SVFᴱᵃˢʸ Device: An Innovative Point-of-Care Device Approach to Isolate SVF from Adipose Tissue. Applicable to Other Tissues to Obtain Single Cell Population
by Jayanthi Boosani1, Rithika Gali1, Dushyanth Kalva2, Radhika Nagre1, Surya S. Singh3, Sandeepta Burgula4, Wasia Rizwani1*
1Genzir Technologies Pvt. Ltd., Asifnagar, Hyderabad-500028, Telangana, India
2Inform Cosmetic Surgery & Medical Aesthetics, Jubilee Hills, Hyderabad, Telangana, India
3Department of Biochemistry, Osmania University, Hyderabad, Telangana, India
4Department of Microbiology, Osmania University, Hyderabad, Telangana, India
*Corresponding author: Wasia Rizwani, Genzir Technologies Pvt. Ltd., Asifnagar, Hyderabad-500028, Telangana, India
Received Date: 25 August 2025
Accepted Date: 01 September 2025
Published Date: 03 September 2025
Citation: Boosani J, Gali R, Kalva D, Nagre R, Singh SS, et al. (2025) SVFEasy Device: An Innovative Point-Of-Care Device Approach to Isolate SVF from Adipose Tissue. Applicable to Other Tissues to Obtain Single Cell Population. J Surg 10: 11429 https://doi.org/10.29011/25759760.011429
Abstract
Adipose-Derived Stromal Vascular Fraction (AD-SVF) represents one of the most clinically promising cell-based materials in regenerative medicine due to its rich content of multipotent and immunomodulatory cells. Despite its therapeutic potential, widespread clinical adoption has been hindered by the complexity, variability, and sterility concerns associated with conventional AD-SVF isolation methods. Manual extraction techniques are often labor-intensive, require specialized facilities, and yield inconsistent cell populations, limiting their practicality in surgical and point-of-care settings. To overcome these limitations, we have developed a patented device that enables surgeons to isolate AD-SVF in a standardized, reproducible, and clinically efficient manner. The system utilizes an optimized enzymatic digestion protocol to process adipose tissue and deliver high-yield AD-SVF within less than two hours, making it compatible with intraoperative workflows. The AD-SVF obtained demonstrates comparable properties to cultureexpanded adipose-derived stromal cells, containing a heterogeneous mix of regenerative cell types, including mesenchymal stem cells with multipotent differentiation potential and a favorable immunophenotypic profile. Beyond adipose tissue, the versatility of the device extends to the enzymatic digestion of solid tissues of varied origins, broadening its potential applications in reconstructive and regenerative surgery. This flexibility allows surgeons to access viable, autologous cell populations at the point of care, without the delays, costs, or contamination risks of cell culture expansion. By streamlining the isolation process, this device bridges the gap between laboratory innovation and surgical practice, offering a practical and surgeon-friendly solution to integrate regenerative cell therapies into real-time clinical care.
Keywords: Adipose-Derived Stem Cells (ADSC); Extracellular Vesicles; GenT2C (Tissue-To-Cells) Device; Lipoaspirate; Organoids; Regenerative Medicine; Stromal Vascular Fraction (SVF); SVFEasy Device
Introduction
Adipose tissue has emerged as an attractive source of stem cells for clinical use, offering surgeons a readily available and minimally invasive alternative to bone marrow. It can be harvested in large quantities through standard liposuction performed under local anaesthesia, with far less morbidity than bone marrow aspiration [1]. Importantly, adipose tissue contains 500–2500 times more mesenchymal stem cells than the same volume of bone marrow, and unlike bone marrow, its stem cell reservoir remains stable throughout life. Adipose-derived cells also demonstrate superior proliferative and differentiation potential, longer telomere length, and lower senescence, ensuring more reliable regenerative outcomes [2]. Adipose-Derived Stem Cells (ADSCs) possess low immunogenicity and immunosuppressive properties, which broaden their clinical applicability in reconstructive and regenerative procedures, while reducing the risk of rejection or autoimmunity [3]. Within adipose tissue, the Stromal Vascular Fraction (SVF) is particularly valuable, containing a heterogeneous population of multipotent cells that have shown therapeutic efficacy in myocardial infarction [4], osteoarthritis [5], multiple sclerosis [6], periodontitis [7], and Parkinson’s disease [8]. To translate these advantages into surgical practice, next-generation devices are being developed to simplify SVF extraction. Our patented SVFeasy (GenT2C) system provides a standardized, point-of-care platform for isolating SVF in a sterile, reproducible, and clinically efficient manner-allowing surgeons to access regenerative cell therapies directly within the operating room.
