Surgical Site Infection Wound Bundles Should Become Routine in Colorectal Surgery: A Meta-Analysis
Authors: Deirdre Foley1, Madga Bucholc2, Randal Parlour3, Caroline McIntyre1, Alison Johnston1, Michael Sugrue3*
*Corresponding Author: Michael Sugrue, Emergency Surgery Outcomes Advancement Project (eSOAP), Letterkenny University Hospital, Donegal, Ireland This project was supported by the European Union’s INTERREG VA Programme, managed by the Special EU Programmes Body (SEUPB) and Donegal Clinical and Research Academy
Received Date: 19 January, 2022
Accepted Date: 24 January, 2022
Published Date: 27 January, 2022
Citation: Foley D, Bucholc M, Parlour R, McIntyre C, Johnston A, et al. (2022) Surgical Site Infection Wound Bundles Should Become Routine in Colorectal Surgery: A Meta-Analysis. J Surg 7: 1465 DOI: https://doi.org/10.29011/2575-9760.001465
Abstract
Background: Surgical Site Infections (SSI) are a major source of post-operative complications and potentially affect oncological outcomes. Reducing SSI is multi-factorial, best served by the additive affect of individual wound bundle elements. With changing strategies and novel innovations ongoing meta-analyses are needed to inform current practice. This study undertook a meta-analysis of existing wound bundles impact on SSI in colorectal surgery.
Methods: A PROSPERO-registered (ID: CRD42018104923) meta-analysis following Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines and using databases PubMed, Scopus and Web of Science, from January 2008 to July 2018, was undertaken. Articles scoring ≥ 17 using Methodological Index for non-randomised Studies (MINORS) criteria were included.
Results: 5,104 articles were reviewed, and 27 studies met inclusion criteria with a total cohort of 23851 patients. Wound bundles significantly decreased SSI rates from 17.5% to 9.7%. Sub-analysis identified greatest impact on superficial SSI (risk reduction of 54%; p<0.00001) and organ-space infections (risk reduction 42%; p=0.0006).Wound bundles also significantly reduced hospital length of stay (MD = −0.79; p<0.00001).
Conclusions: Colorectal wound bundles significantly reduce the risk of SSI and length of hospital stay. They should become routine in colorectal surgery. Future work encompasses the need for standardisation of wound complications, standardised follow-up of patients and internationally agreed research definitions.
Keywords: Care pathway; Colorectal surgery; Surgical site infections; Surgical outcomes; Wound bundles
List of Abbreviations: ASA: American Society of Anesthesiologists; CDC: Centres for Disease Control and Prevention; CI: Confidence Interval; ECDC: European Centres for Disease Control and Prevention; IHI: Institute of Healthcare Improvement; MINORS: Methodological Index for non-randomised Studies; NSQIP: National Surgical Quality Improvement Program; PO: Per Oral; PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines; RR: Relative Risk; SSI: Surgical Site Infection
Introduction
The global impact of Surgical Site Infection (SSI) is increasingly recognised, both in terms of post-operative complications and the effect on patient’s outcomes. SSI rates vary internationally, related in part to variable definitions, different populations, co-morbidities and strategies utilised to reduce surgical site infection [1,2]. Surgical site infection may cause distress and inconvenience to patients, delay their discharge, increase risk of incisional hernia and re-admission to hospital [3,4]. Furthermore, the hospital or patients may be financially penalised. Recently the negative oncological impact of SSI is becoming increasingly reported [5-7]. A key to reducing SSI is a team approach, involving all providers, in every phase of care, with a cumulative additive benefit of each aspect in the bundle. A wound bundle, in general, will have more than three components and extend from pre-operative care through to rehabilitation. Newer concepts in colorectal surgery wound care include negative pressure therapy [8] and wound protective devices [9,10].
While several meta-analyses have been performed looking at bundles and surgical site infection, with the exception of PopVicas, et al. [11], most relate to publications and interventions before 2016. The search strategy used in this paper differed from that of Pop-Vicas, et al. [11] in that it used different keywords and databases. This study therefore undertook a meta-analysis of bundle impact on SSI.
Methods
Search Strategy and Study Eligibility
A detailed meta-analysis of the literature was undertaken to incorporate articles relating to colorectal surgery wound care, surgical wound infection, and surgical site care bundles. Existing research optimizing wound care in colorectal surgery was reviewed to determine current bundle strategies to improve wound outcomes. A systematic review and meta-analysis of all published English articles was conducted using PubMed, Scopus, Web of Science and Cochrane electronic databases from 2008 to July 2018. A literature search was conducted using keywords; colorectal surgery, surgical site infections, wound bundles, compliance, care pathway, and surgical outcomes. Additional studies were identified by searching the reference lists of included articles.
Inclusion and Exclusion Criteria
The methods of the analysis and inclusion criteria were specified in advance and registered with the International Prospective Register of Systematic Reviews (PROSPERO) on 23/07/2018. This meta-analysis adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. The Centres for Disease Control and Prevention (CDC) definitions for surgical site infection were used. They are classified into superficial, deep or organ/space in this study [12]. A wound care bundle was defined as three or more items combined to reduce wound infection as per the Institute for Healthcare Improvement [13]. For this meta-analysis, only studies with pre- and post- intervention SSI data for colorectal surgery were included, while studies that did not compare results to pre-intervention SSI rates were not included. Non-English articles were not included.
Eligibility Assessment and Data Extraction
Eligibility assessment was performed independently in a blinded standardised manner by two reviewers (DF and CMcI). Disagreements between reviewers were resolved by discussion between the two review authors and if no agreement could be reached, it was planned a third reviewer would decide (AJ), however a third reviewer was not required. Two reviewers (DF and CMcI) independently assessed each published study for the quality of study design by using the Methodological Index for nonrandomised Studies (MINORS) score [14]. A MINORS score of ≥ 17 was considered the standard for inclusion. Information was extracted from each included study on SSI classifications, bundle elements, length of stay, bundle adherence rates, study design, country, study length, cohort sizes, and SSI rates pre- and postintervention. The primary outcome was SSI rates following the use of wound bundles. Secondary outcomes were the effect of individual interventions included in the bundles and the SSI rates for superficial, deep and space organ infections.
Statistical Analysis
For comparison of SSI rates pre-and post-intervention risk ratios (RR) were calculated using Review Manager Version Five (RevMan5). Meta-analyses were performed by computing the RR using Mantel-Haenszel method and both fixed-effect models or random-effects models, depending on the heterogeneity of studies. Heterogeneity was assessed using the I2 statistic where a value greater than 50% was considered high and a randomeffect model was then used to combine variables of interest. RR and 95% Confidence Intervals (CI) for each classification of SSI was calculated, along with the p-value for which a value < 0.05 represented statistical significance. For the analysis of wound bundle elements, individual bundle elements in each study were reported in three phases of care: pre, peri- and post-operative care. However, any perioperative intervention that was only used once was not included in the table and was reported separately. The individual elements of each wound bundle were reviewed, and random-effect models were used to further explore the underlying effects of specific methodological features and intervention aspects of the care bundles on the rate of SSI. Some wound bundle features were identified that may explain some of the heterogeneity in the risk of SSI between studies.
