Suspected Molecular Links in Sebaceous Gland Carcinoma of the Eyelid: A scoping review
by Maurya RP1, Maurya AK2*, Ali A3*, Singh CB3 Gupta S1, Prajapat MK, Roy M5 , Yadav A2, Ranjan S1, Manav1, Singh VP1, Al-Mujaini AS6
1Regional Institute of Ophthalmology, Institute of Medical Sciences, Banaras Hindu University, Varanasi, India.
2Department of Zoology, Institute of Science, Banaras Hindu University, Varanasi, India.
3Centre for Genetic Disorders, Institute of Science, Banaras Hindu University, Varanasi, India.
4Department of Ophthalmology, National Institute of Medical Sciences & Research, Jaipur, NIMS University Rajasthan, India.
5Department of Ophthalmology, Hind Institute of Medical Sciences, Mau, Ataria, Sitapur, U.P., India.
6College of Medicine and Health Sciences, Sultan Qaboos University, Muscat, Oman.
*Corresponding author: Ali A, Centre for Genetic Disorders, Institute of Science, Banaras Hindu University, Varanasi, India. Email: akhtar@bhu.ac.in
Maurya AK, Department of Zoology, Institute of Science, Banaras Hindu University, Varanasi, India.
Received Date: 27 August, 2024
Accepted Date: 04 September, 2024
Published Date: 06 September, 2024
Citation: Maurya RP, Maurya AK, Ali A, Singh CB, Singh S, et al. (2024) Suspected Molecular Links in Sebaceous Gland Carcinoma of the Eyelid: A scoping review. J Oncol Res Ther 9: 10245. https://doi.org/10.29011/2574-710X.10245.
Abstract
Objectives: This review aimed to comprehensively analyse molecular mechanisms like mismatch repair genes, cell cycle regulators, and signalling pathways including WNT, SHH, and CASPASE-3/YAP in eyelid sebaceous gland carcinoma (SGC). In silico analysis explored the selectively expressed proteins (SEPs) in SGC patients through network-based analysis. Methods: A thorough literature search was performed using PubMed, Google Scholar and Web of Science databases by using specific and relevant terms. A protein-protein interaction (PPI) network was constructed for selected genes with strong evidence from the literature, using the STRING 11.0 database and Cytoscape 3.7.1 software. Results: This review reports that mutations in MMR genes primarily occur in MLH1 and MSH2, followed by MSH6, PMS2, and tumor suppressor P53 genes in SGC patients. Mutation and dysregulation in genes involved in hedgehog, ß-CATENIN, CASPASE-3/YAP, and C-MYC-AR-P53 signalling are also crucial during tumorigenesis in the sebaceous gland. The network-based approach elucidates the role of MMR genes and experimentally determines interactions, co-expression, and combined scores. Dysregulation of immune checkpoint regulators including PD-1, PD-L1, and CTLA leads to poor cancer cell presentation to immune cells. The lowest combined scores were observed for ß-catenin (CTNNB1), and sonic hedgehog (SHH). Conclusion: This review summarizes and concludes that mutations and dysregulation in the genes involved in MMR, WNT/ß-CATENIN, HEDGEHOG, and CASPASE-3/YAP signalling are crucial for SGC in the eyelid. In silico analysis provides a better understanding of the essential genes involved in the tumorigenesis of the sebaceous gland and is helpful for targeted drug therapy.
Keywords: Eyelid; Sebaceous Gland Carcinoma; Mismatch Repair Genes; Signaling pathway; In silico Analysis; STRING Database.
Introduction
Sebaceous gland carcinoma (SGC) is a relatively rare, slowgrowing cancer, yet it is the most aggressive and life-threatening tumor of the eyelid. SGC originates from the Meibomian glands, glands of Zeis, or glands associated with the caruncle [1]. It is locally invasive and often metastasizes to various parts of the body, including the liver, brain, and lymph nodes [2, 3]. The etiology of SGC is largely unknown, though several risk factors have been identified, including advanced age, Asian or South Asian race, female gender, Muir–Torre syndrome (MTS), colorectal carcinoma/visceral malignancies, previous irradiation of the head and neck, mutations in the Rb and p53 genes, and infections of HIV and HPV [1, 4].
Most of the MTS-associated tumors exhibit mutations in DNA mismatch repair (MMR) genes such as MLH1, MLH2, MSH2, MSH6, PMS2, and p53, as well as microsatellite instability (MSI), often caused by oxidative stress. During replication and recombination, the MMR system corrects mismatches of single nucleotide bases, deletions, and insertions. Due to the crucial role of MMR genes in maintaining replication fidelity and genomic integrity, the MMR system is considered a genomic caretaker. Dysregulation of critical signaling pathways, such as ß-catenin/ Wnt and hedgehog pathways, which regulate tissue differentiation, has been implicated in the development of various skin and noncutaneous tumors [5]. Lower expression levels of the genes: E-cadherin (CDH1) and ß-catenin (CTNNB1) are associated with poor tumor differentiation and increased tumor inflammation in sebaceous eyelid carcinoma, indicating the potential prognostic value of E-cadherin and ß-catenin in patients with SGC.
Additionally, abnormal expression of ß-catenin, hedgehog ligands, and mutations in key regulators of these signaling pathways are linked to various tumors, including lung, colorectal, breast, hepatocellular, oral squamous cell carcinoma, non-melanoma skin tumors, and bladder cancer [6, 7]. While the roles of these pathways and their associated molecules or risk factors are well-documented in several cancers, their involvement in sebaceous carcinoma remains underexplored. The etiology of sebaceous gland carcinoma is still unclear. Bioinformatics approaches are extensively used to identify the genes, proteins, and pathways involved in SGC, enhancing our understanding of its pathogenesis and potential therapeutic targets. Systems biology and computational methods combined can effectively identify an array of genes that may be targeted within potential gene networks.
The clinical signs and symptoms of SGC in each patient that indicate a hereditary predisposition to visceral cancer are of utmost importance for cancer prevention. Therefore, understanding recent molecular advances in sebaceous carcinoma is essential for early prognosis and targeted drug therapy in SGC patients. Currently, detailed information and comprehensive literature on the role of MMR genes associated with the activation of ß-catenin and Hedgehog signalling are not well documented for Indian SGC patients. Surgical excision of sebaceous glands is the only treatment option for patients diagnosed with SGC. In several SGC cases, the affected eye is also removed (exenteration) to protect the patient’s life. Thus, there is an urgent need to understand the molecular pathogenesis of SGC for better prognosis, targeted drug therapies, and free survival of patients.
In this review, we provide a comprehensive analysis of the literature on SGC, focusing on the role of DNA damage repair genes and their mutations that lead to the activation of p53 and hedgehog/ß-catenin pathways in the absence of Wnt signalling during sebaceous gland carcinoma.
Method of Literature Search
In this review, an extensive literature search was conducted using the NCBI databases (PubMed, OMIM, and Gene), Google Scholar, and Medline. Search terms included ‘eyelid tumors’, ‘sebaceous carcinoma’, ‘sebaceous gland carcinoma’, ‘sebaceous neoplasm’, and ‘sebaceous cell carcinoma’. All selected research articles and reviews were published primarily in English. Additional references were gathered from selected articles published in peer-reviewed journals. The study was approved by the institutional human ethics committee and written informed consent was obtained from the patient for publication of the photographs.
