Stroke Associated with Carotid Artery Occlusion Shortly After Radiotherapy in Three Patients: A Case Series
by Alexandra Karampera1, Gianluca Costamagna2, Carl Manuata Tetaria2*, Mahmut Ozsahin3, Andreas F. Hottinger4, Sara Fontanella5, Patrik Michel2
1Stroke Center EOC, Neurology Department, Neurocentre of Southern Switzerland (NSI), Ospedale Civico, Via Tesserete 46, CH-6900 Lugano, Switzerland.
2Stroke Center, Department of Neurology, Centre Hospitalier Universitaire Vaudois and University of Lausanne, Lausanne, Switzerland.
3Department of Radiation Oncology, Department of Oncology, Lausanne University Hospital and Lausanne University, Switzerland.
4Services of Neurology and Oncology, Lausanne University Hospital and University of Lausanne, Switzerland.
5Department of histopathology, Central Institute, Valais Hospital, Sion, Switzerland.
*Corresponding author: Tetaria CM, Stroke Center, Department of Neurology, Centre Hospitalier Universitaire Vaudois and University of Lausanne, Lausanne, Switzerland.
Received Date: 23 September, 2025
Accepted Date: 30 September, 2025
Published Date: 03 October, 2025
Citation: Karampera A, Costamagna G, Tetaria CM, Ozsahin M, Hottinger AF, et al. (2025) Stroke Associated with Carotid Artery Occlusion Shortly After Radiotherapy in Three Patients: A Case Series. Int J Cerebrovasc Dis Stroke 08: 201. https://doi.org/10.29011/2688-8734.100201
Abstract
Background: Carotid stenosis is a late complication of head and neck irradiation, increasing the risk of stroke or transient ischemic attack. Long-term effects of radiotherapy on carotid vessel walls include accelerated atherosclerosis, intimal thickening, and fibrosis. Whether RT can have short-term effects on pre-existing atherosclerotic plaques favouring acute ischemic stroke remains unknown. Case Presentation: We present three patients with acute ischemic stroke due to carotid occlusion shortly after neck radiation therapy for head and neck cancer. Time from radiation therapy to stroke-onset ranged from 1 hour to 10 days. All patients presented pre-existing multiple vascular risk factors. Thus, AIS and RT may have been coincidental. However, the irradiation volume included the internal carotid artery in all three patients, raising the possibility that irradiation may have triggered acute carotid occlusion. Conclusions: We describe three patients with ischemic stroke associated with carotid artery occlusion shortly after radiation therapy for head and neck cancer. Though the association is uncertain and may be coincidental, radiation therapy may cause instability of pre-existing atherosclerotic plaques causing stroke.
Keywords: Radiotherapy; Carotid Artery Occlusion; Stroke; Head And Neck Cancer; Atherosclerotic Plaque.
List of Abbreviations:
ACA: Anterior Cerebral Artery
CT: Chemotherapy
CTA: Computed Tomography Angiography
ICA: Internal Carotid Artery
MCA: Middle Cerebral Artery
MRI: Magnetic Resonance Imaging
RT: Radiation Therapy
TIA: Transient Ischemic Attack
Background
Carotid stenosis is an important consequence of head and neck radiation therapy (RT) that usually occurs several years after exposure, increasing the risk of ischemic stroke or transient ischemic attack (TIA) [1]. Radiation induces vessel damage in two phases. First, it promotes intimal proliferation, medial fibrosis and thrombosis of the vasa vasorum. Subsequently, RT can prompt chronic inflammatory reactions [1]. The severity of vessel damage seems to be dose-dependent, and the spatial distribution of the occlusive vasculopathy corresponds to the irradiated volume [2]. Despite this close relationship between RT and stroke risk, other cardiovascular risk factors are usually present in patients with head and neck neoplasms, particularly smoking [3], making the definition of stroke mechanisms in these cases more complex. We describe three patients with ischemic stroke due to carotid occlusion - occurring shortly after neck RT - and raising the possibility of acute RT-induced endothelial damage as the underlying mechanism.
We searched the Acute STroke Registry and Analysis of Lausanne (ASTRAL) for the combination of ischemic stroke, active cancer, ongoing/recent RT, and acute carotid occlusion. For the identified patients we collected demographics, cerebrovascular risk factors and previous events, oncological and medical history, treatments, details of recent chemotherapy (CT) and RT, stroke characteristics and cerebrovascular imaging performed after the stroke onset.