Four studies provided sufficient raw data to carry out a meta-analysis on risk factors for SSI [15-18]. The following risk factors were analyzed: American Society of Anesthesiologists (ASA) physical status, diabetes mellitus and surgical approach (open vs. laparoscopic).
Results
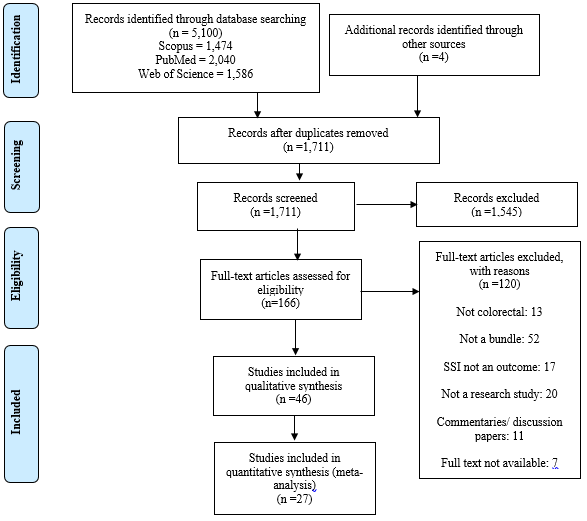
This meta-analysis reviewed 5,104 articles. 46 studies were found to be potentially suitable and 27 studies [19-41] were included in this meta-analysis with a total cohort of 23851 patients (Figure 1).
19/46 studies were excluded from the meta-analysis: Seven studies stratified cohorts based primarily on compliance in using a bundle [42-48], six did not state colorectal specific SSI rates [49-54], 3 were deemed of low quality [55-57] and three did not provide pre-intervention cohort sizes [58-60]. Characteristics of included studies are shown in Table 1.
Wound Bundle and their effect on Surgical Site Infection Rates
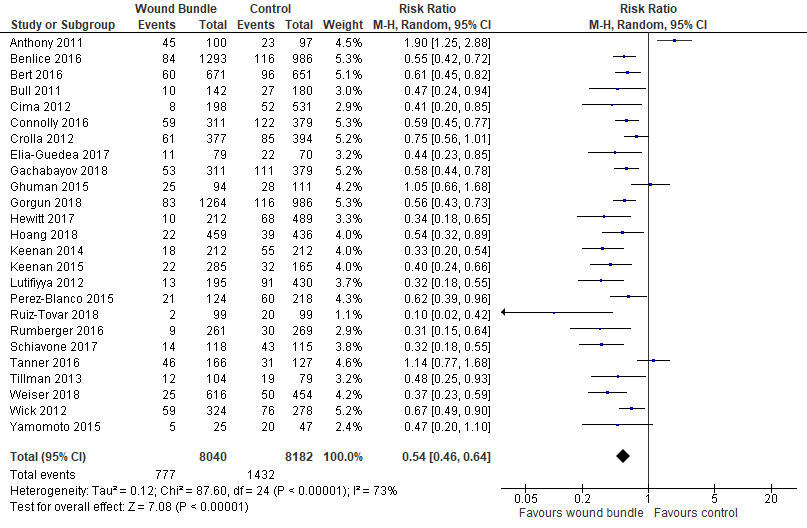
Overall SSI Rates: Of the 27 studies included in the meta-analysis two large studies (almost 8000 patients) only reported superficial SSI rates and were not included in the overall SSI rate analysis [39,41]. There was an overall decrease in SSI rates following the implementation of wound bundles (1432/8182 [17.5%] vs 777/8040 [9.7%]). There was significant heterogeneity between trials (I2=73%) and a random-effects model was used. Despite the heterogeneity there was significant reduction in the risk of SSIs by 46% (RR=0.54; 95% CI, 0.46-0.64; p<.00001, I2=73%) (Figure 2).
22 of the 25 studies had a statistically significant decrease in overall SSI rates following bundle implementation [16-20, 22-38]. Two studies showed no effect [21,40] and Anthony, et al. 2011 [15] reported a statistically significant increase in SSI after bundle implementation.
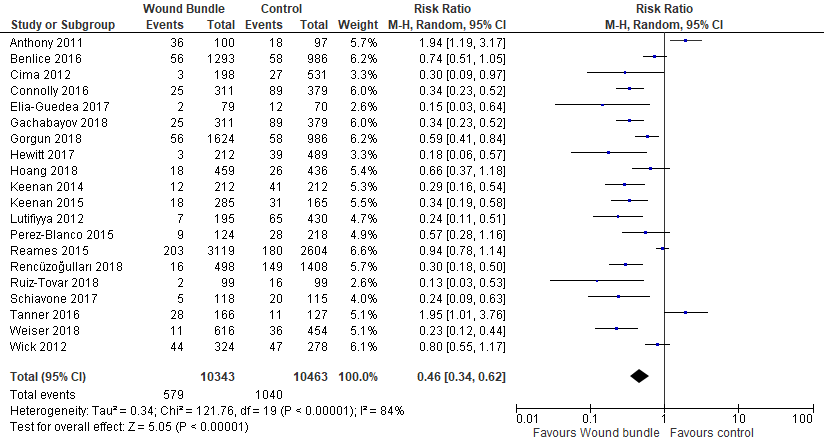
Superficial SSI Rates: Superficial SSI rates were reported in 20 studies with a cohort of 20,806 patients. The meta-analysis showed that wound bundles reduced superficial SSIs by 54% (RR= 0.46; 95% CI, 0.34-0.62; p<.00001, I2=84%) (Figure 3).
Deep SSI Rates: Fifteen studies [16,18,20-22,25,26,28-32,34,36-38] included data on deep SSIs, with only one study [37] showing a statistically significant decrease in deep SSI rates. Overall there was not a statistically significant reduction in the risk of deep SSI and this meta-analysis required a fixed-effect model due to its heterogeneity of I2=0% (RR=0.76; 95% CI, 0.56-1.04; p= 0.09, I2=0%) (Figure 4).