Results
We developed an easy-to-use device to enable high efficiency extraction of SVF. Each SVF isolation device contains three chambers and ports (Figure 1 A). The upper chamber of the container is 80 ml in volume and the second and third chambers are 10 ml in volume. The device also contains various syringe ports for sample and buffer addition and removal-Tissue-In (TI) port for sample and buffer injection, Waste-Out (WO) port for waste removal via a vacuum motor or suction pump, and Cells-Out (CO) port for SVF collection. Inside the device, chamber 1 holds the adipose tissue (solid fat pieces or lipoaspirate). After collagenase type 1 or any other appropriate enzyme digestion, the separated individual cells pass through a filter and settle in the second chamber, while second filter in lower chamber allows only passage of lysed red blood cells and fluids but restricts stromal cells in the middle chamber. The SVF isolation procedure using this device is outlined in Figure 1. Briefly, the lipoaspirate tissue was first injected into the device, followed by washing with Phosphate Buffered Saline (PBS, pH 7) using a vacuum pump. Then the sample was digested with enzyme. In this case, collagenase type 1 was used for 1hr at 370C, washed and lysed with red blood cell lysis buffer for 10 min at RT. Finally, the SVF was collected from the device after washing off the lysis buffer and the cells were pelleted by centrifugation for 5 min. The entire process, from lipoaspirate tissues to ready-to-use SVF, takes less than two hours. We tested isolating SVF from lipoaspirate tissues using our device. Compared to manual extraction of SVF, using the device constantly yields more viable cells (1.3 – 8.4 ± 0.5 folds, p<0.5 folds, Figure 2A and Table 1). We also found that incubating tissue at the time of digestion in humidity or 5% CO2 significantly enhanced the cell number obtained from same amount of tissue. We observed that solid abdominal tissue had significantly higher number of stromal cells compared to lipoaspirate samples. Although amount of enzyme concentration required was twice to achieve similar digestion in both types of tissue samples.
Fluorescent microscopy analysis showed that a major population of cells as positive for CD44 and CD105 markers while negative for CD34 and CD45 markers (Figure 2D), indicating that the isolated SVF contains stem cells. Stemness markers relevant to AD-SVF have been discussed in detail in [9]. Flow cytometry analysis also showed that populations of the extracted cells are positive for CD44. CD31 was similar to unstained cells (Supplementary Figure 1). To further confirm the stemness, we also differentiated the SVF into adipocytes, chondrocytes and osteocytes [10,11]. The differentiated adipocytes, chondrocytes and osteocytes stained positive with Oil red O, Safranin and Alizarin red S respectively (Figures 3A,B). Enzymatic digestion did not alter the differentiation capabilities of AD-SVF. In order to shorten the time for differentiation in 2D cultures, we used a magnetic levitation method by 3D systems to facilitate growth and differentiation of SVF cells in 3D culture. Not only did the cells grow but they differentiated within 3 -4 days into respective phenotypes based on growth factors specific to adipocytes, chondrocytes and osteocytes respectively (Figure 3D). Panel C depicts unstained stromal cells grown in 3D condition. We also tested two samples with PRP (platelet-rich plasma) extracts to check the differentiation capacity of AD-SVF [12]. They had similar effects as fetal bovine serum (contains growth factors) and phenotype-specific differentiation markers (data not shown). We realized that precaution should be exercised when using PRP if only one phenotype is the goal for therapeutic applications. Maybe adding additional phenotypic enhancers or correct microenvironment is essential. Additional studies need to be done to characterize the features and signaling pathways activated in the presence of PRP.

Figure 1: Manual of the SVF isolation device. A) Left, image of the device. Right, schematic of the device. The device has three chambers. The chambers are separated by filters into upper chamber for tissues and the second chamber for SVF, while the third chamber is for waste and for lysed debris. Tissue/ buffers/enzyme are loaded through the Tissue-In (TI) port. Waste is collected through the WasteOut (WO) port into a jar connected to the suction/vacuum pump. And SVF is collected through the Cell-Out (CO) port. B) Procedure of isolating SVF using the device. The lipoaspirate tissue was first injected into the device, followed by washing with phosphate buffered saline using a vacuum pump. Then the sample was digested with appropriate enzyme for 1hr at 370C and lysed with RBC lysis buffer for 10 min at RT. Finally, the SVF was collected from the device and the cells were pelleted by centrifugation and counted by trypan blue staining method.