Epidemiology and Demography Update
International Level
SGC represents 1–3% of all malignant tumors and 0.6–10.2% of eyelid tumors. Recent studies reported 3,360 new cases of eye and orbit cancer in 2019, in which 370 deaths occurred. The incidence rate of SGC was similar in 2018 and 2019 [8]. In 2012, the frequency of SGC in males and females was 3.2 per 1 million and 1.6 per 1 million persons, respectively. However, the incidence rate was lower in 2009, with 1-2 cases per 1 million individuals per year. SGC is the third most common eyelid malignancy after basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) [9]. This trend indicates that the incidence of SGC is increasing significantly.
National Level
The incidence of sebaceous gland carcinoma shows significant variations according to geographic area and appears to have a racial preference. The incidence of SGC varies across different studies in the Asian-Indian population. Eyelid SGC is relatively common cancer, accounting for 28–60% of all eyelid malignancies [4]. In India, SGC is the most predominant malignant ocular lesion (37%), followed by squamous cell carcinoma (SCC, 21%) and basal cell carcinoma BCC (11.1%). A recent study enrolled 536 patients, of whom 285 (53%) were observed with SGC, while BCC and SCC were found in 128 patients (24%) and 99 patients (18%), respectively [10, 11]. Other malignant tumors with similar clinical features were also observed among the enrolled patients.
Geographic variation in trends of extraocular tumors was noted in a study of 52 patients. Among these, 25 patients (48%) had been diagnosed as eyelid tumors with benign (64%) and malignant eyelid neoplasms (36%). Benign lesions were recognized female preponderance with male:female ratio of 1:2.25 [12]. A prospective study of 34 patients had observed that the most frequent tumor site was the eyelid (94.12%), followed by cruncle (5.88%). In the SGC patients, most frequent affected site was observed (41.18%) in the upper eyelid, followed by lower lid (29.41%). However, medial canthus and lateral canthus were minimally (11.76% each) involved in the tumour progression in the eyelid. In extraocular tumors, painless mass was the most common clinical presentation that included nodular type (82.35%) and ulcero-nodular type (17.65 %). Regional lymph node metastasis was observed in 20.59% of patients, and distant metastasis was observed in 8.82%. Approximately 35.29% of patients underwent surgery, while 23.53% patients received neoadjuvant chemotherapy. These results were consistent with previous findings [13].
In the Indian population, SGC is often reported late, in advanced stages of tumor. Several factors contribute to the poor prognosis in patients with SGC, including lymphovascular and orbital invasion, multicentric origin, pagetoid spread to skin and conjunctiva, large tumor size, involvement of both upper and lower eyelids, and poor histopathological differentiation. The increasing burden of SGC on the nation and society is significant that increases the economic burden and health issues on patients, society and nation. Addressing the pathogenesis and treatment of SGC require the investigation of molecular genetic studies, including MMR genes and related signaling pathways, to improve therapeutic outcomes for patients.
Molecular Genetics
Mismatch Repair (MMR) and P53 Genes
In recent decades, our understanding of the molecular basis of SGC tumorigenesis has advanced significantly. Many studies have highlighted the involvement of crucial genes, particularly tumor suppressor, oncogenes, and MMR genes. The MMR system is vital, acting as a guardian of genomic integrity. The identification and repair of mismatches in the genome are essential for normal cellular function. However, failure of the MMR system results in MSI. A deficiency in MMR genes leads to a lack of coordination with other DNA repair genes, frequently found in patients with non-polyposis colorectal cancer and sebaceous carcinoma.
Sebaceous neoplasms, including adenoma, epithelioma, and carcinoma, often demonstrate a high frequency of MSI [5, 12]. MSI is a form of genetic instability associated with DNA mismatch defects and is also found in gastric, endometrial, and skin tumors [14]. Sebaceous gland neoplasms can be associated with autosomal-dominant Muir-Torre syndrome (MTS). MTS is characterized by the occurrence of sebaceous gland neoplasms and/or keratoacanthomas, along with visceral malignancies such as gastrointestinal and genitourinary cancers. Typically, MTS involves at least one sebaceous gland neoplasm and one internal malignancy, most commonly colonic carcinoma.
Given the predisposition of patients with MTS to cancer in various tissues, particularly the colon, urothelium, and endometrium, it is necessary to study the molecular mechanisms underlying these diseases. This is essential for cancer surveillance programs for both patients with MTS and their immediate relatives. MTS patients with different sebaceous and internal tumors often exhibit high-grade MSI (MSI-H). Germline mutations in MMR genes, particularly in MSH2, have been identified in two families suffering from MTS [12, 15].
MMR-deficient cancers have a high risk of mutation, primarily caused by UV radiation or oxidative stress (ROS; Figure 1). The lack of MMR genes often occurs with the loss of other DNA repair genes, leading to deficient expression in cancer. MMR genes are also associated with hereditary non-polyposis colorectal carcinoma (HNPCC), which tends to cause various visceral malignancies along with prominent skin tumors like sebaceous carcinomas. A previous study of 31 patients found that 16 patients had SGC associated with HNPCC and MTS, exhibiting high-grade MSI. Thus, these patients had a high probability of an underlying MMR defect.
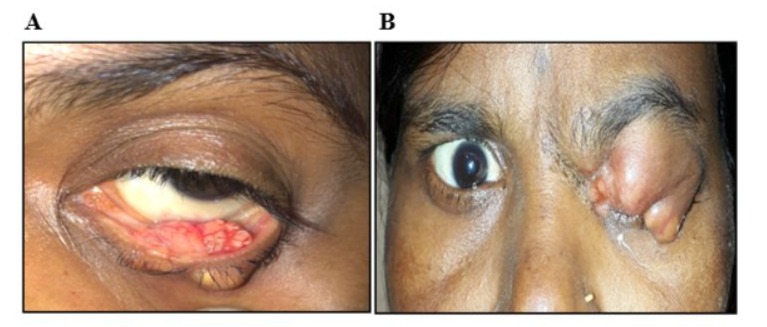
Figure 1: (A) Everted left lower eyelid of the patient showing a large yellowish ulcero-nodular lesion, confirmed by biopsy analysis to represent sebaceous gland carcinoma. (B) Massive, multilobulated mass in the left upper eyelid mass of a 50-year-old woman, diagnosed as an aggressive sebaceous gland carcinoma by histopathology.
MSH2 and MLH1 are the most frequently affected MMR genes in HNPCC. MSH2 and MSH6 deficient mouse models of HNPCC develop sebaceous tumors with MSI, although differentiation in the sebaceous gland was not observed in the MSH6 deficient mouse model. Homozygous Msh2 and Mlh1 knockout mice develop sebaceous skin tumors along with internal malignancies [16]. Alterations in transcript levels of MMR genes are often linked to carcinogenesis. For instance, suspected families developing colorectal cancer (HNPCC) had a 1bp insertion mutation (2269–2270 insT) within the MLH1 gene in 4 out of 11 (36%) cases, resulting in no expression of the MLH1 protein.