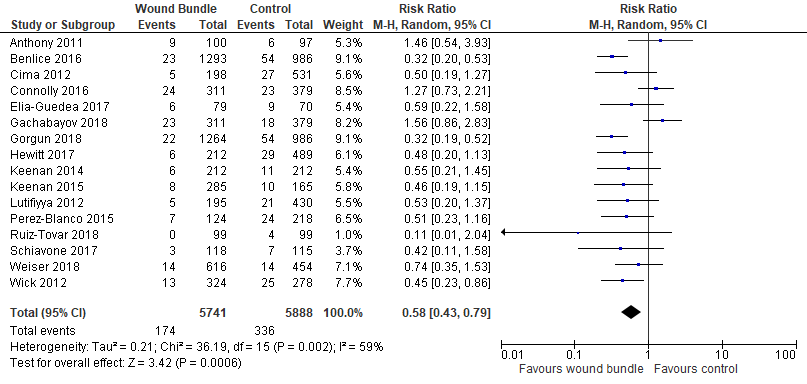
Organ Space SSI Rates: Sixteen studies [15,16,18,20,22,23,25,27-32,34,36,38] reported organ space SSI rates. The meta-analysis showed a statistically significant reduction in the risk of organ/space SSIs by 42% (RR=0.58; 95% CI, 0.43-0.79; p=.0006, I2=59%) (Figure 5).
Bundle Elements Results: We identified the following study features that may explain some of heterogeneity in the risk of SSI between studies: the use of Mechanical Bowel Preparation (MBP) and oral antibiotics, wound protectors, instruments for closure, and the implementation of pre-operative shower/wipes with chlorhexidine. Eight studies used both MBP and oral antibiotics. Care bundles including MBP and oral antibiotics had greater risk reduction in SSI then bundles without but the difference was not statistically significant (RR 0.57 vs 0.61, p-value 0.86) (Figure S1).
Fourteen studies implemented pre-operative shower/wipes with chlorhexidine gluconate. There was a greater risk reduction in SSI in care bundles using chlorhexidine gluconate than bundles without but the difference was not statistically significant (RR 0.51 vs 0.62, p-value 0.31) (Figure S2).
12 studies reported outcomes of a dedicated wound closure instrument tray. There was a greater risk reduction in SSI in care bundles using wound closure tray (RR 0.47 vs 0.74, p-value 0.05) (Figure S3).
9 studies reported outcomes of wound bundles that included wound protectors. There was no greater risk reduction in SSI in these care bundles than bundles without wound protectors (RR 0.69 vs 0.54, p-value 0.44) (Figure S4). Analyses of wound bundle elements are shown in supplementary Tables S1-S2.
Length of Stay Results: There were seven studies that included data on the length of hospital stay in both pre-intervention and post-intervention cohorts [18,22,23,25,27,31,34]. The mean difference between the length of hospital stay pre- and post-intervention was calculated in a meta-analysis. Two studies [22,34] provided the mean and standard deviation (mean ± SD) for the number of hospital days. The other five studies provided the median with either the full range or interquartile range. For these five studies [18,23,25,27,31], the mean ± SD were calculated from the data provided, according to calculations set out in the following studies: Hozo, et al. (2005) [61], Luo, et al. (2017) [62] and Wan, et al. (2014) [63]. This is based on an assumption of normal distribution in these studies. There was a statistically significant mean difference between the two groups in favour of the wound bundle (MD = −0.79; 95% CI: −1.10 to -0.49; p<0.00001) (Figure S5).
Risk Factor Results: Four studies provided sufficient raw data to carry out a meta-analysis on risk factors for SSI [18,20,23,30]. The American Society of Anaesthesiologists Physical Status classification ≥III was found to be a significant preoperative risk factor (OR=1.66, CI=1.32-2.09, p<0.0001) (Figure S6).
A meta-analysis of diabetes was also carried out which showed a statistically insignificant decrease in SSI in patients with diabetes mellitus (OR=.40, CI=.12-1.33, p=0.13) (Figure S7).
Another meta-analysis was carried out on open surgical approach vs. laparoscopic approach which showed an increased incidence in SSI in open approach however it was statistically insignificant. (OR=1.41, CI=.65-3.08, p=0.38) (Figure S8).
A number of studies had insufficient data for forest plot analysis. Bert, et al. [17] reported the following significant risk factors for SSIs; intervention technique (endoscopic vs. open) (OR, 2.07; CI, 1.253.62), ASA score ≥ 3 (OR, 1.80; CI, 1.262.57), urgent procedures (OR, 1.81; CI, 1.222.66) and contamination class ≥3 (OR, 2.32; CI, 1.623.31). Ghuman, et al. [40] found that smoking (OR, 3.75; CI, 1.54-9.13; p = 0.004), diabetes mellitus (OR, 2.75; CI, 1.28-5.95; p = 0.009), and incision location (OR, 1.37; CI, 1.04-1.83; p = 0.03) were significant risk factors. Hewitt, et al. [26] reported that using a laparoscopic approach is a significant factor in reducing SSI (OR, .43; CI, .24-.77). Rencuzogullari, et al. [39] reported that open surgical approach (OR, 2.15; CI, 1.273.60; p=0.004), wound class III-IV (OR 13.2; 95% CI, 8.36-21.0; p<0.001) and BMI (OR 1.30; 95% CI, 1.14-1.49; <0.001) were found to be independent risk factors for SSI occurrence.
Discussion
This meta-analysis evaluated the efficacy of wound bundles in SSI reduction, based on a ten-year literature review, yielded 27 publications meeting quantitative criteria. Two were RCT and 25 were retrospective cohort studies. The overwhelming evidence supports the use of wound bundles, even with the recognised heterogeneity of the studies. Surgical site infection, including superficial, deep and organ space, is one of the most common complications following open and colorectal cancer surgery [64]. At the outset, there is a global challenge in relation to the definition and heterogeneity of both superficial and deep SSIs. The lack of standardization of wound event reporting is common both in colorectal and other areas of surgery [65]. DeBord, in an editorial review of the issue, looks at the concept of proposals to classify surgical site events and surgical site occurrences requiring procedural interventions [66]. Of the 27 papers used in our meta-analysis, 15 papers used the Centres for Disease Control and Prevention (CDC) definitions for SSI [12]. A further ten papers used the National Surgical Quality Improvement Program (NSQIP) which uses the CDC definition for the types of SSI. The two remaining papers used the European ECDC definition which is again the CDC definition.
Definitions and reporting of surgical site occurrences, first defined by the Ventral Hernia Working Group (VHWG) in 2010 [67] to include seroma, wound dehiscence, and enterocutaneous fistula have not been widely adopted thus far [66]. Only one study, Anthony et al. 2011, showed an increase in SSIs following application of wound bundles, which may have been due to their failure to include mechanical bowel preparation or oral antibiotic preparation.
