Study on Changes in Intestinal Flora Caused by Intake of Enterococcus faecalis kawai- in Relation to Developmental Disorders
by Satoshi Kawakami1*, Teruhisa Nemoto2, Yusuke Maeda3, Shin Shimizu4
1Kiryu University, Faculty of Health Care, Department of Nutrition, Gunma, 379-2392, Japan
2Japan Intestinal Flora Health Promotion Association, Tokyo, 152-0011, Japan
3Maeda Clinic, Okayama, 701-0205, Japan
4Shinbiosis Corporation, Senior Researcher. Osaka, 534-0025, Japan
*Corresponding author: Satoshi Kawakami, Kiryu University, Faculty of Health Care, Department of Nutrition, Gunma, 379-2392, Japan
Received Date: 17 July, 2024
Accepted Date: 03 September, 2024
Published Date: 06 September, 2024
Citation: Kawakami S, Nemoto T, Maeda Y, Shimizu S (2024) Study on Changes in Intestinal Flora Caused by Intake of Enterococcus faecalis kawai- in Relation to Developmental Disorders. J Community Med Public Health 8: 466. https://doi.org/10.29011/2577-2228.100466
Abstract
In recent years, the number of children with developmental disorders such as ASD and ADHD have been increasing. It is believed that these changes are due to changes in diet, and one of the causes is an imbalance of the intestinal flora. It is said that there is a close relationship between the intestinal flora and brain function, so if we can improve the intestinal flora, we can approach brain function. Developmental disorders reduce QOL and ADL, so it is not only a problem for the individual, but also a burden on those around them. Developmental disorders are caused by poor transmission of neurotransmitters in the brain, but it is possible that improving the intestinal flora can normalize neurotransmission by working homeostasis in the body. In this study, Enterococcus faecalis kawai was administered for 12 months to children under 10 years old who were diagnosed with developmental disorders, and clinical symptoms improved. In addition, significant improvements were observed when the balance of the intestinal flora was examined from faeces. This is the first time in the world that developmental disorders have been improved by this test substance. The fact that improvement can be seen simply by taking the supplement, without relying on intestinal flora transplantation, is thought to be beneficial as it does not cause pain to the child.
Introduction
In recent years, autism spectrum disorder (ASD) and Attention Deficit Hyperactivity Disorder (ADHD) have become a problem. These disorders affect communication skills, making it difficult for people to adapt to society. ASD and ADHD are classified as neurodevelopmental disorders [1], and symptoms include poor communication skills and difficulty responding when spoken to [2]. For this reason, ASD and ADHD are diagnostic names that encompass a continuum (spectrum) of various conditions, such as stereotypic behavior [3]. The diagnostic criteria for ASD are set out in the DSM-5 as meeting two criteria: “social communication disorder” and “restricted interests [4]. At the same time, ADHD is a neurodevelopmental disorder (developmental disorder) or behavioral disorder characterized by symptoms such as hyperactivity, impulsivity, and inattention [5]. Although these are classified as developmental disorders, the symptoms of developmental disorders vary widely, so it is necessary to provide various types of support tailored to each individual and appropriate environmental adjustment [6]. It is considered effective to provide comprehensive support that combines environmental adjustment, psychosocial support, and drug therapy in collaboration with various support organizations according to the individual’s condition [7]. In this context, one area of research that has been attracting attention in recent years is the causal relationship between intestinal flora and developmental disorders [8]. As the term braingut correlation suggests, intestinal flora also influences the central nervous system [9]. There have been reports of intestinal flora disorders in patients with developmental disorders [10]. There have also been reports of autism symptoms being alleviated by improving intestinal flora [11].
Approximately 1000 types of enterobacteria, totalling approximately 100 trillion bacteria, reside in the intestines and form the intestinal flora [12]. Disturbance in the balance of the intestinal flora is called dysbiosis, and it causes inflammation and abnormal insulin resistance through cell metabolism regulators such as AMPK and NF-κB [11]. It is believed that these abnormalities lead to a vicious cycle in which developmental disorders progress [13]. Enterococcus faecalis Kawai strain lactic acid bacteria (Enterococcus faecalis Kawai strain) was discovered among the lactic acid bacteria living in healthy humans by Dr. Yasuo Kawai while he was searching for lactic acid bacteria that are considered to be most effective in preventing the three major lifestyle-related diseases of cancer, heart disease, and cerebrovascular disease, and is a lactic acid bacterium that has been proven to be particularly effective in treating arteriosclerosis [14]. It is suggested that Enterococcus faecalis Kawai strain lactic acid bacteria may also be highly effective in improving the intestinal environment and preventing developmental disorders.