Case Presentation
We identified 3 patients with atheromatous occlusion of the internal carotid artery, during or shortly after neck RT. Table 1 shows the characteristics of the three patients who fulfilled the inclusion criteria.
|
|
Patient 1 |
Patient 2 |
Patient 3 |
|
Stroke date |
22.1.2003 |
12.3.2010 |
28.08.2015 |
|
Sex, age |
Female, 87y |
Female, 57y |
Male, 68y |
|
Current stroke diagnosis |
Right MCA stroke from right ICA occlusion at its origin partially borderzone |
Right MCA borderzone stroke from right ICA occlusion at its origin |
Right MCA borderzone stroke from right ICA occlusion |
|
Vascular risk factors |
Arterial hypertension |
Arterial hypertension Hypercholesterolemia Smoking |
Arterial hypertension Atrial fibrillation Smoking Diabetes type II |
|
Other medical history |
Left MCA ischemic stroke with right hemiparesis 7y earlier, no sequelae. Aortic valve stenosis. Congestive heart failure |
Facial erysipelas with septic shock (2y). Upper vena cava syndrome (2m). Probable Takotsubo myocardiopathy (2m). |
Probable cirrhosis CHILD A (2y). Chronic alcohol abuse. Sepsis due to digestive tract infection (2y). Right parieto-occipital lacunar sequalae (brain CT in 2013 and 2015). |
|
Oncological history |
Right undifferentiated epidermoid amygdala carcinoma T2N0M0 (4m) |
Neuroendocrine small cell carcinoma of the right lung (2m); type “extensive disease “: bone and liver metastasis |
Left aryepiglottic fold keratinizing squamous cell carcinoma cT3 cN1 cM0 (3m) |
|
Previous surgery and chemotherapy |
Twice incomplete tumor resection 3m. and 1m. before stroke, limited by its proximity to internal carotid. |
3 cycles of carboplatin/etoposide, 3 weeks apart. |
7 cycles of weekly cetuximab target therapy. |
|
RT volume, dose, timing of stroke |
Cervical on the surgical resection site: right amygdala, right velum. Right ICA within the RT volume. 11x2 Gy, daily fractions till stroke. Stroke 2d after the last fraction. |
5x4 Gy to cervical spine (C1 to C7), and 1x8 Gy to dorsal spine (D12-S1). ICA bilaterally within the RT volume. TIA (left leg) 1h after end of first fraction. Stroke 5 hours after the first fraction. |
Cervical, focused on the primary tumor and ipsilateral left internal jugular lymph node (33 x 2.12 Gy) , Bilateral (33 x1.6 Gy) on selected cervical lymph node drainage areas. Both ICA within the RT volume, but more on the left. Stroke 2w after last session. |
|
Acute deficits |
Left hemiparesis, left homonymous hemianopia, left hemineglect |
Left hemiparesis and sensory deficit |
Left hemiparesis and hemineglect |
|
NIHSS on admission à 7 days. |
10 à 4 |
10 à 11 |
8 à 10 (at 48 hours) |
|
Clnical outcome
|
Died of unknown cause after 7 months at home. |
Died after 13 months from pneumonia, sepsis and and multiorgan failure |
Died of cardiorespiratory Insufficiency after 3 d. |
|
Neuroimaging |
Acute CT : Right MCA frontal and parietal stroke, partially borderzone. CTA: occlusion right ICA at its origin with calcified plaque and ring sign extending to skull base. Irregular non-stenosing plaque left ICA. No follow-up imaging. |
Acute CT: acute right anterior superficial borderzone lesion. CTA: Right ICA occlusion with ring sign extending into siphon. Embolic right ACA sub-occlusion (A2). MRI (10d) : right deep and superficial borderzone MCA stroke. MRA: right ICA occlusion at its origin. |
Acute CT: subacute right MCA stroke CTA: Right ICA occlusion |
|
Doppler |
1d: compatible with right ICA occlusion. Atheromatosis of the two carotid bifurcations |
4d: compatible with right ICA occlusion. Atheromatosis of the two carotid bifurcations. Left ICA stenosis (40%). |
3d: right ICA occlusion starting at bifurcation. ECA stenosis |
|
ORL: h: hours; d: days; m: months; ICA: internal carotid artery; MCA: middle cerebral artery; MRI: magnetic resonance imaging; CT: computed tomography; CTA: computed tomography angiography; RT: radiotherapy; ECA: external carotid artery |
|||
Table: Patients’ demographics, clinical features, medical history, imaging findings and oncological therapy.