There is a significant increase in SSI rate in urgent or emergency procedures due to a myriad of confounding factors such as poor preoperative preparation and both clean contaminated and dirty operations [17,68,69]. Watanabe, et al. [70] have suggested that in cases of colon perforation with generalised contamination, delayed primary skin closure or leaving an incision open to heal by secondary intention should be considered. This is increasingly challenged by more use of comprehensive wound bundles that include wound irrigation and incisional negative pressure therapies [71]. However, despite this, a significant proportion of dirty wounds (without fasciitis) are not closed primarily. In a study by Alkaaki, et al. [72], more than half (30/55 [54%]) of the infected patients in their study underwent emergency surgery and they found that emergency surgery increased the risk of SSI fivefold compared to elective surgery. Ensuring strict adherence of preventative wound bundles, especially in emergency procedures, may see a very significant reduction in SSI globally. Successful implementation of clinical guidelines to reduce hospital acquired infections is challenging. Some have evolved using protocol-driven reduction [73] and others have looked at multiple different implementation strategies [74]. The Institute of Healthcare Improvement (IHI) developed a concept of bundles. A bundle generally uses more than three evidence-based measures which implemented together are more effective than in isolation. Recently, Tomsic, et al. suggested that bundle size itself is important and in their analysis suggested that a bundle with more than eleven items have additional standalone benefit in surgical site reduction [75].
In our meta-analysis we identified that surgical site infections were significantly reduced with the use of wound bundles. With sub-analysis of SSI into superficial SSI, deep SSI and organ space SSI, there were differences in outcome. Superficial SSI and organ space SSI were significantly reduced by the bundle, whereas there was only a trend for deep SSI. The reason for this is not entirely clear and may relate to the variability in bundle elements used. Many studies did not use negative wound pressure dressings. Recently, Murphy and colleagues [76] in Canada identified that negative pressure in the Neptune study had no associated effect on SSI. They report a very high SSI rate, approaching 32-34%. However, in their study they did not report or use any wound bundle. This may account for the failure to obtain a significant reduction in infection. Ideally, bundles target areas for reduction in variation in the delivery of care focusing on three key phases pre-operative, intra-operative and post-operative. Bundles should not just involve the patient but also their family. Pop-Vicas and colleagues [11] published a recent meta-analysis on colorectal bundles for surgical site infection prevention in the journal of Infection Control and Hospital Epidemiology. Multiple papers on the same topic are important to reinforce an important clinical issue. Given the potential implications in terms of cost, prolonged hospital stay, patient discomfort, and the potential adverse oncological and survival effects of both superficial and deep SSI, it is important that surgeons and those involved in the primary care of colorectal cancer and colorectal benign patients implement aspects of care bundles that are proven.
Wound protectors are commonly used in colorectal surgery and are recommended in open abdominal surgery in the ACS and SIS Guidelines [60]. However, there are some conflicting results on this in the literature [60,77-79]. The combination of MBP and antibiotic (PO) preparation is recommended for all elective colectomies according to ACS and SIS guidelines [77]. Other surgical techniques such as quilting or killing the dead space to reduce seroma and the use of subcuticular suturing should be looked at with increasing evidence that these may reduce wound infection rates [80,81]. This paper did not specifically look at laparoscopic versus open colorectal surgery and this is something that will need to be done into the future, stratifying cohorts or having separate or comparative studies [82]. Although we found that colorectal wound bundles significantly reduce the risk of SSI and length of hospital stay our study has several limitations. Firstly the vast majority of the included studies were retrospective cohort studies with heterogeneous interventions; no assessment of risk of bias was carried out. Secondly the primary outcome measure of SSI does not have a specified length of follow-up. Thirdly only four studies provided sufficient raw data to carry out a metaanalysis on risk factors for SSI; a small number of patients were included in each analysis. In addition, the effect on wound bindle efficacy in patients with immune compromise, or ongoing Covid infection has not been widely studied.
Conclusion
This meta-analysis has identified significant reductions in wound infections with implementation of wound bundles.
As Surgeons we have the responsibility to ensure we routinely use wound bundles which should become routine in colorectal surgery. Future work encompasses the need for standardisation of wound complications, standardised follow-up of patients and internationally agreed research definitions.
Figures
Tables
|
Author and year |
Country |
Study design |
Sample group |
Data collection period |
Sample size baseline |
Sample size cohort |
SSI definition |
Surveillance |
|
Anthony 2011 |
USA |
RCT |
colorectal |
2 yr, 8 months |
97 |
100 |
CDC |
30 days |
|
Benlice 2016 |
USA |
Cohort |
colorectal |
1 yr, 1 yr |
986 |
1293 |
NSQIP |
30 days |
|
Bert 2016 |
Italy |
Cohort |
colorectal |
1 yr |
651 |
671 |
ECDC |
30 days |
|
Bull 2011 |
Australia |
Cohort |
colorectal |
1yr, 1 yr |
180 |
275 |
CDC |
|
|
Cima 2013 |
USA |
Cohort |
colorectal |
1 yr, 2 yr |
531 |
198 |
NSQIP |
30 days |
|
Connolly 2016 |
USA |
Cohort |
colorectal |
3.5 yr, 3.5 yr |
379 |
311 |
CDC |
30 days |
|
Crolla 2012 |
Netherlands |
Cohort |
colorectal |
1.5 yr, 2.5 yr |
394 |
377 |
CDC |
30 days |
|
Elia-Guedea 2017 |
Spain |
Cohort |
colorectal |
3 mo, 3.5 mo |
70 |
79 |
CDC |
|
|
Gachabayov 2018 |
USA |
Cohort |
colorectal resections |
3 yr,3 yr |
379 |
311 |
CDC |
NSQIP |
|
Ghuman 2015 |
Canada |
Cohort |
Colon resections |
111 |
103 |
CDC |
||
|
Gorgun 2018 |
USA |
Cohort |
Colorectal |
1 yr,1 yr |
986 |
1264 |
NSQIP |
30 days |
|
Hewitt 2017 |
USA |
Cohort |
colorectal |
2 yr, 1 yr |
489 |
212 |
NSQIP |
NSQIP |
|
Hoang 2018 |
USA |
Cohort |
colorectal |
2 yr, 4 yr |
436 |
459 |
NSQIP |
NSQIP |
|
Keenan 2014 |
USA |
Cohort |
Colorectal |
3 yr,1.5 yr |
212 |
212 |
NSQIP |
30 days |
|
Keenan 2015 |
USA |
Cohort |
Colorectal |
16 mo, 20 mo |
165 |
285 |
NSQIP |
|
|
Lutifyya 2012 |
USA |
Cohort |
colorectal |
4 yr, 1.5 yr |
430 |
195 |
NSQIP |
NSQIP-30 days |
|
Perez-blanco 2015 |
Spain |
Cohort |
Colorectal |
3 yr, 1 yr |
218 |
124 |
CDC |
|
|
Reames 2015 |
USA |
Cohort |
colorectal |
2 yr, 2 yr |
2604 |
3119 |
CDC |
|
|
Rencüzoğulları 2018 |
USA |
Cohort |
colorectal |
30 mo, 18 mo |
1408 |
498 |
CDC |
|
|
Ruiz-Tovar 2018 |
Spain |
RCT |
Elective lap CRC cancer |
2 yr |
99 |
99 |
CDC |
30 Days |
|
Rumberger 2016 |
USA |
Cohort |
Colorectal |
1 yr, 10 mo |
269 |
261 |
CDC |
30 days |
|
Schiavone 2017 |
USA |
Cohort |
colorectal |
1 yr, 1 yr |
115 |
118 |
CDC |
30 days |
|
Tanner 2016 |
UK |
Cohort |
Colorectal |
6 mo, 6 mo |
127 |
166 |
HPA |
30 days |
|
Tillman 2013 |
USA |
Cohort |
colorectal |
1 yr, 1 yr |
79 |
104 |
NSQIP |
|
|
Weiser 2018 |
USA |
Cohort |
colorectal |
10 mo,13 mo |
454 |
616 |
CDC |
30 days |
|
Wick 2012 |
USA |
Cohort |
Colorectal |
1 yr, 1 yr |
278 |
324 |
NSQIP |
|
|
Yamamoto 2015 |
Japan |
Cohort |
Colorectal |
3 yr, 2 yr |
47 |
25 |
CDC |
Table 1: Characteristics of studies used in Meta-analysis.
