jamal Zahir2, Nathan Smith1, Oluwatodimu Raji1,2, David Nelles1,2, Ken Hsu1,2, Dimitriy Kondrashov1,2*
1Department of Orthopaedic Surgery, San Francisco Orthopaedic Residency Program, St. Mary’s Medical Center, San Francisco, California, USA
2The Taylor Collaboration Orthopaedic Biomechanics Laboratory, San Francisco, California, USA
*Corresponding Author: Dimitriy Kondrashov, Department of Orthopaedic Surgery, San Francisco Orthopaedic Residency Program, St. Mary’s Medical Center, San Francisco, California, USA
Citation: Martin A, Zahir J, Smith N, Raji O, Nelles D, et al. (2024) The “Hanging Garden” Method of Incidental Durotomy Repair: An Illustrative Case Report and Technique Description. J Surg 9: 11182 DOI: 10.29011/2575-9760.011182
Received Date:
Accepted Date:
Published Date:
Abstract
This article evaluates the utility of the “hanging garden” technique of dural repair, while advocating for formally standardizing and naming the technique. Herein, we describe the illustrative case of a 55- year-old patient who underwent the “hanging garden” technique of dural repair during a Pedicle Subtraction Osteotomy (PSO) for a fixed sagittal deformity. The patient underwent primary suture closure utilizing the “hanging garden” technique. There were no persistent peri/postoperative complications including Cerebrospinal Fluid (CSF) leak, neurological deficit, nerve root injury, vascular injury, nor infection. To the best of our knowledge, we are the first to coin the term “hanging garden” with respect to this technique of dural repair. Previous studies discuss direct (i.e. suture closure) and indirect (i.e. sealant/graft) repair modalities, but have not previously named a systematic method of protecting the nerve rootlets with traction sutures applied to the dura nor have they formally standardized the technique.
Keywords: Adult Spinal Deformity; Cerebrospinal Fluid; Dural Repair; Durotomy; Dural Tear; Spine
Introduction
Dural Tear (DT), a common complication of spine surgery, has a reported incidence ranging from about 2% to 20% [1-3]. The dura mater consists mostly of collagen and fibroblasts and is less than 500 µm thick [4]. In surgery for Adult Spinal Deformities (ASD), iatrogenic DTs are common, requiring prompt identification and treatment. The gold standard involves secure primary suture closure. For larger or irreparable tears, augmentation with a dural patch or sealant is recommended to reduce the risk of complications, including infection, persistent CSF leakage, pseudomeningocele, durocutaneous fistula, and nerve rootlet entrapment [6,7]. Herein, we describe and determine the utility of the “hanging garden”
technique for dural repair which involves tractions sutures pulling the dura safely away from the nerve rootlets. To the best of our knowledge, this is the first standardized description of this DT repair technique in the English-language literature.
Illustrative Case Presentation and Technique Description
An otherwise healthy 55-year-old patient presented to our institution with a fixed sagittal spinal deformity. The deformity was causing pain and affecting the patient’s Quality of Life (QoL) and posture. After thorough history and physical, imaging, preoperative clearance, and discussion of risks and benefits of surgery versus not pursuing treatment, a decision was made to pursue surgical correction via PSO. Informed consent was obtained and the patient was scheduled for surgery. The patient was brought to the operating room and prepped and draped in standard fashion, a posterior muscle splitting midline approach was taken and dissection was carried down to the spinous process of T1-T4, all while obtaining intraoperative neuromonitoring (motor evoked potentials and somatosensory evoked potentials). During the osteotomy a considerable amount of scar tissue was encountered. As dissection of the scar tissue was performed to minimize cauda equina compression and dural buckling, an egress of clear fluid was encountered, signifying an intraoperative DT. The decision was made to pursue primary closure due to the small size of the DT. In order to protect the neural elements and provide adequate closure, the “hanging garden” method of dural closure was performed. That is, 6-0 Prolene® (Ethicon Inc., Cornelia, GA, USA) sutures were placed at the edges of the DT with gentle retraction of the edges, and were raised away from the underlying neural elements. Next, watertight primary closure was achieved using 6-0 Prolene® sutures in an interrupted pattern, Castro Viejo needle drivers, and ring forceps - all while protecting the underlying neural elements (Figure 1 and Figure 2). Adjuncts such as free fat graft, fibrin glues, and bioabsorbable sheets were not required for closure. Intraoperative Valsalva maneuver to 30-40 mmHg did not demonstrate any CSF leak, therefore the closure was deemed watertight. Our patient did not experience any perioperative complications (e.g. persistent CSF leak, spinal headache, etc). Postoperative magnetic resonance imaging (MRI) did not show any CSF leak nor collection. Repeat Valsalva maneuver to 30-40 mmHg confirmed there was no CSF leakage. Postoperative complications such as CSF leak, neurological deficit, nerve root injury, vascular injury, wound dehiscence, and infection were absent at one week, three month, six month, and one year follow up, leading to a normal postoperative course.