Patient 1: A 87-year-old female patient with incomplete resection of an undifferentiated squamous-cell carcinoma of the right tonsil (pT2 cN0 cM0, R1) was hospitalized for postoperative RT. RT planning included a total dose of 66 Gy in 2 Gy/fraction over 33 sessions delivered on the right tonsil, the adjacent lymphatics as well as the vascular axis and the right velum. Two days after the 11th fraction, the patient developed acute left hemiparesis, hemianopia and hemineglect. Brain computer tomography (CT) showed a right middle cerebral artery (MCA) stroke partially in borderzone territories (Figure 1A-B). Computed tomography angiography (CTA) showed right internal carotid artery (ICA) occlusion in its extracranial tract, displaying a “ring sign” characteristic of acute carotid occlusion [4] (Figure 1C). The patient died of unknown cause after 7 months at home.
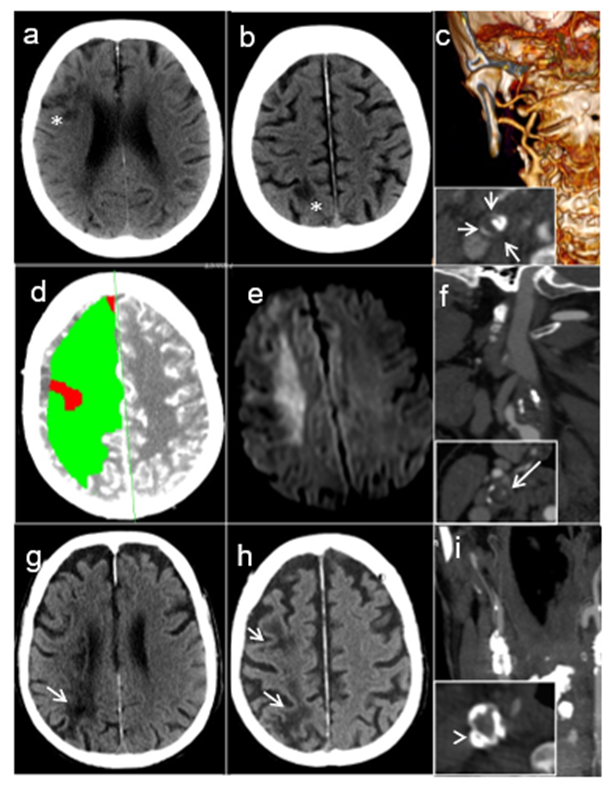
Figure 1: Brain imaging of the three patients: A-C: Patient #1: Plain CT with borderzone infarcts in the anterior (A) and posterior (B) carotid territories (white asterisks). C) Angio-CT with ICA occlusion at its origin (carotid ring sign, short white arrows). D-F: Patient #2. D) Acute perfusion CT with global hypoperfusion of the right carotid territory. B): diffusion-weighted MRI at day 10 showing restriction of diffusion consistent with a right borderzone infarction. C) Angio-CT with ICA occlusion at its origin (carotid ring sign, long white arrow). G-I: Patient #3. G ) Plain CT with borderzone infarcts in the deep (G) and superficial (H) territories (white arrows). I) Angio-CT with right ICA occlusion at its origin (annular calcified plaque in the common carotid artery, white arrowhead).
Patient 2: A 57-year-old female smoker presented recurrent, transient episodes of left extremity shaking and left hemiparesis that were not investigated. Two weeks later after one of these episodes, she presented signs of superior vena cava syndrome. Oncological work-up showed a newly diagnosed small-cell lung carcinoma with bone and liver metastases. Brain MRI displayed a right cortical lesion initially interpreted as a metastasis. She started levetiracetam for presumed focal seizures. She underwent three cycles of palliative chemotherapy with cisplatin and etoposide. Nine days after the last administration, she received RT on cervical metastasis to prevent spine instability. A total dose of 20 Gy in 4 Gy/fraction was planned, but one hour after the first fraction, the patient presented a transient left leg paresis, followed five hours later by progressive left hemiparesis. Her palliative RT was completed. Brain CT showed an acute right MCA borderzone stroke (Figure 1D) confirmed ten days later by MRI (Figure 1E). Acute CTA showed right ICA with a ring sign, suggesting occlusion (Figure 1F). Retrospectively, the limb shaking, and right cortical lesions were interpreted as athero-embolic ischemic events from a preexisting right carotid stenosis. The patient died 3 months after stroke due to community-acquired pneumonia complicated by acute respiratory failure and septic shock.
