Ten Years Follow-Up Using TMJ Prosthesis in Peek Lt1 20%Ba, Retrospective Biomechanical and Clinical Study
by Wladimir Genovesi1*, Iara Comenale2, Moises Veloso Fernandes2, Wladimir Genovesi Filho2
1Department Chief, TMJ Pain Clinic, Hospital 9 de Julho, São Paulo, Brazil
2Assistant, TMJ Pain Clinic, Hospital 9 de Julho, São Paulo, Brazil
*Corresponding author: Wladimir Genovesi, Department Chief, TMJ Pain Clinic, Hospital 9 de Julho, São Paulo, Brazil
Received Date: 30 November, 2023
Accepted Date: 04 December, 2023
Published Date: 06 December, 2023
Citation: Genovesi W, Comenale I, Fernandes MV, Filho WG (2023) Ten Years Follow-Up Using TMJ Prosthesis in Peek Lt1 20%Ba, Retrospective Biomechanical and Clinical Study. J Surg 8: 1945 https://doi.org/10.29011/2575-9760.001945
Abstract
The Temporomandibular Joint (TMJ) is a complex joint, with distinct anatomical and functional characteristics, making it difficult to treat. Many authors, beginning in the early 20th century, reported techniques for TMJ reconstruction, aiming at recovering its shape and ideal function. After conducting studies on CT scans of 50 TMJs, A system for customized TMJ prostheses (temporomandibular joint) was developed in PEEK Lt1 20%Ba with 2/3 of the ramus length. The glenoid cavity was shaped into a flattened plane, with removal of the articular eminence of the temporal bone, at an angle of 90° with the base of the skull. The experimental tests were carried out in dry medium and at room temperature in an electromechanical machine of the Model E1000 of the brand INSTRON®. The analysis of the roughness parameters was evaluated in tracks. The laboratory results were satisfactory. The analysis of the histological synovial tissue in specimen were observed new vessels formation and synovial metaplasia like around prosthesis tissue. Based on 10 years of follow-up (2013 to 2023), with 9 patients and 17 surgeries performed, TMJ reconstruction using full custom TMJ prosthesis using PEEK Lt1 20% Ba, a new surgical technique was performed it is less traumatic than those used by other surgeons worldwide. The design of the prosthesis developed in customized PEEK Lt1 20%Ba presents an anatomical profile, where the center of gravity is preserved which can provide an ideal prosthesis, returning to the aspect physiological mandibular movements, providing less wear on the articular surface of the prosthesis.
Keywords: Customized prosthesis; PEEK Lt1 20%Ba; Reconstruction; TMJ
Introduction
Like other synovial joints, the TMJ is vulnerable to injuries and pathologies inherent in synovial joints. Despite advances the TMJ is a complex joint, making it difficult to reestablish the ideals of form and function, generating controversy regarding the most appropriate technique to carry out this task. With the technological advancement of materials, the development of prototypes that biomechanically resemble normal articulation, a new prosthesis for TMJs was developed. Since the early days, surgeons have tried to recover the loss of function in patients who have diseases or intra-articular injuries in the TMJ, limiting the physiological movements of the jaw affecting its function, not allowing adequate masticatory movement. In 1934, Risdon used laminated sheets of gold in the articular fossa in an attempt to prevent relapse in patients with ankylosis in the joint region of the skull base (labrum). Previously, Eggers [1] used sheets of tantalum on the condylar surface. From these early attempts to prevent or avoid relapse in patients with ankylosis and allow proper physiological function, researchers have studied new materials and surgical techniques to restore jaw function, providing these patients a healthier life. Developing a prosthesis for TMJ has benefited patients with bony ankylosis, fibrous ankylosis, degenerative arthritis, psoriatic arthritis, Sjögren’s syndrome, patients undergoing multiple TMJ surgeries, sequelae of trauma and tumors in the region and other conditions that may affect synovial joints. Mercuri [2,3] reports of the development of a number of prostheses aimed at reconstructing and replacing temporomandibular joints with different materials and patterning, (Christensen TMJ prosthesis system, Endotec Hoffmann Pappas TMJ prosthesis system, Lorenz prosthesis and TMJ Concept/Stryker) [2].
The total or partial reconstruction of the TMJ has its issues; some studies (Leibenger research) show thati t is not possible to use a condylar prosthesis without lining the skull base (Glenoid fossa prosthesis), for a period no longer than 20 months, since it may imply a process of reabsorption of the skull base with or without fistula formation, metallosis and new bone formation (ectopic bone). Many materials have been used to create an ideal prosthesis, but most have had a short shelf life or characteristics that have prevented its use [4]. PEEK is a polymer (Polyetheretherketone), derived from petroleum, developed by Invibio/ UK, subdivision from Victrex Co. Marketed in the 1980s, it is a thermoplastic polymer, interconnected to a ketone and other functional groups attached to an aromatic molecular structure, and is semicrystalline [5]. It was previously used for orthopedic and spinal implants. Its molecular structure confers resistance to high temperatures, providing stability, with an inert compound. It is compatible with fillers such as glass and carbon fibers that have greater strength than many metals. In the 1990s, many researchers evaluated the biocompatibility and in vivo stability of PEEK, classifying it as a high-performance material. Early use of synthetic polymers as a framework for developing biomaterials and producing polymethyl methacrylate bone cement was used in 1943; these polymers continue to successfully compete with the metal and ceramic applications for craniomaxillofacial and orthopedic implants [611].
In recent decades, biomedical engineering has emerged as a single discipline, crossing the traditional boundaries between physical and biological sciences. Engineering has expanded the clinical application of polymers as biomaterials, with great benefit and reaching a position of undeniable relevance [11-16]. Since the 1980s, the polyaryletherketone (PEEK) have been increasingly used as a biomaterial of choice for trauma, orthopedic, and spinal implants. And there is an extensive literature from Kurtz [9] on polymer science, with respect to the structure, mechanical properties and chemical resistance of PEEK as a synthesized biomaterial. Based on this research, it can be understood why this polymer family is inherently strong, inert and biocompatible. Due to its relative inertia, PEEK Lt1 20% Ba is already commonly used in developing new bioactive materials. PEEK Lt1 20% Ba has had the greatest clinical impact in the design field of spinal implants (cage, knee prosthesis), being widely accepted due to its radiopacity. It has been used as the material of choice for replacing synovial joints, such as knee prostheses and recently for TMJ (Genovesi.W). Our studies began in 2006, and recently, after extensive laboratory tests (Labmat and CENIC laboratories, Brasil) a TMJ prosthesis system in PEEK Lt1 20% Ba was developed [17]. Numerous studies document the clinical success in developing new products with PEEK Lt1 20% Ba and or CFR PEEK polymers in orthopedic and craniomaxillofacial surgery. A recent survey conducted by the School of Biomedical Engineering, Science and Health Systems, Drexel University, Philadelphia, PA;[11] studies in bio tribology have also investigated PEEK composites of high strength material for friction, impact, and biocompatibility, as well as for use in arthroplasty implants. Due to the interest in further improving implant fixation, biomaterials in PEEK research[9-10] also focused on the compatibility of the polymer with bioactive materials, including hydroxyapatite (HA) as the composite filler or as a surface coating material. As a result of research into biomaterials, PEEK and related compounds can now be manipulated with a wide range of physical, mechanical, and surface properties depending on the application of the implant. PEEK Lt1 20%Ba, in turn, is a light, sturdy and completely biocompatible, even when using Peek on PEEK in a TMJ or knee prosthesis. If a little fragmentation occurs, it will not cause a bodily reaction, as a function of its biocompatibility. Regarding its micro-particles, a histological study shows microparticles being phagocytized, and new vessel formation around them (not giant cell formation). This new material can be indicated for a new products development, it can be a new alternative for medical devices (Figure 1, Figure 2). Furthermore, PEEK particles were incorporated by macrophages, but no giant-cell reactions could be seen. This applies to all sizes of the PEEK Lt1 20%Ba particles.