It is said that there is a close relationship between the intestinal flora and the immune system [15]. If the balance of the intestinal flora is not maintained, the immune system will be weakened, causing fungi to proliferate, resulting in inflammation, and allowing undigested proteins that should not be absorbed to enter the body through the intestinal wall. This is known as “leaky gut syndrome” [16]. Therefore, improving the intestinal flora is thought to not only improve the immune system, but also be an approach to developmental disorders.
In humans, cells are constantly dividing according to the cell cycle, and senescent cells also appear as a result. Senescent cells may increase in the body because it becomes difficult to remove them due to a decline in the immune system in the body [17]. Therefore, developmental disorders can also be seen as a problem with immune function [18]. Various immune cells act to remove senescent cells, but this effect may be weakened by a decline in immune function, which is correlated with the intestinal flora [19]. Therefore, this study was planned with the aim of elucidating the disease prevention effect of Lactobacillus faecalis Kawai strain against developmental disorders. We believe that this study will contribute to the construction of a new treatment strategy for the prevention and improvement of developmental disorders from the perspective of suppressing the progression of developmental disorders by improving the intestinal environment.
In recent years, the concept of fasting has become popular in Japan. Fasting means to fast, but it means to restrict the intake of solid food. There are types of regular fasting that are applied worldwide based on traditional, cultural, or religious backgrounds. In ancient medicine, fasting has been an established treatment since Hippocrates. Since then, it has been recommended by most old European medical schools for the treatment of acute and chronic diseases [20]. The frequency of food intake in modern people tends to have a long period of energy intake and a short fasting period, and high-calorie diets and sedentary lifestyles affect the body’s metabolism and increase the incidence of obesity, diabetes, cardiovascular disease, stroke, and dementia year by year [21]. At the same time, it is said that fasting changes the balance of the intestinal flora, so it may be useful for preventing lifestyle-related diseases such as strengthening the immune system [22]. However, since the subjects with developmental disorders studied in this study were young people, fasting was not possible. Therefore, this time, the purpose of this study was to analyze changes in the intestinal flora of young people with developmental disorders using Enterococcus faecalis Kawai strain lactobacillus.
Materials and Methods
This study received ethical approval from the International Society for Geriatrics and Gerontology Ethics Committee (ISGN_NI10032023).
Type of study: Single-blind functional evaluation
Intervention method
The subjects were aged 10 years or younger. Each subject orally ingested Lactobacillus faecalis Kawai strain for 12 months according to the dosage (※1) determined based on the subject’s weight. All nine subjects were diagnosed with developmental disorders using the Social Responsiveness Scale Second Edition (SRS-2) before and after ingestion. Two of the subjects dropped out, leaving only seven in the final sample. Stool samples were collected from the seven subjects, and intestinal flora tests were performed. In addition, the balance of intestinal flora was scored for statistical purposes and divided into five groups: A, A’, B, B’, C, C’, and D, with A: 90, A’: 80, B: 70, B’: 60, C: 50, C’: 40, and D: 30, with the minimum and maximum scores set at 30 and 90, respectively. Since the n number for these five groups was 7, a non-parametric test was performed. Based on the obtained results, the statistical analysis software IBM SPSS Statistics version 25 was used, and because there was a pairing, a Wilcoxon signed rank test was used to perform a significance test at a significance level of 5%.