|
Author
and Year |
Preoperative
CHG
Wipes/ Shower |
Risk
assessment for SSI |
Preoperative
infection screen |
Smoking
Cessation |
Omission
of Mechanical
Bowel
Preparation |
Bowel
preparation with oral antibiotics |
Mechanical
Bowel preparation |
Pre-operative
glycemic screen/ control |
preoperative
normothermia |
Pre-operative
Checklist |
Pre-operative
patient education |
|
Anthony
2011 |
|
|
|
|
ü |
|
|
|
ü |
|
|
|
Benlice
2016 |
ü |
|
|
|
|
ü |
ü |
|
|
|
|
|
Bert
2016 |
ü |
|
|
|
|
|
|
|
|
|
|
|
Bull
2011 |
|
|
|
|
|
|
|
ü |
ü |
|
|
|
Cima
2012 |
ü |
|
|
|
|
|
|
|
|
|
ü |
|
Connolly
2016 |
ü |
|
|
|
|
|
|
|
|
|
|
|
Crolla
2012 |
|
|
|
|
|
|
|
|
ü |
|
|
|
Elia-Guedea
2017 |
|
|
|
|
|
|
|
|
|
|
|
|
Gachabayov
2018 |
ü |
|
|
|
|
|
ü |
ü |
|
ü |
|
|
Ghuman
2015 |
|
|
|
|
|
|
|
|
|
|
|
|
Gorgun
2018 |
ü |
|
|
|
|
ü |
ü |
|
|
|
|
|
Hewitt
2017 |
ü |
|
ü |
ü |
|
ü |
|
ü |
ü |
ü |
|
|
Hoang
2018 |
|
|
|
|
|
ü |
|
ü |
|
|
|
|
Keenan
2014 |
ü |
|
|
|
|
ü |
ü |
ü |
|
|
ü |
|
Keenan
2015 |
ü |
|
|
|
|
ü |
ü |
ü |
|
|
|
|
Lutifiyya
2012 |
ü |
|
|
ü |
|
ü |
ü |
ü |
ü |
|
ü |
|
Perez-Blanco |
ü |
|
|
|
|
|
|
ü |
|
|
|
|
Reames
2015 |
|
|
|
|
|
|
|
|
|
|
|
|
Rencüzoğulları
2018 |
|
|
|
|
|
ü |
ü |
|
|
|
|
|
Ruiz-Tovar
2018 |
|
|
|
|
|
|
ü |
|
|
|
|
|
Rumberger
2016 |
ü |
|
|
|
|
|
|
ü |
|
|
|
|
Schiavone
2017 |
|
|
|
|
|
ü |
ü |
ü |
|
|
|
|
Tanner
2016 |
ü ** |
|
ü |
|
|
|
|
ü |
|
|
|
|
Tillman
2013 |
|
|
|
|
|
|
|
|
|
|
|
|
Weiser
2018 |
ü |
ü |
|
|
|
ü |
ü |
|
|
|
|
|
Wick 2012 |
ü |
|
|
|
|
ü |
ü |
|
|
|
|
|
Yamamoto 2015 |
|
|
|
|
|
|
|
|
|
|
|
Table S1: Study and their Preoperative Interventions.
|
Peri-op Glycemic control |
Hair removal with clippers |
Skin Preparation with CHG in alcohol |
Antibiotic prophylaxis <60 minutes before surgery |
Antibiotic re-dose within 2-4 hours if required |
Intra-operative normothermia |
Wound protectors |
Triclosan Sutures |
Double gloving |
Glove and/or Gown change |
New wound closure tray |
Suction tip change and wound washout |
Limited OR Traffic |
Antibiotic irrigation of
Abdomen |
redraping/ draping |
Supple-mental Oxygen |
Checklist fulfilment |
|
|
Anthony 2011 |
|
|
|
ü |
|
ü |
ü |
|
|
|
|
|
|
|
|
ü |
|
|
Benlice 2016 |
|
|
|
ü |
|
|
ü |
|
|
ü |
|
ü |
|
|
|
|
|
|
Bert 2016 |
|
ü |
|
ü |
|
ü |
|
|
|
|
|
|
|
|
|
|
|
|
Bull 2011 |
ü |
|
|
ü |
|
ü |
|
|
|
|
|
|
|
|
ü |
ü |
|
|
Cima 2013 |
|
|
ü |
ü |
ü |
|
|
|
|
ü |
ü |
|
|
|
|
|
|
|
Connolly 2016 |
ü |
ü |
ü |
ü |
|
ü |
ü |
|
|
ü |
ü |
|
ü |
|
|
|
|
|
Crolla 2012 |
|
ü |
|
ü |
|
ü |
|
|
|
|
|
|
ü |
|
|
|
|
|
Elia-Guedea
2017 |
|
|
|
ü |
ü |
|
|
|
|
ü |
ü |
|
ü |
|
|
|
|
|
Gachabayov
2018 |
|
ü |
ü |
|
|
ü |
ü |
|
|
ü |
ü |
|
ü |
|
ü |
|
ü |
|
Ghuman 2015 |
|
|
|
|
|
|
ü |
|
|
ü |
ü |
|
|
|
ü |
|
|
|
Gorgun 2018 |
|
|
ü |
ü |
|
|
ü |
|
|
ü |
ü |
ü |
|
|
|
|
|
|
Hewitt 2017 |
ü |
ü |
ü |
ü |
|
ü |
ü |
|
|
ü |
ü |
|
|
ü |
ü |
ü |
ü |
|
Hoang 2018 |
ü |
ü |
|
ü |
ü |
ü |
|
|
|
|
ü |
|
|
|
|
ü |
|
|
Keenan 2014 |
|
|
ü |
ü |
|
ü |
ü |
|
|
ü |
ü |
|
ü |
|
|
|
|
|
Keenan 2015 |
|
|
ü |
ü |
|
ü |
ü |
|
|
ü |
ü |
|
|
|
|
|
|
|
Lutifiyya
2012 |
ü |
ü |
ü |
ü |
ü |
ü |
|
|
ü |
|
|
|
|
|
|
ü |
|
|
Perez-Blanco |
ü |
|
ü |
ü |
ü |
ü |
|
|
|
ü |
|
|
|
|
|
|
|
|
Reames 2015 |
ü |
ü |
|
ü |
|
ü |
|
|
|
|
|
|
|
|
|
|
|
|
Rencüzoğulları
2018 |
|
|
|
ü |
|
|
ü |
|
|
|
|
|
|
|
|
|
|
|
Ruiz-Tovar
2018 |
|
|
ü |
ü |
|
ü |
|
ü |
ü |
|
|
|
|
ü |
|
|
ü |
|
Rumberger
2016 |
|
ü |
ü |
ü |
ü |
ü |
|
|
|
|
|
|
|
|
|
ü |
|
|
Schiavone
2017 |
ü |
|
ü |
ü |
ü |
|
|
|
|
ü |
ü |
|
|
|
|
|
|
|
Tanner 2016 |
ü |
ü |
ü |
ü |
|
ü |
|
|
|
|
|
|
|
|
ü |
|
|
|
Tillman 2013 |
ü |
ü |
|
ü |
|
ü |
|
|
|
|
|
|
|
|
|
|
|
|
Weiser 2018 |
ü |
ü |
|
ü |
ü |
ü |
|
|
|
|
ü |
|
|
|
|
|
|
|
Wick 2012 |
|
|
ü |
|
|
ü |
|
|
|
ü |
ü |
|
|
|
|
|
|
|
Yamamoto 2015 |
|
|
|
ü |
ü |
|
|
ü |
ü |
|
|
|
|
|
|
|
|
Table S2: Study and their Intraoperative Interventions.
