Management for Plunging Thyroid Goiter Combined with Aortic Valve Replacement : a Clinical Case Study of a Septuagenarian Patient with Symptomatic Severe Aortic Stenosis with Literature Review
by Said Khallikane*1, Hicham Kbiri2, Mehdi Nabil2, Hakim Elbaraka2, Mehdi Didi2, Ayoub Dahioui3, Reda Mounir4, Hamid Jalal5, Noureddine Atmani6, Abdelmajid Bouzerda7, Abdessamad Abdou8, Issam Serghini9, Ayoub Belhadj10, Younes Aissaoui11, Youssef Qamouss12
1Former anesthesiologist-intensivist, Hassan II Military Hospital, Laayoune, Mohammed VI Hospital, Dakhla, Anesthesiology service, Avicenna Military Hospital.
2Faculty of Medicine and Pharmacy, Cadi Ayyad University, Marrakech, Morocco.
3Cardiothoracic and Vascular Surgery, trainee resident in Cardiac Surgery, Avicenna Military Hospital, Faculty of Medicine and Pharmacy, Cadi Ayyad University, Marrakech 40000, Kingdom of Morocco.
4Cardiothoracic and Vascular Surgeon, Avicenna Military Hospital, Faculty of Medicine and Pharmacy, Cadi Ayyad University, Marrakech 40000, Kingdom of Morocco.
5Cardiologist, Cardio-echocardiographic, Professor of Cardiology, Avicenna Military Hospital, Faculty of Medicine and Pharmacy, Cadi Ayyad University, Marrakech 40000, Kingdom of Morocco.
6Cardiothoracic and Vascular Surgeon, Professor of Cardiac Surgery, mini-invasive Cardiac Surgery, Interventional Cardiovascular Surgery, Avicenna Military Hospital, Faculty of Medicine and Pharmacy, Cadi Ayyad University, Marrakech 40000, Kingdom of Morocco.
7Cardiologist, Interventional Cardiologist, Head of the Cardiology Department, Premier Medical-Surgical Center, Professor of High Medical Education in Cardiology-Cardiovascular ICU- Interventional Cardiology University Cadi Ayyad, Faculty of Medicine and Pharmacy of Marrakech 4000, Kingdom of Morocco.
8Head of Cardiothoracic and Vascular Surgeon, Professor of Cardiac Surgery, mini-invasive Cardiac Surgery, Interventional Cardiovascular Surgery, Avicenna Military Hospital, Faculty of Medicine and Pharmacy, Cadi Ayyad University, Marrakech 40000, Kingdom of Morocco.
9Former Anesthesiologist-Intensivist. Professor of High Medical Education in Anesthesiology-Critical Care, at Avicenna Military Hospital, Faculty of Medicine and Pharmacy, Cadi Ayyad University, Marrakech 40000, Kingdom of Morocco.
10Head of Surgical ICU, Professor of High Medical Education in Anesthesiology-Critical Care, Avicenna Military Hospital, Faculty of Medicine and Pharmacy, Cadi Ayyad University, Marrakech 40000, Kingdom of Morocco.
11Head of Medical ICU, Professor of High Medical Education in Anesthesiology-Critical Care, Avicenna Military Hospital, Faculty of Medicine and Pharmacy, Cadi Ayyad University, Marrakech 40000, Kingdom of Morocco.
12Chief of Anesthesiology-ICU-Emergency Department, Anesthesiologist-Intensivist, Professor of High Medical Education Grade C in Anesthesiology-Intensive Medicine, Avicenna Military Hospital, Faculty of Medicine and Pharmacy, Cadi Ayyad University, Marrakech 40000, Kingdom of Morocco.
*Corresponding author: Said Khallikane, Former anesthesiologist-intensivist, Hassan II Military Hospital, Laayoune, Mohammed VI Hospital, Dakhla, Anesthesiology service, Avicenna Military Hospital.
Received Date: 17 November 2024
Accepted Date: 21 November 2024
Published Date: 25 November 2024
Citation: Khallikan S, Kbiri H, Nabil M, Elbaraka H, Didi M, et al. (2024) Management for Plunging Thyroid Goiter Combined with Aortic Valve Replacement : a Clinical Case Study of a Septuagenarian Patient with Symptomatic Severe Aortic Stenosis with Literature Review. Ann Case Report 9: 2089. https://doi.org/10.29011/2574-7754.102089
Abstract
The management of severe aortic stenosis (SAS) and plunging thyroid goiter in high-risk surgical patients presents significant challenges due to the complexity and interdependence of these conditions. This comprehensive case study highlights the simultaneous surgical approach in a 70-year-old female patient with symptomatic SAS and a massive plunging thyroid goiter, characterized by tracheal compression and cardiac involvement. The patient exhibited progressively worsening dyspnea, NYHA class III heart failure, and a massive goiter with significant mediastinal extension. A multidisciplinary team of endocrinology, ENT, cardiology, cardiac surgery, and anesthesia specialists opted for a combined surgical strategy. The procedure began with a total thyroidectomy to address airway and mediastinal compression, followed by immediate aortic valve replacement under extracorporeal circulation. The surgery proceeded without major complications, with meticulous intraoperative monitoring, staged closure of surgical layers, and effective hemostasis management. Postoperatively, the patient demonstrated a favorable recovery, with no recurrent laryngeal nerve damage or hypocalcemia, and was extubated within 12 hours. This case underscores the importance of a multidisciplinary approach for optimal outcomes in high-risk surgical patients with concurrent SAS and plunging goiter. It also emphasizes the need for comprehensive preoperative planning, timely interventions, and seamless coordination between surgical teams. Plunging thyroid goiter and severe aortic stenosis (AS) represent rare but significant comorbidities that challenge perioperative management in high-risk surgical patients. Plunging goiters, characterized by thyroid migration into the chest, can compress vital structures, while severe AS introduces considerable cardiovascular risk during surgery. Comprehensive preoperative evaluation, including imaging of the ascending aorta, aortic arch, and thyroid anatomy, is crucial to guide management. This report highlights the complexities of addressing coexistent plunging thyroid goiter and severe AS, emphasizing the necessity of a multidisciplinary approach. Surgical timing and technique-whether sequential or simultaneous procedures—must be individualized based on anatomical and physiological considerations. Preoperative optimization and tailored strategies, such as transcatheter aortic valve implantation (TAVI) or minimally invasive thyroidectomy, are vital to mitigating risks. This case underscores the importance of collaborative heart and thyroid teams in determining optimal surgical pathways and preempting complications in a high-risk population.
Keywords: Severe Aortic Stenosis (SAS); Plunging Thyroid Goiter; High-Risk Surgical Patients; Multidisciplinary Team; Preoperative Planning; Difficult Airway Management; Surgical Timing and Technique.
Abstract
The management of severe aortic stenosis (SAS) and plunging thyroid goiter in high-risk surgical patients presents significant challenges due to the complexity and interdependence of these conditions. This comprehensive case study highlights the simultaneous surgical approach in a 70-year-old female patient with symptomatic SAS and a massive plunging thyroid goiter, characterized by tracheal compression and cardiac involvement. The patient exhibited progressively worsening dyspnea, NYHA class III heart failure, and a massive goiter with significant mediastinal extension. A multidisciplinary team of endocrinology, ENT, cardiology, cardiac surgery, and anesthesia specialists opted for a combined surgical strategy. The procedure began with a total thyroidectomy to address airway and mediastinal compression, followed by immediate aortic valve replacement under extracorporeal circulation. The surgery proceeded without major complications, with meticulous intraoperative monitoring, staged closure of surgical layers, and effective hemostasis management. Postoperatively, the patient demonstrated a favorable recovery, with no recurrent laryngeal nerve damage or hypocalcemia, and was extubated within 12 hours. This case underscores the importance of a multidisciplinary approach for optimal outcomes in high-risk surgical patients with concurrent SAS and plunging goiter. It also emphasizes the need for comprehensive preoperative planning, timely interventions, and seamless coordination between surgical teams. Plunging thyroid goiter and severe aortic stenosis (AS) represent rare but significant comorbidities that challenge perioperative management in high-risk surgical patients. Plunging goiters, characterized by thyroid migration into the chest, can compress vital structures, while severe AS introduces considerable cardiovascular risk during surgery. Comprehensive preoperative evaluation, including imaging of the ascending aorta, aortic arch, and thyroid anatomy, is crucial to guide management. This report highlights the complexities of addressing coexistent plunging thyroid goiter and severe AS, emphasizing the necessity of a multidisciplinary approach. Surgical timing and technique-whether sequential or simultaneous procedures—must be individualized based on anatomical and physiological considerations. Preoperative optimization and tailored strategies, such as transcatheter aortic valve implantation (TAVI) or minimally invasive thyroidectomy, are vital to mitigating risks. This case underscores the importance of collaborative heart and thyroid teams in determining optimal surgical pathways and preempting complications in a high-risk population.