(※1) Dosage and Administration
Weight 10-19 kg: 1 packet (295 mg)
Weight 20-29 kg: 2 packets (590 mg)
Weight 30-39 kg: 3 packets (885 mg)
Weight 40-49 kg: 4 packets (1180 mg)
Results
Changes in intestinal flora
As shown in Table 1 and Table 2, subjects with developmental disorders have poor intestinal flora balance. However, when the test substance was administered, the intestinal flora improved. Furthermore, when the scores were statistically processed, the result was P=0.026, which satisfied P<0.05, and a significant difference was confirmed (Figure 1).
|
Subjects |
Intestinal floral test |
|||||
|
Be |
fore |
After |
||||
|
1 |
D |
30 |
D |
30 |
||
|
2 |
D |
30 |
C |
50 |
||
|
3 |
C |
40 |
C |
50 |
||
|
4 |
D |
30 |
C |
50 |
||
|
5 |
D |
30 |
B |
60 |
||
|
6 |
D |
30 |
C |
50 |
||
|
7 |
D |
30 |
B |
70 |
||
Table 1: Changes in intestinal flora before and after administration of test substance (score).
|
Test substance |
Before |
12 months after |
p value |
|
Mean ± SD |
Mean ± SD |
||
|
Intestinal floral |
31.4 ± 3.78 |
51.4 ± 12.1 |
0.026 |
Table 2: Intestinal flora average and SD.
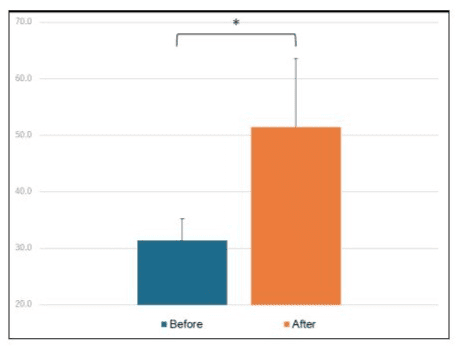
Figure 1: Intestinal flora average and SD (P<0.05).
Changes in the intestinal flora of each subject The target balance of intestinal flora is shown in Figures 2-9.
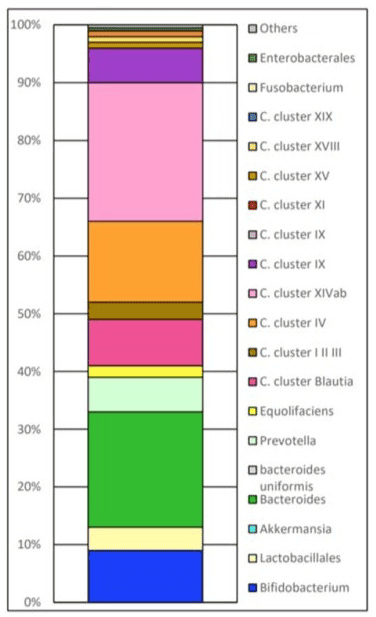
Figure 2: Target Balance.
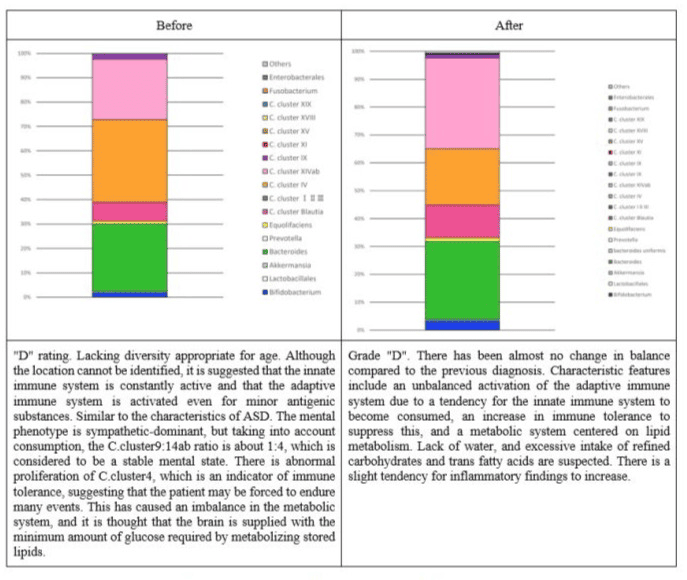
Figure 3: Changes in the intestinal flora of subject 1.