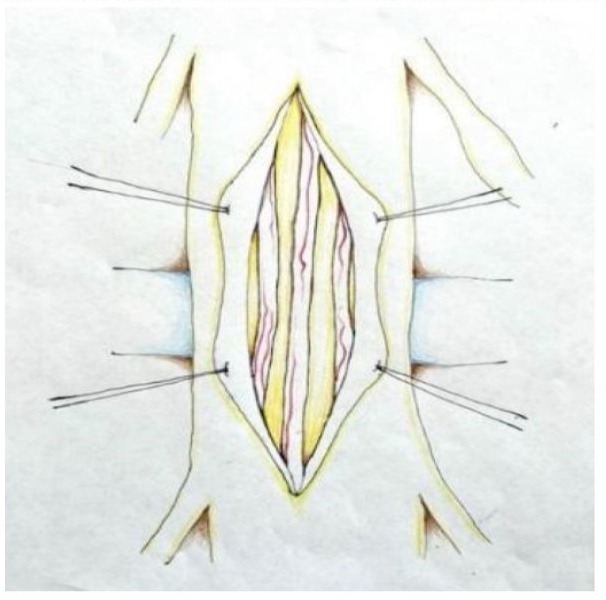
Figure 1: “Hanging Garden” Technique of Traction Suture Placement.

Figure 2: Closure of dural defect during lumbar PSO. Note use of olive tip suction and ring forceps along with utilization of “hanging garden” technique with traction sutures on the sides of the repair.
Discussion
DTs arising from ASD surgery can lead to various complications, including intense headaches, nausea, vomiting, sensitivity to light, continuous CSF leakage, pseudomeningocele, persistent draining fistula, and noncommunicating hydrocephalus. Without timely correction, these issues may lead to lasting consequences such as the formation of an ascending hygroma, subarachnoid hemorrhage, intraventricular hemorrhage, and subdural hematoma [8,9]. Additional complications such as bowel and bladder dysfunction, may also be related to incidental durotomy. The retrospective study by Oshina et al. found a significantly higher rate (p<0.001) of bowel and bladder dysfunction (i.e. sphincter disturbance) among patients with DT who were undergoing microendoscopic surgery of the lumbar spine [10]. This suggests that during the incidental durotomy repair, the sacral nerve rootlets may have been captured and damaged from the suture needle. This makes sense anatomically as the sacral rootlets are more dorsal and central, thereby increasing the risk of damage during primary DT suture repair. We posit that these deleterious complications may be decreased or even avoided with use of the “hanging garden” technique, as the goal of the technique is to pull the dura safely away from the delicate neural structures underneath. This should prompt spine surgeons to utilize the “hanging garden” technique in order to protect underlying neural structures during primary closure. There are also a number of risk factors for DT in the ASD population identified in the existing literature, including older age, osteotomy, revision surgery, and rheumatoid arthritis (RA) [2,6,11-17]. Continually evolving anatomical understanding should also inform future management and repair techniques, such as, recently published literature highlighting the importance of posterior epidural ligaments (PELs) identified both in thoracic and lumbar regions (Figure 3). A study identified PELs in 5 out of 14 cadavers (35.7%) and observed that thoracic PELs possess adequate tensile strength to pose a potential risk to the integrity of the dural sheath during surgery [18].

Figure 3: Posterior epidural ligaments in thoracic spine ( black arrows → point to posterior epidural ligaments).