Keywords: Severe Aortic Stenosis (SAS); Plunging Thyroid Goiter; High-Risk Surgical Patients; Multidisciplinary Team; Preoperative Planning; Difficult Airway Management; Surgical Timing and Technique.
Introduction
Surgical intervention and the management of two complicated disease processes, especially in high-risk surgical patients, are now high on the list of health care providers’ agenda due to an aging society. First of all, degenerative aortic stenosis is a common condition in the elderly, it should be considered that is the most prevalent valvular heart disease in the elderly. It affects approximately 12% of people over the age of 75, with a higher prevalence in women than in men. [1] In particular, severe aortic stenosis (SAS) has one of the worst prognoses among valvular heart diseases. Furthermore, studies have shown that individuals with degenerative aortic stenosis often have comorbidities such as hypertension, diabetes, and coronary artery disease, which increases the complexity of managing this condition in older patients. [2] Although surgical and transcatheter aortic valve implantation (TAVI) improve the prognosis of severe aortic stenosis, SAS with left ventricular dysfunction represents a ‘difficult scenario’ for precise and definitive treatment. [3] Secondly, massive goiter with a cranial-caudal measurement of more than 8 cm or a substantially high cervical extension, so-called ‘plunging goiter’, occurs in less than 10% of cases and may invade structures within the thorax [4]. Anesthesia for thyroid surgery offers unique challenges, and advanced airway settings are often mandatory because of the difficulty of managing airway patency. [5] Severe aortic stenosis and a plunging goiter would be better managed from a comprehensive management point of view. To the best of our knowledge, none of the comprehensive international significant reports included a patient with the combined clinical condition as presented in this study, we have been the first group to describe a patient in whom severe aortic stenosis was surgically managed together with a plunging goiter, apart from a few cases that describe combined surgery of severe aortic stenosis and thyroidectomy [6,7]. Although the prevalence of these two diseases might not be so high, especially in developing countries, this comprehensive review is very useful for improving the management of such patients, optimizing the surgical outcome. Finally, an earlier onset of management with a modern procedure for aortic stenosis seems to be extremely important in receiving the best results. [8] The coordinated workflows and pathways ensure timely patient admissions and access to the specific class of treatments for certain diseases, such as myocardial infarction, acute stroke, heart failure, or sepsis. In the context of high-risk patients that have associated periprocedural complications, some protocols and algorithms have been instituted such as TAVI, a Percutaneous Transseptal Transcatheter Mitral Valve Procedure, mini-invasive surgical mitral valve intervention, and Ebstein’s surgery. [9] This article presents a complex medical situation of a septuagenarian patient, with the description of her medical history, preoperative diagnostics, chosen operative technique with results, and follow-up, who was admitted to the cardiosurgical department with the diagnosis of SAS and large plunging thyroid goiter. The case presents a typical clinical case highlighting the complex relationship between two distinct surgical areas: intracardiac and extracardiac. The extremely severe condition of the hospitalized patient (NYHA III), and the high risk of death during surgical treatment were the reasons for treatment to be carried out according to guidelines for a multidisciplinary approach. The purpose of the present comprehensive review is to provide insights concerning the existing evidence on the concomitance of severe aortic stenosis and plunging thyroid goiter regarding surgical patients, discuss admittance, evaluation of comorbid conditions, the current clinical practice, including a brief summary of pathophysiological mechanisms and reported management strategies, and finally, it analyzes the criteria for the assessment, the patient safety point of view the prevention of serious complications found using periprocedural anesthesia and surgical literature in patients who are unsuitable for the use of standard operative techniques. The important issue is ambiguity about standards during multidisciplinary approaches in this fragile population. [8,9] While cases of SAS in elderly and high-risk patients are still to be found in clinical practice, and much evidence exists on this topic, to the best of our knowledge, a comprehensive review about severe aortic stenosis with a coexistent plunging thyroid goiter in high-risk surgical populations does not exist. The primary objective of the combination of the above two diseases is to present the interdependence between the plunging thyroid goiter and the aortic valve directly connected with the aortic root and aorta. It may also shed some light on the necessity of combined treatment of patients with aortic stenosis and lesions located in the close neighborhood of the aortic root or other vascular structures. This subject is relevant to physicians and anesthesiologists who do not necessarily manage these diseases but are expected to possess knowledge of this issue. The present review summarizes previously published data and is designed to be an easy reference guide for use by a busy practicing clinician and therefore briefly describes the current clinical practice.
The aim of the presented paper is to determine the present management strategies and recent views on the prognosis in polymorbid patients with a coexisting plunging thyroid goiter. [6]
Case Presentation