References
- Collaborative G (2017) Determining the worldwide epidemiology of surgical site infections after gastrointestinal resection surgery: protocol for a multicentre, international, prospective cohort study (GlobalSurg 2). BMJ open 7: e012150.
- Rosenthal V, Richtmann R, Singh S, Apisarnthanarak A, Kübler A, et (2013) Surgical Site Infections, International Nosocomial Infection Control Consortium (INICC) Report, Data Summary of 30 Countries, 2005-2010. Infection Control & Hospital Epidemiology 34: 597-604.
- Limón E, Shaw E, Badia JM, Piriz M, Escofet R, et al. (2014) Post-discharge surgical site infections after uncomplicated elective colorectal surgery: impact and risk factors. The experience of the VINCat Pro Journal of Hospital Infection 86: 127-132.
- Murray BW, Cipher DJ, Pham T, Anthony T (2011) The impact of surgical site infection on the development of incisional hernia and small bowel obstruction in colorectal surgery. The American Journal of Surgery 202: 558-560.
- Beecher S, OʼLeary D, McLaughlin R, Kerin M (2018) The Impact of Surgical Complications on Cancer Recurrence Rates: A Literature Re Oncology Research and Treatment 41: 478-482.
- Sprenger T, Beißbarth T, Sauer R, Tschmelitsch J, Fietkau R, et al. (2018) Long‐term 23 prognostic impact of surgical complications in the German Rectal Cancer Trial 24 CAO/ARO/AIO-94. Brit J Surg 105: 1510-1518.
- Lawler J, Choynowski M, Bailey K, Bucholc M, Johnston A, et al. (2020) Meta-analysis of the impact of postoperative infective complications on oncological outcomes in colorectal cancer surgery. BJS Open 2020.
- Boland P, Kelly M, Donlon N, Bolger J, Mehigan B, et al. (2020) Prophylactic negative pressure wound therapy for closed laparotomy wounds: a systematic review and meta-analysis of randomised controlled trials. Irish Journal of Medical Science 2020.
- Zhang L, Elsolh B, Patel S (2017) Wound protectors in reducing surgical site infections in lower gastrointestinal surgery: an updated meta Surgical Endoscopy 32: 1111-1122.
- Bressan AK, Aubin JM, Martel G, Dixon E, Bathe OF, et al. (2018) Efficacy of a Dual-ring Wound Protector for Prevention of Surgical Site Infections After Pancreaticoduodenectomy in Patients With Intrabiliary Stents: A Randomized Clinical Trial. Annals of surgery 268: 35-40.
- Pop-Vicas A, Abad C, Baubie K, Osman F, Heise C, et al. (2020) Colorectal bundles for surgical site infection prevention: A systematic review and meta-analysis. Infection Control & Hospital Epidemiology 41: 805-812.
- ACH Surveillance for SSI Events | NHSN | CDC
- What Is a Bundle? | IHI - Institute for Healthcare Improvement [Internet]. Ihi.org 2020
- Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, et al. (2003) Methodological index for non-randomized studies (MINORS): development and validation of a new instrument. ANZ journal of surgery 73: 712
- Anthony T, Murray BW, Sum-Ping JT, Lenkovsky F, Vornik VD, et al. (2011) Evaluating an evidence-based bundle for preventing surgical site infection: a randomized trial. Archives of Surgery 146: 263-269.
- Gachabayov M, Senagore AJ, Abbas SK, Yelika SB, You K, et al. (2018) Perioperative hyperglycemia: an unmet need within a surgical site infection bundle. Techniques in coloproctology 22: 201-207.
- Bert F, Giacomelli S, Amprino V, Pieve G, Ceresetti D, et al. (2017) The “bundle” approach to reduce the surgical site infection rate. Journal of evaluation in clinical practice 23: 642-647.
- Elia-Guedea M, de Laspra EC, Echazarreta-Gallego E, Valero-Lazaro MI, Ramirez-Rodriguez JM, et al. (2017) Colorectal surgery and surgical site infection: is a change of attitude necessary? International journal of colorectal disease 32: 967-974.
- Bull A, Wilson J, Worth LJ, Stuart RL, Gillespie E, et al. (2011) A bundle of care to reduce colorectal surgical infections: an Australian experi Journal of Hospital Infection 78: 297-301.
- Lutfiyya W, Parsons D, Breen J (2012) A colorectal “care bundle” to reduce surgical site infections in colorectal surgeries: a single-center The Permanente Journal 16: 10.
- Tanner J, Kiernan M, Hilliam R, Davey S, Collins E, et al. (2016) Effectiveness of a care bundle to reduce surgical site infections in patients having open colorectal surgery. The Annals of The Royal College of Surgeons of England 98: 270-274.
- Pérez-Blanco V, García-Olmo D, Maseda-Garrido E, Nájera-Santos MC, García-Caballero J (2015) Evaluation of a preventive surgical site infection bundle in colorectal surgery. Cirugía Española (English Edition) 93: 222-228.