Another study showed that 50% (12/24) of families carried pathogenic mutations, including point mutations, nonsense mutations, missense mutations, splice site changes, and exon deletions in MLH1, MSH1 and MSH6. Additional common mutations are listed in Table 1. Recent studies have also shown somatic mutations in PI3K signaling components in 52% (14/27) of cases with ocular sebaceous carcinoma. Additional mutations affecting DNA repair activity and the chromatin remodeling pathway were observed in 4 cases. High levels of MSI were found in 3 cases, and targeted sequencing identified somatic mutations in MMR genes such as MLH1 and MSH2, suggesting the involvement of these mutations in tumorigenesis. Based on mutational genetics, sebaceous carcinoma falls into three subtypes: two are defined by MMR-derived insertion and deletion or UV damage mutations, and the third harbours truncating mutations in the ZNF750 transcription factor [12].
|
Mutation |
Detection method |
References |
|
MLH1 |
||
|
Out-of-frame del. codon 347–470 |
RT-PCR and PTT |
Bapat et al.,44 1996 |
|
150 ins T and 1884 del GGAAA |
SSCA |
Kruse et al.,13 1996 |
|
c. 2194A>T Exon 19 p.Lys732X |
PCR and Sanger sequencing |
Švec et al.,45 2014 |
|
MSH2 |
||
|
1985del AG |
PCR and DNA sequencing |
Kolodner et al.,46 1994 |
|
L458X |
PCR, RT-PCR and sequencing |
Liu et al.,47 1996 |
|
289 ins 22bp |
SSCP analysis, PTT and direct sequencing |
Kruse et al.,13 1996 |
|
380del AT |
SSCP analysis, PTT and Direct Sequencing |
|
|
2427 ins G |
Direct sequencing |
Godard et al.,48 1999 |
|
1578delC |
SSCA and direct sequencing |
Mangold et al.,49 2004 |
|
Del exon 15–16 |
SSCA and direct sequencing |
|
|
Del exon 1–6 |
SSCA and direct sequencing |
|
|
Del exon 9–10 |
SSCA and direct sequencing |
|
|
MSH6 |
||
|
p.R911* nonsense mutation |
Whole exome sequencing |
North et al.,50 2018 |
|
CTNNB1 |
||
|
Exon 3 p.Q61Q (c.183A>G) |
Direct sequencing |
Kwon et al.,51 2015 |
|
P53 |
||
|
Point mutation in TP53 exons 5-8 |
SSCP |
Kiyosaki et al.,52 2010 |
|
CGA>TGA Exon 6 Arg>Stop |
PCR and DNA sequencing |
Jayaraj et al.,43 2015 |
|
GAC>TAC Exon 7 Asp>Tyr |
PCR and DNA sequencing |
|
|
GAG>GAA Exon 6 Glu>Glu |
PCR and DNA sequencing |
|
|
MLH1: mutL homolog 1; ins: insertion; del: deletion; SSCA: single strand conformation analysis; RT-PCR: reverse transcriptase-polymerase chain reaction; PTT: protein truncation test; MSH2: mutS homolog 2; bp: base pair; SSCP: single-strand conformation polymorphism; DNA: deoxyribonucleic acid; MSH6: mutS homolog 6; CTNNB1: catenin beta1; P53: Protein53. |
||
Table 1: Common mutations in genes suspected to be associated with sebaceous gland carcinoma [13, 43-52].
The tumorigenesis process is incomplete without considering the role of p53 genes. The transcription factor p53 is a tumor suppressor and master regulator that induces apoptosis during DNA damage. Failure of p53 functions leads to uncontrolled cell proliferation and genomic instability (Figure 2). The specific cause of p53 pathway activation in SGC is not well-understood. However, a previous report showed missense and nonsense mutations in p53 genes in about 67% of SGC tumors (Table 1). Immunoreactivity was observed in 50–100% of SGC cases, indicating significant alterations in p53 signaling. Strong immunoreactivity and overexpression of p53 were found in approximately 100% of intraepithelial sebaceous carcinoma cases, demonstrating its diagnostic potential in differentiating SGC from benign sebaceous proliferation (11%), BCC (20%), and SCC (50–60%) [17]. A similar role has been observed for p16. Mutation in p53 was also identified by Sanger sequencing. Other mutations highlighted for SGC include G:C→A:T (GGA to AGA), where the substitution of arginine with glycine at codon 199 at the dimer-dimer interface significantly affects the ionic environment. Thus, alterations in the p53 signaling appears to be a primary event in SGC [18].
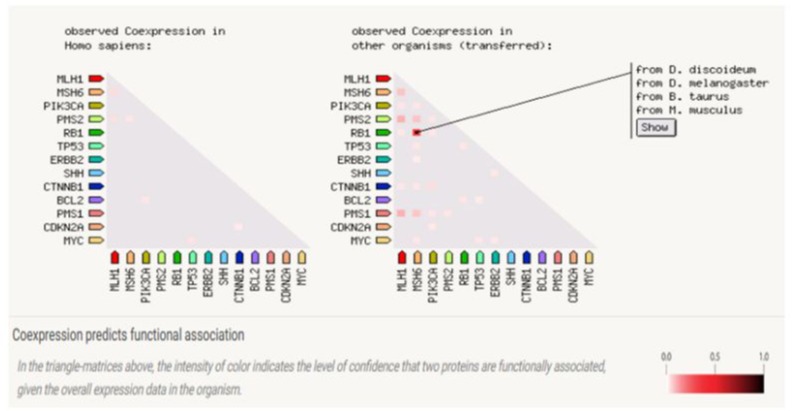
Figure 2: Gene Co-expression: Co-expression score based on RNA expression patterns and protein co-regulation.
Molecular Signaling in Eyelid SGC
Cell Cycle Dysregulation
Global microarray analysis reveals expression patterns of crucial genes associated with eyelid SGC. Cell cycle progression-related genes such as CDKN2A, CDK1, and CCNE1 are highly expressed in patients with SGC. CCNE1, a cell cycle regulator during the G1/S transition, promotes S-phase entry and the formation of DNA replication complexes. Overexpression of CCNE1 has been observed in ovarian cancer and hepatocellular carcinoma. The expression of CDK1 is activated in patients with SGC, promoting the transition of G2-M and G1-S phases. CDKN2A is a tumor-suppressor gene that regulates both CCNE1 and CDK1 to maintain cellular homeostasis. The preventive role of CDK1, CDKN2A, and CCNE1 is also found in patients with SGC through the overexpression of these genes [19, 20]. Although, the role of CDKN2A in targeting CDK1 and CCNE1 is reported to be insufficient for the prevention of SGC, the mutation and functional inactivation of CDKN2A are vital for understanding its interplay in the cell cycle regulation pathway [21].
Immune Checkpoint Regulator
The tumor microenvironment represents the interplay between malignant tumor cells and stromal cells. Stromal cells are a heterogeneous population including immune system cells (T-cells, B-cells, and macrophages) and fibroblasts, which have tumor-promoting properties at the level of multistage carcinogenesis. Targeting stromal cells could be an attractive strategy for chemotherapeutic agents against multiple forms of cancer, including SGC. T-cell-mediated immunity primarily regulates the elimination of pathogens and inhibits abnormally transformed tumor cells to maintain homeostasis in the body. Programmed death-ligand 1 (PD-L1) is encoded by the CD274 gene and is a major co-inhibitory checkpoint molecule. PD-L1 interacts with programmed cell death 1 (PD-1), predominantly expressed in T-cells, suppressing T-cell-mediated elimination of tumor cells. PD-L1 is endogenously expressed by immune cells and stromal cells. PD-1, the primary ligand of PD-L1, is expressed by T cells [22]. Aberrant expression of PD-1 and PD-L1 and their infiltration within the tumor island and stroma have been studied in SGC. Additionally, activated T-cells secrete IFN-γ, upregulating PD-1 expression. Both dendritic and tumor cells in the vicinity of these T-cells respond to IFN-γ by upregulating PD-L1 expression. The engagement of PD-1 with PD-L1 results in the suppression of T-cell activity. High levels of CD3+, CD8+, and PD-1+ T-cells at the tumor periphery promote immunosuppression in SGC, highlighting the potential of anti-PD-1 therapy in patients with locally advanced or metastatic SGC [23].