We report the case of a 70-year-old female patient with a history of poorly controlled arterial hypertension, presenting with two coexisting conditions that required either a two-step approach or simultaneous management, involving a thyroidectomy followed by cardiac surgery under cardiopulmonary bypass (CPB). In our case, a simultaneous approach was chosen following a multidisciplinary consultation. With a medical history of poorly controlled arterial hypertension, a multinodular plunging goiter in hyperthyroidism treated with carbimazole 10 mg/day, and atrial fibrillation associated to severe aortic valve stenosis managed with acenocoumarin 4 mg (¼ tablet/day) and bisoprolol 2.5 mg/day, the patient presented with a progressively worsening condition. The onset of her symptom’s dates back to five months prior, marked by a brief episode of syncope, prompting an emergency consultation. Initial evaluation revealed tachycardia due to atrial fibrillation on a background of severe aortic valve stenosis. She was placed on symptomatic treatment. The course of her illness was characterized by a worsening baseline dyspnea, progressing from NYHA class II to class III, accompanied by exertional palpitations without chest pain. The patient was referred to the ENT consultation for progressively worsening dyspnea, associated with orthopnea and a massive goiter, and cardiology consultation for the evaluation of her heart disease. During her recent assessment she also reported reduced exercise tolerance and occasional dizziness with neck symptoms, including subclavicular pain and swallowing limitations. Clinical examination revealed a large goiter deforming the neck, visible on inspection, with signs of tracheal compression. Cardiac auscultation identified an intense systolic ejection murmur at the aortic focus radiating to the carotids, with no signs of right-sided heart failure or peripheral edema. Thyroid ultrasound showed a multinodular goiter containing hypoechoic cystic areas and calcifications, with a plunging thoracic component compressing cervical and superior mediastinal structures. A cervical-thoracic CT scan, with and without contrast injection, confirmed the plunging nature of the goiter. It extended into the anterior mediastinum, displacing the trachea and major vessels without evidence of invasion. The scan revealed a multinodular goiter characterized by generalized thyroid gland enlargement, predominantly affecting the left lobe, with an irregular, lobulated contour. The goiter is characterized by heterogeneity, macrocalcifications, and uneven enhancement, marking hypodense necrotic and/or cystic fluid-filled areas. The large goiter measures 12.13 x 9.06 x 6.63 cm in the left lobe, 10.5 x 6 x 5.36 cm in the right lobe, and 5 cm in the isthmus. It extends upwards to the submandibular spaces and laterally towards the anterior and posterior spinal spaces, particularly on the left side. The jugulo-carotid vascular axes are displaced laterally and posteriorly, but remain unobstructed. The goiter extends downward into the superior level of the anterior mediastinum, filling the prevascular space and pushing vascular structures posteriorly. It reaches the level of the superior border of the aortic arch, coming into contact with the supra-aortic trunks, exerting a mass effect, but without invasion or obstruction. The upper aerodigestive pathways are unobstructed and symmetrical, although there is posterior and rightward displacement at the larynx and trachea level, without narrowing or impact on their lumen, which remains patent and of normal size. (Figure 1) Transthoracic echocardiography revealed severe degenerative aortic stenosis with high pulmonary arterial hypertension for age (PASP through tricuspid regurgitation at 55 mmHg). The left ventricle was non-dilated, hypertrophied with an indexed mass of 107.11 g/m² (eccentric remodeling), end-diastolic diameter (EDD) at 44 mm, end-systolic diameter (ESD) at 28 mm, and preserved systolic function with an ejection fraction (EF) of 55% (biplane Simpson method). (Figure 2)
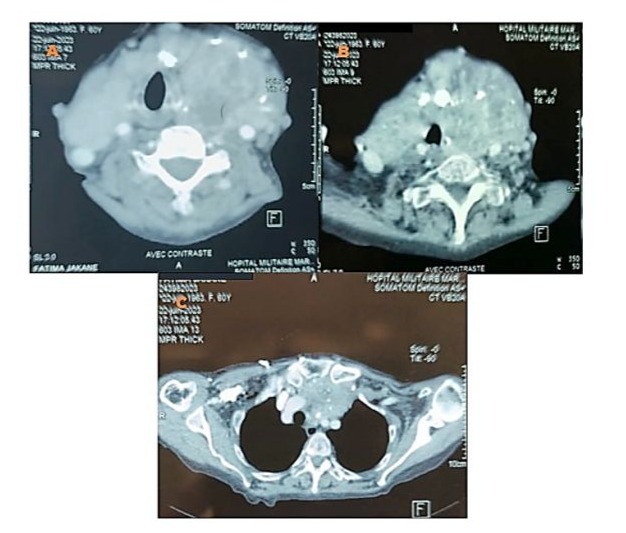
Figure 1: Axial CT scan with contrast injection showing overall hypertrophy of the thyroid gland, predominantly involving the left lobe, with irregular and nodular contours. The gland exhibits spontaneously heterogeneous density with heterogeneous enhancement (A, B). The upper aerodigestive tract remains unobstructed, with posterior and rightward displacement of the larynx and trachea, whose lumen remains patent and of normal caliber. (A, B) It extends to the superior mediastinum through the thoracic inlet, in a retrosternal position, filling the prevascular space up to the level of the superior border of the aorta and the supra-aortic trunk, which remain patent and uninvolved. (C)
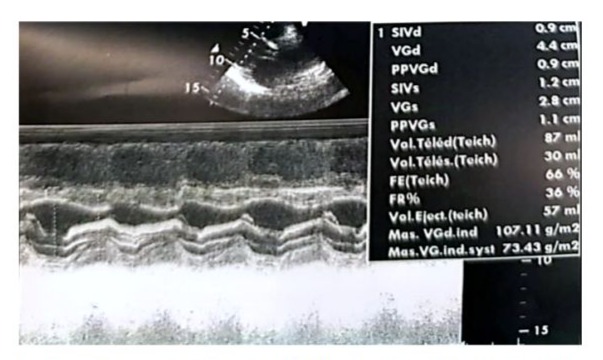
Figure 2: Real-time echocardiographic image showing a non-dilated, hypertrophied left ventricle with an interventricular septum measuring 0.9 cm in diastole and 1.2 cm in systole. The indexed left ventricular mass was 107.11 g/m², indicating eccentric remodeling. The left ventricular end-diastolic diameter (EDD) measured 44 mm, the end-systolic diameter (ESD) measured 28 mm, and systolic function was preserved with an ejection fraction (EF) of 66% (Teichholz method). The shortening fraction was within the normal range at 36%.
Left ventricular filling pressures were normal, and the inferior vena cava was dilated to 16 mm and slightly compliant. (Figure 3,4) The left atrium was dilated, with a surface area of 21.5 cm² and a volume of 44 ml/m². (Figure 5) The right ventricle had preserved systolic function with a TAPSE (Tricuspid Annular Plane Systolic Excursion) of 22 mm and a tissue Doppler S’ velocity of 12 cm/s. The right atrium was dilated at 19.7 cm², free of echoes. Assessment of the valvular apparatus showed a tricuspid aortic valve with degenerative changes, including calcified masses between the right and left cusps, limiting valve opening. Severe stenosis was noted, with a valvular area of 0.8 cm² (indexed area of 0.58 cm²), a Vmax of 3.93 m/s, and a mean gradient of 35 mmHg. The aortic annulus measured 19 mm, and minimal regurgitation was observed. (Figure 6-9)
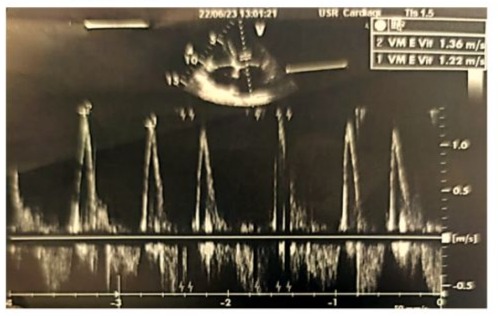
Figure 3: Doppler echocardiographic image of the transmitral antegrade flow showing a non-elevated mitral E-wave velocity of 1.36 m/s (<1.5 m/s), with the absence of an A-wave due to atrial fibrillation.
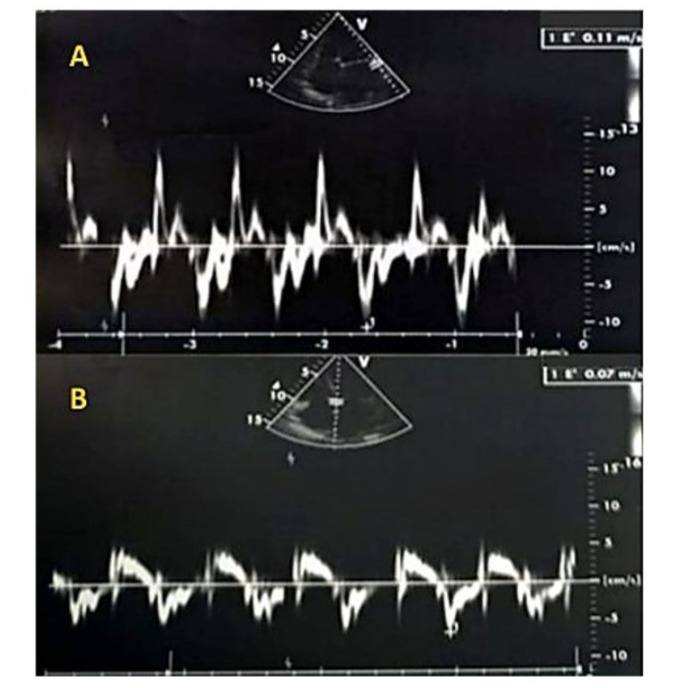
Figure 4: Tissue Doppler imaging (TDI) at the mitral annulus was performed using a 4-chamber view, minimizing filters and gains, and placing the sampling window at the lateral annulus (A) or the septal annulus (B). The TDI recording showed a positive systolic wave followed by a single diastolic Ea wave at 7 cm/s lateral, (A) and 11 cm/s septal, (B), with no Aa wave due to atrial fibrillation. The absence of significant reduction in the Ea wave indicates normal flow, as in healthy individuals, the Ea wave is typically greater than 8 cm/s
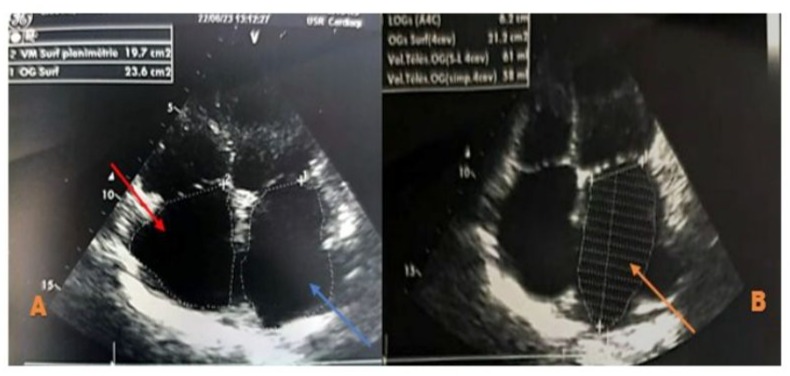
Figure 5: Zoomed echocardiographic image in the apical 4-chamber view focusing on the atria, showing a dilated left atrium with a surface area of 23.6 cm² (Figure A, blue arrow) and a volume of 58 ml/m² (Figure B, orange arrow). The right atrium is also dilated, measuring 19.7 cm², and appears free of echoes (Figure A, red arrow).