Figure 1: Glenoid component.

Figure 2: Mandibular component.
Materials and Methods
After conducting studies on CT scans of 50 TMJs, which measured the size of the mandibular ramus, from the Sigmoid notch to the angle of the jaw, was determined the average length to be 470 mm (Figure 3a). In the glenoid cavity, an average of 0.8 mm concave radius and length of lateral / medial (19 mm), was obtained. (Figure 3b, Figure 3c). A system for customized TMJ prostheses (temporomandibular joint) was developed in PEEK Lt1 20%Ba with 2/3 of the ramus length, understanding that, it is not necessary to cover all the ramus surface for good fixation and preserve all mandible movement. This prosthesis system has been developed by the author since 2012, with PEEK Lt1 20%Ba material, making it possible now to market the product. The Laros Corporation sponsored all projects of developing PEEK LtI 20%Ba, in a customized prosthesis system, by injection molding. In the near future, it will be marketed in three sizes – (small, medium, large). Unlike the existing models on the market, this prosthesis system presents an anatomical / functional design, as shown in the Figures 4a, Figure 4b.
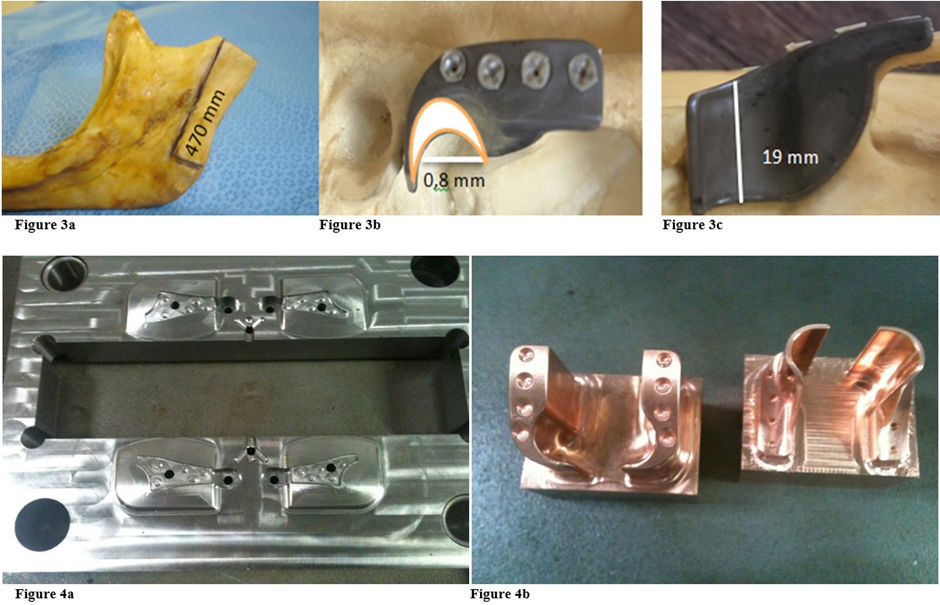
Prototype Prepared for Laboratory Tests
The osteotomy was done at the neck of the condyle, with 15mm under condyle articular surface, maintain horizontal plane at the sigmoid norch, physically it´s not necessary cover all the ramus, 2/3 of the length is enough to fixed it. The ramus component is situated at a 90º angle with the condyle, which must be adapted on the mandibular osteotomy preserving the gravity center with the base of skull. The glenoid cavity was shaped into a flattened plane, with removal of the articular eminence of the temporal bone, at an angle of 90° with the base of the skull, avoiding recurrent subluxation. Thereby, allowing a free (syncopated) joint, avoiding the possibility of dislocation of the prosthesis. (Figure 5a, Figure 5b, Figure 5c, Figure 5d, Figure 6). The tests were carried out in dry medium and at room temperature in an electromechanical machine of the Model E1000 of the brand INSTRON®. The total PEEK Lt1 20%Ba Prosthesis system mounted in a model Poliurethene Skull/ Mandibular, with density 30D (similar human bone density). It was submitted to cyclic loading with a frequency of 3HZ, with a ratio between loading (R=0.1). The number of cycles was recorded until the failure of the test body or until the limit of cycles for interruption of the assay has been achieved.
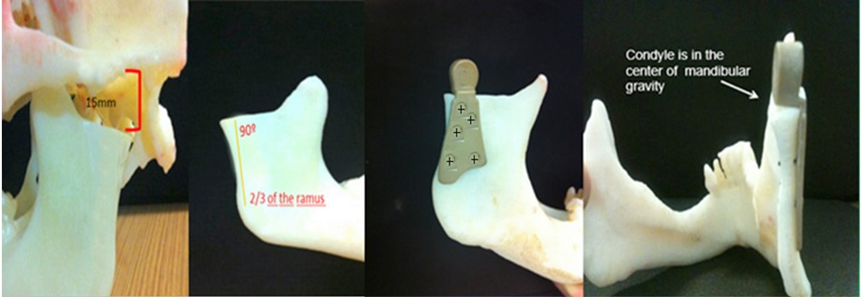
Figure 5: A: Mandibular osteotomy with15mm from the glenoid fossa. B: Horizontal plane at the sigmoid norch and 2/3 of the length cover of the ramus. C: The Ramus component is situated at a 90º angle with the condyle. D: The condile is in the center of mandibular gravity.
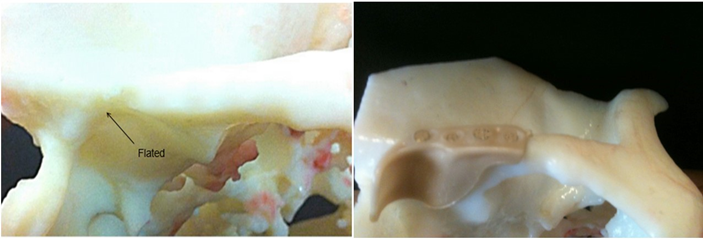
Figure 6: Eminectomy to maintain flatted with 90° with base skull.
After the fatigue test, the system was subjected to a static compression (Figure 7). The PEEK Lt1 20%Ba screws diameters 2.0mm for (Glenoid fossa component) and 2.4mm for (Mandibular component) were submitted a test of Twisting (torsion) insertion/pullout (Figure 8). For analysis of the roughness parameters was using a measuring machine, the glenoid component was evaluated in six (6) tracks, as identified Figure 9 and the mandibular component was evaluated in fourteen (14) tracks as identified in Figure 10. The reported expanded measurement uncertainty U is stated as the standard measurement uncertainty multiplied by the coverage factor k, which for a T distribution with Veff effective degrees of freedom. It was evaluated under Calibration Laboratory conditions according to ABNT NBR ISO/IEC 17025.
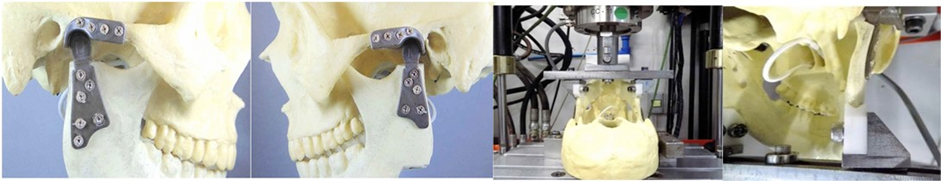
Figure 7: Laboratory experiment with cranio-mandibular model in polyacrylic resin of TMJ Prothesis in PEEK Lt1 20%Ba.

