Testosterone and Prevention: Effects on Renal Function
by Aksam Yassin1-3*, Raidh Talib Alzubaidi1,2, Hatem Kamkoum1, Anas Albudairat1, Hasan Abdallah1, Ahmad Haroon1, Abdulla Al-Ansari 1,2
1Hamad Medical Corporation, Aisha Al Attiyya Hospital, Andrology & Men’s Health Unit, Qatar
2Weill Cornell Medical School NY, Qatar
3Dresden International University, Preventive Medicine Program, Dresden Germany
*Corresponding author: Aksam Yassin, Hamad Medical Corporation, Aisha Al Attiyya Hospital, Andrology & Men’s Health Unit, Qatar
Received Date: 15 July 2025
Accepted Date: 23 July 2025
Published Date: 25 July 2025
Citation: Yassin A, Alzubaidi RT, Kamkoum H, Albudairat A, Abdallah H, et al. (2025) Testosterone and Prevention: Effects on Renal Function. J Urol Ren Dis 09: 1427. https://doi.org/10.29011/2575-7903.001427.
Abstract
Objectives: Testosterone Therapy (TTh) is the main treatment for elderly men with hypogonadism. No evidence of the long-term effectiveness of TTh on renal function is reported to date.
Methods: In this study, we evaluated the long-term TTh of Testosterone Undecanoate (TU) administration on renal function parameters in 496 symptomatic hypogonadal men, with T levels ≤350 ng/dL. The treatment group (T-group) consisted of 312 patients and obtained TU 1000 mg for 12 weeks followed by 6-week intervals and for up to 8 years. The remaining 184 hypogonadal men, who opted against TTh, served as a control group (C-group). The two groups were similar in criteria prior to treatment. We evaluated renal function by calculating serum creatinine, urea, uric acid, and Glomerular Filtration Rate (GFR) according to Mayo Clinic guidelines for 8 years. This study obeys the ethical guidelines of German medical association according to Section 15 of the Professional Code, document for AY- Ref. EK/CH/AU signed on Jun 2015.
Results: During the study period, the T-group exhibited lower levels of urea (47.0 ± 11.8 to 34.0 ± 13.9 mg/dL), uric acid (6.57 ± 1.2 to 5.49 ± 1.5 mg/dL), serum creatinine (0.90 ± 0.10 to 1.12 ± 0.9 mg/dL), and higher-level in GFR (87.0 ± 12.9 to 98.0 ± 8.0 mL/min/1.73 m2), which were significant. Alternatively, the C-group exhibited an increase in their serum creatinine (1.16 ± 0.31 to 1.19 ± 0.58 mg/dL), an increase in uric acid (5.54 ± 1.2 to 5.44 ± 1.7 mg/dL), and a decrease in GFR (92.0 ± 20.1 to 87.0 ± 26.1 mL/ min/1.73 m2). A total of 25 deaths (7.8%) was recorded in the T-group, among them 11 (44%) were cardiovascular. On the other hand, 28 patients (15.2%) died in C-group and all deaths (100%) were found to cardiovascular causes.
Conclusion: The results suggest that long-term TTh could improve renal function in hypogonadal men comparing to slight deterioration observed in patients without intervention. In addition to reducing mortality in cardiovascular patients, it is almost half to half.