C-MYC–AR–P53 Axis
C-MYC is a transcription factor that regulates the differentiation of sebocytes. The tumor suppressor gene p53 is a key modulator of C-MYC activity, with approximately two-thirds of sebaceous carcinomas reporting p53 mutations. Oncogenic levels of C-MYC activity promote p53 activation through the DNA damage pathway. Elevated levels of p53 correlate with poor prognostic outcomes in SGC. Lower levels of C-MYC activation promote sebaceous gland expansion and differentiation, while higher levels stimulate sebaceous gland proliferation and inhibit differentiation. C-MYC is commonly associated with histone modifications marking active genes and may therefore amplify the program of gene expression dictated by other transcription factors [24, 25]. These observations suggest the need for clinicians and researchers to explore the factors influencing the outcome of C-MYC activation in eyelid SGC. Androgen receptors (AR) have been identified as an important target of C-MYC in mouse models. However, p53 has been reported to inhibit the expression of AR by direct association with the AR promoter, thereby inhibiting AR activity. C-MYC activity also regulates AR functions to prevent p53 activation, terminate proliferation, and promote differentiation onset [26]. Additionally, C-MYC is associated with histone modification and promotes the expression of active genes [27]. The transcription factor p53 regulates AR signaling by reducing local androgen synthesis in the skin. A higher number of mutations in the p53 gene causes DNA damage and inhibits AR activity in prostate cells [28, 29]. The C-MYC-AR-p53 axis highlights the importance of its target for better diagnosis and treatments in patients with SGC.
CASPASE-3/YAP Signaling
Caspase-3 is a cysteine-aspartic acid protease that plays a crucial enzymatic role in the intrinsic apoptotic cascade. Apoptosis leads to the activation of caspases, which are present as inactive zymogens in proliferating cells [30]. Several studies have shown the implication of caspase-3 in cell differentiation, dendritic pruning, sperm maturation, and learning and memory [31]. In response to various apoptotic stimuli, caspase zymogens are converted to an active state by proteolytic cleavage, initiating a cascade of reactions that lead to cell death [32, 33]. However, some studies suggest a distinct non-apoptotic role of caspase-3 as a critical regulator of cell proliferation and organ size [34, 35]. A previous study reported that caspase-3 is activated in proliferating cells of SGC but does not instruct cell elimination [36].
An in vivo study revealed that the genetic deletion or chemical inhibition of caspase-3 diminished cell proliferation, decreased cell number, and reduced the size of the eyelid sebaceous gland. Moreover, caspase-3 is also found to modulate the activation and nuclear translocation of YES protein, a vital regulator of organ size. The a-catenin (CTNNA1) remains in the cytosol via interaction with YAP (YES-associated protein) and 14-3-3 protein (cytosolic phospholipase A2), forming a stable complex that inhibits the access of PP2Ac. Consequently, sequestered YAP is in the cytoplasm in an inactivated state. Caspase-3 enables the release and nuclear translocation of YAP by cleaving a-catenin. YAP is responsible for driving the expression of XIAP (X-linked inhibitor of apoptosis protein), which in turn inhibits caspase-3 activity in eyelid SGC (Figure 3). XIAP serves as a feedback antagonist of the caspase-3/YAP circuit.
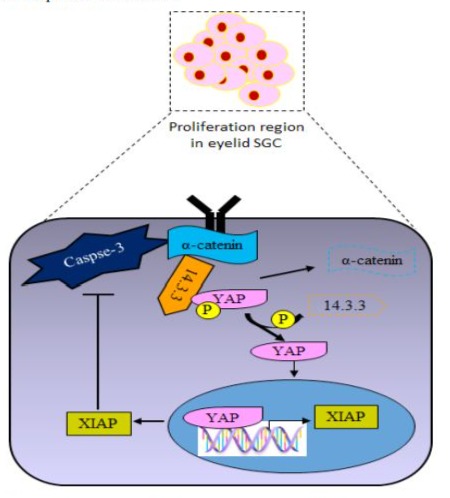
Figure 3: Schematic diagram of caspase-3/YAP signaling in proliferating cells of eyelid sebaceous gland carcinoma (SGC). a-catenin remains in the cytosol via interaction with YAP and 143-3 protein, forming a stable complex. Caspase-3 facilitates the liberation and nuclear translocation of YAP by cleaving a-catenin, where YAP is responsible for driving the expression of XIAP (X-linked inhibitor of apoptosis protein), which in turn inhibits caspase-3 activity in eyelid SGC.
Another study has shown that caspase-3 knockout mice displayed a significant decrease in cell proliferation, consequently reducing the size of the sebaceous gland. Additionally, the administration of caspase-3 inhibitors in mice controls the size and cell proliferation of the sebaceous gland [32, 33]. However, the caspase-3/YAPmediated cell proliferation and its regulation in eyelid SGC are not fully explored. Therefore, a precise and correct understanding of the caspase-3/YAP cascade is needed for future investigation. Together, these comprehensive analyses highlight the potential role of caspase-3 inhibitors as therapeutic agents against eyelid SGC.
Wnt/ß-catenin and Sonic Hedgehog Pathway
The activation of Wnt/ß-catenin and Sonic Hedgehog (SHH) signaling pathways is crucial for the normal development of embryo and adult in the vertebrates. Under normal cellular conditions, the expression of Wnt and SHH is required during stem cell renewal and embryogenesis (Figure 4). Increased expression of Wnt, SHH, and LEF-1 is necessary for the differentiation of multipotent stem cells into hair follicles and sebocyte progenitors, eventually developing into sebaceous glands. Therefore, deficient expression of Wnt and SHH proteins is linked with the pathogenesis of SGC in humans [18, 37].
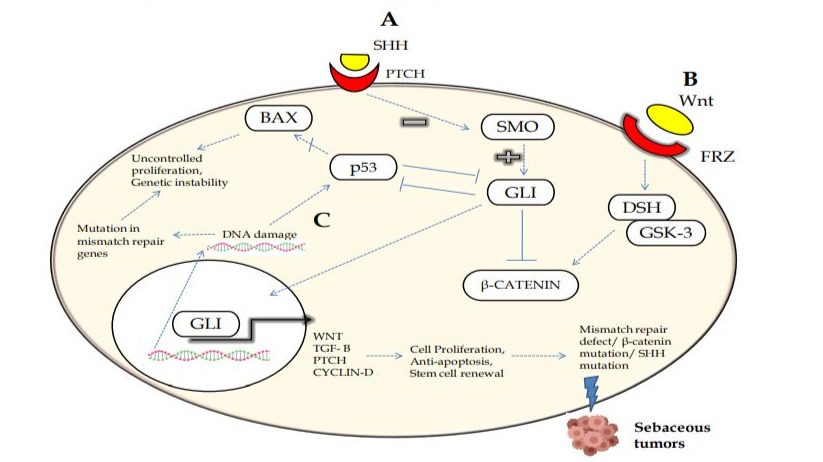
Figure 4: Illustration of the possible mechanisms for the pathogenesis of eyelid sebaceous gland carcinoma (SGC). (A) The downstream cascade of Sonic Hedgehog suppresses Smoothened with activation of Gli protein-dependent transcription of target genes. (B) Wnt signaling activation causes translocation of ß-catenin into the nucleus, leading to gene transcription. (C) Inhibition of the p53 pathway leads to an inability to stop the cell cycle, ultimately causing genetic instability. Thus, any mutations and dysregulations in the mismatch repair genes or alterations in signaling pathways appear to be implicated in SGC.