Figure 8
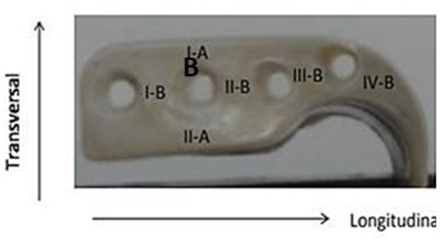
Figure 9
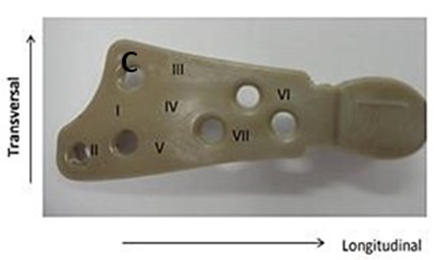
Figure 10
Laboratory results
According to Table 1, was observed that the components of both the joint cavity and the mandibular branch of prosthesis in PEEK Lt1 20%Ba did not present any failure during and after the compression in dynamic and static tests on its surface, of the sample and experimental model. In relation to the resistance curve and maximum strength of PT in PEEK Lt1 20%Ba, was verified according to Figure that the material presents a high degree of resilience when subjected to compression and fatigue loads when compared to other prosthetic materials, reaching in Newtons the maximum resistance of 800N. (Figure 11). According to Figure 12 the TMJ system with 3.000.000 cycles with 350N in dynamic test, compression fatigue did not show any failure. Obs: The brown color of the system in these pictures means that occurred over heating during the injecting molding, but according Invibio Co, UK, it doesn´t alter the PEEK composition, it means that the product it’s in good conditions. Table 2 Shows test results of compression fatigue. The PEEK Lt1 20%Ba screws diameters 2.0mm were submitted a test the according to Figure the curves obtained in the torsion screws test with torque (Nm) axle “x” and Angular Displacement (o) axle “y”, it`s fracture around 351 Newtons (N). (Figure 13a, Figure 13b).
|
STUDY VARIABLES - PARAMETERS |
RESULTS |
||||||||||
|
Mode |
Force |
Freq |
Rason |
Run out |
Force ref |
Speed |
F Max |
F Min |
Cycles |
Contact surface Screws- Twist, insertion/ Pull out |
Contact surface Components Skull/Mandi bular |
|
Static cyclos- compression Fadigue Compression |
300 |
30Hz |
0,1 |
3 x106 |
- |
- |
351,4N |
- |
- |
No fault |
No fault |
|
- |
- |
- |
- |
350 |
5mm |
300 |
30 |
3 x106 |
No fault |
No fault |
|
Table 1: Representation of the study variables and the results of the experimental model of TMJ Prothesis in PEEK submitted to compression and fatigue cycles by compression in the laboratory.
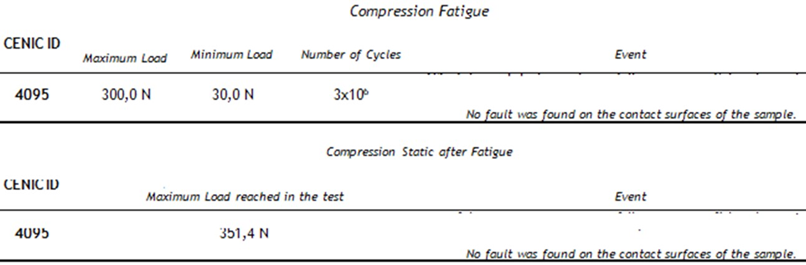
Table 2: Shows test results of compression fatigue.
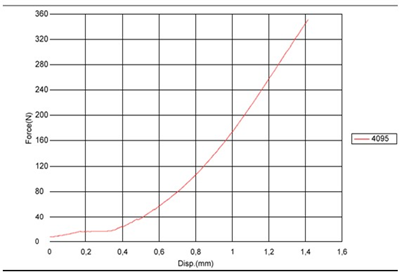
Figure 11: Graph representing the curve of the experimental laboratory test of maximum fatigue and compression of the TMJ PT model components in PEEK Lt1 20%Ba, Maximum force curves (N) x Number of cycles
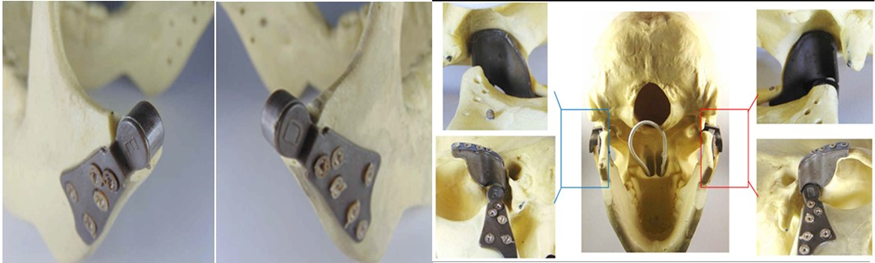
Figure 12
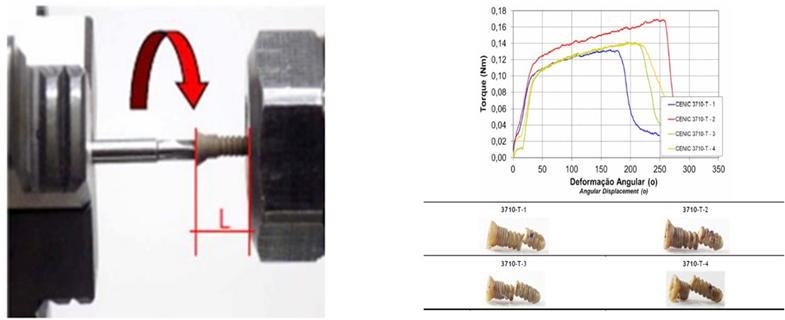
Figure 13: A: Test setup. B: Curves obtained in the torsion test and samples of static test. The mode of failure was rupture for all samples.
Table 3 Results of torsion. The Figure 14 shows the curves obtained of pullout test. Table 4 Results of pullout.
The Figure 15a, Figure 15b shows another test insertion and removal. Table 5 Results of driving torque test. The PEEK Lt1 20%Ba screws diameters 2.4mm for (Mandibular component) were submitted a test of pullout. (Figure 16a, Figure 16b). Table 6 Results of pullout. The Figure 17 shows the test setup used in driving torque test and Figure 18 shows the curves obtained. Table 7 Results of driving torque test. Table 8 Results of torsion.
The results of the roughness parameters were contained in Tables 9, 9.1, 9.2, 9.3. Table 9. Parameters of roughness Ra, Rz e Rmax. (Longitudinal)- Glenoid Component. Table 9.1. Parameters of roughness Ra, Rz e Rmax. (Transversal)-Glenoid Component. Table 9.2. Parameters of roughness Ra, Rz e Rmax. (Longitudinal)-MandibularComponent. Table 9.3. Parameters of roughness Ra, Rz e Rmax. (Transversal) – Mandibular Component.
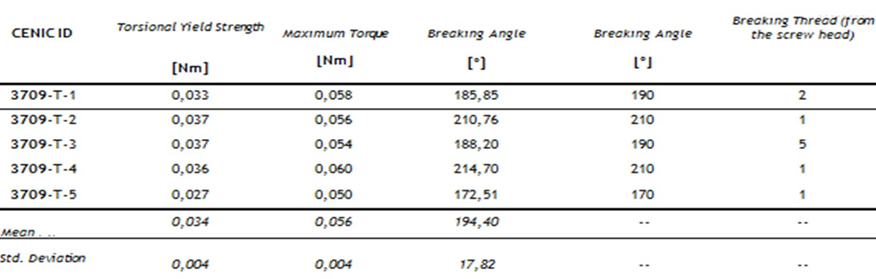
Table 3: Results of torsion.
