Surgical Treatment of Migraines: A Review
by Justin D Sawyer, Danielle Olla, Lauren Woldanski*, Nicole Sommer
Institute for Plastic Surgery Southern Illinois University School of Medicine, Springfield, IL, USA
*Corresponding author: Lauren Woldanski, Institute for Plastic Surgery Southern Illinois University School of Medicine, 747 N Rutledge Ave, 3rd Flr, Baylis Building Springfield IL, 62702IL, USA
Received Date: 27 March, 2025
Accepted Date: 07 April, 2025
Published Date: 10 April, 2025
Citation: Sawyer JD, Olla D, Woldanski L, Sommer N (2025) Surgical Treatment of Migraines: A Review. J Family Med Prim Care Open Acc 9: 280. https://doi.org/10.29011/2688-7460.100280
Abstract
Background and Aims: Migraine headaches affect millions of Americans and remain a large burden on the U.S. healthcare system. Traditional management includes avoidance of triggers and multiple abortive and prophylactic pharmacologic therapies. There have been several advances in treatment in the last decade, most notably calcium-gene related peptide antagonists and migraine surgery. Migraine surgery is relatively new technique targeting peripheral nerves of the head and neck thought to serve as trigger sites for initiating migraine headaches. Our aim is to provide an overview of the current medical and surgical management for refractory migraines. Methods: We review current pharmacologic therapies and describe the foundational evidence for migraine surgery as an end-stage treatment for patients with difficult to treat migraine headaches. Results: There is a myriad of pharmacologic treatments available for migraine headaches. Majority of the selection has sub-optimal symptom resolution rates. The most promising non-surgical intervention includes those that target the calcium-gene related peptide pathway, although long term data is still needed. Surgical intervention provides optimistic outcome data to provide relief for patients with refractory, debilitating migraines. Interpretation: Refractory migraines can be a vexatious problem for the physician and patient alike. Pharmacologic options for migraine headaches are plentiful but success mercurial. The most promising pharmacologic therapies are those targeting the calcium-gene related peptide pathway, which would require ongoing therapy. Surgical intervention is a promising intervention for definitive symptom relief conserved for patients with refractory, debilitating migraines.
Keywords: Migraine; Migraine headaches; Migraine surgery; Migraine trigger zones; Refractory migraines
Introduction
Migraine headaches are a prevalent disorder that cause significant burden to patients, the healthcare system, and the economy at large. It has been estimated that 35 million Americans, constituting to more than fourteen percent of the population, suffer from migraine headaches [1]. The economic consequences are significant, with 112 million collective workdays missed, $1 billion in medical expenses, and $16 million in productivity annually [2-4].
Primary Care providers and Neurologists bear the brunt of the condition, given its long, complicated, and frequently unsuccessful treatment modalities. Plastic Surgeons become involved in refractory cases, offering botulinum toxin injections or surgical intervention.
Traditionally, migraines have been treated with multiple pharmacologic agents for prophylaxis and abortive therapy. Abortive therapies include analgesics, Non-Steroidal Anti-Inflammatory Drugs (NSAIDs), triptans (5-HT serotonin receptor agonists), and ergot derived compounds. Prophylactic interventions range from lifestyle modifications (smoking cessation, regular exercise, caffeine avoidance, stress management, and sufficient sleep) to multiple drug therapies. The most common drugs used are beta-blockers (BBs), anticonvulsants, Calcium Channel Blockers (CCBs), anti-depressants, serotonin antagonists, nutraceuticals [5,6] and Calcium Gene-Related Peptide (CGRP) antagonists, which offer inconsistent and often less than satisfactory symptom control.
A better understanding of the pathophysiology and recent advances in migraine management have led to a repertoire of treatments which are commonly performed by plastic and peripheral nerve surgeons. Recent research has unveiled that irritation of peripheral trigeminal nerve branches may precede central spreading which may be responsible for initiation and progression of the symptoms of migraines [6]. The purpose of this review is to discuss advances in the understanding of migraine pathophysiology and consequential treatments offered by plastic and peripheral nerve surgeons, which may provide additional options to the primary care physician, internist, and neurologists repertoire for referral in the management of refractory migraine patients.
Discussion
Current medical management of migraine headaches can be divided into abortive therapy and prophylactic therapy. This article will focus on prophylactic medical therapies, as the surgical and non-surgical options offered by plastic surgeons are prophylactic measures.