Previous studies have revealed that a defective ß-catenin binding site in LEF-1 leads to the accumulation of ß-catenin in the cytoplasm and its hyperactivation in the eyelid, caused sebaceous skin tumor generation [38]. This leads to the up-regulation of Indian hedgehog (IHH) protein, promoting the proliferation of sebaceous precursor cells. Mutations in ß-catenin have been observed in hair follicle tumors such as pilomatrixomas, along with upregulated SHH signaling. Another study has shown that components of the Wnt signaling pathway are significantly overexpressed in SGCs, with elevated activity associated with cancer including colorectal, hepatocellular, breast, and non-melanoma skin tumors [18, 39]. Similarly, previous studies have also shown an association of SHH signaling activation with the development of human cancers such as medulloblastoma, basal cell carcinoma, gastrointestinal tract tumors, ovarian cancer, and breast cancer [40]. Moreover, increased levels of Hedgehog and HH target gene products: GLI1 and PTCH1 were found in human primary bladder tumors. Therefore, targeting the Hedgehog pathway could be valuable in the clinical management of bladder cancer. Notably, SHH gene mutations are the most common cause of sporadic and inherited holoprosencephaly (HPE), suggesting that variable levels of SHH activity might contribute to some of the phenotypic variations found in HPE patients.
The contribution of Hedgehog and Wnt signaling during eyelid sebaceous carcinoma is poorly understood, except for one immunohistochemistry (IHC) study [37]. This study conducted IHC on 37 cases of eyelid sebaceous gland carcinoma, where 29 patients showed no metastasis, while 8 patients exhibited lymph node or distant metastasis with higher expression of SHH, ABCG2, and Wnt proteins. This report revealed that SHH and Wnt signaling pathways are aberrantly activated in SGC and might be involved in metastasis.
Recently, the involvement of the Hedgehog pathway in SGC has been observed using IHC and immunofluorescence (IF) techniques, showing the unregulated expression of Patched1 (PTCH1), Smoothened (SMO), and glioma-associated zinc transcription factors (Gli1 and Gli2). The expression of PTCH1 and SMO was observed in the cytoplasm, while Gli1 and Gli2 were expressed in both the cytoplasm and nuclei with similar expression levels [41]. Thus, the role of Hedgehog and Wnt signaling was found in almost all types of cancer with its functional implications. To date, the molecular mechanisms of Hedgehog and Wnt signaling in SGC remain unexplored. Moreover, the activation of Hedgehog signaling in SGC raises the possibility of medical treatment. ß-catenin is an essential developmental gene, overexpressed in the cytoplasm in the majority of eyelid SGC cases (66%), correlating positively with tumor size, orbital invasion, and pagetoid spread [42].
This review summarizes previous literatures based on the development of tumors in sebaceous glands. Mutations in MMR genes such as MLH1, MSH2, MSH6, PMS2, and p53 are not fully known in SGC in the Indian patients [43]. Furthermore, the role of Wnt/ß-catenin and the Hedgehog pathway and their dysregulation in patients with SGC pathogenesis in Indian population is still not fully understood. So far, none of the studies have been done on therapeutic treatment via targeting Hedgehog pathway in patients with SGC. Thus, this review suggests that clinicians and researchers should focus on the Hedgehog pathway as a target for therapeutic treatments in patients with SGC (Figure 4). Although, significant research has been done, including mutational analysis and the identification of associated genes and their expression in SGC and other cancers. However, no study has been performed on the interaction and binding of transcriptional factors (e.g., p53) with target candidate genes and their altered transcript and protein levels in sebaceous gland carcinoma [37, 42]. Mutational screening and sequence analysis, including deletion, duplication, and insertion of candidate genes, have not been fully investigated in Indian SGC patients. Only a few studies have reported the role of SHH/Wnt signaling and the expression of crucial proteins involved in SGC, but the mechanistic functions remain unclear. Therefore, the SHH/Wnt signaling pathway could be a causative factor for tumor progression in sebaceous glands. The expression levels of genes involved in SHH/Wnt signaling have not been fully studied at the protein level in patients with SGC. This review suggests that more studies are required for a better understanding of the molecular biology of sebaceous gland development and the pathogenesis of SGC in humans [18, 37].
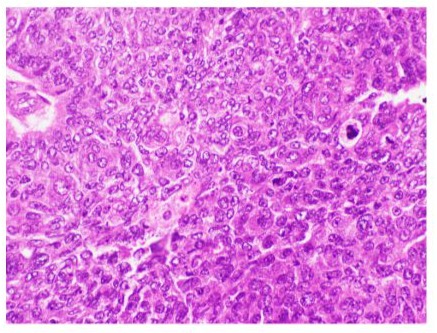
Figure 5: Microphotograph of sebaceous carcinoma showing cytoplasmic vacuoles and mitotic activity (Hematoxylin & Eosin at magnification X20).
Histopathology
In SGC patients, various clinico-pathological factors are associated with a poor prognosis of the tumor. These factors include lymphovascular and orbital invasion, involvement of both upper and lower eyelids, poor differentiation, multicentric origin, long duration of symptoms, large tumor size, an infiltrative pattern, and pagetoid invasion of the epithelium of the skin or conjunctiva. Prognosis is generally better for tumors originating from the glands of Zeis. However, tumors originating from the upper eyelids have a worse prognosis compared to those of the lower lids [2, 3].
In Silico Analysis
Bioinformatics approaches have been widely used to identify the molecular basis of disease pathogenesis. The main aim of the present review is to describe the crucial genes and signaling pathways involved in the pathogenesis of sebaceous gland carcinoma. This review focuses on a comprehensive analysis of candidate genes and signaling pathways along with their functional analysis based on protein-protein interaction (PPI) networks using STRING-version 11.0 (Search Tool for the Retrieval of Interacting Genes/Proteins) database and Cytoscape 3.7.1 software. For example, several genes, including MLH1, MSH2, MSH6, PMS2, p53, SHH, and CTNNB1 are most involved in the tumorigenesis of the sebaceous gland in Indian patients (Figure 7A and B) [43].
To date, no literature is available that lists the role of crucial genes with their mutational spectrum, expression levels (e.g., genes and proteins), and their cross-talk networks or signaling pathways in patients with SGC. The molecular and functional analysis of the Hedgehog pathway and its key components in SGC remain unexplored. Many key regulators or genes are selected and implicated for mutational analysis and dysregulation during SGC pathogenesis. Therefore, we review strong indicators or markers and genes involved in SGC pathogenesis by in-silico analysis via their interaction and binding.
In the current review, in-silico analysis of models has been performed for interacting proteins that are often involved in SGC. The proteins with high tendencies of interaction, homology, and co-expression are shown in the STRING database. These proteins could be selected for further wet lab study to better understand their functions in SGC. The STRING database, a biological database for predicted PPIs, contains information from curated databases, experimental data, text mining, co-expression, co-occurrence, and gene neighbourhood data.