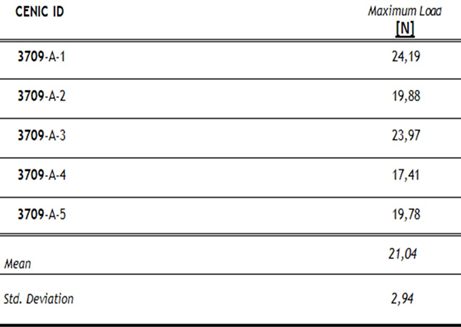
Table 4: Results of pullout.
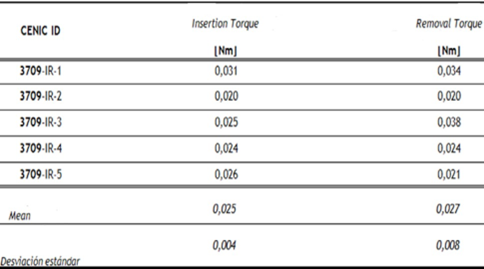
Table 5: Results of driving torque test.
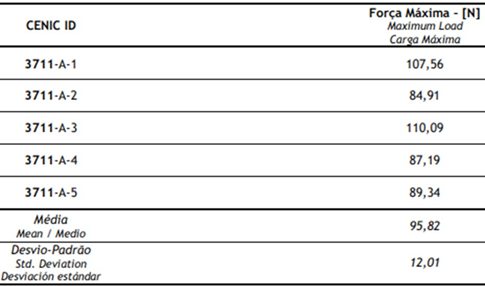
Table 6: Results of pullout.
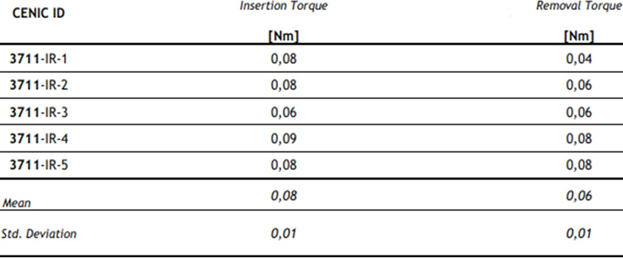
Table 7: Results of driving torque test.
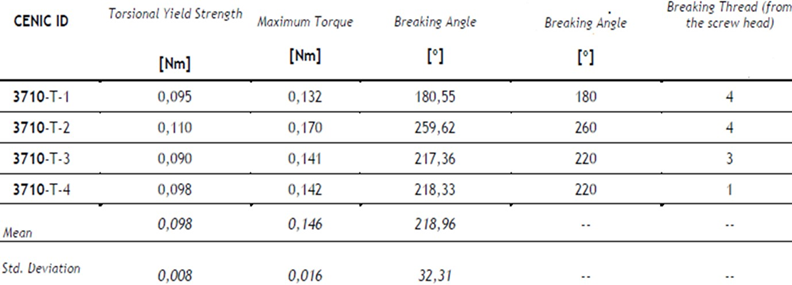
Table 8: Results of torsion.
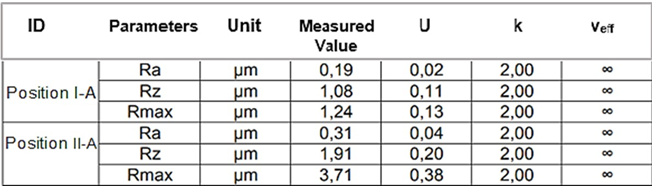
Table 9: Parameters of roughness Ra, Rz e Rmax. (Longitudinal)- Glenoid Component.
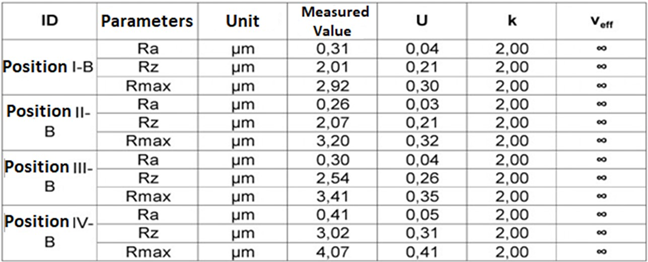
Table 9.1: Parameters of roughness Ra, Rz e Rmax.(Transversal)-Glenoid Component.
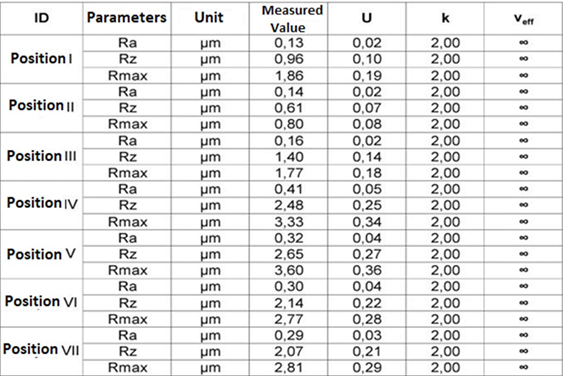
Table 9.2: Parameters of roughness Ra, Rz e Rmax. (Longitudinal)-Mandibular Component.
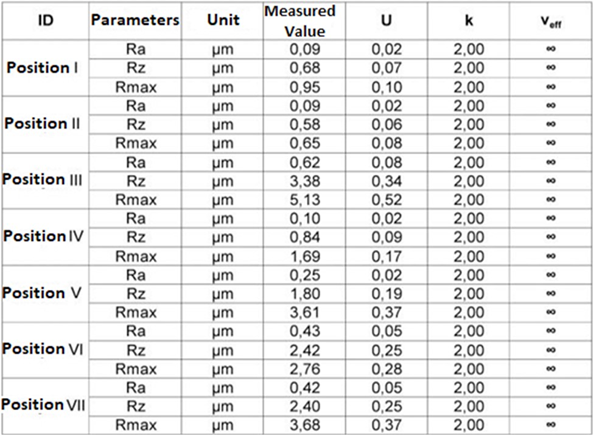
Table 9.3: Parameters of roughness Ra, Rz e Rmax. (Transversal) - Mandibular Component.
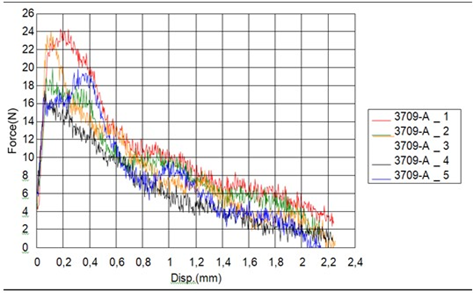
Figure 14: Curves obtained in the pullout test.
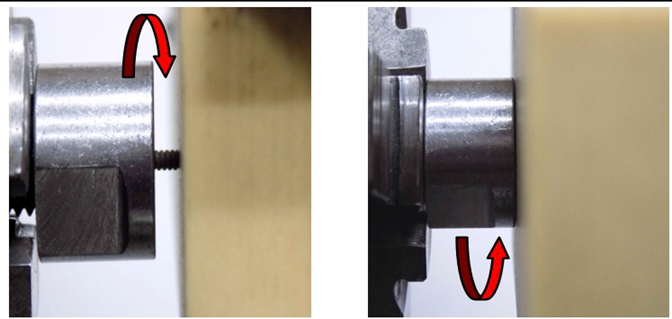
Figure 15

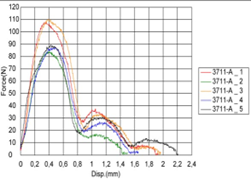
Figure 16: A: Test setup. B: Curves obtained in the pullout test.