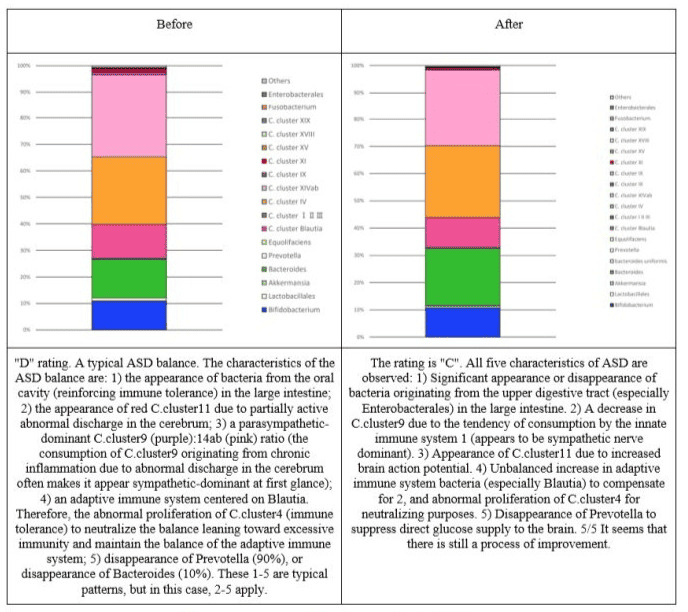
Figure 4: Changes in the intestinal flora of subject 2.
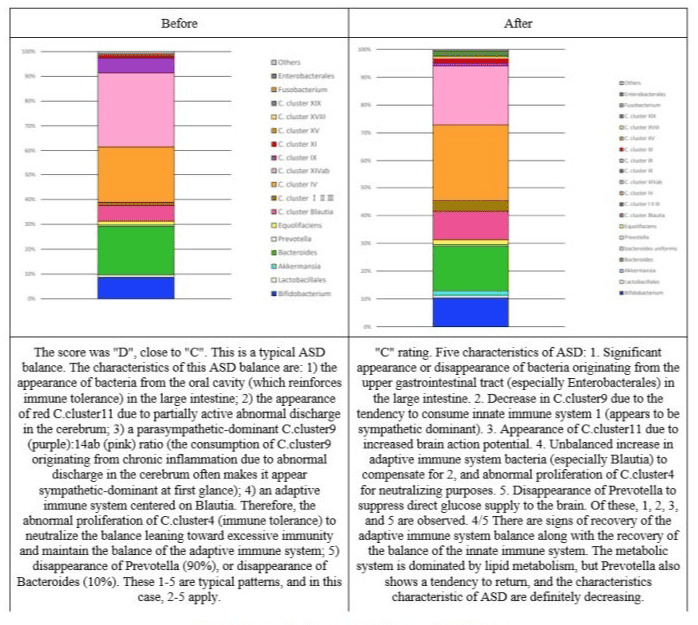
Figure 5: Changes in the intestinal flora of subject 3.
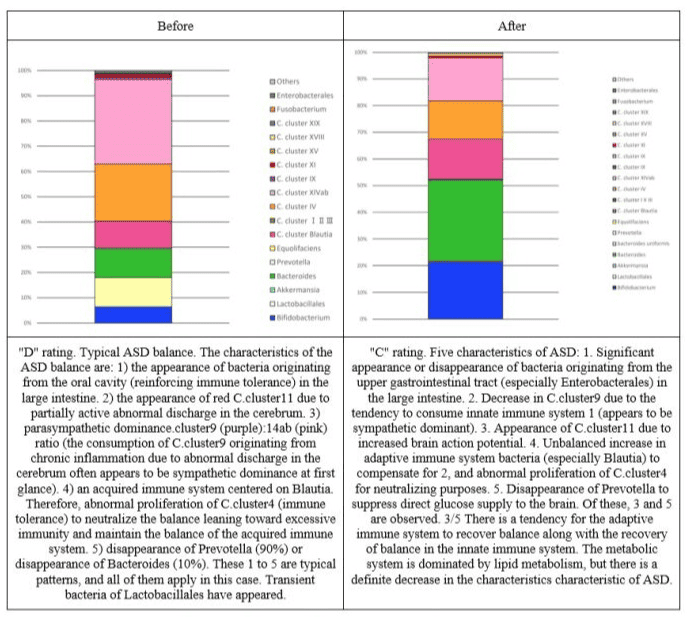
Figure 6: Changes in the intestinal flora of subject 4.