In our review, we propose protein-protein interactions based on available literatures and studies performed. In this analysis, maximum homology, co-expression, experimentally determined interaction, and combined score views were observed among selectively expressed proteins (SEPs) in humans (Table 2). The constructed model using the STRING database proves better interactors of SEPs with other proteins responsible for causing SGC. Some commonly important interactors with SEPs through STRING include proteins like BCL2, MYC, RB1, CDKN2A, ERBB2, and PIK3CA, which are strong interacting proteins. TP53 and CTNNB1, transcription factors that can bind to promoter and regulatory elements, modulate the expression pattern of selected genes. All these proteins are critically important for the development and maintenance of the eye. Mutation or dysregulation in genes or proteins involved in eyelid formation causes ocular tumorigenesis.
|
Node 1 internal Id |
Node 2 internal Id |
Co-expression |
Experimentally determined interaction |
Combined score |
|
MSH6 4433630 |
MLH1 4433549 |
0.182 |
0.639 |
0.999 |
|
PMS2 4435654 |
MSH6 4433630 |
0.161 |
0.552 |
0.999 |
|
PMS2 4435654 |
MLH1 4433549 |
0.22 |
0.869 |
0.993 |
|
TP53 4435880 |
BCL2 4446777 |
0 |
0.525 |
0.984 |
|
PMS1 4448418 |
MSH6 4433630 |
0.141 |
0.552 |
0.986 |
|
TP53 4435880 |
CDKN2A 4448997 |
0 |
0.384 |
0.997 |
|
TP53 4435880 |
RB1 4435747 |
0.061 |
0.379 |
0.986 |
|
TP53 4435880 |
MYC 4451407 |
0.091 |
0.139 |
0.969 |
|
CTNNB1 4441426 |
ERBB2 4435897 |
0 |
0.472 |
0.989 |
|
CTNNB1 4441426 |
MYC 4451407 |
0 |
0.328 |
0.991 |
|
PMS1 4448418 |
MLH1 4433549 |
0.188 |
0.874 |
0.969 |
|
TP53 4435880 |
MLH1 4433549 |
0 |
0 |
0.972 |
|
TP53 4435880 |
PMS2 4435654 |
0 |
0 |
0.963 |
|
TP53 4435880 |
ERBB2 4435897 |
0 |
0.167 |
0.945 |
|
TP53 4435880 |
PIK3CA 4435346 |
0 |
0.06 |
0.907 |
|
TP53 4435880 |
MSH6 4433630 |
0.061 |
0.336 |
0.776 |
|
MLH1 4433549 |
MYC 4451407 |
0 |
0.305 |
0.626 |
|
MLH1 4433549 |
CDKN2A 4448997 |
0.054 |
0 |
0.799 |
|
CTNNB1 4441426 |
TP53 4435880 |
0 |
0 |
0.792 |
|
PMS1 4448418 |
PMS2 4435654 |
0.061 |
0 |
0.705 |
|
PMS1 4448418 |
TP53 4435880 |
0 |
0 |
0.446 |
|
CTNNB1 4441426 |
CDKN2A 4448997 |
0.048 |
0 |
0.67 |
|
MSH6 4433630 |
RB1 4435747 |
0.429 |
0 |
0.501 |
|
CTNNB1 4441426 |
MLH1 4433549 |
0.061 |
0 |
0.652 |
|
CTNNB1 4441426 |
RB1 4435747 |
0.056 |
0.16 |
0.428 |
|
MSH6 4433630 |
CDKN2A 4448997 |
0 |
0 |
0.542 |
|
MLH1 4433549 |
PIK3CA 4435346 |
0.061 |
0 |
0.676 |
|
SHH 4437380 |
TP53 4435880 |
0 |
0 |
0.785 |
|
CTNNB1 4441426 |
SHH 4437380 |
0 |
0 |
0.714 |
|
SHH 4437380 |
ERBB2 4435897 |
0.061 |
0.125 |
0.582 |
|
MLH1: mutL homolog 1; MSH2: mutS homolog 2; MSH6: mutS homolog 6; CTNNB1: catenin beta 1; P53: Protein 53; PMS2: PMS1 homolog 2; TP53: Tumor protein 53; BCL2: B-cell lymphoma 2; CDKN2A: cyclin dependent kinase inhibitor 2A; PIK3CA: phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha; SHH: Sonic Hedgehog; CTNNB1: catenin beta 1; ERBB2: erb-b2 receptor tyrosine kinase 2; RB1: Retinoblastoma; PMS2: PMS1 homolog2; PMS1: PMS1 homolog1; MYC: myc proto-oncogene. |
||||
Table 2: Putative interactors of selected proteins in human using the STRING 11.0 database.
Examining the model generated through STRING, it was observed that the maximum combined score was obtained between MSH6MLH1 (0.999) and MSH6-PMS2 (0.999) along with co-expression (genes correlated in expression in an experiment and strong indicators of functional associations) MSH6-MLH1 (0.154) and MSH6-PMS2 (0.173; Figure 6 and Table 2), but no homology was found between them. However, maximum homology (0.664) and co-expression (0.269) were found between PMS2-MLH1 with a combined score (0.998). Almost similar readings were found between PMS1-MLH1 with a slightly lower combined score (0.986).
Regarding other organisms, human beings show a lack of co-expression analysis due to insufficient literature and experimental proof available (Figure 6). The homology and occurrence were maximum in eukaryotes and minimum in archaea for all 13 interactive proteins (Figure 7). Among all putative interactors, MMR genes show maximum occurrence among phyla. General hypothesis for oxidative damage to DNA repair activity is represented in Figure 9.
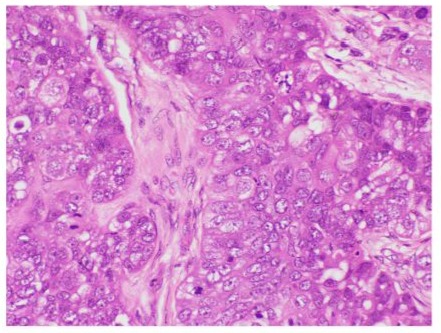
Figure 6: Microphotograph demonstrating lobules with high grade sebaceous gland cells (Hematoxylin & Eosin at magnification X40).
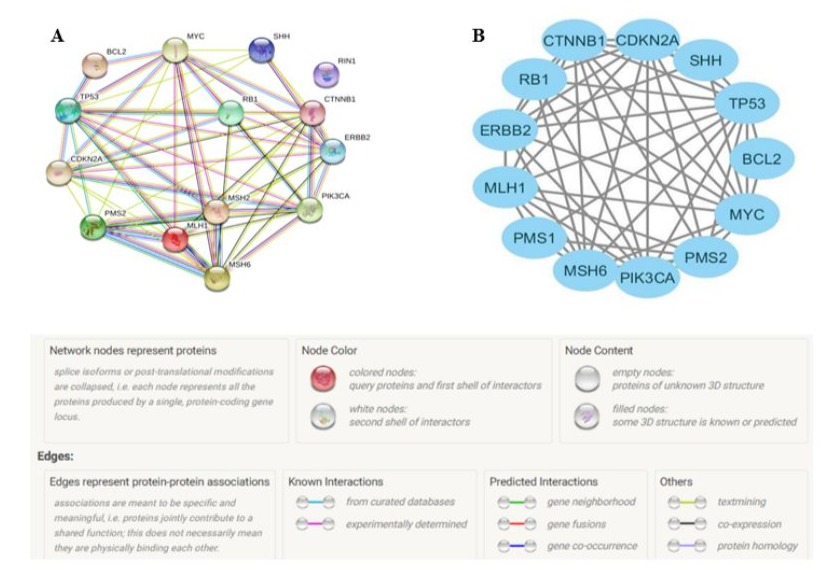
Figure 7: A: In silico analysis of protein-protein interactions constructed using the STRING 11.0 database. B: Cluster analysis of the protein network using Cytoscape 3.7.1 software. Nodes represent proteins, and lines represent interactions between two proteins.