In managing intraoperatively diagnosed DTs, a direct primary repair is the preferred approach, necessitating DT exposure through extended laminectomy. Therefore, it has been argued that, virtually all dural repairs should be performed under an operating microscope [19]. Suture closure with 5-0 to 7-0 nonabsorbable monofilament in a running or interrupted pattern is common, with careful rootlet repositioning if the tear is below the conus medullaris. Additionally, tacking sutures may be utilized at torn areas [20]. Additionally, blood in the dural sac, if present, can be minimized with sterile saline wash to minimize arachnoiditis. Valsalva maneuver to 30-40 mmHg ensures repair adequacy. If no CSF leakage is seen during Valsalva maneuver, then repair is considered watertight. Adjuncts such as free fat graft, fibrin glues (e.g. Duraseal® Integra Life Sciences, Plainsboro, NJ, USA) and bioabsorbable sheets (e.g. Duragen® Integra Life Sciences, Plainsboro, NJ, USA) enhance suboptimal or incomplete closure, however some studies have indicated hydrogel (such as Duraseal®) expansion even after initial deployment [21]. Moreover, caution is vital to prevent stenosis, especially ventrally, laterally, or in tight corners with deployment of Duraseal® or Duragen®. Duraseal® is an absorbable, synthetic polyethylene glycol (PEG) hydrogel used as an adjunct for repair of DT [22]. Duragen®, on the other hand, is a 3 dimensional sheet of collagen matrix used to cover and adhere to a repaired tear, or to augment thin, missing, or highly damaged dura [23]. Patches are crucial for extensive traumatic tears. Additionally, autograft tissues (such as fascia or subdermal fat) provide options for massive or irreparable tears. Nevertheless, all closures must be watertight or as close to watertight as possible [7,20,24]. However, challenging areas, such as ventral tears during posterior surgery or axilla of nerve roots, may require alternative techniques such as nerve root sheath sewing. Meticulous wound closure with interrupted facial sutures (Figure 4 and Figure 5) or running subcuticular and skin stitches is vital to prevent CSF fistulas and other complications [25].

Figure 4: “Hanging Garden” Technique of Traction Suture Placement and Dural Closure.

Figure 5: Watertight closure of dural defect using interrupted sutures.
In cases of postoperative CSF leaks, a closed approach is favored, employing nonsurgical options such as lumbar drains to divert CSF, allowing for secondary intention healing. Lumbar drains are preferable over ventriculostomy, typically diverting CSF for 5-7 days to optimize healing while avoiding additional surgery [25]. An auxiliary method of managing DT and subsequent CSF leakage is the use of carbonic anhydrase inhibitors (CAIs), such as acetazolamide (Diamox). The mechanism of action of carbonic anhydrase inhibitors results in decreased production of CSF by choroid plexus, thereby limiting CSF leakage. Up to a 39%-48% decrease in CSF production after CAI administration has been reported as compared to typical daily production [26]. Some studies have shown that CAI is an effective treatment for CSF leakage due to DT, however other studies did not show the same benefit [27,28].Finally, radiological modalities for diagnosis such as T2 weighted MRI or CT myelography can also aid in diagnosis and localization of DT. Ongoing CSF leakage warrants prolonged bed rest, further surgical exploration, and/or subarachnoid drain placement [22,25,29,30]. As an adjunct to clinical and radiological evaluation, beta 2 transferrin testing of wound discharge is an effective method of diagnosing a CSF leak. Beta 2 transferrin is a protein exclusively found in CSF and perilymph [31]. The study by Shenoy et al. found that a novel double armed suture dural repair device (DuraStat LLC, Exton, PA, USA) was able to repair the dura in significantly less time and with significantly less variation than traditional instrumentation (p=0.013). Moreover, the novel device had significantly higher water tightness (p=0.005) and significantly less trapped nerve roots (p=0.016) [32]. Dong et al. investigated direct and indirect repair methods, stating that one stage direct suture repair with graft augmentation is recommended. Still, indirect repair remains the method of choice for larger DTs with many of the new sealants and grafts only employed in animal models and in vitro [33]. The systematic review by Choi et al. evaluated the various DT repair modalities. They concluded that primary closure with and without graft augmentation is the best method of DT repair. Infection rates between primary closure with and without patch, graft, or sealant were not significantly different.