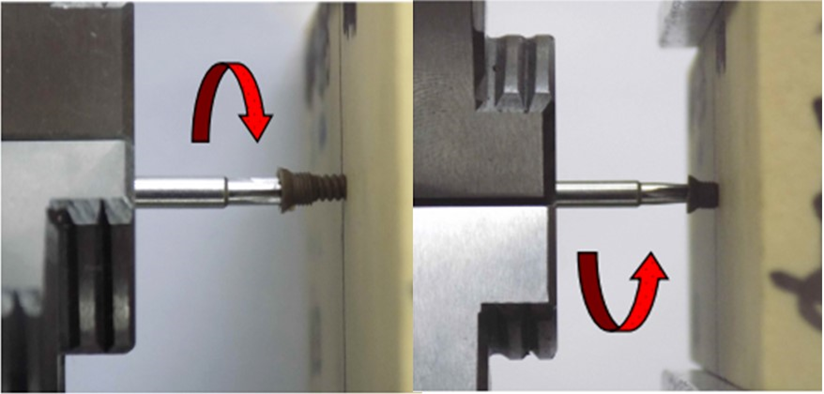
Figure 17: Test setup.
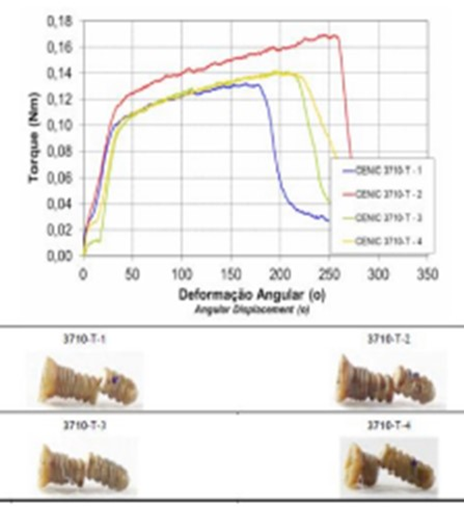
Figure 18: Curves obtained in the torsion.
Surgery Technique
An endaural incision can be made, or an extended preauricular incision, performing soft tissue dissection to reach the joint itself. The osteotomy was done at the neck of the condyle, with 15mm under condyle articular surface or an imaginary line of the ankylosing mass. (at the Sigmoid notch line). The osteotomy must maintain a 90° angle with the mandibular branch, for a perfect adaptation of the condyle on the ramus osteotomy. Articular eminence osteotomy of the temporal bone was performed from the lateral till the medial, maintain 90° with base skull, (flatted) and wearing with a diamond drill, eliminating the remaining bone, spicules, and the planning surfaces of the glenoid cavity at the mandibular branch. The bilaminar zone was removed with electrocauterization, preventing compression of the posterior region, with the glenoid cavity design protecting the anterior wall of the ear. With the patient in normal occlusion, the screws for the intermaxillary block were placed. Installing the glenoid cavity, adaptation was investigated, and it was fixed with four screws in the zygomatic arch. By tunneling processes, we proceeded to detach the masseter muscle, preserving its insertion, as well as the maintenance of the zygomatic process and the insertion of the temporal muscle. Knowing that the insertions of the superficial masseter and temporalis muscles fibers contribute to lateral movements, they were preserved. (Figure 19). Installing the prosthesis by tunneling, while maintaining their long axis parallel to the long axis of the ramus, to adapt the inferior condyle surface at the mandible osteotomy. With proper adaptation, it was fixed with 5 screws. Begin by fixing screws in the cranial direction (third upper branch) to have proper orientation of the long axis of the mandibular branch.
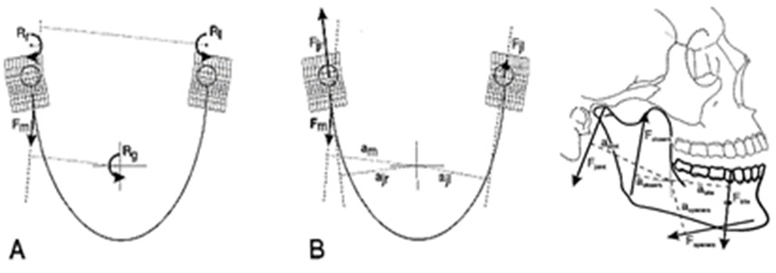
Figure 19: Forces acting on the lower jaw in the sagittal plane. Cross-hairs: center of gravity. F-closers: mean force of the jaw-closing muscles. F-openers: mean force of jaw-opening muscles. F-joint: joint force. F-bite: bite force. a: moment arm of the different forces.
After, the approach angle of the mandible an approximately 1.5cm incision was made, releasing longitudinally through the fibers of the masseter muscle, to view the lower third of the prosthesis and fix it with the screw of the lower third, thereby maintaining alignment parallel to the posterior border of the mandibular branch on its distal portion. Proceed to fix the other screws. It was important to note that a joint space of 2.0 mm/3mm was maintained to allow free jaw movement. This new prosthesis design was based on the physical principles, in which two-thirds is enough to maintain the lever to support all mandible movements. So, the prosthesis designd id not need to cover all the mandibular ramus (Figures 20,21). Based upon 10 years of experience (02/2013) with this model of customized prosthesis, made from PEEK Lt1 20%Ba, 17 surgeries were performed in 11 patients; 7 patients had bilateral procedures and 4 had unilateral surgery. Mandibular movements were preserved in all patients. Genovesi [17]. All 11 patients had some articular pathologies of various causes:
Figure 20: The shape of the condile and the glenoid fossa flatted, allow the condile free movement.
Figure 21: Initial open - 10mm.
. Pathologies = Degenerative arthritis.
. Multiple TMJ surgeries = Fibrosis ankylosis.
. Condylar resorption after orthognathic surgery.
Considering the same model of the prosthesis made in the PEEK Lt1 20%Ba, material that meets all the ideal biomechanical requirements for the human body, combined with the previous experience of our research.
Case Report
A female patient, 35 years old, was referred to our service by neurology, with painful symptoms in the bilateral pre-auricular region, with an opening limitation, of approximately 10mm. A 3D CT scan of the temporomandibular joint was requested, where bilateral osseous fibrosing ankylosis was observed. Proposed joint reconstruction surgery with a customized PEEK Lt1 20%Ba, the patient was informed that would be participating in a pilot study of a new prosthesis model. The patient agreed and with formalized and signed consent, the surgery was performed. This study was approved by 9 de Julho hospital Scientific Committee and ANVISA. (Figure 21, Figure 22, Figure 23, Figure 24a, Figure 24b, Figure 24c, Figure 24d, Figure 25). The patient from our study, after 4 years had a fracture at the neck of the condyle surface, it was proposed to remove and change the prosthesis (Ramus Component), the patient agreed with it. The surgery was performed, and this review surgery allow us observe and remove the tissue over the prosthesis and make histological study. According to Histological study in the synovial tissue specimen, was observed new vessels formation and synovial metaplasia like around prosthesis. tissue. (Figure 26).
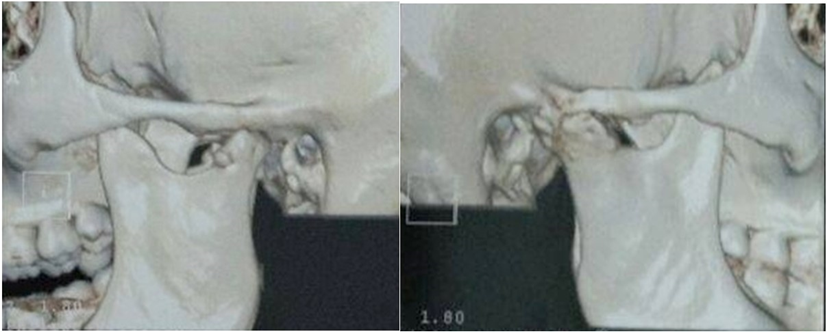
Figure 22: Ankylosis L and R (3D CT scan).