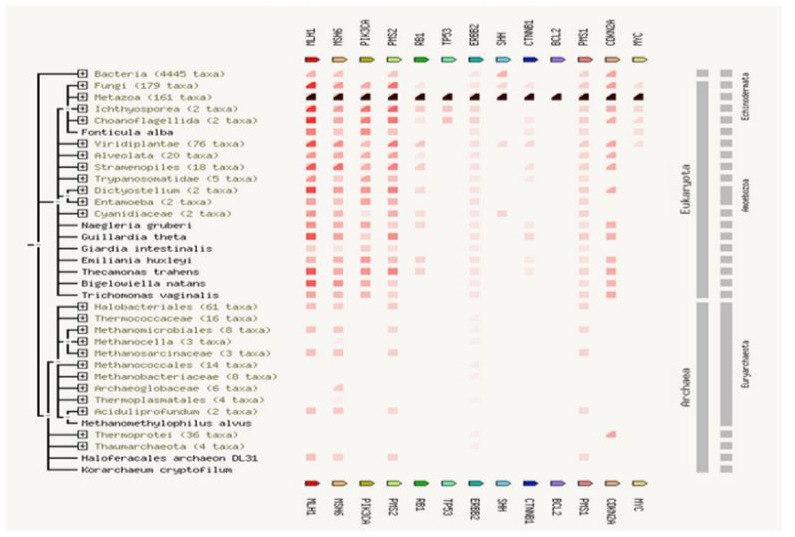
Figure 8: Gene concurrence: Gene families similarity occurrence is highest in eukaryotes and lowest in archaea.
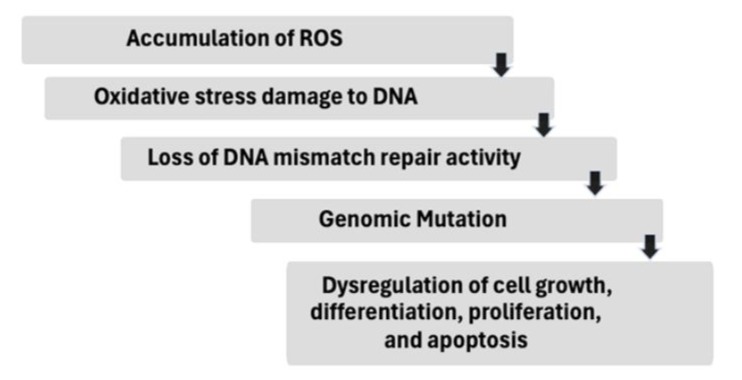
Figure 9: General hypothesis for oxidative damage to DNA repair activity.
Conclusion
In this review, we have investigated and summarized the role of selectively expressed genes (SEGs) such as MLH1, MSH2, MSH6, PMS2, p53, SHH, and CTNNB1 in the pathogenesis of eyelid sebaceous gland carcinoma. Our analysis showed that MMR genes are significantly correlated with a poor survival rate in SGC patients, like other cancers. Additionally, pathways including WNT/ß-CATENIN, CAPSPASE-3/YAP, C-MYC-AR-P53, and Hedgehog are major contributors to cancer cell proliferation.
The references in this review confirm the potential role of SEGs in both animal models and humans. Computational-based analysis helps to prioritize gene networks and their potential roles, enhancing our understanding of molecular genetics and cellular mechanisms underlying the progression and development of sebaceous gland carcinoma. The results from the PPI network analysis suggest that the SEPs and dysregulated pathways play important roles in SGC, providing theoretical knowledge for the search for effective drug targets. This review remarkably covers the noteworthy points, but further studies are still necessary to justify these findings in disease pathways, progression and management.
Conflict of Interest
We declare that no conflict of interest for this manuscript. All authors have confirmed and approved the manuscript for submission. The authors confirm that the manuscript has been submitted solely to this journal and it is not published elsewhere.
References
- Knackstedt T, Samie FH (2017) Sebaceous Carcinoma: A review of the scientific literature.Current Treatment Options in Oncology 8:47.
- Straatsma BR (1956) Meibomian gland tumors. AMA Arch Ophthalmol 56:71-93
- Shields JA, Demirci H, Marr BP, Eagle Jr. RC, Shields CL (2005) Sebaceous carcinoma of the ocular region: A review. Surv Ophthalmol 50:103–122.
- Kaliki S, Ayyar A, Dave TV, Ali MJ, Mishra DK, et al. (2015) Sebaceous gland carcinoma of the eyelid: clinicopathological features and outcome in Asian Indians. Eye (Lond) 29:958-63.
- Umar A, Risinger JI, Hawk ET, Barrett JC (2004) Testing guidelines for hereditary non-polyposis colorectal cancer. Nat Rev Cancer 4:153-8.
- Deo SVS, Shukla NK, Singh M, Jha D, Khanna P, et al. (2012) Locally advanced sebaceous cell carcinoma (T3) of eyelid: incidence and pattern of nodal metastases and combined modality management approach. Orbit 31:150-4.
- Kyllo RL, Brady KL, Hurst EA (2015) Sebaceous carcinoma: Review of the literature. Dermatol Surg 41:1-15.
- Kaliki S, Das AV (2021) Ocular and Periocular Tumors in India: An EyeSmart Electronic Medical Record Analysis of 9633 Cases from a Referral Center. Middle East Afr J Ophthalmol 27:199-203.
- Kass LG, Hornblass A (1989) Sebaceous carcinoma of the ocular adnexa. Surv Ophthalmol 33:477-90.
- Jahagirdar SS, Thakre TP, Kale SM, Kulkarni H, Mamtani M (2007) A clinicopathological study of eyelid malignancies from central India. Indian J Ophthalmol 55:109-12.
- Kale SM, Patil SB, Khare N, Math M, Jain A, et al. (2012) Clinicopathological analysis of eyelid malignancies - A review of 85 cases. Indian J Plast Surg 45:22-8.
- Gupta P, Gupta RC, Khan L (2017) Profile of eyelid malignancy in a Tertiary Health Care Center in North India. J Cancer Res Ther 13:484486.
- Singh R, Maurya RP, Bosak S, Singh MK, Singh VP (2016) Sebaceous gland carcinoma and its correlation with different signaling pathways with emphasis on p53. Int J Ocul Oncol Oculoplasty 1:34-41
- Ponti G, Longo C (2013) Microsatellite instability and mismatch repair protein expression in sebaceous tumors, keratocanthoma, and basal cell carcinomas with sebaceous differentiation in Muir-Torre syndrome. J Am Acad Dermatol 68:509-10.
- Abdel-Rahman WM, Mecklin JP, Peltomäki P (2006) The genetics of HNPCC: application to diagnosis and screening. Crit Rev Oncol Hematol 58:208–220.
- Wei K, Clark AB, Wong E, Kane MF, Mazur DJ, et al. (2003) Inactivation of Exonuclease 1 in mice results in DNA mismatch repair defects, increased cancer susceptibility, and male and female sterility. Genes Dev 17:603-14.