Sealants did not significantly reduce the rate of CSF leakage when combined with primary suture closure. They also did not find a significant difference between the interrupted suture and locked suture techniques. The rates of neurological deficit were not significantly different between the groups. Again, this highlights that direct repair should be performed if possible [7]. Haque et al. utilized a minimally invasive surgery (MIS) retractor system for novel use of a CV-20 taper ½ circle, 10 mm diameter needle and Scanlan® (Scanlan International Inc., Saint Paul, MN, USA) dural closure set. The CV-20 needle is typically utilized in pediatric neurosurgery, and in this case its small size allowed for successful dural repair in difficult to reach working areas. No complications were appreciated at the 24-week postoperative follow up. This may represent a cost-effective option for institutions already utilizing this instrumentation and emphasizes the importance of extreme diligence in tight areas when repairing DTs [34]. Another method of DT repair was described by Heo et al. which involves placing a nonpenetrating titanium vascular anastomosis clip during Biportal Endoscopic Spine Surgery (BESS). They concluded that clipping may be an effective alternative for incidental durotomy. There were no complications of the clipping discussed [35]. As endoscopic surgery continues to gain traction, this technique may increase in its use. Finally, and perhaps most importantly, Rahyussalim et al. employed a very similar method to the “hanging garden” technique, however they utilized this for herniated nerve rootlets. They also placed suture anchors at the dural edges and applied traction to engulf the herniated nerve rootlets. Nevertheless, they did not formally name this technique [36]. Although there are numerous methods of DT repair, it is apparent that a watertight seal and coverage of the defect by means of direct or indirect repair is required and is the most effective method of closure for DTs amenable to this type of repair. We offer here our preferred technique of dural repair with our suggested name “hanging garden” inspired by the remarkable Babylonian creation and one of the Seven Wonders of the Ancient World.
Conclusion
In our experience, we found that the “hanging garden” technique of DT repair is a safe and effective primary suture closure method, all while protecting the underlying neural structures. While there are various methods of DT repair, primary closure with or without augmentation remains the gold standard. As such, we are the first to name the “hanging garden” technique and formally standardize this method of dural tear repair.
References
- Wang JC (1998) Dural tears secondary to operations on the lumbar spine. Management and results after a two-year-minimum follow-up of eighty-eight patients. JBJS 80: 1728-1732
- Iyer S, Klineberg EO, Zebala LP (2018) Dural Tears in Adult Deformity Surgery: Incidence, Risk Factors, and Outcomes. Glob Spine J 8: 2531.
- Williams BJ, Sansur CA, Smith JS (2011) Incidence of Unintended Durotomy in Spine Surgery Based on 108 478 Cases. Neurosurgery 68: 117-124.
- Kwon S, Suh SW, Kim D (2018) Analysis of dural sac thickness in the human cervical spine. Anat Sci Int 93: 284-290.
- Kalevski S, Peev N, Haritonov D (2010) Incidental Dural Tears in lumbar decompressive surgery: Incidence, causes, treatment, results. Asian J Neurosurg 5: 54-59.
- Jo DJ, Kim KT, Lee SH, Cho MG, Seo EM (2015) The Incidence and Management of Dural Tears and Cerebrospinal Fluid Leakage during Corrective Osteotomy for Ankylosing Spondylitis with Kyphotic Deformity. J Korean Neurosurg Soc 58: 60-64.
- Choi EH, Chan AY, Brown NJ (2021) Effectiveness of Repair Techniques for Spinal Dural Tears: A Systematic Review. World Neurosurg 149: 140-147.
- Endriga DT, Dimar JR, Carreon LY (2016) Communicating hydrocephalus, a long-term complication of dural tear during lumbar spine surgery. Eur Spine J 25: 157-161.
- Tan LA, Kasliwal MK, An HS, Byrne RW (2018) Obstructive Hydrocephalus Due to Intraventricular Hemorrhage After Incidental Durotomy During Lumbar Spine Surgery. Spine 43: E316-E319.
- Oshina M, Segawa T, Manabe N, Oshima Y, Tanaka S, et al. (2020) Incidence, prognosis, and risk factors for bladder and bowel dysfunction due to incidental dural tears in lumbar microendoscopic surgery. Spine J 20: 688-694.
- Bernstein DN, Kurucan E, Menga EN, Molinari RW, Rubery PT, et al. (2018) Comparison of adult spinal deformity patients with and without rheumatoid arthritis undergoing primary non-cervical spinal fusion surgery: a nationwide analysis of 52,818 patients. Spine J 18: 18611866.
- Skovrlj B, Cho SK, Caridi JM, Bridwell KH, Lenke LG, et al. (2015) Association Between Surgeon Experience and Complication Rates in Adult Scoliosis Surgery: A Review of 5117 Cases From the Scoliosis Research Society Database 2004–2007. Spine 40: 1200-1205.
- Shaw R, Skovrlj B, Cho SK (2016) Association Between Age and Complications in Adult Scoliosis Surgery: An Analysis of the Scoliosis Research Society Morbidity and Mortality Database. SPINE 41: 508514.
- Nakajima K, Nakamoto H, Kato S (2020) Influence of unintended dural tears on postoperative outcomes in lumbar surgery patients: a multicenter observational study with propensity scoring. Spine J 20: 1968-1975.