Keywords: Hypogonadism; Prevention; Renal Function, cardiovascular Diseases, Mortality, Testosterone Therapy
Introduction
Functional hypogonadism is known as age-related decrease in Total Testosterone (TT) levels with sexual symptoms [1-4]. The prevalence of functional hypogonadism range is estimated from 12% for 50-year-old, to 30% for 70-year-old men [5], and the chance increased with obesity and aging [6]. Symptoms of hypogonadal syndrome such as infertility, impaired libido, fatigue, and risk of depression have a great impact on the Quality Of Life (QoL) [7,8]. Additionally, several components of Metabolic Syndrome (MetS) such as dyslipidemia, hypertension, obesity, decrease in muscle mass, insulin resistance, and dysregulation of glucose metabolism are also associated with the low TT levels [911], and hence increased the risk of Cardiovascular Disease (CVD) [12,13]. Many studies have shown that Testosterone Therapy (TTh) in hypogonadal men improved body composition, sexual function and reduces risk of CVD [14-27]. It is worth noting that long-term treatment of TTh and the effect on renal have not been reported to date. Hypogonadism in men with Chronic Kidney Disease (CKD) has significant morbidity with CVD and anemia [28]. The link between hypogonadism and CKD is likely to be multifactorial. Other comorbidities with LOH are known as CVD, diabetes, and obesity, which can be linked to CKD with a dysregulation in the hypothalamic-pituitary-gonadal axis [29-31]. Although many studies were concentrated on the sexual function and the effects of TTh on CKD, limited protocols on the direct effects of TTh on renal function were published so far.Herein, we present the renal function data of hypogonadal men with 8-years of long-term TTh.
Methods
Design and setting
A cohort prospective observational study was conducted at Institute of Urology and Andrology, Segeberger Kliniken, Norderstedt-Hamburg, Germany, and Men’s Health Department, Hamad Medical Cooperation, Doha, Qatar. A convenience sampling method were used to include 496 men (mean age: 59 ± 9.5 years) who suffers from low testosterone level ≤350 ng/ dL (≤12.1 nmol/L) and hypogonadism. Any other patients were excluded. Observational data registry started in November 2004 till January 2015.
Sample
A total of 312 men has enrolled in the treatment group (T-group) and received 1000 mg Testosterone Undecanoate (TU) every 12 weeks, followed for 6 weeks and for up to 8 years. Among them, 140 men had discontinued treatment for 17 months after 5.5 years for reimbursement issues. The remaining 184 patients were selected for the control group (C-group). The two groups were mostly similar medically and demographically prior to treatment. The assessment of the long-term effect of TU on renal function as per Mayo Clinic test guidelines was evaluated by measuring uric acid, serum creatinine, urea and Glomerular Filtration Rate (GFR).
Ethical Consideration
Institutional Review Board (IRB) approval was obtained from the ethics committee in the German medical association (¨Arztekammer) (EK/CH/AU/June 1, 2015). All patients have signed the informed consent before enrolling in this study. In accordance with the declaration of Helsinki, the research was registered at ResearchRegistry.com with unique identifying number (researchregistry6769) Yassin A, et al. (2025). This study is written under STROCSS 2019 Guideline R. Agha, et al. (2019).
Statistical Analysis
Statistical analysis was performed using the SPSS (Statistical Package for Social Sciences, v.12, Windows package, USA) and GraphPad Prism V.8.4.3 (GraphPad Software, La Jolla, California, USA). Data were expressed as mean ± SD or as a simple number. Clinical parameters were studied between both groups over the treatment periods by linear mixed effect, repeated-measures model with period, group and their interaction considered as fixed effects. Data were compared using the Х2 test or Fisher’s exact test as appropriate. Correlations were performed using Spearman’s correlation coefficient. Regression was used for multivariate test analysis and as designated. A p-value < 0.05 was considered statistically significant.