The array of medications available for the prophylactic treatment of migraines illustrates the historical lack of understanding regarding migraine pathophysiology. Traditional drugs include BBs, CCBs, anti-depressants, anti- epileptics, serotonin antagonists, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, and a variety of supplements [5-7]. BBs, CCBs, and anti-convulsant are the mainstays of migraine prophylaxis treatment [5,7]; however, data regarding their efficacy is inconsistent. In addition, each of the aforementioned drugs carry an array of adverse drug reactions and potentially lifelong medication dependence (Table 1).
|
Therapeutic Sub-Class |
Examples |
Dosing |
Efficacy |
Side Effects |
|
Beta Blockers |
||||
|
Propranolol Metoprolol |
BID |
50% efficacy of reducing migraine frequency, duration, or intensity by 50% |
Drowsiness Fatigue Hypotension Bradycardia impotence Gastrointestinal upset Depression Memory loss |
|
|
Calcium Channel Blockers |
||||
|
Verapamil |
TID-QID |
Decreased migraine frequency from 6.7 to 3.8 migraines per patient per month. Mean decrease of 49% [37] |
Bradycardia Heart block |
|
|
Anti-depressants |
||||
|
TCAs |
Amitriptyline |
QHS |
50% efficacy in 55.3% (vs 34.0% of placebo subjects) [38] |
Antimuscarinic: Dry mouth Constipation Dizziness Blurred vision Urinary retention Prolonged QT Lowered seizure threshold |
|
SSRIs |
Fluoxetine |
Daily |
Mixed data as to whether efficacious or not [39] |
Nausea Diarrhea Constipation Sleep problems Decreased libido/erectile dysfunction Dry mouth drowsiness |
|
SNRIs |
Venlafaxine |
Four less headache days in 2-month period vs one less with placebo [40] |
||
|
Antiepileptics |
||||
|
Gabapentin |
TID |
50% efficacy in 46% of patients vs 16% placebo [41] |
Dizziness Somnolence Anesthesia |
|
|
Valproic Acid* |
Daily |
Divalproate – reduction of 4.4 to 3.2 per week (vs 4.2 to 3.6 placebo) |
Weight gain Nausea/vomiting GI upset Pancreatitis Pancytopenia Liver Failure Requires OP lab work |
|
|
Topiramate* (most frequently used) |
qDay- BID |
54% have 50% efficacy vs. 23% with placebo [42] |
Paresthesia Memory problems Fatigue Decreased appetite Nausea Diarrhea Renal stone |
|
|
Calcium Gene-related Peptide Antagonists |
||||
|
Monoclonal Antibodies |
Galonezumab |
Once monthly |
50% reduction of migraines 70- 77% |
Injection site irritation |
|
*FDA approved for migraine prevention |
||||
Table 1: Pharmacologic Therapies for Migraine Prophylaxis.
Beta-Blockers and Calcium Channel Blockers
The BBs propranolol and metoprolol are commonly used for migraine prophylaxis. Literature suggests that these drugs have about a 50% efficacy, defined by reducing migraine frequency, duration, or intensity by 50% [7]. BBs' common side effects include drowsiness, fatigue, hypotension, bradycardia, impotence, gastrointestinal upset, depression, and memory loss [7]. Unlike BBs, CCBs have become less favourable for migraine prevention in recent years. Their use was based on early evidence that has since been determined insufficient according to multiple evidence-based guidelines by the American Academy of Neurology [7, 8].
Anti-Depressants
Multiple classes of anti-depressants are used for migraine prevention with a wide array of mechanisms of action. Tricyclic Anti-Depressants (TCAs) have long been used for migraine prophylaxis with level II evidence; however, these drugs have an extensive profile of adverse reactions. These include anti- muscarinic symptoms such as dry mouth, constipation, dizziness, blurred vision, urinary retention, prolonged QT interval, and lowered seizure threshold [8].
Selective Serotonin Reuptake Inhibitors (SSRIs) and serotonin- norepinephrine reuptake inhibitors (SNRIs) have been studied with mixed results regarding efficacy in migraine prevention. A 1999 review examined placebo- controlled trials and have found no benefit in patients treated with the SSRI fluoxetine vs. placebo [9]. The SNRI, venlafaxine, has been shown to significantly reduce the number of headache days in treated patients vs. placebo; however, in this study 14% of patients withdrew secondary to adverse drug reactions including nausea, vomiting, and drowsiness [10].
Anti-Epileptics
The most common anti-epileptic drugs used in the treatment of migraine prevention are gabapentin, valproic acid, and topiramate, all of which have different mechanisms. Topiramate is likely the most frequently used, given the high-quality data supporting its use. Topiramate and valproate are the only anti- epileptics with Food and Drug Administration (FDA) approval for migraine prevention [7].
A 2008 Cochrane review concluded that there is not sufficient evidence to inform clinical practice regarding gabapentin in migraine prevention [11].