- Chen X, Feng F, Yu X (2020) Robot-assisted orthopedic surgery in the treatment of adult degenerative scoliosis: a preliminary clinical report. J Orthop Surg 15: 282.
- Kwan KYH, Bow C, Samartzis D (2019) Non-neurologic adverse events after complex adult spinal deformity surgery: results from the prospective, multicenter Scoli-RISK-1 study. Eur Spine J 28: 170-179.
- Louie PK, Iyer S, Khanna K (2022) Revision Strategies for Harrington Rod Instrumentation: Radiographic Outcomes and Complications. Glob Spine J 12: 654-662.
- Rimmer C, Adds P (2015) The posterior epidural ligament in the thoracic region: a cadaveric and histological study. Folia Morphol 77: 748-751.
- Rodríguez D, Amin U, Bartolomé D (2023) Management of incidental durotomies in an integrated Orthopaedic and Neurosurgical Spinal Unit. Brain Spine 3: 102682.
- Dafford EE, Anderson PA (2015) Comparison of dural repair techniques. Spine J 15: 1099-1105.
- Yeh KL, Wu SH, Fuh CS, Huang YH, Chen CS, et al. (2022) Cauda equina syndrome caused by the application of DuraSeal TM in a microlaminectomy surgery: A case report. World J Clin Cases 10: 11178-11184.
- Kim KD, Ramanathan D, Highsmith J (2019) DuraSeal Exact Is a Safe Adjunctive Treatment for Durotomy in Spine: Postapproval Study. Glob Spine J 9: 272-278.
- Narotam PK, José S, Nathoo N, Taylon C, Vora Y (2004) Collagen Matrix (DuraGen) in Dural Repair: Analysis of a New Modified Technique: Spine 29: 2861-2867.
- Suter A, Spirig JM, Fornaciari P (2019) Watertightness of wound closure in lumbar spine—a comparison of different techniques. J Spine Surg 5: 358-364.
- Brodano GB (2014) Is lumbar drainage of postoperative cerebrospinal fluid fistula after spine surgery effective? J Neurosurg Sci 58: 23-27.
- Carrion E (2001) Use of acetazolamide to decrease cerebrospinal fluid production in chronically ventilated patients with ventriculopleural shunts. Arch Dis Child 84: 68-71.
- Chaaban MR, Illing E, Riley KO, Woodworth BA (2013) Acetazolamide for high intracranial pressure cerebrospinal fluid leaks. Int Forum Allergy Rhinol 3: 718-721.
- Kankam SB (2022) Investigating acetazolamide effectiveness on CSF leak in adult patients after spinal surgery. Neurocir Engl Ed 33: 293299.
- Battal B, Kocaoglu M, Bulakbasi N, Husmen G, Tuba Sanal H, et al. (2011) Cerebrospinal fluid flow imaging by using phase-contrast MR technique. Br J Radiol 84: 758-765.
- Patel DM, Weinberg BD, Hoch MJ (2020) CT Myelography: Clinical Indications and Imaging Findings. RadioGraphics 40: 470-484.
- Haft GF, Mendoza SA, Weinstein SL, Nyunoya T, Smoker W (2004) Use of beta-2-transferrin to diagnose CSF leakage following spinal surgery: a case report. Iowa Orthop J 24: 115-118.
- Shenoy K, Donnally CJ, Sheha ED, Khanna K, Prasad SK (2021) An Investigation of a Novel Dural Repair Device for Intraoperative Incidental Durotomy Repair. Front Surg 8: 642972.
- Dong RP, Zhang Q, Yang LL, Cheng XL, Zhao JW (2023) Clinical management of dural defects: A review. World J Clin Cases 11: 29032915.
- Haque RM, Hashmi SZ, Ahmed Y, Uddin O, Ogden AT, et al. (2013) Primary Dural Repair in Minimally Invasive Spine Surgery. Case Rep Med 2013: 1-6.
- Heo DH, Ha JS, Lee DC, Kim HS, Chung HJ (2022) Repair of Incidental Durotomy Using Sutureless Nonpenetrating Clips via Biportal Endoscopic Surgery. Glob Spine J 12: 452-457.
- Rahyussalim AJ, Djaja YP, Saleh I, Safri AY, Kurniawati T (2016) Preservation and Tissue Handling Technique on Iatrogenic Dural Tear with Herniated Nerve Root at Cauda Equina Level. Case Rep Orthop 2016: 1-6.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.