Patient 3: A 68-year-old male active smoker with a history of arterial hypertension, diabetes, chronic alcohol abuse, was diagnosed with a left aryepiglottic keratinizing squamous cell carcinoma (cT3 cN1 cM0) after a routine CT-scan performed as part of liver cirrhosis follow-up. Brain CT showed a chronic minor right posterior borderzone stroke and further investigations with CTA displayed a 90% calcified asymptomatic stenosis at the origin of the right internal carotid. He received RT with a total dose of 69.36 Gy in 33 fractions of 2.12Gy on the primary tumor and internal jugular lymph node, as well as 52.8Gy in 33 fractions of 1.6 Gy on the cervical lymph node drainage areas bilaterally over 6 weeks. He received immunotherapy with cetuximab during the same period. Ten days after the last RT session and 16 days after the last immunotherapy administration, he presented with progressive left hemiparesis and hemineglect. CT and CTA demonstrated a subacute right borderzone MCA stroke and an internal carotid occlusion (Figure 1 G-H). ECG showed new-onset atrial fibrillation. 72 hours upon admission, the patient deceased due to aspiration pneumonia with sudden cardiordiorespiratory arrest. Autopsy demonstrated a right carotid artery thrombus (Figure 2A) and a left carotid artery atheromatous plaque with no signs of inflammation in the carotid walls (Figure 2B).
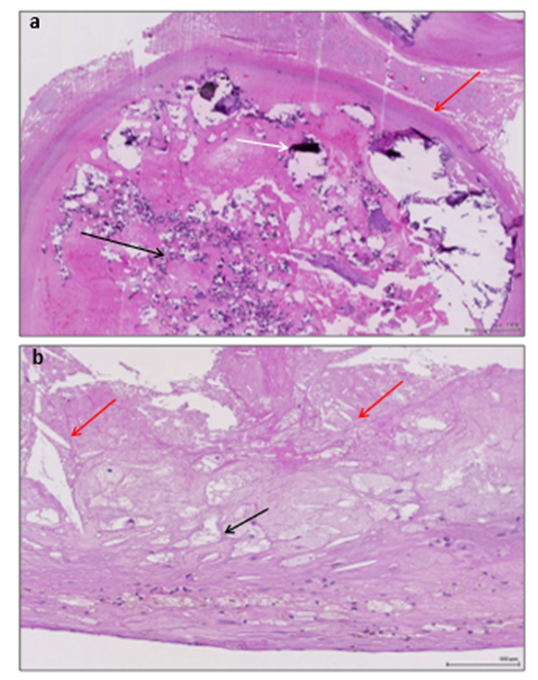
Figure 2: Post-mortem findings of the internal carotid arteries of patient 3. A: Histology of the right (occluded, symptomatic) internal carotid artery showing the thrombus (red arrow) with severe calcifications (black arrows). B: Histology of the left (asymptomatic) internal carotid - included in the irradiation territory - showing an atherosclerotic plaque with lipid-laden foam cells (black arrow) and cholesterol crystals (red arrows).
Discussion
We describe three patients with several vascular risk factors who presented ischemic stroke due to an acute carotid occlusion temporally related with local RT for head and neck cancer. Radiological findings confirmed preexisting local atherosclerosis in all patients, whereas post-mortem results displayed carotid atherosclerotic plaques in patient #3. The underlying physiopathological mechanisms are unknown, but the close temporal relationship suggests that RT may have promoted acute vascular damage in atherosclerotic vessels.
Carotid stenosis as a long-term consequence of head and neck RT is well described [5]. Proposed mechanisms include both direct and indirect effects of RT. Direct effects encompass irradiation damage of the endothelium leading to platelet and macrophage accumulation as well as intimal thickening, medial necrosis and fibrosis on the vessel wall. Indirect effects include the thrombosis of the vasa vasorum leading to adventitia thrombosis and fibrosis. In addition, a persistent upregulation of pro-inflammatory cytokines causing chronic inflammatory changes to the vessel wall seems to lead to the clinically relevant vascular stenosis [1, 2, 5, 6]. Hypothetically, though not present in our case, endothelial damage and inflammatory activation may occur already in the acute stage of RT [1, 5]. However, the exact timing from RT-induced damage to disease onset remains unknown [7]. To our knowledge, all previously reported cases of carotid artery stenosis or occlusion occurred several years after radiation exposure. Brandt-Zawadzki et al. reported one case of left MCA and anterior cerebral artery (ACA) stenosis four months after RT in a patient treated for ependymoma of the fourth ventricule [8].