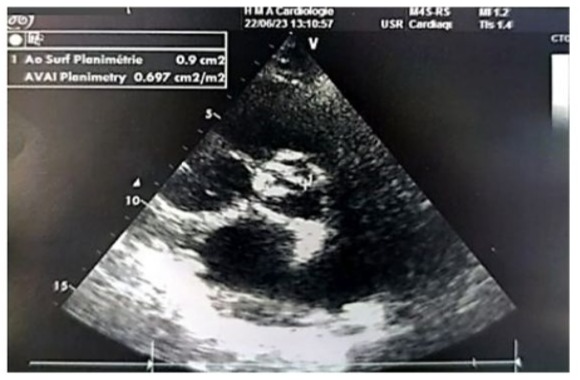
Figure 6: Parasternal short-axis echographic image showing a valvular area of 0.9 cm² by planimetry (indexed area of 0.7 cm²/m²).
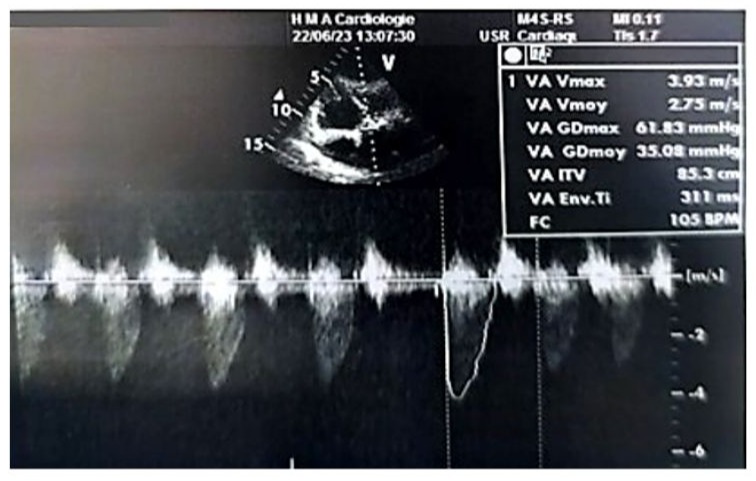
Figure 7: Doppler echocardiographic image in the 5-chamber view at the level of the aortic valve, showing a mean transvalvular gradient of 35 mmHg.
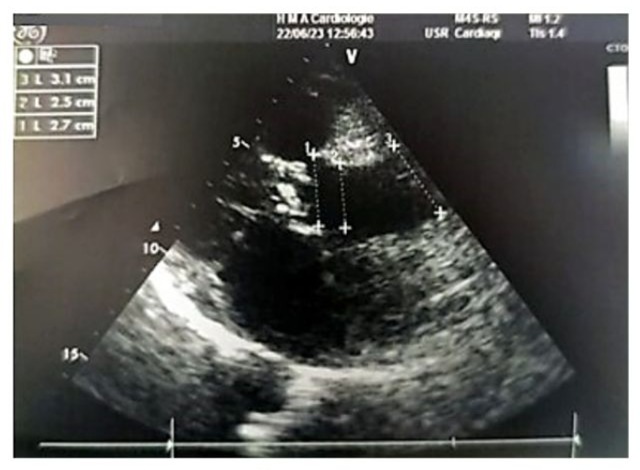
Figure 8: 2D echocardiographic image in the parasternal long-axis view showing a non-dilated proximal aorta. The aortic annulus measures 2.7 cm, the sinus of Valsalva 2.5 cm, and the tubular segment 3.1 cm.
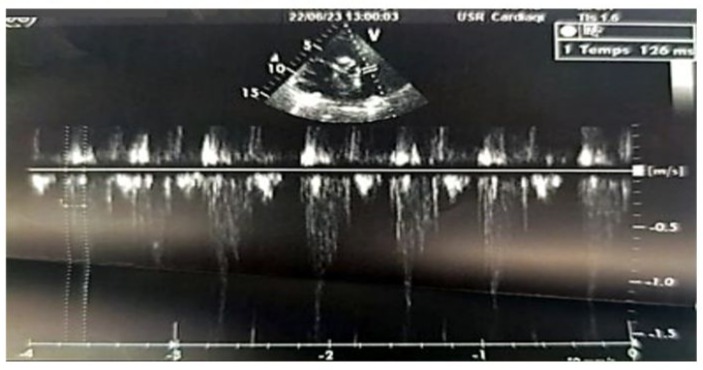
Figure 9: 2D echocardiographic image in the parasternal short-axis view, with pulsed Doppler pointed a few millimeters below the pulmonary valve (in the right ventricle), showing a pulmonary acceleration time to reach the peak velocity with an intermediate value of 126 ms, which is neither short (<90 ms) nor long (>130 ms).
The laboratory workup revealed an NT-proBNP level of 900 pg/ml, with no renal insufficiency, normal thyroid function and inflammatory markers. The preoperative coronary angiography showed atheromatous coronary arteries without significant lesions. The echodoppler of the supra-aortic trunks showed atheromatous changes without significant lesions. The rest of the biological, serological, and radiological assessments were normal. The therapeutic decision was based on combined expertise in both pathologies. Since symptomatic severe aortic stenosis was suspected to be the primary cause of the patient’s dyspnea, reduced walking distance, and markedly decreased quality of life, an isolated aortic valve intervention might have been considered. However, the left lobe of the retrosternal thyroid goiter plunged significantly into the left thoracic cavity, reaching the level of the superior border of the aortic arch and coming into contact with the supra-aortic trunks, exerting a mass effect. This made it impossible to exclude involvement due to this intrathoracic localization. Considering the rapidly appearance of Ortner’s syndrome and the almost hemodynamically significant stenosis, the presented symptomatic manifestations and predominantly affected valvular components led to an interdisciplinary evaluation. This evaluation concluded that the overall cardiopulmonary prognosis was severely compromised. A multidisciplinary meeting was organized, bringing together teams from endocrinology, ENT surgery, cardiology, cardiac surgery, and cardiovascular anesthesia and intensive care. It was decided to perform both surgical interventions in a single procedure rather than in two stages. A total thyroidectomy was performed to eliminate any risk of postoperative tracheal compression and to facilitate access to mediastinal structures, followed immediately during the same operative period by aortic valve replacement under Extracorporeal Circulation (ECC). The first stage consists of performing a total thyroidectomy which was performed by a specialized cervical surgery team. Nasotracheal intubation using a fibroscopic technique in a conscious patient was carried out, after performing bilateral superior laryngeal nerve blocks, a tracheal block via the cricothyroid membrane, and topical anesthesia through oral nebulization of 2% lidocaine and topical application of 5% lidocaine-naphazoline. (Figure 10) The surgery allowed for the complete removal of the goiter, with meticulous postoperative hemostasis and closure of only the skin layer with interrupted sutures. The total closure of the surgical layers was postponed until after ECC, following neutralization of sodium heparin with protamine and restoration to a near-normal coagulant state, which allowed for proper surgical hemostasis, verification, and clot removal before final closure. (Figure 11)
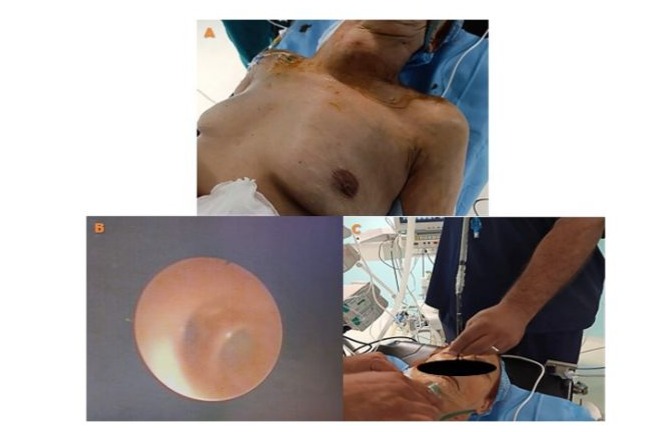
Figure 10: Image before anesthetic induction for the first surgical stage (total thyroidectomy), showing a large goiter and the beginning of awake nasotracheal intubation using a nasofibroscope after regional anesthesia. The image also shows the visualization of the carina with the right and left mainstem bronchi after successful passage through the glottis.