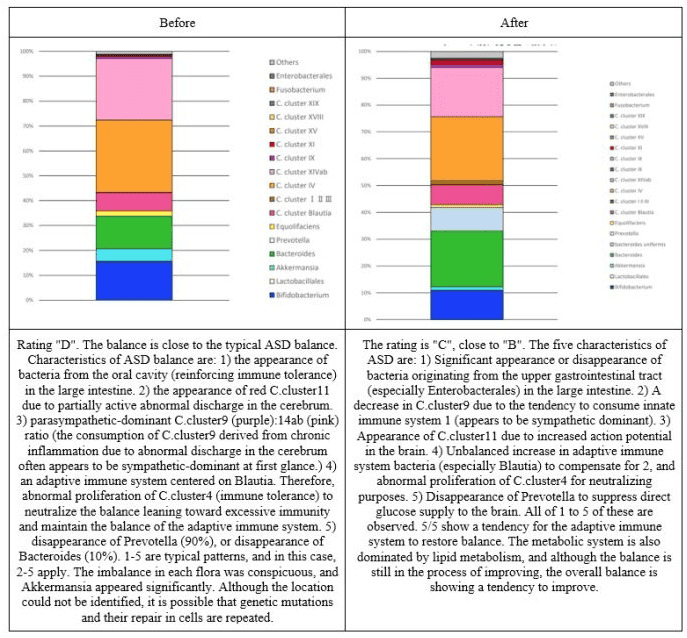
Figure 7: Changes in the intestinal flora of subject 5.
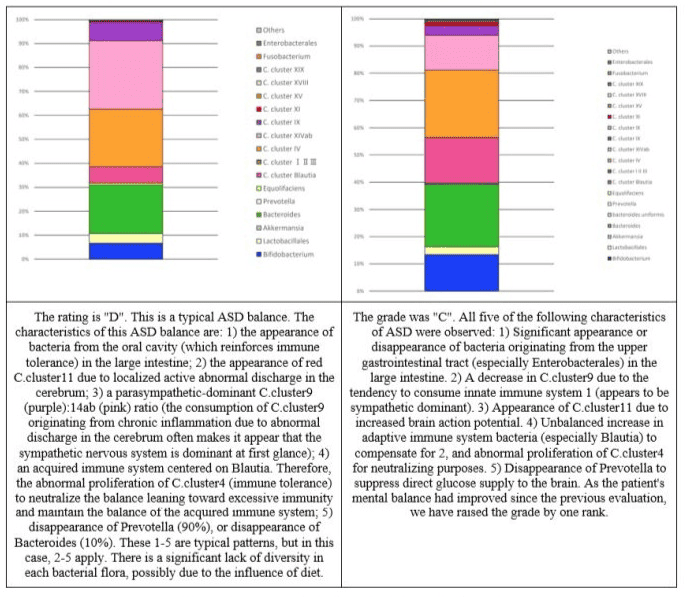
Figure 8: Changes in the intestinal flora of subject 6.
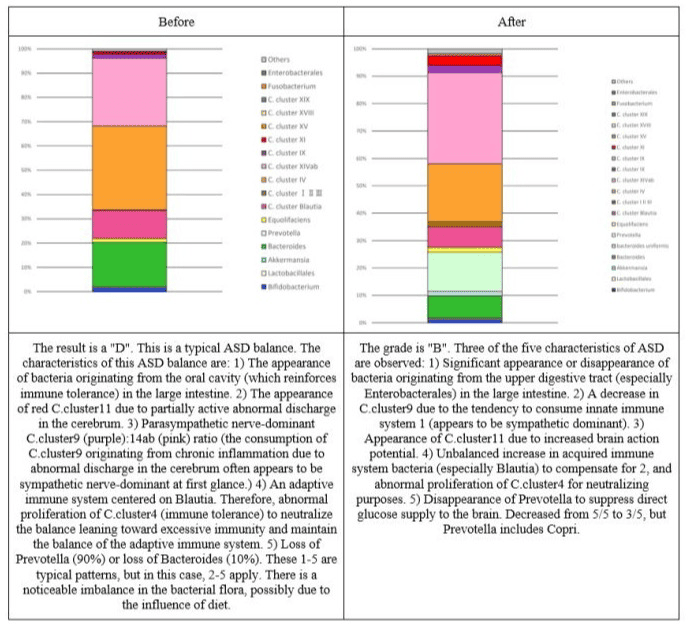
Figure 9: Changes in the intestinal flora of subject 7.