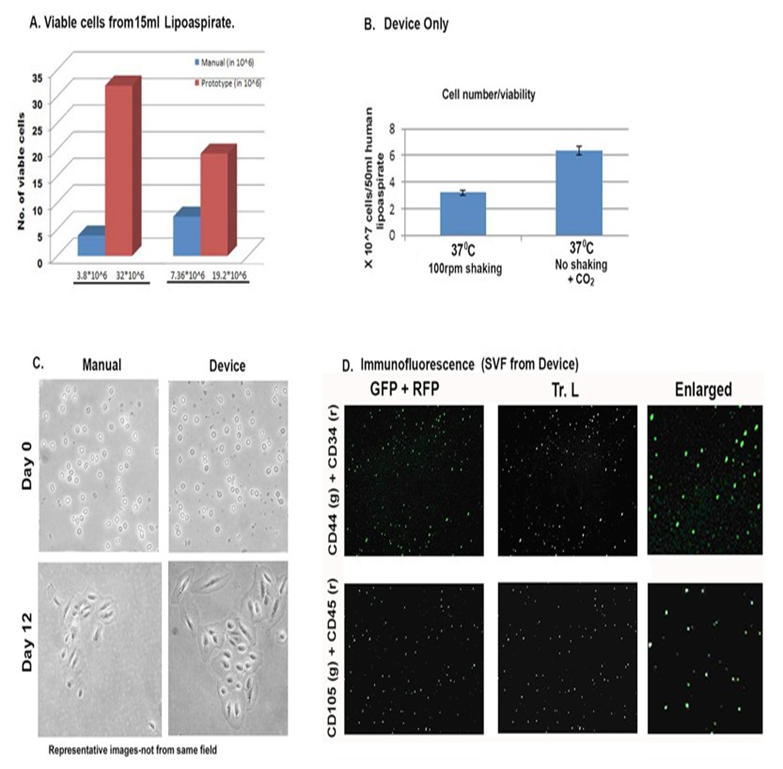
Figure 2: Comparison of manual and device isolated AD-SVF. A) Fold change in number of viable cells isolated comparing manual and device methods. B) Fold change in number of viable cells from device only but under different isolation conditions of AD-SVF. C) ADSVF from manual and device methods in cell culture. D). Immunofluorescence microscopic images of freshly isolated AD-SVF using SVFeasy device. Cells were positive for CD44 and CD105 (g-green fluorescence) and negative for CD34 and CD45 (r-red fluorescence). Tr. L. (Transmitted light) images depict the number of total cells present in each field. Scale bar represents 150μm. All figures are representative of experiments done with multiple lipoaspirate samples.
|
Experiments |
Volume of lipoaspirate tissue (ml) |
Manual isolation viable cell count (x 106) |
Device isolation viable cell count (x 106) |
|
1 |
30 (6ml actual fat) |
5.2 |
6.8 |
|
2 |
15 (4ml) |
3.8 |
32 |
|
3 |
30 (7ml) |
8.5 |
12.5 |
|
4 |
25 (6ml) |
12.3 |
32 |
Table 1: Viable cell counts comparing manual isolation of SVF and with the device. Note the actual amount of lipoaspirate reduces by ~5 times after washing.
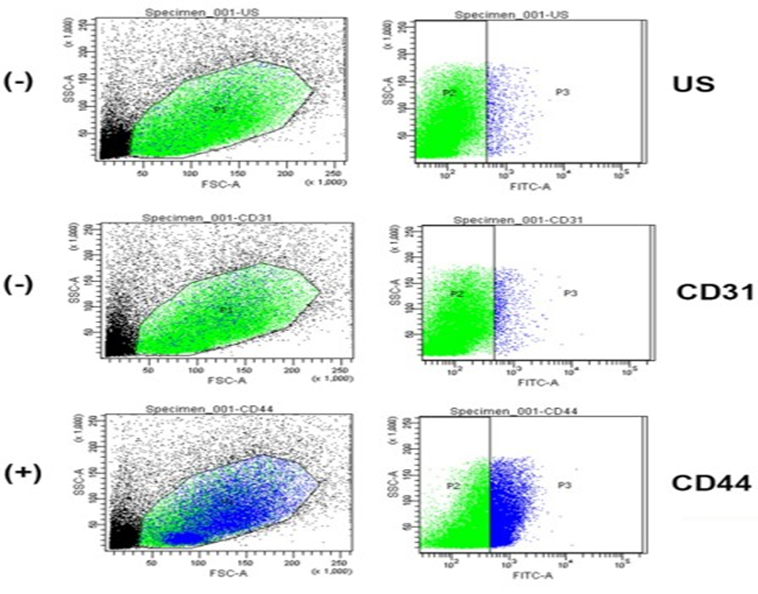
Supplementary Figure 2: Adipose-derived stromal Cells were isolated from human lipoaspirate using the SVFEasy device. Cells were washed twice with ice cold FACS buffer (PBS with 1% Fetal Bovine Serum (FBS) and 0.1% sodium azide) by centrifuging at 2000 rpm for 5 min. The cells were then stained with CD31 and CD44 antibodies at 1:1,000 dilution and incubated for 1hr at 4 ⁰C in the dark. Then, the cells were washed once with ice cold FACS buffer and fixed with 10% formalin at room temperature for 10min. Finally, the cells were washed twice with FACS buffer and resuspended in FACS buffer. The samples were kept in the dark at 4 ⁰C until the flow cytometer analysis. Unstained cells (US) were used to set the scale. CD44 cells are positive for AD-SVF and CD31 are majorly negative for AD-SVF.