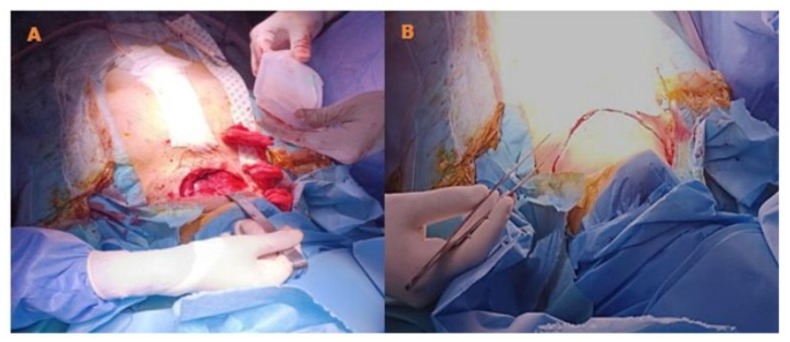
Figure 11: Intraoperative image after the closure of the sternotomy, showing verification of hemostasis in the thyroid bed, placement of a drain after reopening the separate skin sutures, before the final closure of the muscle, subcutaneous, and skin layers.
The second stage consisted of achieving cardiac surgery which was performed immediately afterward (Figure 12). The ETO probe was inserted after the thyroidectomy using a video-laryngoscope to avoid trauma to the mouth, esophagus, or accidental intubation. A mechanical aortic valve was implanted under ECC. Perioperative Doppler ETO monitoring was performed, as well as post-ECC monitoring. (Figure 13-20) (Video 1-8).
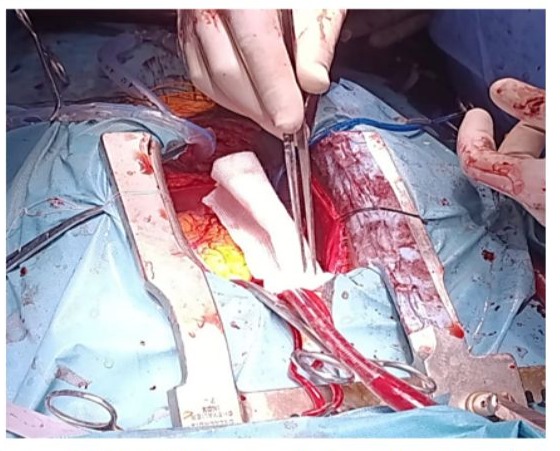
Figure 12: Intraoperative image after total thyroidectomy, showing the sternal retractor in place with the pericardial sac opened.
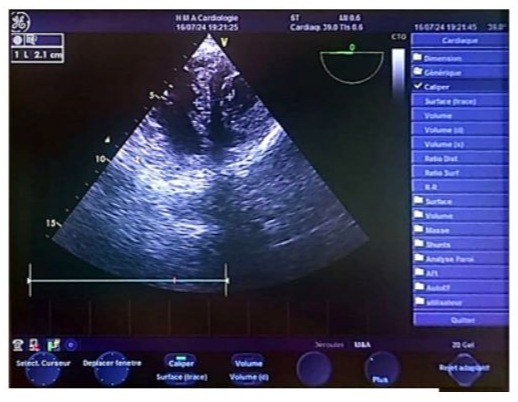
Figure 13: Transgastric biventricular short-axis echocardiographic image showing a thickened interventricular septum measuring 2.1 cm, suggestive of left ventricular hypertrophy.
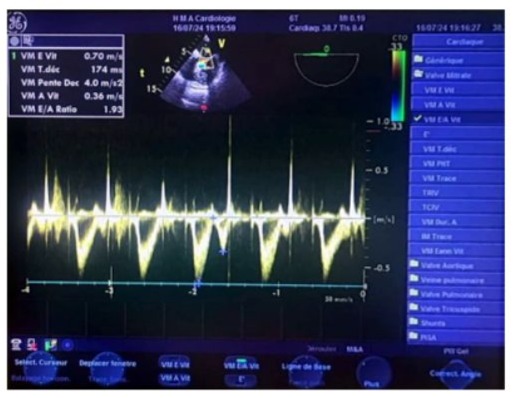
Figure 14: Transesophageal echocardiography (TOE) at the mid-esophageal level, with the sampling window positioned at the distal tips of the mitral leaflets, showing during a transient postoperative passage in sinus rhythm a pseudo-normal flow pattern with an E-wave velocity of 0.70 m/s, an A-wave velocity of 0.30 m/s, an E/A ratio of 1.93 (>1.5), and a calculated deceleration time of 174 ms.
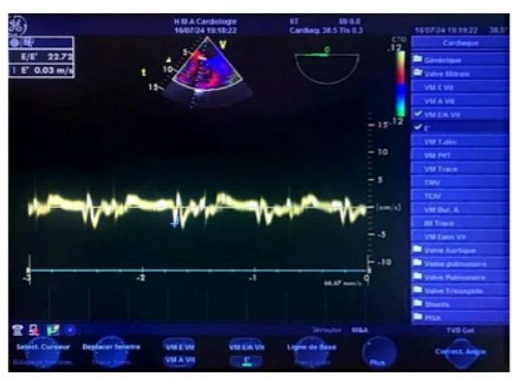
Figure 15: Transesophageal echocardiography (TOE) tissue Doppler at the mid-esophageal level in a 4-chamber view, during a transient postoperative passage in sinus rhythm. The sampling window is positioned at the septal mitral annulus, showing an Ea/Aa ratio <1, indicating impaired relaxation (normalized profile), and an E/Ea ratio of 22, suggesting significantly elevated left ventricular filling pressure.
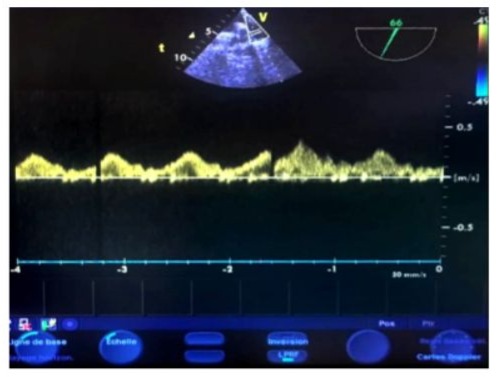
Figure 16: Transesophageal echocardiography (TOE) at the upper esophageal level, with an antih clockwise rotation and a 60° plane of section. The sampling window is positioned 1 cm before the left superior pulmonary vein confluence, showing normal pulmonary flow with a positive systolic wave (S), a positive diastolic wave, and a small retrograde negative A-wave in the late diastole.