Our patients presented an acute internal carotid occlusion resulting in a borderzone stroke, during or very shortly after RT, after having received 22 Gy (11x2 Gy), 20 Gy (5x4 Gy) and 52.8 Gy (33x 1.6 Gy) respectively. Cardiovascular risk factors were present in all patients, and Doppler showed bilateral atheromatosis. These findings suggest that all our patients had preexisting moderate to severe atherosclerosis related to traditional risk factors; furthermore, patient #2 had already suffered from TIA and minor strokes distal to the later carotid occlusion before RT was started. In patient #3 post-mortem findings showed no signs of vessel wall inflammation, a lipid plaque in the left carotid artery and a severely calcified thrombus and atherosclerotic plaque in the right (occluded, symptomatic) carotid artery.
Our cases are open to different interpretations. First, acute RT on the endothelium covering the preexisting atherosclerotic plaque may have prompted cell apoptosis and destabilized the plaques. Indeed, in a murine model, a single dose of RT induced early apoptosis of endothelial cells regardless of the dose with a peak at about 12 hours after administration [9]. Second, though lacking in the histopathological findings of patient 3, RT may have caused local acute intra-plaque inflammation - as shown in vitro and in vivo studies [5] - with subsequent plaque destabilization in patient 1 and 2. As a third possibility, stroke may have been coincidental.
In summary, we describe three patients with acute stroke from atherosclerotic carotid occlusion that occurred shortly after local RT. Although we cannot rule out the possibility of stroke being coincidental to RT, the potential of RT destabilizing vulnerable plaques should warrant as careful planning as possible of the irradiation volumes and territories in patients known to be at risk.
Declarations:
Ethics approval and consent to participate/ for publication:
This short case series presents clinical and radiological data from routine clinical use. For this reason, and given that data are presented in a non-identifiable format, there was not no need for a local ethical commission approval according to the local and national laws. Furthermore, given that all described patients have died more than 5 years before the finalization of this publication, obtaining written consent from next of kin would not be feasible.
Availability of Data and Materials:
The anonymized dataset used and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing Interest:
The authors declare that they have no competing interests
Funding: None
Authors' Contributions:
AK carried out the patient search in the database, interpreted the data and wrote the manuscript. GC interpreted the data and wrote the manuscript. All authors read and approved the final manuscript.
Acknowledgements: Melanie Price Hirt, PhD, for English language correction and editing.
References
- Plummer C, Henderson RD, O’Sullivan JD, Read SJ (2011) Ischemic stroke and transient ischemic attack after head and neck radiotherapy: a review. Stroke 42: 2410-8.
- Zou WX, Leung TW, Yu SC, Wong EH, Leung SF, et al. (2013) Angiographic Features, Collaterals, and Infarct Topography of Symptomatic Occlusive Radiation Vasculopathy: A Case-Referent Study. Stroke 44: 401-406.
- Pelucchi C, Gallus S, Garavello W, Bosetti C, La Vecchia C (2008) Alcohol and tobacco use, and cancer risk for upper aerodigestive tract and liver. Eur J Cancer Prev 17: 340-344.
- Michel P, Ntaios G, Delgado MG, Bezerra DC, Meuli R, et al. (2012) CT angiography helps to differentiate acute from chronic carotid occlusion: the “carotid ring sign”. Neuroradiology 54: 139-46.
- Weintraub NL, Jones WK, Manka D (2010) Understanding Radiation-induced vascular disease. J Am Coll Cardiol 55: 1237-9.
- Russell NS, Hoving S, Heeneman S, Hage JJ, Woerdeman LA, et al. (2009) Novel insights into pathological changes in muscular arteries of radiotherapy patients. Radiother Oncol 92: 477-83.
- Steele SR, Martin MJ, Mullenix PS, Crawford JV, Cuadrado DS, et al. (2004) Focused high-risk population screening for carotid arterial stenosis after radiation therapy for head and neck cancer. Am J Surg 187: 594-598.
- Brant-Zawadzki M, Anderson M, DeArmond SJ, Conley FK, Jahnke RW (1980) Radiation-induced large intracranial vessel occlusive vasculopathy. AJR Am J Roentgenol 134: 51-55.
- Peña LA, Fuks Z, Kolesnick RN (2000) Radiation-induced apoptosis of endothelial cells in the murine central nervous system: protection by fibroblast growth factor and sphingomyelinase deficiency. Cancer Res 60: 321-327.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.