Sodium valproate and its derivatives have been shown to be clinically effective in the prevention of migraines with response rates ranging from 43-48% [7]. A randomized, placebo-controlled trial showed that extended-release Divalproex reduced migraine frequency from 4.4 per week to 3.2 per week vs. 4.2 to 3.6 per week in the placebo group [12]. Like the medications mentioned above, valproate does come with a significant side effect profile, including weight gain, nausea, vomiting, gastrointestinal upset. Patients must also be followed with outpatient labs for the possibility of complications such as pancreatitis, pancytopenia, and liver failure [7].
Topiramate has been shown to be effective in multiple high-quality clinical trials in reducing the frequency of migraine attacks [7,8]. For this reason, it is commonly prescribed for migraine prophylaxis. Like similar drugs, there are several adverse reactions that may decrease compliance including paraesthesia, memory problems, fatigue, decreased appetite, nausea, diarrhoea, renal stone, and several others [7].
CGRP Antagonists and Monoclonal Antibodies
The most significant advance in medical migraine management consists of two classes of medications targeting the CGRP system. CGRP is a small peptide that has been well studied and implicated in the pathophysiology of migraine headaches. It has been noted that nearly half of the trigeminal system’s neurons express CGRP [13]. The first class of drugs targeting CGRP were small peptides that blocked the CGRP receptor called Gepants, all of which have been tested and shown efficacy in reducing the number and duration of migraine attacks. However, some of these drugs had undesirable side effects including hepatic toxicity [14]. Ubrogepant is currently the only drug of this class approved by the FDA and is approved for abortive therapy, although numerous others are currently under investigation.
The CGRP system has also been targeted with Monoclonal Antibodies (MAbs) specific for either the CGRP receptor (erenumab) or the CGRP molecule itself (galconezumab, erenumab, fremanezumab). These drugs have the advantage of extrahepatic metabolism, averting toxicity, and have all shown efficacy in migraine relief [14]. The most common adverse drug reactions include injection site irritations [13]. Trials have shown 50% reduction in migraine days in as high as 77% (eptinezumab 1000 mg) and 70% (galconezumab 150 mg) [15]. These drugs also have the advantage of infrequent dosing (once monthly for galconezumab, erenumab, and fremanezumab and once quarterly for eptinezumab), facilitating greater patient compliance. However, paralleling the relative novelty of these drugs is the lack of long-term data.
While multiple medical agents exist for the prophylactic treatment of migraine headaches, data remain controversial regarding many of the drugs. All drugs used for migraine prophylaxis have many possible adverse drug reactions. Drugs targeting the CGRP system offer the most promising non-surgical therapy for migraine headaches with minimal side effects and will likely overshadow other medical therapies in the future.
Pathophysiology
Understanding of migraine pathophysiology has evolved a great deal in the last decade. Historically, an exclusively vascular causation was the leading hypothesis for migraine pathophysiology; however, recent hypotheses implicate irritation of extracranial nerves, including branches of the trigeminal and the occipital nerve [6,16,17]. It is thought that the peripheral nerve branches endure irritation secondary to compression or traction at defined trigger sites, evidenced by migraine relief with decompression and reduction of traction on the nerves at the trigger sites. The earliest evidence of the peripheral trigger point hypothesis was the discovery that manipulation of the supraorbital and supratrochlear nerves during an endoscopic brow lift incidentally achieved relief of migraine symptoms [17].
It is hypothesized that the peripheral irritation and mechanical stimulation of these nerves lead to the generation of vasoactive peptides such as substance P and CGRP. The peptides may then travel proximally where they have their effect on the dura and vasculature causing the central symptoms of a migraine headache [16-18]. With the success of peripheral nerve releases and decompression in treating migraine headaches, it is convincing that peripheral nerve irritation plays a vital role in the initiation of migraine headaches, although the complete mechanism remains poorly understood. Migraine surgery has been shown to decrease the frequency of migraine headaches approximately 10 headache days per month and decreases pain severity and duration of headaches when refractory episodes occur [19]. There is a reported 79.5 percent rate of immediate improvement or elimination of symptoms [20].
Anatomical Considerations
The trigger sites that have been identified as initiation points for migraines headaches include branches of the trigeminal nerve system (supraorbital, supratrochlear, zygomaticotemporal, and terminal nasal branches) and the greater and lesser occipital nerves (Table 2). These are easily identifiable on a detailed history and migraine diary. Compression of the supraorbital and supratrochlear nerves is implicated in frontal migraines with the corrugator supercilii, depressor supercilii, and procerus muscles as well as adjacent bony and fascial structures serving as the compression and traction sites (Figure 1) [20,21]. The most common trigger points for temporal migraines are the trigeminal nerve's zygomaticotemporal and auriculotemporal branches. The zygomaticotemporal nerve can be irritated/compressed by the temporal bone, the temporalis muscles, and the deep temporal fascia, while the auriculotemporal is more commonly provoked by the superficial temporal artery and preauricular fascial bands (Figure 2) [22,23]. Irritation of the greater occipital nerve is primarily responsible for occipital migraine headaches, typically compressed by semispinalis capitis, occipitalis capitis, trapezius, and nuchal fascia (Figure 3) [24,25]. Finally, rhinogenic migraines are associated with intranasal irritation points of terminal branches of the trigeminal nerve, which can sometimes be identified on computed tomography of the face [26].