Discussion
The above results statistically confirmed the improvement of intestinal flora. It is said that there is a relationship between intestinal flora, brain function, and the immune system [23]. Since the distribution of intestinal flora is said to be abnormal in developmental disorders, [24] the fact that the improvement of intestinal flora was observed in this study suggests that the test substance may lead to the improvement of developmental disorders.
In order to treat and prevent the symptoms of developmental disorders, it is important to adjust the balance of neurotransmitters in the brain [25]. In fact, the majority of existing medicines prescribed for developmental disorders act on neurotransmitters in the brain.
There are 1,000 types of intestinal bacteria, totalling 100 trillion bacteria, present in the intestine, forming the intestinal flora [12]. The intestinal flora is known to be deeply involved in the balance of neurotransmitters in the brain, as mentioned above in the gutbrain correlation, and developmental disorders can also be viewed as a problem of the intestinal environment [26]. In fact, it is known that there are patterns of intestinal flora that are characteristic of developmental disorders [27].
Medicines prescribed for developmental disorders that act directly on the brain’s neurotransmitters have strong side effects, and there is a need to develop safer treatments [28]. Compared to medicines, improving the intestinal environment itself adjusts neurotransmitters in a natural balance and is expected to have a comprehensive effect on improving human biological functions, so it is expected to be a safe treatment that can address the root cause [29].
The test substance, Lactobacillus Faecalis Kawai strain, has many reports of improvement in digestive symptoms such as bowel movements and mental symptoms from those who have been vaccinated, and there have also been reports of improvement in symptoms in children with developmental disorders, suggesting that it may be highly effective in improving the intestinal environment and adjusting the balance of neurotransmitters. As a result, a doctor’s diagnosis was that the clinical symptoms of developmental disorders were clearly improved.
In addition, a significant increase in adiponectin was confirmed when the test substance was used, although this was not part of the current study. Adiponectin is a type of protein secreted from fat cells and is also known as a good hormone [30] and increasing the concentration of adiponectin in the blood is thought to be beneficial for the prevention and treatment of lifestyle-related diseases and cardiovascular diseases [31].
Currently, there are reports that intestinal flora transplants are being used to treat developmental disorders [32]. However, it is believed that there is a high risk in transplanting intestinal flora into children with developmental disorders. However, it is believed that intestinal flora transplants can regulate the balance of the immune system and be beneficial [33]. Therefore, it is not invasive or painful, and simply ingesting the food is a simple method for children to improve their intestinal flora, and it is thought that there is a possibility of improving the intestinal flora. Furthermore, improving the intestinal flora not only improves developmental disorders, but also activates the immune system, which is predicted to lead to the prevention and improvement of various diseases, including lifestyle-related diseases [34]. Therefore, this study is considered to be meaningful because it is predicted that improving the intestinal flora from the child stage will lead to an extension of lifespan and healthy lifespan in the future [35]. Developmental disorders are thought to be not only a problem of the intestinal flora, but also a problem of the immune system [36]. Looking to the future, this study considers that ingesting the food over a long period of time will maintain homeostasis and approach biological functions. This time, the study was conducted on children, but in addition, significant improvements were also observed in CRP, the aforementioned adiponectin, blood glucose levels, and other factors in adults. The results of this study have already been reported [37]. There are many substances that are said to improve the intestinal flora, [38] but this test substance, which approaches the intestinal flora from a multifaceted perspective, is of great significance.