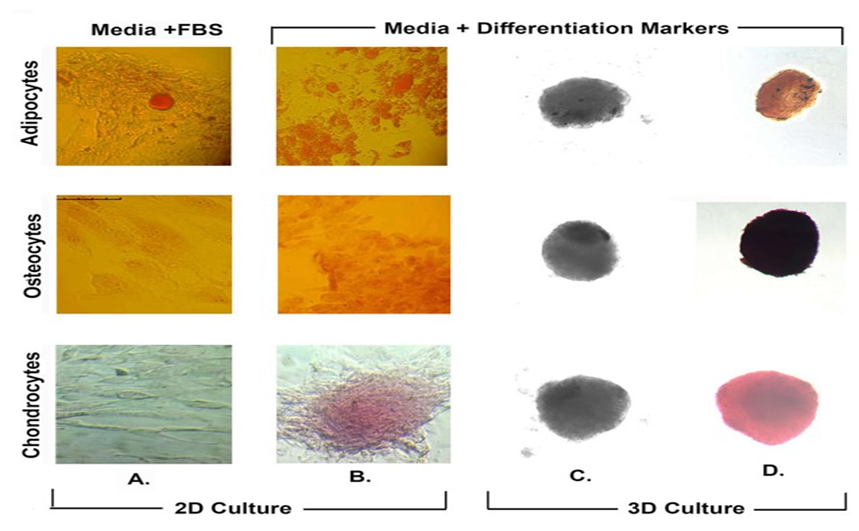
Figure 3: Histochemical analysis of differentiated AD-SVF cells. Stromal cells were grown in 2D culture (panel A &B) and 3D culture (panel C & D) set-up either with FBS (panel A) or respective differentiation markers (panel B, C, D). Following which, cells were independently stained with Oil red O, Alizarin red S and Safranin for differentiated adipocytes, osteocytes and chondrocytes respectively. Panel C represent unstained cells in 3D culture. Scale bar represents 150μm.
We presented here a user-friendly, point-of-care device to isolate adipose-derived SVF in a fast and efficient way. The isolated SVF contains a population of cells that include adipose-derived stem cells capable of multipotent differentiation and immunophenotype [13]. Our device achieved better performance as popular isolation systems available on the market in viable cell count (1 – 6 x 107 cells/ml of lipoaspirate tissues) and processing time (65 – 120 min). Filtration system of the SVFeasy device gets rid of lysed RBC and debris that otherwise settles as a pellet upon centrifugation in the manual method of isolation. Where the majority of the isolation systems on the market require a large device or work station [14], our device is a small disposable unit that integrates with existing gear in a surgical unit. The portable design will allow much easier implementation of our device in clinical settings.
Discussion
Stem cells have the ability of both self-renewal and differentiation. For regenerative medicinal applications, these cells should be found in abundant quantities; be acquired by a minimally invasive procedure; differentiate into multiple cell lineage pathways with reproducibility; should be safely and effectively transplanted to either an autologous or allogeneic host [2]. Since their discovery, Adipose-Derived Stem Cells (ADSC) or Adipose-Derived Stromal Vascular Fraction (AD-SVF) are being explored in many therapeutic areas in regenerative medicine with success. ADSCs demonstrate morphological and immunophenotypic characteristics of MSCs (Mesenchymal Stem Cells) [15]. The great advantage of ADSCs is that they can be harvested through a less invasive method and in larger quantities with proper ethical measures. However, ADSCs are not a homogenous cell population, and a specific CD marker cannot characterize ADSCs explicitly. Surface markers of ADSCs have been identified in 2013, including
CD13+/CD29+/CD44+/CD73+/CD90+/CD105+ and CD31-/CD45-/CD235a- [16]. It is important to understand that the phenotype of ADSCs during culture is dynamic; some markers are expressed de novo, while the expression of others can be lost [17]. For example, it has been noticed that early-passage ADSCs express CD34 that decreases throughout the culture period [18]. Therefore, the mechanisms underlying their regenerative effects need to be fully elucidated with each specific disease indication studies. Multiple pre-clinical and clinical studies are underway and have revealed remarkable therapeutic success in fat grafting, wound healing, bone regeneration, skeletal muscle repair, tendon reconstruction, cartilage regeneration, cardiac repair, and nerve regeneration [9,19]. We have observed in vitro differentiation of AD-SVF into adipocytes, chondrocytes and osteocytes with respective differentiation markers and also with growth-factors present in fetal bovine serum. Growth-factor reduced serum was used to maintain stem cells in culture. We also studied the effect of AD-SVF in dental defects and will be presented in an ensuing publication. Unlike culture-expanded stem cells, ADSVF can be used immediately, minimizing contamination risk and reducing costs [20]. Our device, SVFeasy offers viable AD-SVF in high numbers that can be re-injected safely and efficaciously into patients for autologous regenerative therapeutic applications.