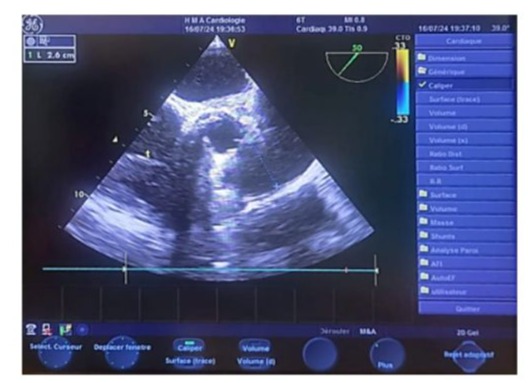
Figure 17: Biplane transesophageal echocardiography (TOE) image in the upper esophageal view centered on the aortic valve, showing a normal diameter of the pulmonary artery trunk measuring 2.6 cm.
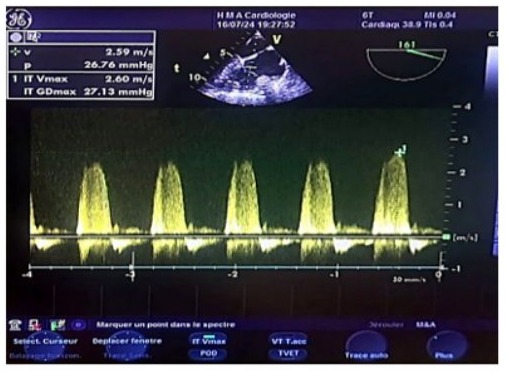
Figure 18: Continuous wave Doppler transesophageal echocardiography (TOE) image of the vena contracta in tricuspid regurgitation (reflux within the tricuspid annulus), showing a normal systolic pulmonary artery pressure estimated at 38 mmHg, with a maximum tricuspid regurgitation velocity of 2.60 m/s. The estimated pulmonary artery diastolic pressure (POD) is 10-15 mmHg, and the tricuspid gradient is 27 mmHg.
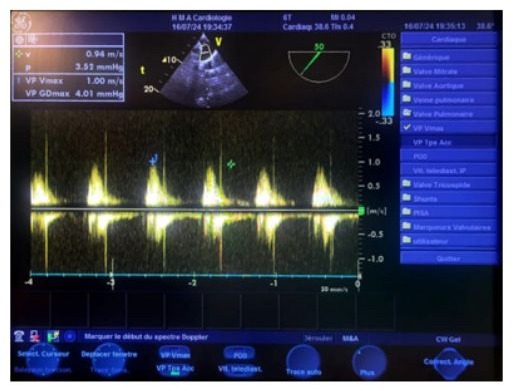
Figure 19: Transesophageal echocardiography (TOE) at the upper esophageal level, at a 50° oblique plane, with continuous Doppler pointed a few millimeters below the pulmonary valve, showing a maximum average velocity of 0.95 m/s, an average velocity of 0.56 m/s, a maximum mean gradient of 4 mmHg, an isovolumic time (IVT) of 20 cm, and the absence of any detectable pulmonary.
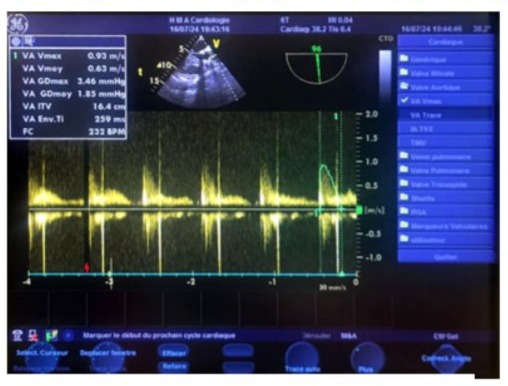
Figure 20: Transgastric continuous Doppler transesophageal echocardiography (TOE) image, at an oblique 135° plane (close to the parasternal long-axis view in transthoracic echocardiography), aligning the ultrasound beam with the aorta, showing a normal postoperative aortic isovolumic time (IVT) of 16.4 cm.
Video 1: Perioperative transesophageal echocardiography (TOE) at the mid-esophageal level at 0° with the probe in contact with the left atrium, with a slight counterclockwise axial rotation due to the upward and leftward tilt of the mitral annulus axis. The anterior mitral valve is visualized at the heart cross with the A2A1 segments of the anterior mitral valve, and the posterior valve at the junction of the lateral walls of the left ventricle and left atrium with the P1-P2 segments of the posterior mitral valve. Alignment with the mitral valve allows for precise analysis of the transmitral antegrade flow, with color Doppler showing a micro mitral regurgitation in the form of a small flame-like jet with a narrow base and very limited extension. By positioning the pulsed Doppler sampling window at the distal tips of the mitral leaflets, the postoperative sinus rhythm passage shows an E-wave velocity of 0.70 m/s (pseudo-normal negative diastolic antegrade flow), with an A-wave velocity of 0.30 m/s, an E/A ratio of 1.93 (>1.5), and a calculated deceleration time of 174 ms.
Video 2: Perioperative transesophageal echocardiography (TOE) in transventricular cuts at 0°, after positioning the probe at the lower esophageal level at the tricuspid valve, with a progressive angulation and gradual withdrawal. The inferior walls of the left ventricle are seen at the top of the screen, with their anterior walls opposite, showing preserved global and segmental biventricular contractility. The interventricular septum is thickened, measuring 2.1 cm.
Video 3: Perioperative transesophageal echocardiography (TOE) in transventricular cuts at 0°, after positioning the probe at the lower esophageal level, with progressive angulation and gradual withdrawal. This shows preserved global and segmental contractility of the left ventricle, with the inferior wall and the posteromedial papillary muscle at the top of the screen, and the anterior wall and anterolateral papillary muscle opposite.
Video 4: Perioperative transesophageal echocardiography (TOE) after positioning the probe at the transgastric level and rotating the sensor to 120°, showing the anterolateral and posteromedial papillary muscles. The image also demonstrates left ventricular hypertrophy, with preserved motion of the inferior and anterior walls.
Video 5: Perioperative transesophageal echocardiography (TOE) at approximately 30 cm from the dental arches, at a 45° to 50° plane, centered on the aortic annulus, showing free mobility of the two leaflets of the mechanical aortic valve (Saint-Jude Medical).
Video 6: Perioperative transesophageal echocardiography (TOE) after positioning the probe at the mid-esophageal level, with a slight counterclockwise axial rotation at an oblique 135° plane (equivalent to the transthoracic long-axis view), showing the functional leaflets of the mechanical aortic valve (Saint-Jude Medical), and the large mitral valve beneath the aorta with its A2 segment, while the P2 segment is located opposite.
Video 7: Perioperative transesophageal echocardiography (TOE) after positioning the probe at the mid-esophageal level with a 45° oblique rotation, showing the papillary muscles of the left ventricle and the left atrium, including the left atrial appendage. At the mitral level, the commissural view is clearly visualized, with the anterolateral commissure beneath the atrium and the posteromedial commissure opposite. The left atrial appendage is seen free of echoes.
Video 8: Perioperative transesophageal echocardiography (TOE) in a four-chamber view, after positioning the probe at the midesophageal level, 35 cm from the dental arches, centered on the right cavities. The image shows an eccentric retrograde flow on continuous Doppler, corresponding to the vena contracta of tricuspid insufficiency (reflux within the tricuspid annulus during systole).
The surgery proceeded without major complications, with a smooth weaning from CBP at a flow rate of 0.2 gamma/min/kg of norepinephrine with the start of paced rhythm, a clamping duration of 60 minutes, and an CBP duration of 95 minutes. At the end of the ECC, after heparin reversal, aortic decannulation, the ENT surgeons performed the final hemostasis of the thyroid bed after sternal and skin closure, and a drain was placed in the thyroid space. (Figure 11) At the end of the procedure, the patient was reintubated orotracheally using video laryngoscopy and transferred to the cardiovascular intensive care unit for anticoagulant therapy management and to monitor for potential hypocalcemia or complications related to the recurrent laryngeal nerves, which were absent, On the ABG, pH was 7.37 mmHg, pCO2 40 mmHg, pO2 120 mmHg, serum K+ level 3.4 mEq/L, Ca2+ level 1,30 mmol/ L. The postoperative course was favorable; the patient was extubated after 12 hours, weaned off norepinephrine, and transferred to the standard care unit the day after the procedure. (Figure 21).