Developmental disorders not only lead to a decline in QOL but also to a decline in ADL [39]. When this happens, the burden on the family increases and the home environment may change significantly. For this reason, it is urgent to improve developmental disorders. In the future, we plan to use this test substance to further improve developmental disorders, focus on the causal relationship between intestinal flora and developmental disorders, and confirm the improvement of developmental disorders by this test substance from a multifaceted perspective.
Conclusion
It is possible that this test substance improved the intestinal flora and the scale of developmental disorders. However, since the developmental disorder itself was not completely improved, we plan to continue taking this test substance and consider whether the developmental disorder is improving. If the use of this test substance can be confirmed to improve developmental disorders, it may be possible to improve them in daily life without relying on pharmaceuticals, which may be the first such report in the world. In addition, because it was found to significantly improve the intestinal flora, it was also suggested that this could lead to the prevention and improvement of lifestyle-related diseases in the future.
Acknowledgements
We would like to express our gratitude to the staff and researchers who cooperated in conducting this study.
Conflict of Interest
There are no conflicts of interest in this study.
References
- Antshel KM, Russo N (2019) Autism Spectrum Disorders and ADHD: Overlapping Phenomenology, Diagnostic Issues, and Treatment Considerations. Curr Psychiatry Rep 21: 34.
- Chen S, Zhang Y, Zhao M, Du X, Wang Y, et al. (2022) Effects of Therapeutic Horseback-Riding Program on Social and Communication Skills in Children with Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Int J Environ Res Public Health 19: 14449.
- Sharma SR, Gonda X, Tarazi FI (2018) Autism Spectrum Disorder: Classification, diagnosis and therapy. Pharmacol Ther 190: 91-104.
- Doernberg E, Hollander E (2016) Neurodevelopmental Disorders (ASD and ADHD): DSM-5, ICD-10, and ICD-11. CNS Spectr 21: 295299.
- Tan TX, Liu Y, Damjanovic V, Ledford E, Li G, et al. (2022) Inattention, hyperactivity/impulsivity, and academic competence: Findings from three cohorts. Br J Educ Psychol 92: 82-104.
- Beqiraj L, Denne LD, Hastings RP, Paris A (2022) Positive behavioural support for children and young people with developmental disabilities in special education settings: A systematic review. J Appl Res Intellect Disabil 35: 719-735.
- van der Gaag RJ, van Wijngaarden-Cremers PJ, Staal WG (2012) The challenge of staging developmental disorders. Tijdschr Psychiatr 54: 965-972.
- Valenzuela-Zamora AF, Ramírez-Valenzuela DG, Ramos-Jiménez A (2022) Food Selectivity and Its Implications Associated with Gastrointestinal Disorders in Children with Autism Spectrum Disorders. Nutrients 14: 2660.
- Naufel MF, Truzzi GM, Ferreira CM, Coelho FMS (2023) The braingut-microbiota axis in the treatment of neurologic and psychiatric disorders. Arq Neuropsiquiatr 81: 670-684.
- Famitafreshi H, Karimian M (2018) Overview of the Recent Advances in Pathophysiology and Treatment for Autism. CNS Neurol Disord Drug Targets 17: 590-594.
- Kang DW, Adams JB, Gregory AC, Borody T, Chittick L, et al. (2017) Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome 5: 10.
- Sebastián Domingo JJ, Sánchez Sánchez C (2018) From the intestinal flora to the microbiome. Rev Esp Enferm Dig 110: 51-56.
- Weiss GA, Hennet T (2017) Mechanisms and consequences of intestinal dysbiosis. Cell Mol Life Sci 74: 2959-2977.
- Kawai stocks and arteriosclerosis. (in Japanese).
- Wastyk HC, Fragiadakis GK, Perelman D, Dahan D, Merrill BD, et al. (2021) Gut-microbiota-targeted diets modulate human immune status. Cell 184: 4137-4153.e14.
- Di Tommaso N, Gasbarrini A, Ponziani FR (2021) Intestinal Barrier in Human Health and Disease. Int J Environ Res Public Health 18: 12836.
- Kowald A, Passos JF, Kirkwood TBL (2020) On the evolution of cellular senescence. Aging Cell 19: e13270.
- Ray D, Yung R (2018) Immune senescence, epigenetics and autoimmunity. Clin Immunol 196: 59-63.