|
Peripheral Nerve |
Compressed Branch |
Migraine Distribution |
Structures of compression |
|
Trigeminal |
Supraorbital |
Frontal |
Corrugator supercilii |
|
Supratrochlear |
Depressor supercilii Procerus Bony/Fascial Structures |
||
|
Zygomaticotemporal |
Temporal |
Temporal bone Temporalis muscles Deep temporal fascia Superficial temporal artery |
|
|
Terminal branches |
Rhinogenic |
Intranasal irritation points |
|
|
C2 |
Greater occipital (medial branch of dorsal ramus) |
Occipital |
Semispinalis capitus Occipitalis capitus Occipital artery Trapezius Nuchal fascia |
|
Lesser Occipital (ventral ramus branch) |
Occipital |
Occipital Artery Fascial bands along its course |
Table 2: Common Trigger Sites and Involved Structures for Migraine Headaches.
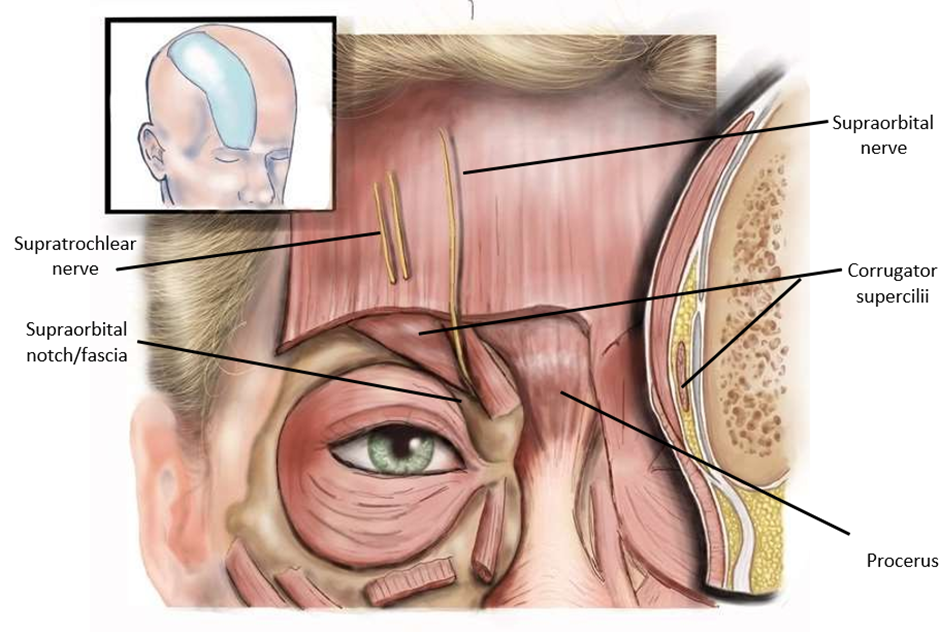
Figure 1: Anatomy of Supratrochlear and Supraorbital Nerves and Compression Sites.
Figure 1 demonstrates the anatomy of the supraorbital and supratrochlear nerves and their compression sites. Both nerves may be compressed by the corrugator supercilii, depressor supercilii, procerus, and surrounding bony and fascial structures.