Chart 1 presents the potential of AD-SVF in biological and regenerative applications [19,21]. In veterinary medicine, intraarticular administration of AD-SVF has shown significant benefits in managing canine osteoarthritis, improving lameness scores, gait, and pain when used alone or in combination with platelet-rich plasma [22,23]. Beyond joint disease, AD-SVF has demonstrated potential in enhancing wound healing, promoting angiogenesis, and supporting bone and cartilage regeneration, including treatment of tendon injuries and meniscus tears [24, 5]. Its accessibility, regenerative properties, and multi-lineage potential highlight AD-SVF as a promising, clinically relevant tool in veterinary regenerative therapies. We tested the ability of AD-SVF isolated from rat fat pads to differentiate into different cell types, presented in Supplementary Figure 2. This is where the handy, user-friendly SVFeasy device we developed comes in practical use in veterinary regenerative medicine. Adipose-Derived Stem Cells (ADSCs) mediate tissue repair through both direct cellular interactions and paracrine mechanisms, with the latter largely attributed to their secretome, comprising Extracellular Vesicles (EVs), cytokines, and a spectrum of soluble bioactive factors. Increasing attention has shifted toward exploiting the ADSC secretome as a cell-free therapeutic modality, given its capacity to circumvent limitations inherent to whole-cell transplantation, including tumorigenic potential, poor engraftment, and cryopreservation challenges [25]. Relative to ADSCs themselves, the secretome exhibits superior stability, diminished immunogenicity, and enhanced suitability for allogeneic applications, thereby positioning it as an attractive candidate for translational development and commercialization [26]. In vitro, ADSCs release a wide array of trophic factors into the culture supernatant, collectively termed Conditioned Medium (CM). ADSC-CM encompasses the entirety of the secretome and has been shown to exert multifaceted protective and reparative effects across diverse systems, including the promotion of cutaneous wound healing [27], attenuation of ischemic myocardial injury [28], and mitigation of neurodegenerative processes [29]. Central to these effects are Extracellular Vesicles (EVs), a heterogeneous class of lipid bilayer–enclosed vesicles subdivided into exosomes, microvesicles, and apoptotic bodies, which function as critical mediators of intercellular communication and tissue remodeling [30]. Among these, ADSC-Derived Exosomes (ADSC-Exos) have emerged as particularly potent effectors, with regenerative efficacy demonstrated in contexts such as cutaneous repair [31], bone regeneration [32], and cartilage restoration. Concurrently, innovations in Three-Dimensional (3D) culture systems have catalyzed advances in organoid biology and bioprinting technologies, thereby expanding the therapeutic horizon of ADSCs. In our studies, magnetic levitation of freshly isolated AD-SVF cells followed by exposure to appropriate differentiation markers led to quick growth and differentiation in 3D cultures. Organoids, generated through the self-organizing properties of stem cells, recapitulate key structural and functional attributes of native tissues and have proven instrumental in disease modeling, pharmacological screening, and tissue engineering [33]. The convergence of 3D bioprinting with organoid culture offers a means to surmount the scalability and reproducibility limitations of conventional methods, enabling precise spatial control over cellular architecture. Incorporating ADSCs into organoid bioprinting platforms holds particular promise for engineering functional tissue constructs, thereby broadening the translational potential of ADSC-based therapies within regenerative medicine.
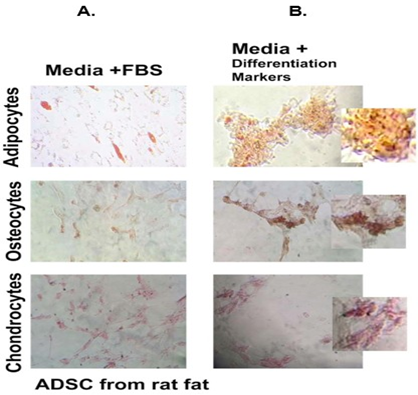
Supplementary Figure 2: AD-SVF was isolated from rat fat pad using the SVFEasy device and allowed to differentiate into adipocytes, chondrocytes and osteocytes respectively in FBS (Panel A) and specific differentiation markers (Panel B). Cells were stained with Alizarin red, Safranin and Oil red O respectively. Pictures were taken using Leica microscope under 20X magnification, scale-150μm. Enzymatic digestion did not alter the stemness of AD-SVF.
Methodology
Sample collection: Lipoaspirate (10) or abdominal fat (5) from different patients was collected in 1X PBS, pH 7.2 (Sigma) with 1X Antibiotic-Antimycotic (AB-AM) solution (Sigma) and processed the same day for various experiments.