- Goldberg EL, Shaw AC, Montgomery RR (2020) How Inflammation Blunts Innate Immunity in Aging. Interdiscip Top Gerontol Geriatr 43: 1-17.
- Michalsen A (2010) Prolonged fasting as a method of mood enhancement in chronic pain syndromes: a review of clinical evidence and mechanisms. Curr Pain Headache Rep 14: 80-87.
- Gill S, Panda S (2015) A Smartphone App Reveals Erratic Diurnal Eating Patterns in Humans that Can Be Modulated for Health Benefits. Cell Metab 22: 789-798.
- Patterson RE, Sears DD (2017) Metabolic Effects of Intermittent Fasting. Annu Rev Nutr 37: 371-393.
- Asadi A, Mehr NS, Mohamadi MH, Shokri F, Heidary M, et al. (2022) Obesity and gut-microbiota-brain axis: A narrative review. J Clin Lab Anal 36: e24420.
- Kang DW, Adams JB, Coleman DM, Pollard EL, Maldonado J, et al. (2019) Long-term benefit of Microbiota Transfer Therapy on autism symptoms and gut microbiota. Sci Rep 9: 5821.
- Pivina L, Semenova Y, Dosa MD, Dauletyarova M, Bjørklund G (2019) Iron Deficiency, Cognitive Functions, and Neurobehavioral Disorders in Children. J Mol Neurosci 68: 1-10.
- Kwak MJ, Kim SH, Kim HH, Tanpure R, Kim JI, et al. (2023) Psychobiotics and fecal microbial transplantation for autism and attention-deficit/hyperactivity disorder: microbiome modulation and therapeutic mechanisms. Front Cell Infect Microbiol 13: 1238005.
- Zhang L, Xu Y, Li H, Li B, Duan G, et al. (2022) The role of probiotics in children with autism spectrum disorders: A study protocol for a randomised controlled trial. PLoS One 17: e0263109.
- Charlot LR, Doerfler LA, McLaren JL (2020) Psychotropic medications use and side effects of individuals with intellectual and developmental disabilities. J Intellect Disabil Res 64: 852-863.
- Thursby E, Juge N (2017) Introduction to the human gut microbiota. Biochem J 474: 1823-1836.
- Wang ZV, Scherer PE (2018) Adiponectin, the past two decades. J Mol Cell Biol 8: 93-100.
- Iwabu M, Okada-Iwabu M, Yamauchi T, Kadowaki T (2019) Adiponectin/ AdipoR Research and Its Implications for Lifestyle-Related Diseases. Front Cardiovasc Med 6: 116.
- Caputi V, Hill L, Figueiredo M, Popov J, Hartung E, et al. (2024) Functional contribution of the intestinal microbiome in autism spectrum disorder, attention deficit hyperactivity disorder, and Rett syndrome: a systematic review of pediatric and adult studies. Front Neurosci 18: 1341656.
- Zhou B, Yuan Y, Zhang S, Guo C, Li X, et al. (2020) Intestinal Flora and Disease Mutually Shape the Regional Immune System in the Intestinal Tract. Front Immunol 11: 575.
- Lone JB, Koh WY, Parray HA, Paek WK, Lim J, et al. (2018) Gut microbiome: Microflora association with obesity and obesity-related comorbidities. Microb Pathog 124: 266-271.
- Badal VD, Vaccariello ED, Murray ER, Yu KE, Knight R, et al. (2020) The Gut Microbiome, Aging, and Longevity: A Systematic Review. Nutrients 12: 3759.
- Hou K, Wu ZX, Chen XY, Wang JQ, Zhang D, et al. (2022) Microbiota in health and diseases. Signal Transduct Target Ther 7: 135.
- Kawakami S, Nemoto T, Maeda Y (2024) Examination of Changes in Various Biomarkers by Intake of Enterococcus faecalis Kawai. Ann Clin Med Case Rep 13: 1-16.
- Markowiak P, Slizewska K (2017) Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 9: 1021.
- Chan KL, Lo CKM, Ho FK, Ip P (2019) Disability-Specific Associations with Child Health and Functioning. Int J Environ Res Public Health 16: 1024.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.