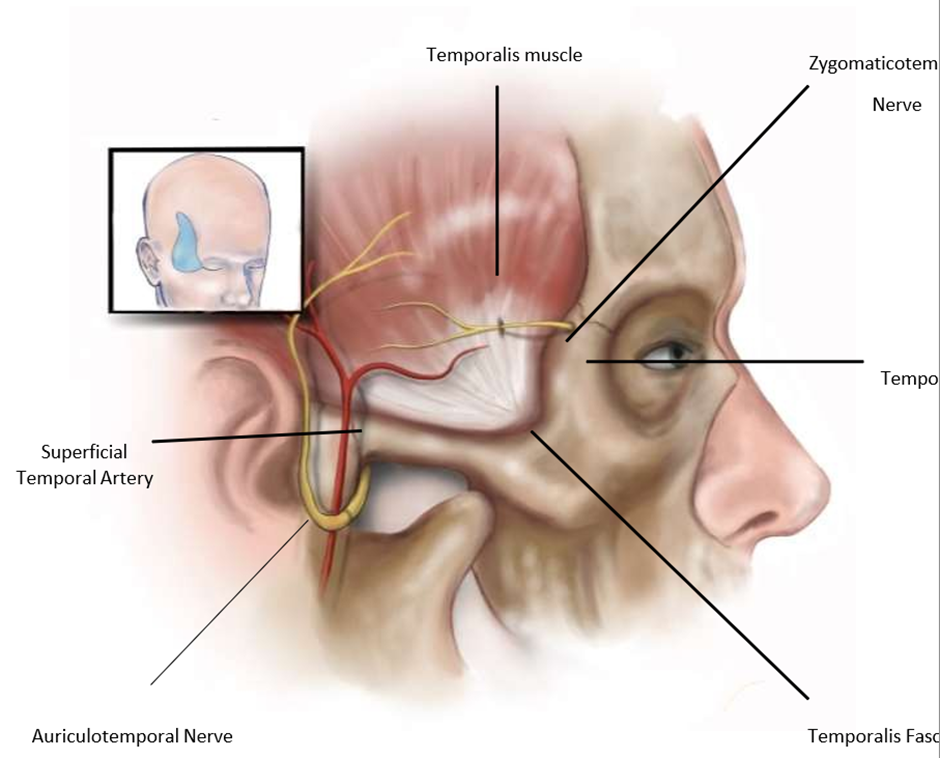
Figure 2: Anatomy of the Zygomaticotemporal Nerve and Compression Sites.
Figure 2 shows the anatomy of the temporal branch
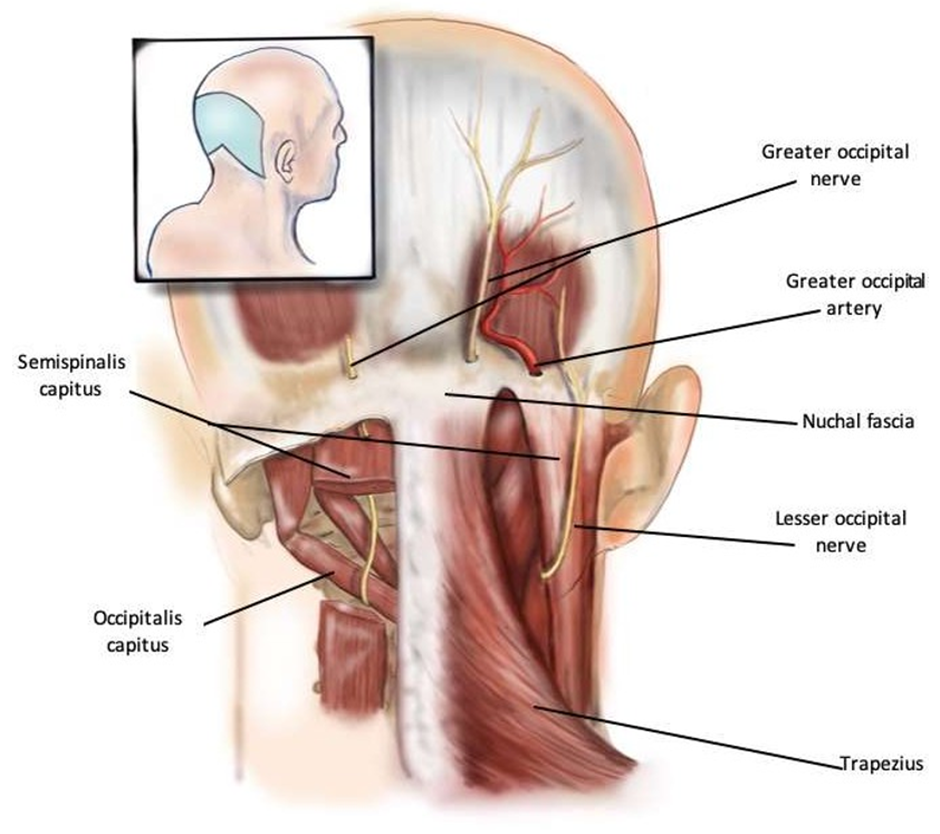
Figure 3: Anatomy of Greater and Lesser Occipital Nerves and Compression Sites.
Figure 3 demonstrates the anatomy of the greater and lesser occipital nerves and their sites of compression. The greater occipital nerve can be compressed by the occipitalis capitus, the semispinalis capitus, the trapezius, the nuchal fascia, and occasionally the occipital artery. The lesser occipital nerve is typically compressed by sternocleidomastoid and the nuchal fascia.
An in-depth understanding of this anatomy provides the basis for the treatment of migraines by plastic surgeons. The peripheral nerve trigger points can be precisely treated and diagnosed with botulinumtoxin A (BTX-A) and local anesthetic blocks prior to surgical intervention.