Manual extraction of SVF
15 – 30ml of lipoaspirate tissue sample was washed twice with 3060ml of Phosphate Buffered Saline (PBS, pH 7.0) by centrifuging the sample at 2000rpm (REMI-8C-100ml tube rotor) for 5 min. After each wash, the upper oily layer and bottom supernatant was discarded and the tissue was transferred to a new falcon tube. Enzyme Cocktail (Collagenase type I (0.05-0.5% + 0.36mM CaCl2 + 1% AB-AM in 10mM PBS, pH 7.2) added to 5ml clean lipoaspirate for tissue dissociation. The tissue was then digested with 7 – 15 ml 0.05% collagenase type 1 (Sigma, #C0130) for 1hr at 37⁰C and 5%CO2, shaking the tube up and down a few times during the digestion. Following the digestion, the pellet was collected after centrifugation for 5 min at 2000rpm. Then the pellet was suspended in and incubated with 1ml 1x RBC lysis buffer (0.15M NH4Cl, 0.01M NaHCO3 and 1.3mM EDTA) at room temperature for 10min. Then the cell pellet was collected again after centrifugation for 5 min at 2000rpm. Finally, the pellet was washed once with 1ml PBS by centrifuging at 2000rpm for 5 min and dissolved in 1ml complete DMEM media. Cell number was counted with trypan blue staining [9].
SVF extraction using the device
15 – 30 ml lipoaspirate tissue was injected into the device through the Tissue-In (TI) port using a syringe. The tissue was washed twice by injecting 15 – 40ml PBS into the device through the TI port and removing the waste from the Waste-Out (WO) port using a vacuum motor pump. The tissue was then digested by injecting 20 – 40ml collagenase type 1 cocktail through the TI port. The device was incubated at 37⁰C and 5%CO2 for an hour. Then 10 – 20ml 1x RBC lysis buffer was injected into the device using the TI port and incubated at room temperature for 10min. The isolated cells were collected in a falcon tube using a syringe through the Cells Out (CO) port and placed in a falcon tube; centrifuged at 2000rpm for 5 min (REMI-8C-15ml tube rotor) and the pellet was collected. Finally, the pellet was washed once with 1ml PBS by centrifuging at 2000rpm for 5 min and dissolved in 1ml complete DMEM media. Cells were counted with trypan blue staining.
Immunofluorescence Microscopy
Once SVF was isolated, cells were fixed with 70% ethanol for 15 min at RT. Centrifuged at 2000rpm in Remi-8C centrifuge for 7min. The pellet was re-suspended in 1X PBS, pH 7.2 for microscopy. Cells were either directly stained with antibodies or permeabilized with 0.1% Triton-X-100 in PBS for 5 min prior to staining and counter-stained with DAPI. 1 million cells each were stained with antibody combination (1) CD44 (FITC-conjugate) and CD34 (RPE conjugate) or (2) CD105 (Alexa Fluor 488) and CD45 (PECy 5.5 conjugate). CD44 and CD105 are positive markers for Adipose-Derived Stem Cells (ADSC) whereas CD34 and CD45 are negative markers for ADSC. Antibody staining was carried out in the dark for 1hr in cold (40C) following manufacturer’s instructions. Antibodies were purchased from ICON Biosystems. The stained cells were washed and resuspended in 50μl PBS and 50μl glycerol, mixed and spotted on slide with a cover slip to capture images under 10X magnification using Leica Fluorescent microscope [9]. For differentiation studies, cells were plated and maintained in growth factor reduced FBS (10%)/ DMEM with 1% AB-AM solution.
Adipocyte Differentiation and Staining with Oil red O
Stromal cells (1.5*10^5) were grown in adipocyte differentiation media (DMEM low glucose, 10% FBS, 1% AB-AM, 0.5mM isobutyl methyl xanthine, 1mM Dexamethasone, 10mM insulin, 200mM indomethacin) for 14 days, changing media every 3rd day. After the 14-day differentiation, the cells were fixed with 10% formalin at room temperature for 20min. The cells were then washed with 60% isopropyl alcohol and left to dry completely. Then the cells were incubated with Oil red O at room temperature for 10min. Finally, the dye was removed and the cells were washed with distilled water four times before imaging [34].
Chondrocyte Differentiation and Staining with Safranin
For chondrogenic differentiation, 1.5*10^5 cells were grown in chondrogenic media (DMEM low glucose with 10%FBS, 1% AB-AM, 6.25mg/ml insulin, 10ng/ml TGF-b, 50nM ascorbic-2phosphate). Cells were maintained till 21 days changing media every third day. For staining of chondrocytes, cells were grown in chondrocyte differentiation media and washed with 1x PBS for 3 times and fixed in 10% formaldehyde for 20min at room temperature after which the cells were washed with 1x PBS 3 times. The cells were stained with Safranin, rinsed with 1% acetic acid for 5 min and rinsed twice with 1x PBS and images were taken [34].
Osteocyte differentiation and staining with Alizarin red S
Cells (1*10^5) were grown in osteocyte Differentiation Media (DMEM high glucose, 10% FBS, 1% AB-AM, 0.01mM 1,25-Dihydroxyvitamin D3, 50mM ascorbic acid, 2mM β-glycerophosphate) for 14 days, changing media every 3rd day. After the 14-day differentiation, the cells were washed with PBS three times and fixed with 4% formaldehyde for 15 min at room temperature. Following fixation, the cells were stained with 1ml of 40mM Alizarin red S for 20 – 30 min at room temperature with gentle shaking. Finally, the dye was removed and the cells were washed with distilled water five times before imaging [34].