Results
The characteristics of patients included in this study are described in Table 1. No significant differences between both groups are found using
Table 1 : Baseline characteristics of testosterone treatment group (T-group) and the control group (c-group). BMI body mass index
(GFR). The mean for T-group including 312 patients were 59 ± 9.5 years, whereas the mean for C-group including 184 patients were 66.1 ± 7.6 years. The elevation in TT levels for hypogonadal men with TTh (T-group) wa observed during 1-year follow-up period (7.90 nmol/L - 15.98 nmol, P < 0.0001) and compared to 9.10 nmol/L to 9.20 nmol/L (C-group) as shown in Figure 1.The change in serum creatine levels was also investigated between the two groups for an 8-years period. Clearly, it shows a lowering level of serum creatine in the T-group (0.90 ± 0.10 to 1.12 ± 0.9 mg/dL) compared to C-group, where it shows an increase in serum creatine levels in C-group from 1.16 ± 0.31 to 1.19 ± 0.58 mg/dL with p < 0.05 and especially for years 4-7 compared to C-group as described in Figure 2. The uric acid level dropped dramatically within the first 5-years for the T-group and from 6.57 mg/dL to 5.49 mg/dL (Figure 3), whereas a steady decrease in uric acid levels for an 8-year study period for both groups giving (6.57 ± 1.2 to 5.49 ± 1.5 mg/dL, p < 0.0001) for T-group and (5.54 ± 1.2 to 5.44 ± 1.7 mg/dL, p < 0.01) for C-group. Furthermore, the dropping of uric acid levels in the T-group is likely engaged with the decrease of serum urea and from 47.0 ± 11.8 to 34.0 ± 13.9 mg/dL (p < 0.0001) as described in Figure 4.Moreover, the results also showed a continuous decrease in GFR levels over the 8-years follow-up in C-group patients and from 92.0 ± 20.1 to 87.0 ± 26.1
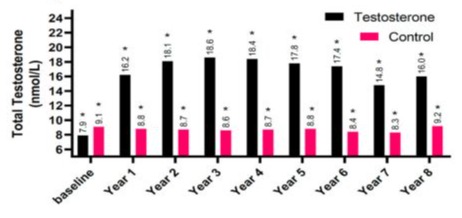
Figure 1: The long-term treatment of testosterone undecanoate versus total testosterone (nmol/L) for 312 hypogonadal men and 184 untreated hypogonadal controls (*p < 0.0001).
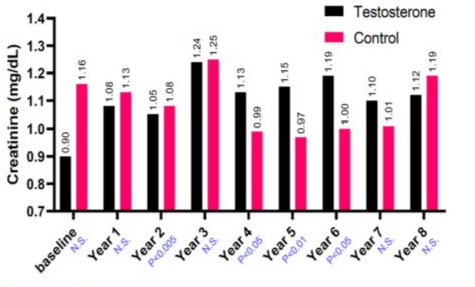
Figure 2: The long-term treatment of testosterone undecanoate versus serum creatinine (mg/dL) for 312 hypogonadal men and 184 untreated hypogonadal controls. N.S. = not significant.
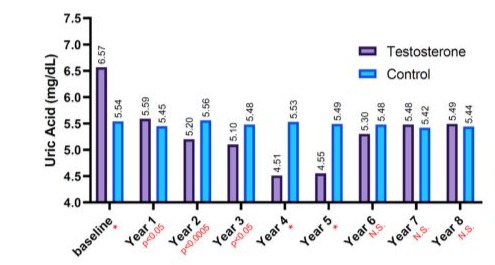
Figure 3: The long-term treatment with testosterone undecanoate versus Serum Uric acid (mg/dL) for 312 hypogonadal men and 184 untreated hypogonadal controls, (*p < 0.001), N.S. = not significant.
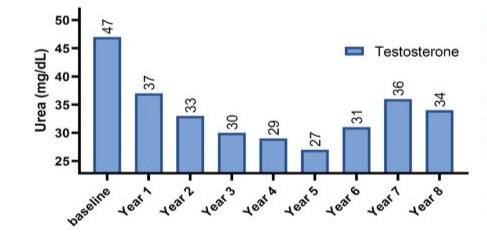
Figure 4: The long-term treatment with testosterone undecanoate versus serum urea (mg/dL) for 312 hypogonadal men.