Botulinum Toxin Type A Injections
BTX-A has become commonplace in the realm of migraine therapy, as indicated by its FDA approval for chronic migraines. The commonly known and cited BTX-A action mechanism includes inhibition of acetylcholine release via the cleavage of the cell surface N-ethylmaleimide-sensitive factor attachment protein receptors, and consequent inhibition of acetylcholine release at the neuromuscular junction [27]. Initially, the theory for BTX-A relieving migraine symptoms was quite simple, in that it was believed to relax the muscles that serve as trigger points of the peripheral branches of the trigeminal and occipital nerve branches, therefore reducing irritation of the nerves implicated in migraine initiation. While this mechanism is likely partially responsible for the role BTX-A plays in migraine alleviation, recent literature suggests that the mechanism may be more complex than simply muscle relaxation at trigger points, also involving central action via axonal transport, modulation of neurotransmitter release, and alterations of nociceptive cell surface receptors and cytokines, and interaction with opioid receptors [28].
Regardless of the precise mechanism, a large body of high-quality evidence exits pertaining to the effectiveness of migraine symptom relief with the use of BTX-A injections. Binder and colleagues reported the first trial results using BTX-A for migraine headaches showing a complete response rate of 51% and partial reduction in 38% of patients [29]. This trial was quickly supported by a randomized controlled trial by Silberstein and colleagues in which 25 units of BTX-A injected into muscle trigger points was shown to significantly reduce the frequency and severity of migraine headaches in trial subjects, as well as reduce the need for acute migraine abortive medications and migraine associated vomiting [27]. Behmand, et al. reported excellent response rates in single trigger site injections for migraines, with 55% of patients reporting complete resolution and an additional 28% reporting at least 50% reduction in frequency or intensity [30]. In 2010, Dodick and colleagues pooled the results of the two Phase 3 Research Evaluating Migraine Prophylaxis studies and again found that the BTX-A groups benefited in both the frequency and severity of the migraine attacks amongst multiple other secondary end points [31]. The high quality of evidence put forth by this group was quintessential in the eventual FDA approval of BTX-A for prophylactic treatment of adults with chronic migraine headaches.
When a patient is evaluated for BTX-A injections in the setting of chronic migraines, trigger points are identified through a series of questions and often a headache diary. It is most useful for a patient to present for BTX-A evaluation with at least a one-month log of their headaches, including the site of origin, location of pain, duration, associated symptoms, presence or absence of aura, and any associated external triggers. This information will allow the injecting physician to identify the trigger points that will most likely lead to the migraines’ successful treatment. BTX-A injections’ success is a good indicator that the patient would benefit from migraine surgery at the identified trigger site.
However, in some patients, serial BTX-A injections will suffice for migraine management, and surgery will not be required. Typically, insurance will cover this therapy if the patient has migraines for at least fifteen days per month, lasting for four or more hours.
Migraine Surgery
Migraine surgery involves the surgical release and neurolysis of targeted peripheral nerve trigger sites identified by history, physical exam, and often prior site-specific BTX-A or local anesthetic injections. In simple terms, this is similar to the surgical release of the median nerve carpal tunnel; although, as previously discussed, peripheral nerve contribution to migraines is undoubtedly more complicated than simply a compression neuropathy.
The advent of migraine surgery was an incidental finding discovered after patients undergoing resection of the corrugator supercilii muscles for facial rejuvenation reported relief of migraine symptoms. This finding was reported by Guyuron et al. in 2000, who showed symptomatic relief in 80% of patients [21]. The group then followed this retrospective review with a prospective trial involving 22 patients undergoing resection of the corrugators with or without transection of the zygomaticotemporal branch of the trigeminal nerve, this time reporting improvement in migraine symptoms in 95.5% of patients, complete resolution of migraines in 45.5%, and significant improvement in 50%. In addition, the number of migraine events per month decreased from 5.2 to 0.8 in the surgical cohort [32].
These studies were followed by the first prospective migraine surgery trial reported in 2005, in which 100 patients were randomized to the surgical group, and 89 underwent either glabellar muscle (corrugators and procerus) resection, resection of the zygomaticotemporal branch of the trigeminal nerve or both, depending on their trigger sites [20]. 92% of the treatment group patients benefited from at least a 50% reduction in migraine frequency severity or duration compared to 15.8% in the control group.
Additional studies have demonstrated significant alleviation of migraine symptoms following migraine surgery, reporting nearly 70% success rates [33,34]; however, none more rigorous than the randomized controlled trial reported by Guyuron and colleagues in 2009 [35]. In this landmark study, patients were randomized to undergo either migraine surgery with release or transection of the involved nerve at the trigger sites (frontal, temporal, or occipital) or a sham procedure in which the trigger site nerve was exposed but not manipulated, released, or transected. 84% of patients in the treatment group had at least a 50% reduction in migraine frequency, severity, or duration compared to 57.7% in the sham surgery group. The finding of symptomatic improvement in the sham group is interesting and possibly attributed to the placebo effect, a known phenomenon in migraine research, or simply inadvertent manipulation of the target nerves during the sham procedure. Regardless, this study provided high- quality evidence for the efficacy of migraine surgery for refractory migraines.