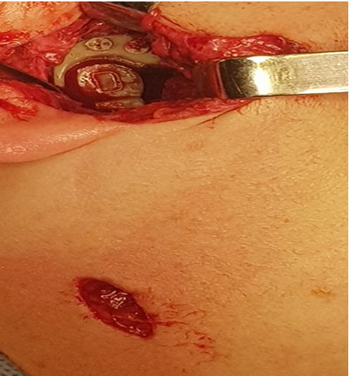
Figure 23: Surgery picture. See surgery technique endaural incision and a small incision retromandibular.
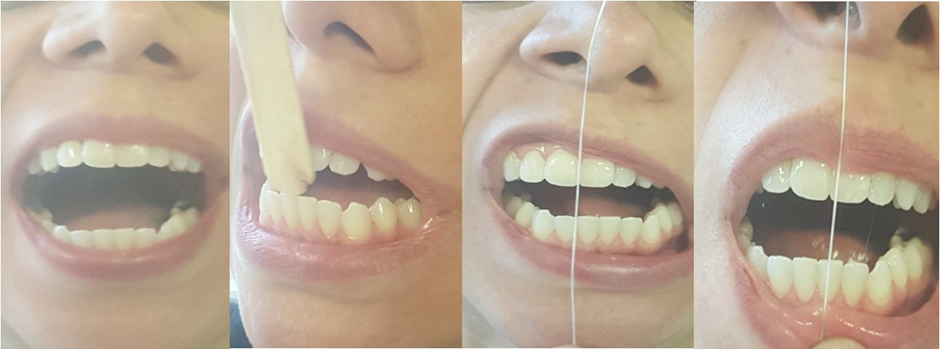
Figure 24: a: Final Open 35mm. b: Protrusion movement. C: Lateral Movement L. d: Lateral Movement R.
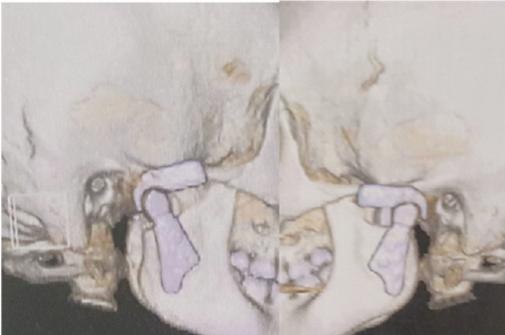
Figure 25: Final Prosthesis in occlusion L and R (3D CT scan).
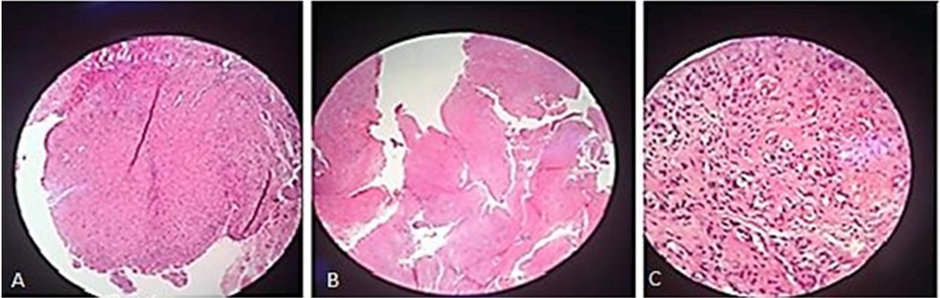
Figure 26: New vessels formation.
In Figure 27showing the toxicity of conventional prosthesis system. Pinto-Borges [18]. The most important difference between TiCrCo/UHMWPE and PEEK Lt1 20%Ba is that the particles released from UHMWPE are not phagosited and allow Gigants Cells formation, PEEK Lt1 20%Ba particles released are phagosited and eliminated by natural via. Avoiding heart, kidney and Brain toxicity. (Figure 28). For the analysis of the histological study was used the electronic microscope of the NIKON ECLIPSE model E200, with magnification of histological wool mines between 40 per 400x per image. The synovial tissue in specimen was removed from temporomandibular joint with PEEK Lt1 20%Ba prosthesis installed “in vivo” in a patient who had undergone total TMJ arthroplasty 4 years ago. The specimen was put on a blade for analysis with Hematoxylin and Eosin chemical dyes. (Figure 29a, Figure 29b, Figure 29c). It doesn’t happen with UHMWPE, which allows a giant cells formation around the particles released, causing a big problem to the patient after 5 years or less of the surgery.
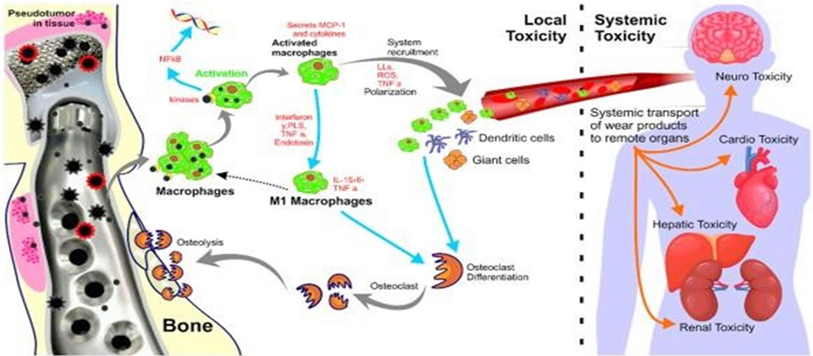
Figure 27: Toxicity of conventional prosthesis system.
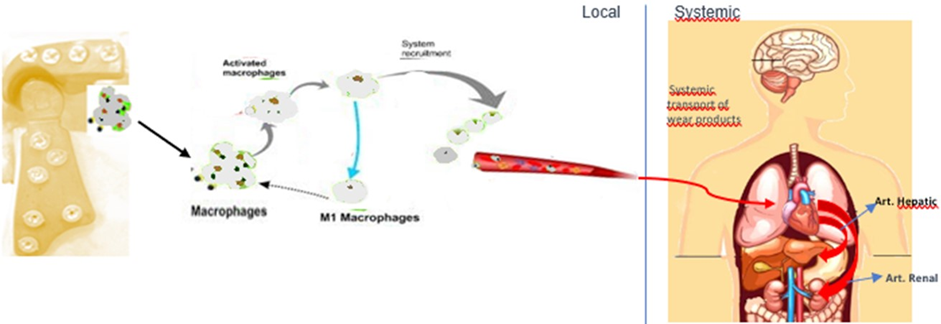
Figure 28: Study showing “No Toxicity” of prosthesis in PEEK system.
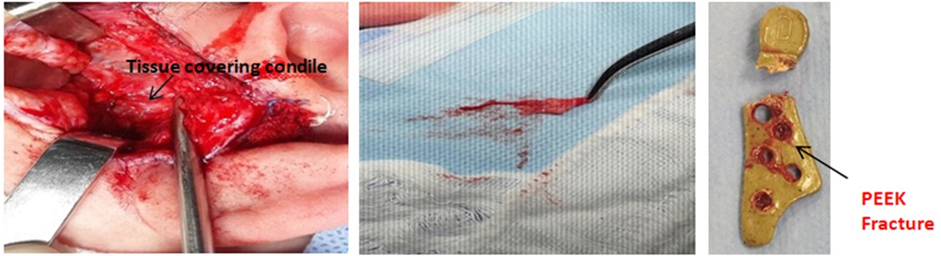
Figure 29: a: The synovial tissue; b: The synovial tissue. c: PEEK Fracture.