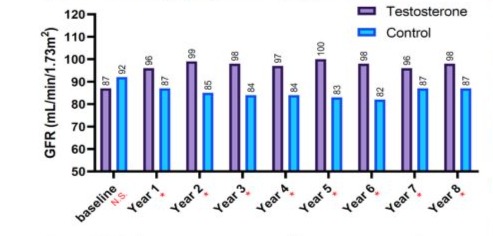
Figure 5: The long-term treatment with testosterone undecanoate versus glomerular filtration rate (GFR, mL/min/1.73m2) for 312 hypogonadal men and 184 untreated hypogonadal controls (*p < 0.0001), N.S. = not significant.
7.8%, p = 0.0351), in which most reported deaths from both groups were attributed to CVD (Table 2).
|
Adverse Events |
||
|
Testosterone Group |
Control Group |
|
|
N |
312 |
184 |
|
Death (%) |
25 (7.8%) |
28 (15.2%)* |
|
Death due to CVD (%) |
11 (44%) |
28 (100%)** |
Table 2: Adverse events observed in testosterone treatment group (T-griuo) and control group (C-group). CVD, Cardiovascular Disease. *P<0.05, **P<0.0001.
Discussion
The low testosterone levels in aging men and the subsequent hypogonadism are associated with different complications such as Metabolic Syndrome (MetS) and increase risk of Cardiovascular Disease (CVD) [10-13]. Also, others are significantly higher in men such as Chronic Kidney Disease (CKD) and renal complications even though a well-documented causal link with CVD, in accordance with cardiorenal syndrome [32-33]. While the long-term effects of TTh on MetS and CVD have been widely reported [34-37], the effect of TTh on renal function in CKD is still limited. Herein, we presented an observational study on the long-term effect of TTh on renal function biomarkers like uric acid, serum creatinine, serum urea and glomerular filtration rate in hypogonadal men. Most of the published protocols of hypogonadism effect on renal function focused on the association with mortality and morbidity of patients including End-Stage Renal Function (ESRD) and CKD [38-40]. The major objectives of TTh in these studies have been sexual function restoration (Treatment of functional hypogonadism) and enhancement of metabolic parameters (lean muscle mass and insulin resistance) [41-42] and no consideration for renal function parameters were mentioned. For instance, in 2009, Tomaszewski et al. reported a baseline study on the correlation between serum testosterone levels and creatinine as an indicator for renal function on eugonadal healthy young men (mean age 18.5 years) [43]. It revealed a normal range for total testosterone concentrations for selected young population; thus it suggests that the effect of testosterone on renal function may endure autonomously of age and beyond the limitations of hypogonadism. Another study by Kurita and co-workers revealed a similar protective effect of endogenous levels of testosterone on kidney function beyond elderly men in Japan [44]. Furthermore, Fukami et al. reported that testosterone levels were significantly lower in the T-group compared to the C-group for patients with renal disease receiving hemodialysis, all the were consistent with our study [45]. The reduction in uric acid levels for patients receiving TTh in this study, along with the increase in GFR, revealed a strong and direct effect of testosterone on renal function. Uric acid, urea, creatine and creatinine are the four non-protein nitrogen fractions in circulations. Uric acid is known because of catabolism, while urea is a by-product of protein metabolism, and both are excreted by the kidney and their serum concentrations reflect the status renal function. Testosterone has a large implication on altering uric acid reabsorption in kidneys by downregulation the transporter protein channel GLUT-9 [39-40]. Moreover, creatinine levels were also found to be lower in patients under testosterone treatment, which is supported by the larger number of published cohort studies [48-50]. The improvement of various components of MetS such as obesity, hypertension and dyslipidemia could be also used as an indication on the effect of TTh on renal function as previously reported [15,16]. However, it was reported that reducing the serum urea concentration in hypogonadal men by increasing the protein anabolism and inhibiting the hepatic urea cycle, which could be in part the cause for having lower levels of serum urea in this study. The deficiency of testosterone has a direct effect on kidney function; it could result from renal ischemia induced by endothelial dysfunction as testosterone demonstrated vasodilatory effects