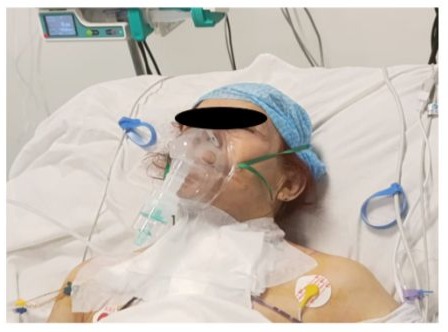
Figure 21: Photo of the patient on postoperative day 1 (J1) after extubation, hemodynamically and respiratory stable with low oxygen requirements.
Discussion
A small percentage of all goiters can migrate to the chest and are referred to as plunging thyroid goiters. Severe symptomatic aortic stenosis uniformly warrants surgical aortic valve replacement. Valvulo-arterial impedance is associated with high mortality, as it encompasses both valvular and arterial loads, making it necessary to replace the valve preceded by a preoperative assessment of the ascending aorta and aortic arch in elderly patients with both conditions. This assessment aims to determine the appropriate therapeutic strategy based on the presence or absence of thoracic aortic dilation. However, these patients have been excluded from most prospective cohorts, limiting the generalizability of the results. [10,11] This text focuses on the admission and management of SAS in a high-risk surgical patient after a timely diagnosis of plunging thyroid goiter. The current literature is mostly devoid of studies, except for single clinical case studies showing excellent postoperative evolution. Besides the planned sequential twin-operating protocols, it is the thorough preoperative assessment and requirements that determine the confidence and further promotion of such interventions. A faster functional impact may be uninterpretable, but together with the extended surgical and anesthetic timings, the principal demand underlines the benefit of timely diagnosis and combined treatment. [12] SAS and plunging thyroid goiter add complexity to the care of a highrisk surgical population. Severe calcific AS is the most common etiology of native SAS in high-risk surgical patients, while thyroid enlargement due to non-toxic multi-nodular goiter accounts for the most common thyroid enlargements seen in the elderly. The prevalence of aortic stenosis and plunging thyroid goiter increases with advancing age in patients now living longer and undergoing more non-cardiac operations for remediable pathology due to perioperative advances in immediate perioperative care. SAS is often discovered on preoperative evaluation, contributing additional surgical risk due to the increased risk of death from surgical intervention. Patients with SAS and structural valve deterioration can demonstrate the ultimate manifestations of valvular heart disease, including severe left ventricular systolic and diastolic dysfunction, a high prevalence of systemic and pulmonary hypertension, compromised arterial stretch from valvular myocardial fibrosis, diminished left ventricular stroke volume, and a limited cardiac output reserve. Complications of SAS include maintenance of systemic blood pressure and coronary perfusion pressure, fixed obstruction to left ventricular ejection, and left ventricular hypertrophy, with an increased prevalence of supraventricular and ventricular brady-dysrhythmias, pulmonary edema, and atherosclerotic coronary artery disease. [11,13]
Goiter development represents the most common neoplastic endocrine disorder, and its removal is the most common extrinsic neck operation worldwide interconnected with a range of thyroid and surgical system comorbidities and complications. [14] Enlargement of descending deep prevertebral and retrotracheal thyroid tissue masses can exacerbate the difficulties of surgical airway management. [5] Coexistence of AS and plunging thyroid goiter and the impact on perioperative hemodynamic stability are important aspects of perioperative evaluation. The presence of a severe pre- or para-tracheal thyroidectomy is highly associated with marked atherosclerotic changes of the ascending and descending aorta and coronary arteries; any available information on the thyroid and the aortic valve can help in the decision-making and the performance of the combined procedure and the selection of anesthetic techniques and hemodynamic monitoring. [11]
Severe aortic stenosis is a critical medical condition that can seriously compromise the function of the cardiovascular system. [15] Plunging thyroid goiter is a mediastinal extension of thyroid enlargement descending into the chest. It may cause significant compression impairment on distal structures of the chest, including the trachea. [16] The classic clinical presentation of aortic stenosis includes angina, dyspnea, heart failure, and relevant exertional syncope, all of which can occur individually or in different combinations. Physical examination should always include the presence of a continuous murmur, which is frequent in severe aortic stenosis, and it is caused by rapidly increased left ventricle pressure receding during the diastolic phase to the left ventricle pressure. [15] The growth rate of plunging goiter is usually slow, and remote compression or invasion of surrounding structures rarely occurs. Still, the pressure from tracheal narrowing and collapse can lead to dyspnea, and considering reducing the goiter size surgically according to the guidelines will need to be well studied. [11] In patients with huge plunging goiters and severe aortic stenosis undergoing open surgeries, an interesting point arises: “First, carry out thoracic surgery, then sternotomy for aortic valve replacement” or “First, carry out aortic valve replacement, then perform thoracic surgery in the chest via sternotomy”? or “Should thyroidectomy and sternotomy be performed simultaneously?” A comprehensive view of the heart, neck, and thorax anatomy and physiology should be mandatory in the evaluation findings to determine the appropriate medical approach on a case-by-case basis for the correct medical course. [2,6,17]
Analysis SAS in high-risk patients has been a pitfall for management because of its magnitude and implications. Integration of various parameters pertaining to the severity of stenosis and periprocedural and procedural risks is important to guide further decisions. Various imaging tools have been proposed in management and cannot actually replace each other. The different stresses and limitations imposed by these imaging modalities might hold importance in depicting the appropriate evaluation of variability between patients. In a similar fashion, a patient’s risk profile also differs widely, and thereby, a tailored approach to management is imperative with regard to the surgical management of SAS. Various surgical options have been depicted in valve replacement procedures with several efficacies and implications. A non-surgical option for the relief of SAS, which is the mainstay for today’s management scenario, can be performed by the transcatheter approach. [3,8,9]
However, certain surgical adjuncts like preoperative optimization of the patient have to be performed safely to derive improved surgical outcomes. Consequently, the aortic stenosis patient with routine presentation has a more or less algorithm-derived approach in today’s time. There might be minimal challenges in deciding about the timing of surgical relief and even in the type of surgical relief with the aid of a multidisciplinary discussion. [3] [8] Though the scenario with the presence of plunging thyroid goiter in such patients is quite gloomy, as the disease and its crooked course might not permit time for the heart team to decide the type and final venue of disease management. Thereby, it is important for the surgical team to work closely with the endocrinologist to ablate the thyroid glands preoperatively in as short a time frame as possible to avoid any further complications due to the stenosis and concomitantly release the pressure effects of the goiter before the definitive procedure for the relief of stenosis is anticipated. The patient still remains hospitalized until the final diagnosis is made and the future course of action is decided between the thyroid and cardiac teams to avoid last-minute catastrophe during hospitalization for elective routine surgery. [6,16] Many patients with severe comorbid conditions and those with a lower left ventricular ejection fraction are at a higher risk for aortic valve replacement. As such, a high-risk calculator has been developed to risk-stratify patients undergoing surgery. [17] Preoperative assessment is of utmost importance for patients planning to undergo a surgical procedure. Multidisciplinary evaluations provide detailed and comprehensive assessments of underlying cardiac problems. Stress tests, biomarkers, and imaging tests are necessary to provide in-depth assessments of the underlying condition. Cardiovascular imaging that has implications for the AVR procedure should be tailored, especially in cases of concomitant circulatory failure. The aortic valvuloplasty and TAVI Heart Team is mandatory, but a double Heart Team is necessary in cases of thyroid goiter to evaluate the indications for neo-adjuvant interventional or emergency interventional invasive thyroid embolization. [6,8,9,16] The aim of stratifying the risk is to adapt the management of the procedure itself and, much more, the management of associated comorbidities in order to optimize the patient before surgery by choosing an ideal timing that allows for better modulation of any future complications. [17] It is clear that the physical assessment and the preparation time before surgical operation and the modality of carrying out a potential tracheal tactic significantly influence the type of surgical approach. Assessment is often asymptomatic despite the severe degree; the process of preoperative patient preparation is primarily aimed at controlling comorbidities and is useful to provide preoperative training, better timing for active endoscopic posture, and obtaining informed consent in the surgical experience.