They reported 5-year outcomes in 2011, demonstrating a persistent positive response to the surgery in 88% of patients in their treatment group [36].
No surgery comes without risks, and while the risks are minimal for migraine surgery, they should be discussed with the patient preoperatively. Reported adverse events include numbness in the head and face, temporal hollowing, post-operative itching, temporary hair loss, neck stiffness, and persistent function of the glabellar muscles; however, many of these were not persistent when studied at five years postoperatively [35,36]. An additional risk is that the patient may not respond to the surgical treatment and may no longer benefit from BTX-A. As with any surgical procedure, bleeding, infection, and injury to nearby structures are additional risks of migraine surgery.
Conclusions
Migraine treatment is a persistently prevalent problem that can involve multiple medical specialties. In the last two decades, the understanding of migraine headaches, their pathophysiology, and treatment has significantly evolved. There is a growing body of high-quality evidence to support the treatment of migraine headaches using BTX-A and surgical intervention. This understanding and ability to refer patients to plastic surgeons will enhance opportunities to offer successful intervention for a challenging problem.
Conflicts of Interest: The authors have no conflicts of interest to disclose.
References
- Burch RC, Loder S, Loder E, Smitherman TA (2015) The prevalence and burden of migraine and severe headache in the United States: updated statistics from government health surveillance studies. Headache 55: 21-34.
- Hu XH, Markson LE, Lipton RB, Stewart WF, Berger ML (1999) Burden of migraine in the United States: disability and economic costs. Arch Intern Med 159: 813-818.
- Stewart WF, Lipton RB, Celentano DD, Reed ML (1992) Prevalence of migraine headache in the United States. Relation to age, income, race, and other sociodemographic factors. JAMA 267: 64-69.
- Goldberg LD (2005) The cost of migraine and its treatment. Am J Manag Care 11: S62-S67.
- Goadsby PJ, Sprenger T (2010) Current practice and future directions in the prevention and acute management of migraine. Lancet Neurol 9: 285-298.
- Olla D, Sawyer J, Sommer N, Moore 4th JB (2020) Migraine Treatment. Clin Plast Surg 47: 295-303.
- Silberstein SD (2015) Preventive Migraine Treatment. Continuum (Minneap Minn) 21: 973-989.
- Silberstein SD, Holland S, Freitag F, Dodick DW, Argoff C, et al. (2012) Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology 78: 1337-1345.
- Gray RN, Goslin RE, McCrory DC, Eberlein K, Tulsky J, et al. (1999) AHRQ Technical Reviews, in Drug Treatments for the Prevention of Migraine Headache. Agency for Health Care Policy and Research (US): Rockville (MD).
- Liu F, Ma T, Che X, Wang Q, Yu S (2017) The Efficacy of Venlafaxine, Flunarizine, and Valproic Acid in the Prophylaxis of Vestibular Migraine. Front Neurol 8: 524.
- Mulleners WM, Chronicle EP (2008) Anticonvulsants in migraine prophylaxis: a Cochrane review. Cephalalgia 28: 585-597.
- Freitag FG, Collins SD, Carlson HA, Goldstein J, Saperet J, et al. (2002) A randomized trial of divalproex sodium extended- release tablets in migraine prophylaxis. Neurology 58: 1652- 1659.
- Edvinsson L, Haanes KA, Warfvinge K, Krause DN (2018) CGRP as the target of new migraine therapies - successful translation from bench to clinic. Nat Rev Neurol 14: 338-350.
- Edvinsson L (2019) Role of CGRP in Migraine. Handb Exp Pharmacol 255: 121-130.
- Schuster NM, Rapoport AM (2017) Calcitonin Gene-Related Peptide- Targeted Therapies for Migraine and Cluster Headache: A Review. Clin Neuropharmacol 40: 169-174.
- Olesen J, Burstein R, Ashina M, Tfelt-Hansen P (2009) Origin of pain in migraine: evidence for peripheral sensitisation. Lancet Neurol 8: 679-690.
- Lv X, Wu Z, Li Y (2014) Innervation of the cerebral dura mater. Neuroradiol J 27: 293-298.
- Fusco M, D'Andrea G, Miccichè F, Stecca A, Bernardini D, et al. (2003) Neurogenic inflammation in primary headaches. Neurol Sci 24: S61-S64.