- Weiser M, Gonen M, Usiak S, Pottinger T, Samedy P, et al. (2018) Effectiveness of a multidisciplinary patient care bundle for reducing surgical-site infections. British Journal of Surgery 105: 1680-1687.
- Crolla RM, van der Laan L, Veen EJ, Hendriks Y, van Schendel C, et (2012) Reduction of surgical site infections after implementation of a bundle of care. PloS one 7: e44599.
- Keenan JE, Speicher PJ, Thacker JK, Walter M, Kuchibhatla M, et (2014) The preventive surgical site infection bundle in colorectal surgery: an effective approach to surgical site infection reduction and health care cost savings. JAMA surgery 149: 1045-1052.
- Hewitt DB, Tannouri SS, Burkhart RA, Altmark R, Goldstein SD, et al. (2017) Reducing colorectal surgical site infections: a novel, residentdriven, quality initiative. The American Journal of Surgery 213: 36-42.
- Yamamoto T, Morimoto T, Kita R, Masui H, Kinoshita H, et al. (2015) The preventive surgical site infection bundle in patients with colorectal BMC surgery 15: 128.
- Connolly TM, Foppa C, Kazi E, Denoya PI, Bergamaschi R (2016) Impact of a surgical site infection reduction strategy after colorectal Colorectal Disease 18: 910-918.
- Schiavone MB, Moukarzel L, Leong K, Zhou QC, Afonso AM, et al. (2017) Surgical site infection reduction bundle in patients with gynecologic cancer undergoing colon surgery. Gynecologic oncology 147: 115-119.
- Wick EC, Hobson DB, Bennett JL, Demski R, Maragakis L, et al. (2012) Implementation of a surgical comprehensive unit-based safety program to reduce surgical site infections. Journal of the American College of Surgeons 215: 193-200.
- Ruiz-Tovar J, Llavero C, Morales V, Gamallo C (2018) Effect of the application of a bundle of three measures (intraperitoneal lavage with antibiotic solution, fascial closure with Triclosan-coated sutures and Mupirocin ointment application on the skin staples) on the surgical site infection after elective laparoscopic colorectal cancer surgery. Surgical Endoscopy 32: 3495-3501.
- Cima R, Dankbar E, Lovely J, Pendlimari R, Aronhalt K, et al. (2013) Colorectal surgery surgical site infection reduction program: a national surgical quality improvement program-driven multidisciplinary singleinstitution experience. Journal of the American College of Surgeons 216: 23-33.
- Tillman M, Wehbe-Janek H, Hodges B, Smythe WR, Papaconstantinou HT (2013) Surgical care improvement project and surgical site infections: can integration in the surgical safety checklist improve quality performance and clinical outcomes? 1. journal of surgical research 184: 150-156.
- Keenan JE, Speicher PJ, Nussbaum DP, Adam MA, Miller TE, et al. (2015) Improving outcomes in colorectal surgery by sequential implementation of multiple standardized care programs. Journal of the American College of Surgeons 221: 404-414.
- Rumberger LK, Vittetoe D, Cathey L, Bennett H, Heidel RE, et al. (2016) Improving outcomes in elective colorectal surgery: a singleinstitution retrospective review. The American Surgeon 82: 325-330.
- Benlice C, Gorgun E (2016) Using NSQIP Data for Quality Improvement: The Cleveland Clinic SSI Experience. Seminars in Colon and Rectal Surgery 27: 74-82.
- Hoang SC, Klipfel AA, Roth LA, Vrees M, Schechter S, et al. (2019) Colon and rectal surgery surgical site infection reduction bundle: to improve is to change. Am J Surg 217: 40‐45.
- Gorgun E, Rencuzogullari A, Ozben V, Stocchi L, Fraser T, et al. (2018) An Effective Bundled Approach Reduces Surgical Site Infections in a High-Outlier Colorectal Unit. Diseases of the Colon & Rectum 61: 89-98.
- Rencüzoğulları A, Trunzo J, Vogel J, Khoshknabi D, Stocchi L, et al. (2018) Superficial Surgical Site Infection after Colorectal Surgery: Targeting High-Risk Patients Increases the Efficacy of Prevention Bun Turkish Journal of Colorectal Disease 2018.
- Ghuman A, Chan T, Karimuddin AA, Brown CJ, Raval MJ, et al. (2015) Surgical site infection rates following implementation of a colorectal closure bundle in elective colorectal surgeries. Diseases of the Colon & Rectum 58: 1078-1082.
- Reames BN, Krell RW, Campbell DA, Dimick JB (2015) A checklistbased intervention to improve surgical outcomes in Michigan: evaluation of the Keystone Surgery program. JAMA surgery 150: 208-215.
- Vu JV, Collins SD, Seese E, Hendren S, Englesbe MJ, et al. (2018) Evidence that a regional surgical collaborative can transform care: surgical site infection prevention practices for colectomy in Michigan. Journal of the American College of Surgeons 226: 91-99.
- Pastor C, Artinyan A, Varma MG, Kim E, Gibbs L, et al. (2010) An increase in compliance with the Surgical Care Improvement Project measures does not prevent surgical site infection in colorectal surgery. Diseases of the Colon & Rectum 53: 24-30.
- Wick EC, Gibbs L, Indorf LA, Varma MG, Garcia-Aguilar J (2008) Implementation of quality measures to reduce surgical site infection in colorectal patients. Diseases of the colon & rectum 51: 1004-1009.
- Hechenbleikner EM, Hobson DB, Bennett JL, Wick EC (2015) Implementation of surgical quality improvement: auditing tool for surgical site infection prevention practices. Diseases of the Colon & Rectum 58: 83-90.
- Jaffe TA, Meka AP, Semaan DZ, Okoro U, Hwang C, et al. (2017) Optimizing value of colon surgery in Michigan. Annals of surgery 265: 1178-1182.
- Larochelle M, Hyman N, Gruppi L, Osler T (2011) Diminishing surgical site infections after colorectal surgery with surgical care improvement project: is it time to move on?. Diseases of the Colon & Rectum 54: 394-400.
- Waits SA, Fritze D, Banerjee M, Zhang W, Kubus J, et al. (2014) Developing an argument for bundled interventions to reduce surgical site infection in colorectal surgery. Surgery 155: 602-606.
- Losh JM, Gough A, Rutherford R, Romero J, Diaz G, et al. (2017) Surgical Site Infection Reduction Bundle: Implementation and Challenges at Ventura County Medical Center. The American Surgeon 83: 1147-1151.
- Kwaan MR, Weight CJ, Carda SJ, Mills-Hokanson A, Wood E, et al. (2016) Abdominal closure protocol in colorectal, gynecologic oncology, and urology procedures: a randomized quality improvement trial. The American Journal of Surgery 211: 1077-1083.