Conclusion
The study showed the necessity for trials on this coexisting pathology. High-risk surgical therapy seems to be the choice for a limited number of patients. While nowadays percutaneous Aortic Valve Implantation or Aortic Valve Intervention (AVI), typically TAVI, also known as Transcatheter Aortic Valve Replacement (TAVR). performed under local anesthesia may be a solution for these patients, another group should undergo an intraluminal or minimally invasive thyroidectomy by the cardiothoracic or general surgeon anesthetized using general inhalation or general intravenous anesthesia, particularly if hyperthyroidism is diagnosed. A careful analysis, in fact, proves that all the bad outcomes are clearly related to the postoperative course of the patient, even if the preoperative assessment by the heart team was confirmed. The management of patients preoperatively must be an overall decision between the guidelines and the philosophy of the center, therefore the heart team. We think that every high risk with the possibility to reverse the situation is also a “heart team decision”. The guideline-supported procedures for the most appropriate preoperative approach, perioperative techniques, and postoperative recovery time of the patient without or with severe assessment are also revised to support the present clinical case report.
Participants Consent
All participants (patient and her family) have given their explicit consent for the publication of personal data concerning themselves as part of this study. They understand that this data may include information that could identify them in the context of the research findings. They have been informed about the purpose of this publication, the type of data that will be disclosed, and the potential implications. They acknowledge that this information will be publicly accessible after publication. They confirm that their consent is voluntary and that they have the right to withdraw it at any time prior to publication.
Data availability statement: The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement: The studies involving humans were approved by the ethics committee of Avicenne Military Hospital, chaired by Colonel Major Dr. Radouane Niamane, Professor of Higher Education at the Faculty of Medicine and Pharmacy, Cadi Ayyad University, Marrakech, Morocco, and Head of the Rheumatology Department at Avicenne Military Hospital.The studies were conducted in accordance with the local legislation and institutional requirements. The participant provided their written informed consent to participate in this study.
Author agreement and contribution: All authors contributed to the production of this article. They also declare that they have read and approved the final version of this manuscript. Khallikane S: Conceptualization, Investigation, Methodology, Writing– original draft; Kbiri H: Curation, Formal Analysis, Writing– review and editing; Nabil M: Supervision, Writing–review and editing; Hakim Elbaraka: Funding acquisition; Writing–review and editing; Didi M: Supervision, Writing–review and editing. Dahioui A, Mounir R, Jalal H, Atmani N, Abdou A, Bouzerda A: Investigation, Validation, Writing–review and editing; Serghini I: Funding acquisition, Writing–review and editing. Belhadj A: Conceptualization, Investigation, Supervision, Writing–review; Aissaoui Y: Supervision, Guidance, Mentorship, Final review; Qamouss Y: Proposal of the clinical case idea, drafting of the article outline, Assistance in obtaining references, Supervision, and Editing
Funding: The author(s) declare that no financial support was received from any organization for the submitted work.
Acknowledgments: The authors want to express their appreciation and gratitude to the following medical professionals involved in this investigation: members of the Perfusionists’ Team (El hilali Otmane )-for their cooperation and meritorical support; members of the Anesthesia Team (Aziz Akhiri, Atif Bouhadane, Jaafari Mohamed, Lhbib) – for their contribution in obtaining images and data for this study; operating room instrument technician team members ( El hilali Otmane) – for his cooperation and support and members of the Postoperative Care Team (Abderrazzak echchahed, Azeroual Hamza, aiabbir Mohamed) - for their invaluable help in collecting samples for this investigation.
Conflict of Interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: that they have no conflicts of interest. The authors declare that the study was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. And that there are no other relationships or activities that could appear to have influenced the submitted work.
Publisher’s Note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material: The is No Supplementary Material for this article to declare.
References
- Kontogeorgos S, Thunström E, Basic C, Hansson PO, Zhong Y, et al. (2020). Prevalence and risk factors of aortic stenosis and aortic sclerosis: a 21-year follow-up of middle-aged men. Scand Cardiovasc J. 54(2):115-123.
- Mantovani F, Fanti D, Tafciu E, Fezzi S, Setti M, et al. (2021). When Aortic Stenosis Is Not Alone: Epidemiology, Pathophysiology, Diagnosis and Management in Mixed and Combined Valvular Disease. Front Cardiovasc Med. 8:744497.
- Mengi S, Januzzi JL Jr, Cavalcante JL, Avvedimento M, Galhardo A, et al. (2024) Aortic Stenosis, Heart Failure, and Aortic Valve Replacement. JAMA Cardiol.
- Unlu MT, Aygun N, Kostek M, Isgor A, Uludag M. (2022) Substernal Goiter: From Definitions to Treatment. Sisli Etfal Hastan Tip Bul. 56(2):167-176.
- Unar AA, Shamim F, Deewani MH, Wasif M, Ikram M. (2023) Successful management of a difficult airway in a case of advanced thyroid cancer. J Pak Med Assoc. 73(5):1095-1099.
- Andrzej G, Włodzimierz G, Olga J, Wojciech P, Lech A, et al. (2024) The evaluation of simultaneous combined surgery of the heart and thyroid - own experience. Journal of Education, Health and Sport. Online. 64: 162-175.
- Wee JJ, Tay KJ, Sudirman SRB. Loh SRH (2024) Total Thyroidectomy while on Extracorporeal Membrane Oxygenation for Thyroid Storm. Indian J Otolaryngol Head Neck Surg 76: 2108–2112.
- Costa GNF, Cardoso JFL, Oliveiros B, Gonçalves L, Teixeira R (2023) Early surgical intervention versus conservative management of asymptomatic severe aortic stenosis: a systematic review and metaanalysis. Heart. 109(4):314-321.
- Khokhar AA, Curio J, Sticchi A, Hartley A, Demir OM, et al. (2024) Transcatheter Aortic Valve Implantation to Treat Degenerated Aortic, Mitral and Tricuspid Bioprosthesis. J Clin Med. 2024 Jan 19;13(2):592.
- Unlu MT, Aygun N, Kostek M, Isgor A, Uludag M (2022) Substernal Goiter: From Definitions to Treatment. Sisli Etfal Hastan Tip Bul. 56(2):167-176.
- Avgoustou C, Jannussis D, Avgoustou E (2024) Evaluation of Secondary Retrosternal Goiter and Challenges in Management: A review. Anna Clin Rev Cas Rep: ACRCR-122.
- Sakr M (2024). Midline Neck Swellings: Clinical Approach. In: Midline Neck Swellings. Springer.
- Oukessou Y, Mennouni MA, Douimi L, Rouadi S, Abada RL, et al. (2021) Cervical approach to cervico-mediastinal goiters: Experience of a Moroccan ENT tertiary center - Case series. Ann Med Surg (Lond). 62:353-357.
- Gurushantappa Y, Nishanth L, Sanjay M (2020). Prevalence of nodular goiter in patients with breast diseases. Journal of Clinical and Investigative Surgery. 5: 91-95.
- Saeed S, Scalise F, Chambers JB, Mancia G (2020) Hypertension in aortic stenosis: a focused review and recommendations for clinical practice. J Hypertens. 38(7):1211-1219.
- Kwok CS, Bagur R, Rashid M, Lavi R, Cibelli M, et al. (2017) Aortic stenosis and non-cardiac surgery: A systematic review and metaanalysis. Int J Cardiol. 240:145-153.
- Pittams AP, Iddawela S, Zaidi S, Tyson N, Harky A (2022) Scoring Systems for Risk Stratification in Patients Undergoing Cardiac Surgery. J Cardiothorac Vasc Anesth. 36(4):1148-1156.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.