Discussion
The reconstruction of the TMJ using a prosthesis remains controversial. Surgical treatment with the use of autografts is an option. Van Loon [12] report that the history of attempts at TMJ reconstruction with alloplastic materials must achieve outcomes that follow biomechanical principles as close as possible. Van Loon et al [13],in their review of the literature on TMJ prosthesis, report that researchers have not yet developed a material that allows for a more effective use period, surpassing the 10 to 12 years of use, as well as designs of prostheses that allow physiological mandibular movements. According, L M Wolford [16] materials for making TMJ prostheses, must meet the following criteria: 1) Composed of biocompatible materials; 2) Functionally compatible materials; 3) Have low wear, flow, and fatigue coefficients; 4) Adaptable to anatomic structures; 5) Rigidly stabilized components; 6) Corrosion resistant and nontoxic; 7) Malleable to facilitate adaptability to the anatomic structures; 8) Have a posterior stop in the fossa component; and 9) Close tolerance of the screw and prosthesis hole diameter. Laboratory studies comparing the different properties of the prosthetic materials used in the most varied joint regions are widespread in the surgical clinic, however, analyzes within this context involving the temporomandibular joint, as well as its components and prosthetic materials are less widespread and scarce in terms of to the use and efficacy of PEEK Lt1 20%Ba. For Cowie [18,19] Janssen[20] in experimental conditions of knee/hip prostheses, the material has been considered an alternative in arthroplasty with high potential and low biological activity against wear particles, when compared to TiCrCo/UWMPE. The beneficial potential of PEEK Lt1 20%Ba was observed in the reduction of stress in the region where the prosthesis joints the bone tissue due to its low rigidity. Therefore, long-term preservation of the bone was noted, and the loads generated by the prostheses are similar to those that occur in intact joints. We corroborate with the studies by Janssen [20] and Cowie[19] where after 10 years of evolution of cases with PEEK Lt1 20%Ba prosthesis and conventional prostheses, it was noted bone resorption exerted in the mandibular ramus and at the root of the zygomatic arch with conventional prostheses, compared to PEEK Lt1 20%Ba where it was not observed any bone resorption.
According to Genovesi [17], “PEEK LT1 20% Ba material has all these properties; it is a promising material for new product development for orthopedic, craniomaxillofacial, oral & maxillofacial and other specialties.” Focusing on these material characteristics, companies that manufacture orthopedic and maxillofacial products, especially for TMJ, have aimed to do the same for a complex joint. The choice of formulation PEEK Lt1 20%Ba it is in function it is radiopacity. The particles released by PEEK Lt1 20%Ba will be phagosited by capillary neoformation of this formed film, histological studies from periprosthetic tissue show this. According to Ashley [21], Hammouche [22] concluded that in comparative studies PEEK Lt1 20%Ba produces particles between 0.93μm in Ø(diameter), which are similar compared to other materials, therefore it does not generate biological reactions and are phagosited. The particles produced in UHMWPE, which are more bioactive, therefore produced a greater biological reaction in 1 year after implantation. PEEKLtI20%Ba demonstrated preservation of joint spaces without the occurrence of foreign body reaction, it presented only scratches on its surface, not altering its shape. It was observed in the study the low toxicity due to the biocompatibility response of PEEK Lt1 20%Ba and its physical properties, compromising less in the long and short term the systemic conditions, as well as the reactive immunological responses in the patient [18].
Metallic particles and ions released from the materials degradation can induce inflammatory response and also the internalization through cell membranes with high risks to alter cellular metabolic functions. Inflammatory reactions induced by metallic debris can cause a series of toxic effects with mutagenic risks to the patients. The use of materials with high wear resistance and less release of toxic debris is one of the main goals of current research on TMJ prosthesis. Thus, TMJ prostheses are still the focus of development concerning pre-and post- surgical situations, taking into account technological advancements in orthopedic prostheses. In the study, it was verified that both components of the joint cavity and the mandibular prostheses in PEEK Lt1 20%Ba material, which presents a high degree of resilience when subjected to compression and fatigue, when compared to other prosthetic materials. For K. Rankin [23-26] PEEK Lt1 20%Ba showed excellent mechanical properties and a modulus of elasticity of 4Gpa, equal to that of bone tissue which is around 1 to 20Gpa. Therefore, the fixation of prostheses in PEEK Lt1 20%Ba is not liable to undergo shearing during joint movements. Mercuri4 reported in the literature that in the currently existing models, where bone lysis occurs around the titanium screws in function of the micro movements, and I state that by compression it has bone resorption in the mandible body and in the zygomatic arch. (Figure 30, Figure 31, Figure 32, Figure 33, Figure 34).
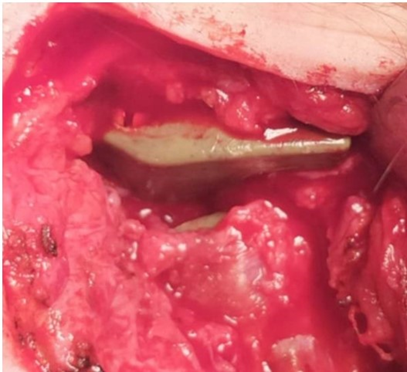
Figure 30: No tissue formation between glenoid fossa and condyle PEEK Lt1 20%Ba Prosthesis.
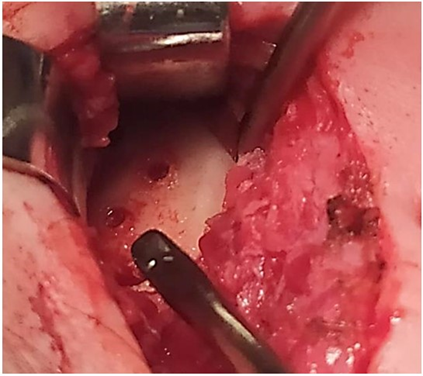
Figure 31: No bone formation and no bone PEEK Lt1 20%Ba Prosthesis (7 years postop).
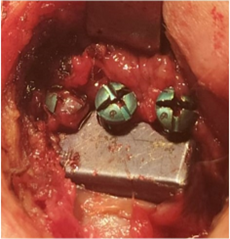
Figure 32: Loosing Screws Conventional Prosthesis TiCrCo/ UWMPE (4 years 8 months Postop).
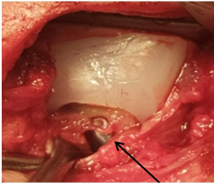
Figure 33: Hyaline tissue between the component of the glenoid cavity and the condyle of the prosthesis Conventional Prosthesis TiCrCo/UWMPE (4 years 8 months Postop).
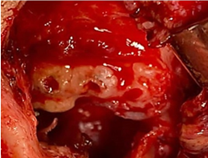
Figure 34: Zygomatic arch resorption Conventional Prosthesis TiCrCo/UWMPE (4 years 8 months Postop).
In addition, the design of the prosthesis developed in customized PEEK Lt1 20%Ba presents an anatomical profile, where the center of gravity is preserved (a characteristic recommended by Van Loon et al.), which can provide an ideal prosthesis, returning to the aspect physiological mandibular movements, providing less wear on the articular surface of the prosthesis. Pinto-Borges [18] reports that standard prostheses provide high loads and friction, and the sliding of the prosthesis support components causes wear of the materials and, consequently, the release of metallic debris into the surrounding tissues, causing metallosis in the patient. In his study Crosbie [24], compared PEEK Lt1 20%Ba with CrCo, and PEEK Lt1 20%Ba presented a compression model similar to the subchondral bone, in that the CrCo is superior, which causes greater bone compression, leading to its resorption. The great concern of current prostheses, both, customized or stock, has been with the material (TiCrCoMo), particularly when it allows aggregation of fibrocytes on its surface causing ectopic bone formation and bone resorption, by compression. (Figure 35, Figure 36). Additionally, there is concern with the weight of materials (average 15Gr) being too heavy as well as materials that do not allow natural physiological mandibular movement in function it´s (design), especially lateral movement, only hinge movement, as a function of the proposed technique.