- Thompson KM, Oldenburg WA, Deschamps C, Rupp WC, Smith CD (2011) Chasing zero: the drive to eliminate surgical site infections. Annals of surgery 254: 430-437.
- Forbes SS, Stephen WJ, Harper WL, Loeb M, Smith R, et al. (2008) Implementation of evidence-based practices for surgical site infection prophylaxis: results of a pre-and postintervention study. Journal of the American College of Surgeons 207: 336-341.
- Hawn MT, Vick CC, Richman J, Holman W, Deierhoi RJ, et al. (2011) Surgical site infection prevention: time to move beyond the surgical care improvement program. Annals of surgery 254: 494-501.
- Koek MB, Hopmans TE, Soetens LC, Wille JC, Geerlings SE, et al. (2017) Adhering to a national surgical care bundle reduces the risk of surgical site infections. PloS one 12: e0184200.
- Berenguer CM, Ochsner Jr MG, Lord SA, Senkowski CK (2010) Improving surgical site infections: using National Surgical Quality Improvement Program data to institute Surgical Care Improvement Project protocols in improving surgical outcomes. Journal of the American College of Surgeons 210: 737-741.
- DeHaas D, Aufderheide S, Gano J, Weigandt J, Ries J, et al. (2016) Colorectal surgical site infection reduction strategies. The American Journal of Surgery 212: 175-177.
- Ng WK, Awad N (2015) Performance improvement initiative: prevention of surgical site infection (SSI). BMJ Open Quality 4: u205401
- Lin D, Carson K, Lubomski L, Wick E, Pham J (2018) Statewide Collaborative to Reduce Surgical Site Infections: Results of the Hawaii Surgical Unit-Based Safety Program. Journal of the American College of Surgeons 227: 189-197.
- Serra-Aracil X, Espin-Basany E, Biondo S, Guirao X, Orrego C, et al. (2011) Surgical site infection in elective operations for colorectal cancer after the application of preventive measures. Archives of Surgery 146: 606-612.
- Liau KH, Aung KT, Chua N, Ho CK, Chan CY, et al. (2010) Outcome of a strategy to reduce surgical site infection in a tertiary-care hospital. Surgical infections 11: 151-159.
- Hozo SP, Djulbegovic B, Hozo I (2005) Estimating the mean and variance from the median, range, and the size of a sample. BMC medical research methodology 5: 13.
- Luo D, Wan X, Liu J, Tong T (2018) Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile Statistical methods in medical research 27: 1785-1805.
- Wan X, Wang W, Liu J, Tong T (2014) Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC medical research methodology 14: 135.
- Kobayashi M, Mohri Y, Inoue Y, Okita Y, Miki C, et al. (2008) Continuous Follow-up of Surgical Site Infections for 30 Days After Colorectal World Journal of Surgery 32: 1142-1146.
- Haskins I, Horne C, Krpata D (2018) A call for standardization of wound events reporting following ventral hernia repair. Hernia 22: 729-736.
- DeBord J, Novitsky Y, Fitzgibbons R, Miserez M, Montgomery A (2018) SSI, SSO, SSE, SSOPI: the elusive language of complications in hernia surgery. Hernia 22: 737-738.
- Breuing K, Butler C, Ferzoco S, Franz M, Hultman C, et al. (2010) Incisional ventral hernias: Review of the literature and recommendations regarding the grading and technique of repair. Surgery 148: 544-558.
- Segal C, Waller D, Tilley B, Piller L, Bilimoria K (2014) An evaluation of differences in risk factors for individual types of surgical site infections after colon surgery. Surgery 156: 1253-1260.
- Imai E, Ueda M, Kanao K (2008) Surgical site infection risk factors identified by multivariate analysis for patient undergoing laparoscopic, open colon, and gastric surgery. Am J Infect Control 36: 727-731.
- Watanabe M, Suzuki H, Nomura S (2014) Risk Factors for Surgical Site Infection in Emergency Colorectal Surgery: A Retrospective Anal Surg Infect (Larchmt) 15: 256-261.
- Frazee R, Manning A, Abernathy S (2018) Open vs Closed Negative Pressure Wound Therapy for Contaminated and Dirty Surgical Wounds: A Prospective Randomized Comparison. J Am Coll Surg 226: 507-512.
- Alkaaki A, Al-Radi O, Khoja A (2019) Surgical site infection following abdominal surgery: a prospective cohort study. Canadian Journal of Surgery 62: 111-117.
- Martinez C, Omesiete N, Pandit V, Villalvazo Y, Nocera M, et al. (2018) Protocol-Driven Reduction in Surgical Site Infection after Colon Sur Journal of the American College of Surgeons 227: e111.
- Ariyo P, Zayed B, Riese V, Anton B, Latif A, et al. (2019) Implementation strategies to reduce surgical site infections: A systematic review. Infection Control & Hospital Epidemiology 40: 287-300.
- Tomsic I, Chaberny I, Heinze N, Krauth C, Schock B, et al. (2018) The Role of Bundle Size for Preventing Surgical Site Infections after Colorectal Surgery: Is More Better?. Journal of Gastrointestinal Surgery 22: 765-766.
- Murphy P, Knowles S, Chadi S, Vogt K, Brackstone M, et al. (2018) Negative Pressure Wound Therapy Use to Decrease Surgical Nosocomial Events in Colorectal Resections (NEPTUNE): A Randomized Controlled Trial. Journal of the American College of Surgeons 227:
- Ban KA, Minei JP, Laronga C, Harbrecht BG, Jensen EH, et al. (2017) American College of Surgeons and Surgical Infection Society: surgical site infection guidelines, 2016 update. Journal of the American College of Surgeons 224: 59-74.
- Zhang L, Elsolh B, Patel S (2017) Wound protectors in reducing surgical site infections in lower gastrointestinal surgery: an updated meta Surgical Endoscopy 32: 1111-1122.
- Pinkney TD, Calvert M, Bartlett DC, Gheorghe A, Redman V, et al. (2013) Impact of wound edge protection devices on surgical site infection after laparotomy: multicentre randomised controlled trial (ROSSINI Trial). Bmj 347: f4305.
- Flick KF, Simpson RE, Soufi M, Fennerty ML, Yip-Schneider MT, et (2020) Comparison of skin closure techniques in patients undergoing open pancreaticoduodenectomy: A single center experience. The American Journal of Surgery 220: 972-975.
- Myint ST, Khaing KS, Yee W, Mon SM, Lwin T (2020) Quilting suture versus conventional closure in prevention of seroma after total mastectomy and axillary dissection in breast cancer patients. ANZ journal of surgery 90: 1408-1413.
- Kulkarni N, Arulampalam T (2020) Laparoscopic surgery reduces the incidence of surgical site infections compared to the open approach for colorectal procedures: a meta-analysis. Techniques in coloproctology 2020: 1-8.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.