3D Cultures and Staining with Respective Dyes
3d cultures by using 24-well bioassembler kit by n3d biosciences : T-25 flask that was 80% confluent with human adipose derived stromal cells was used for this experiment. 25μl nanoshuttles along with 3ml DMEM media were added and incubated overnight at 370C and 5%CO2. The next day cells were trypsinized and 40,000 cells per well were counted and plated in the cell repellant 24 well plate given in the kit. Cells were plated in 9 wells from each of the flasks, 3 wells kept as controls, 3 wells kept for differentiating with FBS and 3 wells for differentiating with PRP for each of adipocytes, osteocytes and chondrocytes respectively. The plate was closed with the custom lid with the 24 well levitating drive kept above it and the regular lid kept above the levitating drive. This set up was kept overnight for incubation. The next day this set up was removed and the 24 well plate was kept on the concentrating drive that was provided and again incubated it for overnight. Next day concentrating drive was removed and only the plate was incubated overnight. Following this, pictures of 3D cultures were taken in 10x before and after staining for adipocytes, chondrocytes and osteocytes.
Statistical Analysis: p-value was calculated using standard T.TEST for cell counts from samples in duplicate. P<0.5 was considered significant since these are different patient samples and procedural variation was taken into account.
Safety and sterility studies of the sterile packed devices were carried out in cells and animal models according to ISO standards (ISO 10993-5; ISO 10993-10; ISO 10993-11; ISO 11737-1) by Bioneeds (I) Private Limited, Bangalore and Astute Labs, Pune (Data not shown).
Ethical Guidelines: Ethical permit to collect lipoaspirates from patients undergoing liposuction at any three different clinical sites was obtained from the institutional ethical committee of Krishna Institute of Medical Sciences, Hyderabad.
Conflict of Interest: There is no conflict of interest to disclose.
Acknowledgements: We acknowledge the efforts of our support team members and collaborators associated with this study, especially Dr. Vasundara from Krishna Institute of Medical Sciences, Dr. Noor Uddin Afzal from Shadan Institute of Medical Sciences, Arif P. from Genzir & Ashwin Kumar from Win Designs.
Funding and Patent: Funding support came from BIRAC/
IKP0396/BIG-09/16 (PI- Dr. Wasia Rizwani) and from Genzir Technologies Pvt. Ltd., Asifnagar, Hyderabad, TG, India. Indian Patent (No. 322555, dated 21/06/2017) was awarded to Genzir Technologies Pvt. Ltd. for the Isolation Device for AdiposeDerived Stromal Vascular Fraction.
References
- Bunnell BA (2008) Adipose tissue: a source of multipotent adult stem cells for tissue engineering and regenerative medicine. Methods 45: 115-120.
- Bansal H, Comella K, Leon J, (2017) Intra-articular injection in the knee of adipose derived stromal cells (stromal vascular fraction) and platelet rich plasma for osteoarthritis. J Transl Med 15: 141.
- Pikula M, Marek-Trzonkowska N, Wardowska A, (2013) Adipose tissue-derived stem cells in clinical applications. Expert Opin Biol Ther 13: 1357-1370.
- Menasché P (2005) Skeletal myoblasts and cardiac repair. J Mol Cell Cardiol 38: 897-905.
- Pak J, Lee JH, Lee SH (2013) Regenerative repair of damaged meniscus with autologous adipose tissue–derived stem cells. J Med Case Rep 7: 229.
- Riordan NH, Ichim TE, Min WP (2009) Non-expanded adipose stromal vascular fraction cell therapy for multiple sclerosis. J Transl Med 7: 29.
- Park JB, Lee MS, Cha JM (2012) Regenerative effect of adipose tissue-derived stem cells on damaged periodontal ligament in a dog periodontitis model. J Periodontal Res 47: 775-783.
- Venkataramana NK, Kumar SK, Balaraju S (2010) Open-labeled study of unilateral autologous bone-marrow-derived mesenchymal stem cell transplantation in Parkinson’s disease. Transl Res 155:62-70.
- Rizwani W, Kalva D, Boosani J (2024) GenAdipose Device gives Clean Lipoaspirate for Generation of Nanofat or Microfat for Autologous Reinjections. CD44-Expressing Adipocytes Observed in Some Human Lipoaspirates. Adv Prev Med Health Care 7: 1067.
- Zuk PA, Zhu M, Ashjian P (2002) Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13: 4279-4295.
- Guilak F, Erickson GR, Gimble JM (2002) Chondrogenic potential of adipose tissue-derived stromal cells in vitro and in vivo. Biochem Biophys Res Commun 290: 763-769.