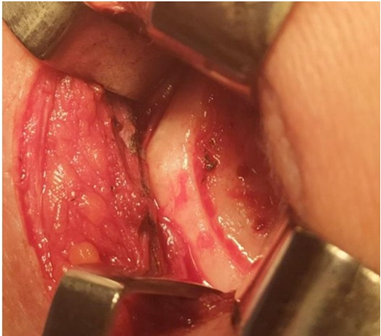
Figure 35: Conventional Prosthesis after 4 years 8 months. removed observe the bone absorption of the mandibular ramus.
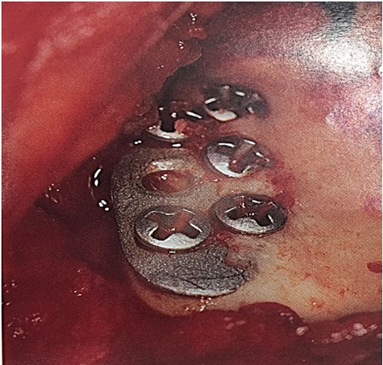
Figure 36: Ectopic bone formation over the prosthesis.
Conclusion
This is a pioneer study in laboratory and clinical with TMJ prosthesis PEEK Lt1 20%Ba, our experience with this TMJ prosthesis design over the last ten years allowed us to conclude that it benefits patients with intra-articular pathologies, especially those with ankylosis. This new model of prosthesis and surgery technique performed, allows the function of the masticatory movements very close to natural physiological mandibular movements. Knowing that PEEK Lt1 20%Ba has all of these features of biocompatibility, biomechanical strength, lightness (3,49g the system) and subchondral bone compressibility, several studies on PEEK at the University of Oxford, (Oxford, England, UK) and Drexel University, (Philadelphia, PA, USA) aim to develop a cage for the spinal column and knee prosthesis. All TMJ prosthesis tests were performed in the Labmat Laboratory, Rio Claro, Brazil and CENIC laboratorie, Rio Claro- Laboratory – Brazil, to confirm their results. We wish to thank the Laros Corporation for sponsoring this study. Pat: 10 2021 0037314 4 (P E E K , PEEKLtI20%Ba, PEEK CFR, PEEK HA, as well as in UHMWPE, Titanium, CrCoMo, Ceramic and Gold).
References
- Eggers GWN (1946) Arthroplasty of the temporomandibular joint in children with interposition of tantalum foil: preliminary report. J Bone Joint Surg 28: 603-606.
- Christensen RW (1971) The temporomandibular joint prothesis eleven years later. Oral Implantol 2: 125-33.
- Mercuri LG (2000) The use of alloplastic prostheses for temporomandibular joint reconstruction. J Oral Maxillofac Surg 58: 70-75.
- Schuurhuis MJ, Dijkastra UP, Stegenga B, Bont GML, Spijkervet FKL (2012) Groningen temporomandibular total joint prosthesis: An 8-year longitudinal follow-up on function and pain. J of CranioMaxilloFac Surg 40: 815-820.
- Schliephake H, Schmelzeisen R, Maschek H, Haese M (1999) Longterm results of the use of silicone sheets after diskectomy in the temporomandibular joint: clinical, radiographic and histopathological findings. Int J Oral Surg Maxillo 28: 323-329.
- Morgan DH (1988) Evaluation of alloplastic TMJ implants. Practice craniomandibular J 6: 224-239.
- Wolford LM, Pitta MC, Reichel-Fischel O, Franco PF (2003) TMJ concepts/ Techmedica custom made TMJ prothesis Total: 5-year follow-up study.Int. J. Oral Maxillofac Surg 32: 268-274.
- Poulsson AHC (2014) Osseointegration of machined, injection molded and oxygen plasma modified PEEK implants in a sheep model. Biomaterials 35: 3717-3728.
- Kurtz SM, Devine JN (2007) PEEK biomaterials in trauma, orthopedic, and spinal. Biomaterials 28: 4845-4869.
- Skinner HB (1988) Composite technology for the total hip arthroplast. Clin Orthop Relat Res 1988: 224-236.
- Blundell DJ, Osborn BN (1983) The morphology of poly(aryletherketone). Polymer 24: 953.
- Van Loon JP, LGM DeBont G (1995) Evaluation Boering the Temporomandibular joint PROTHESES: Review of the literature from 1946 to 1994 and implications for the prothesis designs. J Oral Maxillofac Surg 53: 984-996.
- Van Loon JP, DeBont LGM, Stegenga B, Spijkervet FKL, Verkerke GJ (2002) Groningen temporomandibular joint prosthesis. Development and first clinical application. Int. J. Oral Maxillo Surg 31: 44-52.
- Morgan DH (1992) Development of alloplastic materials for temporomandibular joint prothesis: a historical perspective with clinical illustration. Cranio 10: 192-204.
- Quinn P (2000) Alloplastic reconstruction of the Temporomandibular Joint. IN Fonseca RJ. Oral and Maxillofacial Surgery:Temporomandibular disorders, Philadelphia: WB Saunders Company 17: 316-332.
- Wolford LM (2006) Factors to consider in joint prosthesis systems. Proc (Bayl Uni Med Cent) 19: 232-238.
- Genovesi W, Comenale IC, Filho WG, Fernandes MV (2022) Biomechanical comparative analysis of temporomandibular joint, glenoid fossa and head of the condyle of conventional models prothesis with new PEEK design. J of Oral Biol and Craniofac Res 12: 529-541.
- Pinto-Borges H, Carvalho O, Henriques B, Silva F, Ramos A, et al. (2021) A Preliminary Analysis of the Wear Pathways of Sliding Contacts on Temporomandibular Joint Total Joint Replacement Prostheses. Metals 11: 1-13.
- Cowie RM, Briscoe A, Fisher J, Jennings LM (2017) The Wear of an All-Polymer Knee Replacement under Different Environmental Conditions. 3rd International PEEK Meeting, Washington, D.C 2017.
- Janssen D, De Ruiter L, Rankin K, Briscoe A, Verdonschot N (2020) Pre-clinical Biomechanical Evaluation of Fixation of a Cemented PEEK Femoral Component in TKA. Orthop Procs 102: 52-52.
- Ashley A, Kinga MP, Claire LB, Joanne LT (2016) The Biologic Response to Polyetheretherketone (PEEK) Wear Particles in Total Joint Replacement: A Systematic Review. Clin Orthop Relat Res 474: 2394-2404.
- Hammouche S, Tipper JL, Fisher J, Williams S (2015) PEEK-based Materials as Hip Replacement Bearing Surfaces: A Comparison of Wear and Wear Particles Generated by Injection Molded PEEK-based Materials with Cross Linked Polyethylene Sliding Against Metal and Ceramic Counterfaces. 2nd International PEEK Meeting. Washington 2015.
- Rankin K, Bah MT, Dickinson AS, Sinclair I, Briscoe A, et al. (2013) Characterisation of the Fixation of a Novel Polyetheretherketone Implant for Total Knee Replacement. First International Meeting. The Union League Philadelphia 2013: 25-26.
- Crosbie SH, Jeffrey CF, Mehawej JM, Markman DA, Rydell MA (2013) Particulate and Gravimetric Wear Rate Analysis and Flexural Fatigue Testing of a PEEK Medial Knee Interpositional Arthroplasty Implant. First International Meeting. The Union League Philadelphia 2013: 2526.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.