on the renal by the production of nitric acid [51]. Furthermore, testosterone administration suggests that deficient testosterone levels may protect from inflammationinduced by kidney injury by reducing levels of inflammatory markers [52]. Lastly, the reduction in deaths related to CVD in the T-group suggests a direct contribution to the improvement in renal function (Cardiorenal syndrome) [53-55]. Although, it is inline with previously published studies of reduced mortality and improved cardiovascular health in hypogonadal patients receiving TTh. Yalmiz et al. reported a cross-sectional study of elderly men with Chronic Kidney Disease (CKD). It revealed that the progressiveness of CKD stage related to increased hypogonadism, as well as testosterone levels were inversely related to endothelial dysfunction and the risk of CVD in non-dialysis CKD patients [56]. Although, we found that 100% deaths from C-group due to CVD causes in comparison to 44% in T-group, which emphasis the protective role of testosterone in CVD. Moreover, a retrospective cohort study published by Khurana and co-workers revealed that higher CKD-related deaths in men patients were associated with low testosterone levels [57-65]. Even though this observational study is not a Randomized Controlled Trial (RCT) and has few limitations such as the nature of registry design and scope of interpretation, the large patient cohort study and the long-term follow-up for 8 years provide essential clinical facts. Other ethical issues could be raised for not treating hypogonadal patients at our clinic. As mentioned in our methodology, the large number of discontinuing TTh patients during the treatment course and the hard-to-follow-up due to reimbursement issues limited the group differences in renal function and explained some of the parameters in the treatment group between 4and 7 years.
Conclusion
This study highlights the long-term TTh beneficial effects on renal function in men with low levels of testosterone. Moreover, it also highlights the low CVD-related mortality rate with improvement in renal function. Indeed, RCT with placebo-controlled trials is still needed to elucidate the impact of TTh on cardiovascular and renal functions in hypogonadal men.
References
- Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR (2001) Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging, J Clin Endocrinol Metab 86: 724-31.
- Morley JE, Kaiser FE, Perry HM, patrick P, Morley PMK (1997) et al. Longitudinal changes in testosterone, luteinizing hormone, and folliclestimulating hormone in healthy older men, Metabolism 46: 410-413.
- Snyder PJ (2001) Effects of age on testicular function and consequences of testosterone treatment, J Clin Endocrinol Metab. 86: 2369-2372.
- Süer E, Yaman O (2008) Testosterone therapy and prostate cancer, Turk J Urol 34: 27-30.
- Rhoden EL, Morgentaler (2004) A Risks of testosterone-replacement therapy and recommendations for monitoring, N Engl J Med. 350: 482492.
- Seftel AD (2006) Male hypogonadism. Part I: epidemiology of hypogonadism, Int. J. Impot. Res. 18: 115-120.
- Barrett-Connor E, Von Muhlen DG, Kritz-Silverstein D (1999) Bioavailable testosterone and depressed mood in older men: the Rancho Bernardo Study, J Clin Endocrinol Metab. 84: 573-577.
- Monteagudo PT, Falcao AA, Verreschi IT, Zanella MT (2016) The imbalance of sexhormones related to depressive symptoms in obese men, Aging Male 19: 20-26.
- Mohr BA, Bhasin S, Link CL, Donnell ABO, McKinlay JB (2006) The effect of changes in adiposity on testosterone levels in older men: longitudinal results from the Massachusetts Male Aging Study, Eur J Endocrinol 155: 443-452.
- Derby CA, Zilber S, Brambilla D, Morales KH, McKinlay JB (2006) Body mass index, waist circumference and waist to hip ratio and change in sex steroid hormones: the Massachusetts Male Ageing Study, Clin. Endocrinol. 65: 125-131.
- Kawano H (2010) The relationship between testosterone and metabolic syndrome, Hypertens. Res. 33: 537-538.
- Boden WE, Miller MG, McBride R, Harvey C, Snabes MC (2020) et al. Testosterone concentrations and risk of cardiovascular events in androgendeficient men with atherosclerotic cardiovascular disease, Am Heart J. 224: 65-76.