- Torabi R, Bourn L, Veith J, Wisecarver I, Briley Jr K, et al. (2020) Population-Based Health Utility Assessment of Migraine Headache Symptoms before and after Surgical Intervention. Plast Reconstr Surg 145: 210-217.
- Guyuron B, Kriegler JS, Davis J, Amini SB (2005) Comprehensive surgical treatment of migraine headaches. Plast Reconstr Surg 115: 1-9.
- Guyuron B, Varghai A, Michelow BJ, Thomas T, Davis J (2000) Corrugator supercilii muscle resection and migraine headaches. Plast Reconstr Surg 106: 429-434; discussion 435-7.
- Chim H, Okada HC, Brown MS, Alleyne B, Liu MT, et al. (2012) The auriculotemporal nerve in etiology of migraine headaches: compression points and anatomical variations. Plast Reconstr Surg 130: 336-341.
- Janis JE, Hatef DA, Thakar H, Reece EM, McCluskey PD, et al. (2010) The zygomaticotemporal branch of the trigeminal nerve: Part II. Anatomical variations. Plast Reconstr Surg 126: 435-442.
- Mosser SW, Guyuron B, Janis JE, Rohrich RJ, et al. (2004) The anatomy of the greater occipital nerve: implications for the etiology of migraine headaches. Plast Reconstr Surg 113: 693-697; discussion 698-700.
- Janis JE, Hatef DA, Ducic I, Reece EM, Hamawy AH, et al. (2010) The anatomy of the greater occipital nerve: Part II. Compression point topography. Plast Reconstr Surg 126: 1563-1572.
- Guyuron B (2012) Surgical management of migraine headaches. In: Guyuron B., Kinney B, editors. Aesthetic plastic surgery video atlas. 313-323.
- Silberstein S, Mathew N, Saper J, Jenkins S (2000) Botulinum toxin type A as a migraine preventive treatment. For the BOTOX Migraine Clinical Research Group. Headache 40: 445-450.
- Do TP, Hvedstrup J, Schytz HW (2018) Botulinum toxin: A review of the mode of action in migraine. Acta Neurol Scand 137: 442-451.
- Binder WJ, Brin MF, Blitzer A, Schoenrock LD, Pogoda JM (2000) Botulinum toxin type A (BOTOX) for treatment of migraine headaches: an open-label study. Otolaryngol Head Neck Surg 123: 669-676.
- Behmand RA, Tucker T, Guyuron B (2003) Single-site botulinum toxin type a injection for elimination of migraine trigger points. Headache 43: 1085-1089.
- Dodick DW, Turkel CC, DeGryse RE, Aurora SK, Silberstein SD, et al. (2010) OnabotulinumtoxinA for treatment of chronic migraine: pooled results from the double-blind, randomized, placebo- controlled phases of the PREEMPT clinical program. Headache 50: 921-936.
- Guyuron B, Tucker T, Davis J (2002) Surgical treatment of migraine headaches. Plast Reconstr Surg 109: 2183-2189.
- Dirnberger F, Becker K (2004) Surgical treatment of migraine headaches by corrugator muscle resection. Plast Reconstr Surg 114: 652-657; discussion 658-9.
- Poggi JT, Grizzell BE, Helmer SD (2008) Confirmation of surgical decompression to relieve migraine headaches. Plast Reconstr Surg 122: 115-122.
- Guyuron B, Reed D, Kriegler JS, Davis J, Pashmini N, et al. (2009) A placebo-controlled surgical trial of the treatment of migraine headaches. Plast Reconstr Surg 124: 461-468.
- Guyuron B, Kriegler JS, Davis J, Amini SB (2011) Five-year outcome of surgical treatment of migraine headaches. Plast Reconstr Surg 127: 603-608.
- Solomon GD, Steel JG, Spaccavento LJ (1983) Verapamil prophylaxis of migraine. A double-blind, placebo-controlled study. JAMA 250: 2500-2502.
- Couch JR, Ziegler DK, Hassanein RS (1974) Evaluation of amitriptyline in migraine prophylaxis. Trans Am Neurol Assoc 99: 94-98.
- Modi S, Lowder DM (2006) Medications for migraine prophylaxis. Am Fam Physician 73: 72-78.
- Ozyalcin SN, Talu GK, Kiziltan E, Yucel B, Ertas M, et al. (2005) The efficacy and safety of venlafaxine in the prophylaxis of migraine. Headache 45: 144-152.
- Mathew NT, Rapoport A, Saper J, Magnus L, Klapper J, et al. (2001) Efficacy of gabapentin in migraine prophylaxis. Headache 41: 119-128.
- Silberstein SD (2004) Topiramate in migraine prevention: evidence-based medicine from clinical trials. Neurol Sci 25: S244-S245.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.