- Niemann C, Owens DM, Huelsken J, Birchmeier W, Watt FM (2002) Expression of DeltaNLef1 in mouse epidermis results in differentiation of hair follicles into squamous epidermal cysts and formation of skin tumours. Development 129:95–109.
- Niemann C, Unden AB, Lyle S, Zouboulis CC, Toftgård R, et al. (2003) Indian hedgehog and beta-catenin signaling: role in the sebaceous lineage of normal and neoplastic mammalian epidermis. Proc Natl Acad Sci U S A 100 (Suppl 1):11873–80.
- Kim N, Kim JE, Choung HK, Lee MJ, Khwarg SI (2014) Expression of cell cycle regulatory proteins in eyelid sebaceous gland carcinoma:low p27 expression predicts poor prognosis. Exp Eye Res 118:46-52.
- Yunoki T, Hirano T, Tabuchi Y, Furusawa Y, Torigoe M, et al. (2020) CDKN2A, CDK1, and CCNE1 overexpression in sebaceous gland carcinoma of eyelid. Int Ophthalmol 40:343-350.
- Wuarin J, Nurse P (1996) Regulating S phase: CDKs, licensing and proteolysis. Cell 85:785-7.
- Tomonari M, Shimada M, Nakada Y, Yamamoto I, Itoh M, et al. (2019) Muir-Torre syndrome: Sebaceous carcinoma concurrent with colon cancer in a kidney transplant recipient; a case report. BMC Nephrol 20:394.
- Cook Jr BE, Bartley GB (2001) Treatment options and future prospects for the management of eyelid malignancies: an evidence-based update. Ophthalmology 108:2088-98.
- Rahl RB, Lin CY, Seila AC, Flynn RA, McCuine S, et al. (2010) c-Myc regulates transcriptional pause release. Cell 141:432-45.
- Nascimento em, Cox cl, MacArthur s, Hussain s, Trotter m, et al. (2011) The opposing transcriptional functions of Sin3a and c-Myc are required to maintain tissue homeostasis. Nat Cell Biol 13:1395-405.
- Cottle DL, Kretzschmar K, Schweiger PJ, Quist SR, Gollnick HP,et al. (2013) c-MYC-induced sebaceous gland differentiation is controlled by an androgen receptor/p53 axis. Cell Rep 3:427-41.
- Markova MS, Zeskand J, McEntee B, Rothstein J, Jimenez SA, et al. (2004) A role for the androgen receptor in collagen content of the skin. J Invest Dermatol 123:1052-6.
- Arnold I, Watt FM (2001) c-Myc activation in transgenic mouse epidermis results in mobilization of stem cells and differentiation of their progeny. Curr Biol 11:558-68.
- Watt FM, Frye M, Benitah SA (2008) MYC in mammalian epidermis: how can an oncogene stimulate differentiation?. Nat Rev Cancer 8:234-42.
- Taylor RC, Cullen SP, Martin SJ (2008) Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol 9:231-41.
- Amelio MD, Cavallucci V, Cecconi F (2010) Neuronal caspase-3 signaling: Not only cell death. Cell Death Differ 17:1104-14.
- Fuchs Y, Steller H (2015) Live to die another way: modes of programmed cell death and the signals emanating from dying cells. Nat Rev Mol Cell Biol 16:329-44.
- Bell RAV, Megeney LA (2017) Evolution of caspase-mediated cell death and differentiation: twins separated at birth. Cell Death Differ 24:1359-1368.
- Eskandari E, Eaves CJ (2022) Paradoxical roles of caspase-3 in regulating cell survival, proliferation, and tumorigenesis. J Cell Biol 221:e202201159.
- Dehkordi MH, Munn RGK, Fearnhead HO (2022) Non-Canonical Roles of Apoptotic Caspases in the Nervous System. Front Cell Dev Biol 10:840023.
- Yosefzon Y, Soteriou D, Feldman A, Kostic L, Koren E, et al. (2018) Caspase-3 Regulates YAP-Dependent Cell Proliferation and Organ Size. Mol Cell 70:573-587.e4.
- Kim N, Kim JE, Choung HK, Lee MJ, Khwarg SI (2013) Expression of Shh and Wnt signaling pathway proteins in eyelid sebaceous gland carcinoma: clinicopathologic study. Invest Ophthalmol Vis Sci 54:3707.
- Lang CMR, Chan CK, Veltri A, Lien WH (2019) Wnt Signaling Pathways in Keratinocyte Carcinomas. Cancers (Basel) 11:1216.
- Arce L, Yokoyama NN, Waterman ML (2006) Diversity of LEF/TCF action in development and disease. Oncogene 25:7492-504.
- Iriana S, Asha K, Repak M, Sharma-Walia N (2021) Hedgehog Signaling: Implications in Cancers and Viral Infections. Int J Mol Sci 22:1042.
- Cheng AY, Lan J, Lee CH (2022) Impaired Wnt/beta-catenin and protein patched homolog 1 signaling in extraocular sebaceous carcinoma: A clinical and histopathological study. J Dermatol 49:600-606.
- Taipale J, Beachy PA (2001) The Hedgehog and Wnt signalling pathways in cancer. Nature 411:349-54.
- Jayaraj P, Sen S, Sharma A, Chosdol K, Kashyap S, et al. (2015) Eyelid sebaceous carcinoma: a novel mutation in lymphoid enhancerbinding factor-1. Br J Dermatol 173:811-4.
- Bapat B, Xia L, Madlensky L, Mitri A, Tonin P, et al. (1996) The genetic basis of Muir-Torre syndrome includes the hMLH1 locus. Am J Hum Genet 59:736-9.
- Svec J, Schwarzova L, Janosíkova B, Stekrova J, Mandys V, et al. (2014) Synchronous gastric and sebaceous cancers, a rare manifestation of MLH1-related Muir-Torre syndrome. Int J Clin Exp Pathol 7:5196-202.
- Kolodner RD, Hall NR, Lipford J, Kane MF, Rao MR, et al. (1994) Structure of the human MSH2 locus and analysis of two Muir-Torre kindreds for msh2 mutations. Genomics 24:516-26.
- Liu B, Parsons R, Papadopoulos N, Nicolaides NC, Lynch HT, et al. (1996) Analysis of mismatch repair genes in hereditary non-polyposis colorectal cancer patients. Nat Med 2:169-74.
- Godard V, Coulet F, Bernaudin JF, Housset M, Soubrier F (1999) [Mutation in the MSH2 gene in Muir-Torre syndrome]. Ann Dermatol Venereol 126:600-3.
- Mangold E, Pagenstecher C, Leister M, Mathiak M, Rutten A, et al. (2004) A genotype-phenotype correlation in HNPCC: strong predominance of msh2 mutations in 41 patients with Muir-Torre syndrome. J Med Genet 41:567-72.
- North JP, Golovato J, Vaske CJ, Sanborn JZ, Nguyen A, et al. (2018) Cell of origin and mutation pattern define three clinically distinct classes of sebaceous carcinoma. Nat Commun 9:1894.
- Dong F, Jiang J, McSweeney C, Zou D, Liu L, et al. (2016) Deletion of CTNNB1 in inhibitory circuitry contributes to autism-associated behavioral defects. Hum Mol Genet 25:2738-2751.
- Kiyosaki K, Nakada C, Hijiya N, Tsukamoto Y, Matsuura K, et al. (2010)Analysis of p53 mutations and the expression of p53 and p21WAF1/ CIP1 protein in 15 cases of sebaceous carcinoma of the eyelid. Invest Ophthalmol Vis Sci 51:7-11.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.