- Çalıs S¸ Koca O, ¨Oztürk M, Akyüz M, Karaman MI (2012) et al. Hormonal evaluation in erectile dysfunction, Turk J Urol 38: 19-22.
- Yassin DJ, Doros G, Hammerer PG, Yassin AA (2014) Long-term testosterone treatment in elderly men with hypogonadism and erectile dysfunction reduces obesity parameters and improves metabolic syndrome and health-related quality of life, J Sex Med. 11: 1567-1576.
- Permpongkosol S, Khupulsup K, Leelaphiwat S, Pavavattananusorn S, Thongpradit S (2016) Effects of 8-year treatment of long-acting testosterone undecanoate on metabolic parameters, urinary symptoms, bone mineral density, and sexual function in men with lateonset hypogonadism, J Sex Med. 13: 1199-1211.
- Yassin AA, Nettleship AA, Almehmadi Y, Salman M, Saad F (2016) Effects of continuous long-term testosterone therapy (TTh) on anthropometric, endocrine and metabolic parameters for up to 10 years in 115 hypogonadal elderly men: real-life experience from an observational registry study, Andrologia 48: 793-799.
- Vartolomei MD, Kimura S, Vartolomei L, Shariat SFS (2020) Systematic review of the impact of testosterone replacement therapy on depression in patients with lateonset testosterone deficiency, Eur Urol Focus. 6: 170-177.
- Kataoka T, Hotta Y, Kimura K (2021) A Review of foods and food supplements increasing testosterone levels, J Mens Health 7: 4-14.
- Morgentaler A, Miner MM, Caliber M, Guay AT, Khera M (2015) et al. Testosterone therapy and cardiovascular risk: advances and controversies, Mayo Clin Proc. 90: 224-251.
- Aykan DA, Aykan AÇ (2021) The interaction of drugs to treat cardiovascular diseases and testosterone therapy, their effects and characteristics, J Mens Health 25-31.
- Carrero JJ, Qureshi AR, Nakashima A, Arver S, Parini P (2011) et al. Prevalence and clinical implications of testosterone deficiency in men with endstage renal disease, Nephrol. Dial. Transplant. 26: 184-190.
- Barton CH, Mirahmadi MK, Vaziri ND (1982) Effects of long-term testosterone administration on pituitary-testicular axis in end-stage renal failure, Nephron 31: 61-64.
- Canguven O, Aykose G, Albayrak S, Goktas C, Horuz R (2010) et al. Efficacy of testosterone gel in the treatment of erectile dysfunction in hypogonadal hemodialysis patients: a pilot study, Int. J Impot Res. 22: 140-145.
- Andrew Peel A, Martin S, Vincent A, Turnbull D, Wang X (2020) Testosterone and self-perceived masculinity in an Australian cohort of community-dwelling men, J. Mens Health 16: 28-44
- Shigehara K, Kato Y, Kawaguchi S, Ijima M, Izumi K (2020) Shortterm effects of testosterone replacement therapy on risk predictors for arteriosclerosis among men with late-onset hypogonadism: a casecontrol study, J Mens Health 16: 65-71.
- Park M, Kim S, Won Y (2020) Relationship between testosterone deficiency and the cardiovascular risk factors, diabetes, and hypertension, J. Mens Health 16: 97-106.
- Chatterjee R, Wood S, McGarrigle HH, Lees WR, Ralph DJ (2004) et al. A novel therapy with testosterone and sildenafil for erectile dysfunction in patients on renal dialysis or after renal transplantation, J Fam Plann Reprod. Health Care 30: 88-90.
- Duygun Altıntas¸ Aykan, Ahmet Ça˘grı Aykan (2021)The interaction of drugs to treat cardiovascular diseases and testosterone therapy, their effects and characteristics, J. Mens Health 17: 25-31.