- Van Pham P, Bui KH, Ngo DQ (2013) Activated platelet-rich plasma improves adipose-derived stem cell transplantation efficiency in injured articular cartilage. Stem Cell Res Ther 4: 91.
- Bunnell BA (2021) Adipose Tissue-Derived Mesenchymal Stem Cells. Cells 10: 3433.
- Aronowitz JA, Lockhart RA, Hakakian CS, Birnbaum ZE (2016) Adipose Stromal Vascular Fraction Isolation: A Head-to-Head Comparison of 4 Cell Separation Systems #2. Annals of Plastic Surgery 77: 354-662
- Kern S, Eichler H, Stoeve J (2006) Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 24: 1294-1301.
- Bourin P, Bunnell BA, Casteilla L (2013) Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT).Cytotherapy 15: 641648
- Baer PC (2014) Adipose-derived mesenchymal stromal/stem cells: An update on their phenotype in vivo and in vitro. World J Stem Cells 6: 256-265.
- Mitchell JB, McIntosh K, Zvonic S (2006) Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers.Stem Cells 24: 376-385.
- Yi Qin, Gaoran Ge, Peng Yang (2023) An Update on Adipose-Derived Stem Cells for Regenerative Medicine: Where Challenge Meets Opportunity. Adv. Sci 10: 2207334.
- Bora P, Majumdar AS (2017) Adipose tissue-derived stromal vascular fraction in regenerative medicine: a brief review on biology and translation. Stem Cell Res Ther 8: 145.
- Khan Sharuna, Kaveri Jambagib, Rohit Kumara (2022) Clinical applications of adipose-derived stromal vascular fraction in veterinary practice. Veterinary Quaterly 42: 151-166.
- Black LL, Gaynor J, Gahring D (2007) Effect of adipose-derived mesenchymal stem and regenerative cells on lameness in dogs with chronic osteoarthritis of the coxofemoral joints: a randomized, doubleblinded, multicenter, controlled trial. Vet Ther 8: 272-284.
- Carrillo JM, Manera ME, Rubio M (2018) Posturography and dynamic pedobarography in lame dogs with elbow dysplasia and cranial cruciate ligament rupture. BMC Vet Res 14: 108.
- Fraser JK, Wulur I, Alfonso Z, Hedrick MH (2006) Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol 24: 150-154.
- PK, Kandoi S, Misra R, S V, K R, Verma RS (2019) The mesenchymal stem cell secretome: A new paradigm towards cell-free therapeutic mode in regenerative medicine. Cytokine Growth Factor Rev 46:1-9; b) Trzyna A, Banaś-Ząbczyk A (2021) Adipose-Derived Stem Cells Secretome and Its Potential Application in “Stem Cell-Free Therapy”. Biomolecules 11: 878.
- Cai Y, Li J, Jia C (2020) Therapeutic applications of adipose cell-free derivatives: a review. Stem Cell Res Ther 11: 312.
- Gregorio D, Contador C, Diaz D (2020) Human adipose-derived mesenchymal stem cell-conditioned medium ameliorates polyneuropathy and foot ulceration in diabetic BKS db/db mice. Stem Cell Research & Therapy 11: 168.
- Lee TL, Lai TC, Lin SR (2021) Conditioned medium from adiposederived stem cells attenuates ischemia/reperfusion-induced cardiac injury through the microRNA-221/222/PUMA/ETS-1 pathway. Theranostics 11: 3131-3149.
- Jha KA, Pentecost M, Lenin R (2018) Concentrated Conditioned Media from Adipose Tissue Derived Mesenchymal Stem Cells Mitigates Visual Deficits and Retinal Inflammation Following Mild Traumatic Brain Injury. Int J Mol Sci 19: 2016.
- van Niel G, D’Angelo G, Raposo G (2018) Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol 19: 213-228.
- Li X, Xie X, Lian W, (2018) Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp Mol Med 50: 1-14. b) Liu K, Chen C, Zhang H (2019) Adipose stem cell‐derived exosomes in combination with hyaluronic acid accelerate wound healing through enhancing re‐epithelialization and vascularization. British Journal of Dermatology 181: 854-856.
- Li W, Liu Y, Zhang P (2018) Tissue-Engineered Bone Immobilized with Human Adipose Stem Cells-Derived Exosomes Promotes Bone Regeneration. ACS Appl Mater Interfaces 10: 5240-5254. b) Kang Y, Xu C, Meng L (2022) Exosome-functionalized magnesium-organic framework-based scaffolds with osteogenic, angiogenic and antiinflammatory properties for accelerated bone regeneration. Bioact. Mater 18: 26-41
- Clevers H (2016) Modeling Development and Disease with Organoids. Cell 165: 1586-1597.
- Francis MP, Sachs PCLynne W Isolating adipose-derived mesenchymal stem cells from lipoaspirate blood and saline fraction. Organogenesis 6:1, 11-14; © 2009 Landes Bioscience.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.