- Min Hyuk Park, Seong Eon Kim, Youngin Won (2020) Relationship between testosterone deficiency and the cardiovascular risk factors, diabetes, and hypertension, J. Mens Health 16: 97-106.
- Min Gu Park, Dae Yeon Cho, Yong Sung, Cho Jeong, Kyun Yeo (2020) Effect OF a combination OF OMEGA-3 and oral testosterone undecanoate ON serum testosterone levels IN patients with testosterone deficiency, J. Mens Health 16: 43-51.
- Du Geon Moon, Hyun Jun Park (2020) Prostate and testosterone - the most important keywords in men’s health and healthy aging, J. Mens Health 16: 1-3.
- Cattran DC, Reich HN, Beanlands HJ, Miller JA, Scholey JW (2008) et al. The impact of sex in primary glomerulonephritis, Nephrol. Dial. Transplant. 23: 2247-2253.
- Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJL (2013) chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention, Lancet 382: 339-352.
- Kelly DM, Jones TH (2013) Testosterone: a metabolic hormone in health and disease, J. Endocrinol. 217: R25-R45.
- Kelly DM, Jones TH (2013) Testosterone: a vascular hormone in health and disease, J. Endocrinol. 217 : R47-R71.
- Traish AM, Haider A, Haider KS,Doros G, Saad F (2017) Long-term testosterone therapy improves cardiometabolic function and reduces risk of cardiovascular disease in men with hypogonadism: a real-life observational registry study setting comparing treated and untreated (control) groups, J. Cardiovasc. Pharmacol. Therapeut. 22: 414-433.
- Halilo˘glu AH, G¨okçe I, ¨Ozcan C , Baltacı S, Yaman O (2013) Serum testosterone levels, testis volume, and the risk of prostate cancer: are these factors related? Turk J Urol 39: 12-5.
- Bao Y, Johansen KL (2014) Diagnosis and treatment of low testosterone among patients with end-stage renal disease, Semin. Dial 28: 259-265.
- Peco-Anti´c A, Spasojevi´c B (2014) Growth retardation in children with chronic renal disease, Srp. Arh. Celok. Lek 142: 614-620.
- Hruska KA, Mathew S (2011) The roles of the skeleton and phosphorus in the CKD mineral bone disorder, Adv. Chron. Kidney Dis 18: 98-104.
- Thirumavalavan N, Wilken NA,Ramasamy R (2015) Hypogonadism and renal failure: an update, Indian J Urol 31: 89-93.
- Snyder G,Shoskes , DA (2016) Hypogonadism and testosterone replacement therapy in end-stage renal disease (ESRD) and transplant patients, Transl. Androl. Urol 5: 885-889.
- Tomaszewski M, Charchar FJ, Maric C, Kuzniewicz R, Gola M (2009) et al. Inverse associations between androgens and renal function: the Young Men Cardiovascular Association (YMCA) study, Am. J. Hypertens. 22: 100-105.
- Kurita N, Horie S, Yamazaki S, Otani K, Sekiguchi M et al (2016). Low testosterone levels and reduced kidney function in Japanese adult men: the locomotive syndrome and health outcome in aizu cohort study, J. Am. Med. Dir. Assoc. 17: 371 e1-6.
- Fukami K, Yamagishi S, Sakai K, Kaida Y, Minami A (2014) et al. Carnitine deficiency is associated with late-onset hypogonadism and depression in uremic men with hemodialysis, Aging Male 17: 238-242.
- Goel A, Oni O, Wiegmann P. Testosterone replacement therapy (TRT) delays progression of CKD and ESRD. National Kidney Foundation’s 2017;Spring Clinical Meetings; Orlando, Florida - Poster 239.
- R. Sharma, O. Oni, P. Wiegmann, M. Sharma, M.G. Touza, A. Goel, et al.,Testosterone replacement therapy (TRT) is associated with delayed progression of chronic kidney disease: a retrospective analysis of testosterone normalization in US Veterans, Annals of Nephrology (2020) 51-59.
- Nam JK, Lee KS, Kim TN (2020) Use of testosterone replacement therapy after radical prostatectomy might kill two birds with one stone from the perspective of men’s health and disease control, J. Mens Health 16: e52-e56.
- Molinari C, Battaglia A, Grossini E, Mary DASG, Vassanelli C (2002) et al. The effect of testosterone on regional blood flow in prepubertal anaesthetized pigs, J Physiol 543: 365-372.
- Malkin CJ, Pugh PJ, Jones RD, Kapoor D, Channer KS (2004) et al., The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men, J. Clin. Endocrinol. Metab 89: 3313-3318.
- Shores MM, Smith NL, Forsberg CW, Anawalt BD, Matsumoto AM (2012) Testosterone treatment and mortality in men with low testosterone levels, J. Clin. Endocrinol. Metab 97: 2050-2058.
- Muraleedharan V, Marsh H, Kapoor D,Channer KS, Jones TH (2013) Testosterone deficiency is associated with increased risk of mortality and testosterone replacement improves survival in men with type 2 diabetes, Eur. J. Endocrinol 169: 725-733.
- Hyde Z, Norman PE, Flicker L, Hankey GJ,Almeida OP (2012) et al. Low free testosterone predicts mortality from cardiovascular disease but not other causes: the Health in Men Study, J. Clin. Endocrinol. Metab 97: 179-189.
- Yilmaz MI, Sonmez A, Qureshi AR, Saglam M, Stenvinkel P (2011) et al. Endogenous testosterone, endothelial dysfunction, and cardiovascular events in men with nondialysis chronic kidney disease, Clin. J. Am. Soc. Nephrol 6: 1617-1625.
- Khurana KK, Navaneethan SD, Arrigain S, Jesse DS, Joseph VNJr (2014) et al. Serum testosterone levels and mortality in men with CKD stages 3-4, Am. J. Kidney Dis 64: 367-374.
- Agha R, Abdall-Razak A, Crossley E, Dowlut N, Iosifidis C (2019) et al. for the STROCSS Group, The STROCSS 2019 guideline: strengthening the reporting of cohort studies in surgery, Int. J. Surg 72: 156-165.
- Yassin A, Abdallah H, Kamkoum H, Alzubaidi RT, Albudairat A (2025) Intramuscular Testosterone and the Gel in the Current Treatment Era. Biomed J Sci & Tech Res 61: 53877-53882.
- Yassin A, Kamkoum H, Alzubaidi RT (2025) Is There a Need for Testosterone Therapy in Older Men? J Advances Med Sci 2: 1-3.
- Yassin A, Alzubaidi RT, Kamkoum H, Alzubaidi RT, Ramadan A (2025) Recent Update on Advancements in Testosterone Therapy (TTh). J Urol Ren Dis 10: 1413.
- Yassin A, Alzubaidi RT, Kamkoum H, Akkad MSE, Mahdi (2025) Effect of Testosterone Therapy (TTh) on Liver Function and Steatosis. Gastroint Hepatol Dig Dis 8: 1-8.
- Yassin A, Kamkoum H, Alzubaidi RT, Ansari AA (2025) Testosterone Prevention Role in Men’s Health: Diabetes Mellitus. J Diabetes Treat 10: 10141.
- Yassin A, Albaba B, Kamkoum H, Alzubaidi RT, Al-Qudimat AR (2025) Testosterone Prevention Role in Men’s Health: Cardiovascular Dieseases. Cardiol Res Cardio vasc Med 10: 280.
- Yassin A, Kamkoum H, Alzubaidi RT, Abdallah H, Assad O (2025) How Long Should We Treat with Testosterone: Stopping Testosterone Therapy (TTh) Wat is Next? Effects of Withdrawal and resumption of TTh. Ann Rev Resear 12: 555843.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.